Published online Mar 28, 2015. doi: 10.5320/wjr.v5.i1.34
Peer-review started: September 20, 2014
First decision: November 27, 2014
Revised: December 15, 2014
Accepted: January 15, 2015
Article in press: January 17, 2015
Published online: March 28, 2015
Processing time: 184 Days and 19.1 Hours
Chronic obstructive pulmonary disease (COPD) is characterized by the presence of airflow limitations that are not fully reversible and is a major cause of chronic morbidity and mortality worldwide. Although there has been extensive research examining the molecular mechanisms underlying the development of COPD, there is no proven clinically effective treatment for promoting recovery from established COPD. At present, regeneration is the only hope for a cure in patients with COPD. In this article, we review current treatments for COPD, focusing particularly on recent advances in lung regeneration based on two major approaches: regeneration-promoting agents and cell therapy. Retinoic acids are the major focus among regeneration-promoting agents, while mesenchymal stem cells are the main topic in the field of cell-based therapy. This article aims to provide valuable information for developing new therapies for COPD.
Core tip: There is currently no proven clinically effective treatment for achieving recovery from established emphysema. At present, regeneration is the only hope for a cure in patients with chronic obstructive pulmonary disease (COPD). In this article, we review current treatments for COPD, focusing particularly on recent advances in lung regeneration. This article aims to provide valuable information for developing new therapies for COPD.
- Citation: Fujita M. New therapies for chronic obstructive pulmonary disease, lung regeneration. World J Respirol 2015; 5(1): 34-39
- URL: https://www.wjgnet.com/2218-6255/full/v5/i1/34.htm
- DOI: https://dx.doi.org/10.5320/wjr.v5.i1.34
Chronic obstructive pulmonary disease (COPD) is a slowly progressive respiratory disease characterized by irreversible airflow limitations[1-3]. COPD is a major cause of morbidity and mortality worldwide and is COPD primarily characterized by two distinctive criteria: chronic bronchitis and pulmonary emphysema. Chronic bronchitis results from the inhalation of toxic particles, gases and/or cigarette smoke, which subsequently produces a cough and sputum. Pulmonary emphysema is defined as enlargement of the distal airspace due to destruction of the airway wall[4]. Cigarette smoking is the main etiological factor in this condition, although only 10% to 20% of smokers develop clinically significant COPD. Factors associated with the degree of susceptibility to COPD are considered to be responsible for this variation[5]. Although there has been extensive research examining the molecular mechanisms underlying the development of emphysema, the clinical management of patients with pulmonary emphysema remains mostly supportive. In addition, there is currently no proven clinically effective treatment for achieving a recovery from established emphysema[6,7].
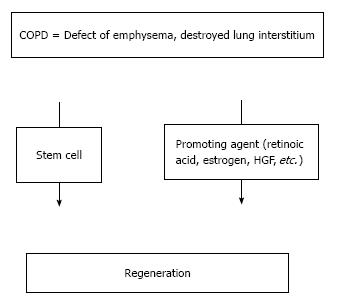
At present, regeneration of the lungs provides the only hope for a cure for COPD. Methods to promote lung regeneration have the potential to alter the natural history of COPD. Such methods include the use of retinoids and mesenchymal stem cell therapy (Figure 1). All-trans-retinoic acid (ATRA) has been reported to rescue the lungs in rats with elastase-induced emphysema and was the first agent reported to promote lung regeneration in a model of emphysema[8]. Recently, stem cell therapy was shown to promote lung regeneration. In this article, we review current treatments for COPD, focusing particularly on recent advances associated with lung regeneration.
Some treatments can be used to control the symptoms and/or sometimes slow the progression of COPD. However, unfortunately, the symptoms of COPD cannot be completely relieved with currently available treatments and typically progresses gradually. One of the most important treatments for COPD is for current smokers to stop smoking.
Bronchodilators are the mainstay of treatment for COPD. These drugs open the airways and decrease sputum production; inhaled bronchodilators are usually administered. There are several types of bronchodilators: short-acting beta agonists and anticholinergics are used to treat mild COPD, while long-acting treatments, such as long-acting beta agonists, anticholinergics or a combination of these agents, are often recommended in cases of moderate COPD. Theophylline can be given orally, and inhaled glucocorticoids may be used for frequent COPD exacerbation and/or in patients with bronchial asthma. In cases of advanced COPD, patients exhibiting hypoxemia are often supplied oxygen therapy, which may improve their survival and quality of life. Pulmonary rehabilitation programs are also important and can be effective in relieving shortness of breath. Lung volume reduction surgery and/or lung transplantation are performed in selected patients with COPD, and vaccination against the flu and pneumococci is also recommended[7].
Novel therapies are currently being developed for COPD. Smoking cessation is fundamental, and new treatments in this field include antinicotine vaccines, cannabinoid receptor antagonists and dopamine D3 receptor antagonists. Anti-inflammatory drugs are also in development to reduce airway inflammation, including kinase inhibitors, chemokine receptor antagonists, innate immune mechanism inhibitors and statins. Antioxidants, mucolytics, antiproteases and antifibrotics are all under active development as well[9]. However, these new treatments are still considered to be insufficient to completely cure COPD, since they are merely modifications of previously established therapies. Nevertheless, lung regeneration may make it possible for damaged lung tissue to recover, eventually becoming healthy.
Regeneration is the process of replacing, engineering or regenerating human cells, tissues or organs to restore or establish a normal function. Approaches to aid in lung regeneration in patients with COPD aim to correct the defects associated with emphysema and replace the destroyed lung interstitium. However, inducing regeneration in the lungs has proven to be difficult, as these organs display a complex three-dimensional system. Moreover, the lungs consist of more than 40 different cell types.
In experimental animal models, the degree of lung recovery depends on species and age. Generally speaking, small animals show more capacity for healing than large animals[10]. For example, the weight of the remaining lung doubles within 14 d after pneumonectomy in rats[11], whereas a period of 28 d 5 mo period is needed in dogs[13]. Age is another important factor affecting the capacity for lung recovery. Kenzaki et al[14] implanted fetal lung tissue fragments into adult rat lungs and found regeneration by the fetal lung tissue, but not the adult lung tissues.
Human lungs have a regenerative capacity, as demonstrated in Nepalese children given maternal vitamin A supplements[15]. In addition, lung regeneration was observed in an adult patient treated with pneumonectomy[16]. The possibility of lung regeneration in cases of COPD has been sought in several settings[17,18]. For example, Butler demonstrated a case in which the patient showed an increase in vital capacity after undergoing resection of lung cancer. The authors hypothesized that, reminiscent of the role of stretching in lung development[16,19], cyclic stretching may be an important trigger for new lung growth. These findings suggest that new lung growth may occur in adult humans.
At present, regeneration is possible. In fact, there are two treatments for inducing regeneration in the lungs: regeneration-promoting agents, such as retinoic acid, and cell therapy, such as that using stem cells.
Several lines of evidence support the concept that alveolar repair, including the formation of a new alveolar wall, is possible in adult mammals. ATRA has been reported to recover elastase-induced emphysema[8] based on results showing the attenuation of alveolar destruction and increases in the number of alveoli. RA is known to play a variety of roles in embryonic branching morphogenesis[20] and is required for the formation of normal alveoli and alveolar elastic fibers in mice[21] as well as elastin synthesis[22]. However, the degree of RA-induced lung regeneration is dependent on age. We previously reported that RA does not produce alveologenesis in adult mice[23,24]. Aging is one cause of the discrepancies observed among different studies employing RA administration.
In addition, the results of a clinical trial of the efficacy of ATRA in the treatment of COPD were recently reported in which a double-blind placebo-controlled clinical trial of ATRA was performed in patients with moderate to severe COPD[25,26]. Notably, ATRA reduced the MMP-9 level and increased the TIMP-1 level, resulting in modulation of the protease/antiprotease balance in COPD patients[27]. However, neither physiologic nor CT measurements changed appreciably in response to the therapy. The REPAIR (Retinoid treatment of Emphysema in Patients on the Alpha-1 antitrypsin International Registry) trial was an investigator-initiated, double-blind, placebo-controlled randomized study performed to assess the safety and efficacy of a selective agonist of the gamma-type retinoic acid receptor in emphysema patients with alpha-1 antitrypsin deficiency. However, no significant treatment differences were found in most of the functional parameters[28]. The TESRA (Treatment of Emphysema with a Selective Retinoid Agonist) study, another trial using a retinoid agonist in COPD patients, was recently conducted and found that the administration of palovarotene, a retinoic agonist, significantly reduced the decline in DLco and FEV1 in patients with lower lobe emphysema[29]. The results of these clinical studies indicate that retinoic acid treatment does not result in any improvements in human COPD patients.
Although estrogens are considered to be responsible for sexual development, their effects beyond the reproductive system are becoming increasingly recognized. A recent study demonstrated that estrogen has a regulatory role in alveolar formation in which estrogen receptor-alpha and estrogen receptor-beta are required for the formation of a full complement of alveoli in female mice. This loss of alveoli may be reversed by estrogen replacement[30], and estradiol replacement slows the rate of decline in the lung function in females with COPD[31].
Adrenomedullin (AM) was initially identified to be a vasodilator. It is found in many tissues, including airway basal cells and type II cells of the lungs[32] and promotes angiogenesis in addition to having protective effects on the cardiovascular and respiratory systems[33]. AM antagonists decrease the lung capillary density and impair alveolar development, whereas AM attenuates arrested lung angiogenesis[34]. Furthermore, treatment with AM improves elastase-induced emphysema in mice[35] and AM has recently been shown to be effective for treating pulmonary hypertension in humans[36,37].
Although hepatocyte growth factor (HGF) is a growth factor for hepatocytes in vitro[38], it also stimulates type II cells and endothelial cells both in vitro and in vivo in the lungs[39,40]. Intranasal treatment with HGF reverses the physiological and morphometric changes associated with lung emphysema in mice[41].
Granulocyte colony-stimulating factor (G-CSF) stimulates the bone marrow to produce granulocytes and stem cells. Treatment with G-CSF promotes recovery of the exercise capacity and regeneration of alveolar structural alterations in emphysematous mice[42]. However, G-CSF has no effect in a dexamethasone model of alveolar insufficiency[43].
Keratinocyte growth factor (KGF), also known as fibroblast growth factor-7, favorably influences alveolar maintenance and repair and possesses anti-inflammatory properties. The administration of KGF before, but not after, treatment protects against elastase-induced pulmonary inflammation, MMP activation, alveolar cell DNA damage and subsequent emphysema in mice[44].
The 3-hydroxy-3 methyl glutaryl coenzyme A reductase inhibitor, simvastatin has been shown to reverse emphysema in adult mice with elastase-induced emphysema, with a reduction in the mean linear intercept[45]. However, a human clinical study did not demonstrate any beneficial effects on the acute exacerbation of COPD[46].
Stem cells (SC), including mesenchymal stem cells (MSCs), and embryonic stem (ES) cells, are mainly used for regeneration as cell therapy. Mouse embryonic stem cells differentiate into alveolar type II cells following endotracheal injection[47]. Alveolar type II cells are important because they differentiate into alveolar epithelial type I cells in damaged lungs and are successfully derived from human ES cells[48]. The use of induced pluripotent stem (iPS) cells is also hopeful in areas of regeneration, similar to human ES cells.
MSCs are stromal cells that can be readily attained from adult bone marrow and adipose tissue in addition to umbilical cords. MSCs have been shown to be capable of differentiating into a variety of cell types, including endothelial, epithelial and neuronal cells as well as adipocytes, depending on the culture conditions[18,49]. MSCs are able to proliferate and migrate to sites of injury and can differentiate into a variety of cell types in the lungs, including type I and type II pneumocytes and myofibroblasts[50-52]. Human amniotic fluid stem cells are produced in response to lung damage in order to express specific alveolar versus bronchiolar epithelial cell lineage markers, such as thyroid transcription factor 1, surfactant protein C and Clara cell 10-kDa proteins[53]. Since the rates of engraftment obtained using exogenous MSCs are too low to achieve cellular replacement of damaged tissue[54,55], the paracrine effect of MSCs is now suggested to be the major mechanism of action. The administration of MSCs results in anti-inflammatory and immunomodulatory activities both in vitro and in vivo. These anti-inflammatory effects are mediated by transforming growth factor beta, prostaglandin E2, interleukin 10 and indoleamine 2; 3-dioxygenase[49,56]. MSCs also have immunomodulatory effects via the inhibition of T-cell and B-cell proliferation, natural killer cell and cytotoxic T lymphocyte activation and antigen-presenting functions[49,56].
A clinical trial to assess COPD regeneration using MSCs was recently conducted in which the safety and efficacy of an IV preparation of allogeneic MSCs (Prochymal) was evaluated[57]. Although there were no significant differences in lung function parameters, the levels of C-reactive protein, an indicator of systemic inflammatory responses, were decreased in some subjects. Another approach applying MSCs is to populate a biological connective tissue scaffold, which can then be used to grow autologous tissue prior to surgical implantation[58].
Research on lung-specific stem cells is also ongoing. Alveoli consist of many type I epithelium cells and a small amount of type II epithelium cells; type II epithelium cells differentiate into type I epithelium cells. Damage to type II epithelium cells has been reported in lungs exhibiting COPD[59,60], and the intratracheal instillation of type II cells attenuates bleomycin-induced fibrosis in rats[61]. In a previous study, progenitor cells were isolated from adult human lungs with the ability to differentiate into alveolar type II cells[62]. In addition, the role of endothelial progenitor cells (EPCs) in the pathogenesis of pulmonary hypertension has been investigated[63]. The use of these lung-specific cell approaches is hopeful, as endothelial dysfunction and reduced levels of circulating EPCs are observed in COPD patients[64,65].
All tissues and organs are made up of cells and the associated extracellular matrix- a secreted product of the resident cells consisting of a unique tissue-specific three-dimensional environment containing structural and functional molecules. Due to the complex three-dimensional architecture and structure-function relationships observed in the lungs, as well as the large number of differentiated cell types present in lung tissues, ex-vivo lung bioengineering is expected to be a difficult task compared with bioengineering of the trachea or larynx.
Hence, a number of trials involving decellularization, recellularization, biomechanical stabilization and implantation approaches for application in the lungs are under investigation[66,67].
At present, the investigation of many regenerative approaches is underway, although none of these therapies are able to recover functional impairments in COPD patients. Several breakthroughs are clearly needed, especially with respect to rebuilding the three-dimensional organ architecture and identifying lung stem cell populations. It is necessary to clarify the inflammatory processes underlying the modulation of inflammation and promotion of tissue repair, and care should be taken to address issues regarding the optimal source, methodology, route and timing of administration as well as costs[10,49]. We are hopeful that these problems will be overcome in the future and that therapies promoting lung regeneration will make it possible for patients to recover from COPD.
We appreciate the assistance of Dr. Brian Quinn in editing the use of the English language.
P- Reviewer: Abdelmobdy Abdelrahim ME, Boots R, Pereira-Vega A S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2626] [Cited by in RCA: 2765] [Article Influence: 125.7] [Reference Citation Analysis (0)] |
| 2. | Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. |
| 3. | Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 859] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 4. | Snider GL, Kleinerman LJ, Thurlbeck WM, Bengali ZH. The definition of emphysema. Report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases workshop. Am Rev Respir Dis. 1985;132:182-185. [PubMed] |
| 5. | Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278:1355-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 865] [Cited by in RCA: 888] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 6. | Hogg JC. A stimulating treatment for emphysema. Nat Med. 1997;3:603-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Ferguson GT, Make B. Management of stable chronic obstructive pulmonary disease. Waltham, MA: UpTo Date 2014; . |
| 8. | Massaro GD, Massaro D. Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats. Nat Med. 1997;3:675-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 374] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 9. | Ross CL, Hansel TT. New drug therapies for COPD. Clin Chest Med. 2014;35:219-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Kubo H. Concise review: clinical prospects for treating chronic obstructive pulmonary disease with regenerative approaches. Stem Cells Transl Med. 2012;1:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Nijjar MS, Thurlbeck WM. Alterations in enzymes related to adenosine 3’,5’-monophosphate during compensatory growth of rat lung. Eur J Biochem. 1980;105:403-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Cowan MJ, Crystal RG. Lung growth after unilateral pneumonectomy: quantitation of collagen synthesis and content. Am Rev Respir Dis. 1975;111:267-277. [PubMed] |
| 13. | Hsia CC, Herazo LF, Fryder-Doffey F, Weibel ER. Compensatory lung growth occurs in adult dogs after right pneumonectomy. J Clin Invest. 1994;94:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Kenzaki K, Sakiyama S, Kondo K, Yoshida M, Kawakami Y, Takehisa M, Takizawa H, Miyoshi T, Bando Y, Tangoku A. Lung regeneration: implantation of fetal rat lung fragments into adult rat lung parenchyma. J Thorac Cardiovasc Surg. 2006;131:1148-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | Checkley W, West KP, Wise RA, Baldwin MR, Wu L, LeClerq SC, Christian P, Katz J, Tielsch JM, Khatry S. Maternal vitamin A supplementation and lung function in offspring. N Engl J Med. 2010;362:1784-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 16. | Butler JP, Loring SH, Patz S, Tsuda A, Yablonskiy DA, Mentzer SJ. Evidence for adult lung growth in humans. N Engl J Med. 2012;367:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 17. | Rennard SI, Wachenfeldt Kv. Rationale and emerging approaches for targeting lung repair and regeneration in the treatment of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2011;8:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Hind M, Maden M. Is a regenerative approach viable for the treatment of COPD? Br J Pharmacol. 2011;163:106-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Brody JS, Thurlbeck WM. Development, growth, and aging of the lung. Handbook of physiology - the respiratory system: mechanics of breathing. Bethesda, MD: American Physiological Society 1986; 355-386. |
| 20. | Malpel S, Mendelsohn C, Cardoso WV. Regulation of retinoic acid signaling during lung morphogenesis. Development. 2000;127:3057-3067. [PubMed] |
| 21. | McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, Snyder JM. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol. 2000;23:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 154] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Liu B, Harvey CS, McGowan SE. Retinoic acid increases elastin in neonatal rat lung fibroblast cultures. Am J Physiol. 1993;265:L430-L437. [PubMed] |
| 23. | Fujita M, Ye Q, Ouchi H, Nakashima N, Hamada N, Hagimoto N, Kuwano K, Mason RJ, Nakanishi Y. Retinoic acid fails to reverse emphysema in adult mouse models. Thorax. 2004;59:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Fujita M, Nakanishi Y. The pathogenesis of COPD: lessons learned from in vivo animal models. Med Sci Monit. 2007;13:RA19-RA24. [PubMed] |
| 25. | Mao JT, Goldin JG, Dermand J, Ibrahim G, Brown MS, Emerick A, McNitt-Gray MF, Gjertson DW, Estrada F, Tashkin DP. A pilot study of all-trans-retinoic acid for the treatment of human emphysema. Am J Respir Crit Care Med. 2002;165:718-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Roth MD, Connett JE, D’Armiento JM, Foronjy RF, Friedman PJ, Goldin JG, Louis TA, Mao JT, Muindi JR, O’Connor GT. Feasibility of retinoids for the treatment of emphysema study. Chest. 2006;130:1334-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Mao JT, Tashkin DP, Belloni PN, Baileyhealy I, Baratelli F, Roth MD. All-trans retinoic acid modulates the balance of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in patients with emphysema. Chest. 2003;124:1724-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Stolk J, Stockley RA, Stoel BC, Cooper BG, Piitulainen E, Seersholm N, Chapman KR, Burdon JG, Decramer M, Abboud RT. Randomised controlled trial for emphysema with a selective agonist of the γ-type retinoic acid receptor. Eur Respir J. 2012;40:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Jones PW, Rames D. TESRA (Treatment of Emphysema with a Selective Retinoid Agonist) study results. Am J Respir Crit Care Med. 2011;183:A6418. |
| 30. | Massaro D, Massaro GD. Estrogen regulates pulmonary alveolar formation, loss, and regeneration in mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1154-L1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Carlson CL, Cushman M, Enright PL, Cauley JA, Newman AB. Hormone replacement therapy is associated with higher FEV1 in elderly women. Am J Respir Crit Care Med. 2001;163:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Martínez A, Miller MJ, Catt KJ, Cuttitta F. Adrenomedullin receptor expression in human lung and in pulmonary tumors. J Histochem Cytochem. 1997;45:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Tokunaga N, Nagaya N, Shirai M, Tanaka E, Ishibashi-Ueda H, Harada-Shiba M, Kanda M, Ito T, Shimizu W, Tabata Y. Adrenomedullin gene transfer induces therapeutic angiogenesis in a rabbit model of chronic hind limb ischemia: benefits of a novel nonviral vector, gelatin. Circulation. 2004;109:526-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Vadivel A, Abozaid S, van Haaften T, Sawicka M, Eaton F, Chen M, Thébaud B. Adrenomedullin promotes lung angiogenesis, alveolar development, and repair. Am J Respir Cell Mol Biol. 2010;43:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Murakami S, Nagaya N, Itoh T, Iwase T, Fujisato T, Nishioka K, Hamada K, Kangawa K, Kimura H. Adrenomedullin regenerates alveoli and vasculature in elastase-induced pulmonary emphysema in mice. Am J Respir Crit Care Med. 2005;172:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Nagaya N, Nishikimi T, Uematsu M, Satoh T, Oya H, Kyotani S, Sakamaki F, Ueno K, Nakanishi N, Miyatake K. Haemodynamic and hormonal effects of adrenomedullin in patients with pulmonary hypertension. Heart. 2000;84:653-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | O'Callaghan DS, Gaine SP. Combination therapy and new types of agents for pulmonary arterial hypertension. Clin Chest Med. 2007;28:169-185, ix. [PubMed] |
| 38. | Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122:1450-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 833] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 39. | Mason RJ, Leslie CC, McCormick-Shannon K, Deterding RR, Nakamura T, Rubin JS, Shannon JM. Hepatocyte growth factor is a growth factor for rat alveolar type II cells. Am J Respir Cell Mol Biol. 1994;11:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Panos RJ, Patel R, Bak PM. Intratracheal administration of hepatocyte growth factor/scatter factor stimulates rat alveolar type II cell proliferation in vivo. Am J Respir Cell Mol Biol. 1996;15:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Hegab AE, Kubo H, Yamaya M, Asada M, He M, Fujino N, Mizuno S, Nakamura T. Intranasal HGF administration ameliorates the physiologic and morphologic changes in lung emphysema. Mol Ther. 2008;16:1417-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Fortunato G, Vidal DT, Klein W, Neto A, Angrizani A, Vasconcelos JF, Kaneto C, Souza BS, Ribeiro-dos-Santos R, Soares MB. Recovery of pulmonary structure and exercise capacity by treatment with granulocyte-colony stimulating factor (G-CSF) in a mouse model of emphysema. Pulm Pharmacol Ther. 2014;27:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Maden M. Retinoids have differing efficacies on alveolar regeneration in a dexamethasone-treated mouse. Am J Respir Cell Mol Biol. 2006;35:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Plantier L, Marchand-Adam S, Antico Arciuch VG, Boyer L, De Coster C, Marchal J, Bachoual R, Mailleux A, Boczkowski J, Crestani B. Keratinocyte growth factor protects against elastase-induced pulmonary emphysema in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1230-L1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Takahashi S, Nakamura H, Seki M, Shiraishi Y, Yamamoto M, Furuuchi M, Nakajima T, Tsujimura S, Shirahata T, Nakamura M. Reversal of elastase-induced pulmonary emphysema and promotion of alveolar epithelial cell proliferation by simvastatin in mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L882-L890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Criner GJ, Connett JE, Aaron SD, Albert RK, Bailey WC, Casaburi R, Cooper JA, Curtis JL, Dransfield MT, Han MK. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N Engl J Med. 2014;370:2201-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 47. | Roszell B, Mondrinos MJ, Seaton A, Simons DM, Koutzaki SH, Fong GH, Lelkes PI, Finck CM. Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tissue Eng Part A. 2009;15:3351-3365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Wang D, Haviland DL, Burns AR, Zsigmond E, Wetsel RA. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:4449-4454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 49. | Rankin S. Mesenchymal stem cells. Thorax. 2012;67:565-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Ishizawa K, Kubo H, Yamada M, Kobayashi S, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett. 2004;556:249-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 181] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 51. | Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 629] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 52. | Aliotta JM, Keaney P, Passero M, Dooner MS, Pimentel J, Greer D, Demers D, Foster B, Peterson A, Dooner G. Bone marrow production of lung cells: the impact of G-CSF, cardiotoxin, graded doses of irradiation, and subpopulation phenotype. Exp Hematol. 2006;34:230-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Carraro G, Perin L, Sedrakyan S, Giuliani S, Tiozzo C, Lee J, Turcatel G, De Langhe SP, Driscoll B, Bellusci S. Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells. 2008;26:2902-2911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 54. | Chang JC, Summer R, Sun X, Fitzsimmons K, Fine A. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol. 2005;33:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 55. | Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 201] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 56. | Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1175] [Cited by in RCA: 996] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 57. | Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143:1590-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 319] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 58. | Badylak SF, Weiss DJ, Caplan A, Macchiarini P. Engineered whole organs and complex tissues. Lancet. 2012;379:943-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 306] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 59. | Yokohori N, Aoshiba K, Nagai A. Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest. 2004;125:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 203] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 60. | Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med. 2006;174:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 370] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 61. | Serrano-Mollar A, Nacher M, Gay-Jordi G, Closa D, Xaubet A, Bulbena O. Intratracheal transplantation of alveolar type II cells reverses bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2007;176:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 62. | Fujino N, Kubo H, Suzuki T, Ota C, Hegab AE, He M, Suzuki S, Suzuki T, Yamada M, Kondo T. Isolation of alveolar epithelial type II progenitor cells from adult human lungs. Lab Invest. 2011;91:363-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 63. | Fadini GP, Avogaro A, Ferraccioli G, Agostini C. Endothelial progenitors in pulmonary hypertension: new pathophysiology and therapeutic implications. Eur Respir J. 2010;35:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Fadini GP, Schiavon M, Cantini M, Baesso I, Facco M, Miorin M, Tassinato M, de Kreutzenberg SV, Avogaro A, Agostini C. Circulating progenitor cells are reduced in patients with severe lung disease. Stem Cells. 2006;24:1806-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 65. | Takahashi T, Suzuki S, Kubo H, Yamaya M, Kurosawa S, Kato M. Impaired endothelial progenitor cell mobilization and colony-forming capacity in chronic obstructive pulmonary disease. Respirology. 2011;16:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Daly AB, Wallis JM, Borg ZD, Bonvillain RW, Deng B, Ballif BA, Jaworski DM, Allen GB, Weiss DJ. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng Part A. 2012;18:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 67. | Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A. 2010;16:2581-2591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 260] [Article Influence: 17.3] [Reference Citation Analysis (0)] |