Published online Sep 19, 2025. doi: 10.5316/wjn.v11.i1.110105
Revised: July 12, 2025
Accepted: September 10, 2025
Published online: September 19, 2025
Processing time: 111 Days and 13.5 Hours
Comorbid non-insulin-dependent diabetes mellitus (NIDDM) and stroke sign
To analyze trends in AAMRs due to comorbid NIDDM and stroke among older adults. The study aims to identify demographic, geographic, and age-related di
Mortality data from the Centers for Disease Control records were analyzed. AAMRs per 100000 and annual per
Between 1999 and 2022, 209001 deaths among adults aged 55+ were attributed to comorbid NIDDM and stroke, with women accounting for 111481 and men 97520 deaths. Urban-rural disparities revealed distinct patterns, with a sharper rise in metropolitan areas post-2014 (APC: 8.6) as compared to non-metropolitan areas. Racial disparities were pronounced, particularly among the Asian/Pacific Islander population, with a steep increase post-2018 (APC: 17.6). Age-stratified analysis showed a marked rise in mortality for ages 55-64 and 85+ from 2015 onwards (APC: 14.3 and 13.5, respectively). Regional trends highlighted the West as having the highest AAMR (14.4), while the Northeast exhibited the lowest (7.0). State-level analysis showed West Virginia with the highest AAMR (18.7) and Nevada the lowest (3.8).
Rising mortality from comorbid NIDDM and stroke underscores increasing disparities across gender, race, age, and regions. Urgent, tailored interventions are required to mitigate these inequities.
Core Tip: The growing mortality burden from coexisting type 2 diabetes and stroke highlights widening disparities across demographic and regional lines. Targeted, context-specific interventions are urgently needed to address these inequities.
- Citation: Khan SMI, Asif H, Raza MH, Waqas M, Ali SZ, Khawar M, Saifullah M, Mehdi AM. Trends and disparities in mortality from comorbid non-insulin-dependent diabetes mellitus and stroke (1999-2022): A retrospective analysis. World J Neurol 2025; 11(1): 110105
- URL: https://www.wjgnet.com/2218-6212/full/v11/i1/110105.htm
- DOI: https://dx.doi.org/10.5316/wjn.v11.i1.110105
Diabetes mellitus (DM), regardless of specific subtype, has an estimated prevalence of more than 38 million people in the United States, although a significant portion remains undiagnosed[1]. The estimated cost of diagnosed diabetes in the United States in 2022 was $412.9 billion[2], making it a substantial burden on the healthcare system. Similarly, stroke is the leading cause of disability in the United States, with more than 795000 people experiencing a stroke each year. The estimated stroke-related costs in the United States between 2019 and 2020 were $56.2 billion[3]. Notably, the incidence of stroke in diabetic patients is 2 to 3 times higher than in non-diabetic individuals. Both non-insulin dependent DM (NIDDM) and stroke are major health concerns, particularly in the elderly population, where these comorbidities significantly increase mortality risk.
Previous studies have shown increasing trends in all-cause mortality due to diabetes, with a projected 38% increase in mortality by 2030[4]. Additionally, higher mortality rates have been observed in rural areas[5], and diabetes-related deaths are more likely to occur in medical or nursing facilities than at home or in hospice care[6]. In contrast, stroke-related deaths have shown a decline, decreasing from 41.1 per 100000 people in 2021 to 39.5 per 100000 people in 2022[7]. However, stroke mortality rates have increased among individuals younger than 35 years and those aged 35 to 64 years[8].
Despite the high prevalence of NIDDM and stroke, no previous study has specifically examined the mortality rates of individuals with both comorbidities. Understanding the effect of coexisting NIDDM and stroke on mortality is essential for initiating comprehensive care strategies and improving patient outcomes. Furthermore, analyzing trends and disparities by demographics, age, and region will help address healthcare inequities. Therefore, this study aims to assess trends and disparities in mortality from comorbid non-insulin-dependent diabetes mellitus and stroke in the United States from 1999 to 2022.
This study used the Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research (CDC WONDER) database[9]. The focus of the study was on mortality related to comorbid NIDDM and stroke in adults between 1999 and 2022. Mortality data were retrieved from the CDC WONDER Multiple Cause of Death database, which captures all conditions listed on death certificates, not just the underlying cause. The International Statistical Classification of Diseases and Related Health Problems-10th Revision codes E11 for NIDDM and I60, I61, I63, I64, I69.0, I69.1, I69.3, and I69.4 for stroke were implemented to identify death records in which both conditions were documented, regardless of their position or causal hierarchy on the certificate. This study sample included adults aged 55 and older at the time of death. Since the study used a publicly available government database, institutional review board approval was not required. The dataset included cause-of-death information from death certificates across all 50 states and the District of Columbia. The study was done by the STROBE guidelines[10]. Type 1 DM (T1DM) was excluded due to its distinct autoimmune etiology, earlier age of onset, and different risk profile. The study focused on adults aged 55 and older, where NIDDM is more prevalent and closely linked to stroke through modifiable metabolic risk factors. Including T1DM could have introduced confounding.
We extracted a range of population-level variables, including population size, demographic characteristics, urban vs rural designation, census regions, age categories, and state-specific data. Demographic variables such as gender and overall mortality trends were compiled for the years 1999 through 2022. In contrast, data stratified by race/ethnicity, census region, urban-rural status, state, and age group were limited to the years 1999 through 2020. Race and ethnicity classifications comprised Non-Hispanic (NH) White, NH Black or African American, Hispanic or Latino, NH American Indian or Alaska Native, and NH Asian or Pacific Islander. Age was grouped into four categories: 55-64, 65-74, 75-84, and 85 years or older.
Urban-rural status was determined using the United States 2013 urbanization framework: Areas with populations over one million were designated as large metropolitan; populations ranging from 50000 to 999999 as small to medium metropolitan; and populations below 50000 as rural[11]. Census regions were categorized as Northeast, Midwest, South, and West according to United States Census Bureau classifications.
Age-adjusted mortality rates (AAMRs) per 100000 population were calculated for each year and stratified by gender, race/ethnicity, state, and urban-rural classification, along with their corresponding 95%CIs. Additionally, crude mortality rates (CMRs) were computed for each defined age group. Both AAMRs and CMRs were standardized using the 2000 United States standard population as the reference[12].
Temporal mortality trends were analyzed using Joinpoint regression analysis (Version 5.0.2, National Cancer Institute) to estimate the annual percentage changes (APC) and associated 95%CIs. This technique enabled the assessment of temporal patterns in mortality associated with comorbid NIDDM and stroke[13]. The statistical significance of APC trends was determined using two-tailed t-tests. An APC with a P value less than 0.05 was considered statistically significant and was interpreted as an increasing or decreasing trend depending on the slope direction.
Mortality rates across various demographic groups revealed significant trends throughout the study period. Between 1999 and 2022, there were 209001 total deaths among older adults (ages 55-85+) attributed to comorbid NIDDM and stroke. During this period, men accounted for 97520 deaths, while women had 111481 deaths (Supplementary Table 1). In race-stratified groups from 1999-2020, the total deaths recorded were 1387 for the American Indian or Alaska Native population, 7133 for the Asian or Pacific Islander group, 24226 for the NH Black people, 129375 for the NH White population, and 16033 for the Hispanic or Latino group (Supplementary Table 2). In age-stratified groups, cumulative deaths from 1999-2020 were 16916 for those aged 55-64, 38972 for those aged 65-74, 65439 for those aged 75-84, and 57320 for those aged 85+ (Supplementary Table 3).
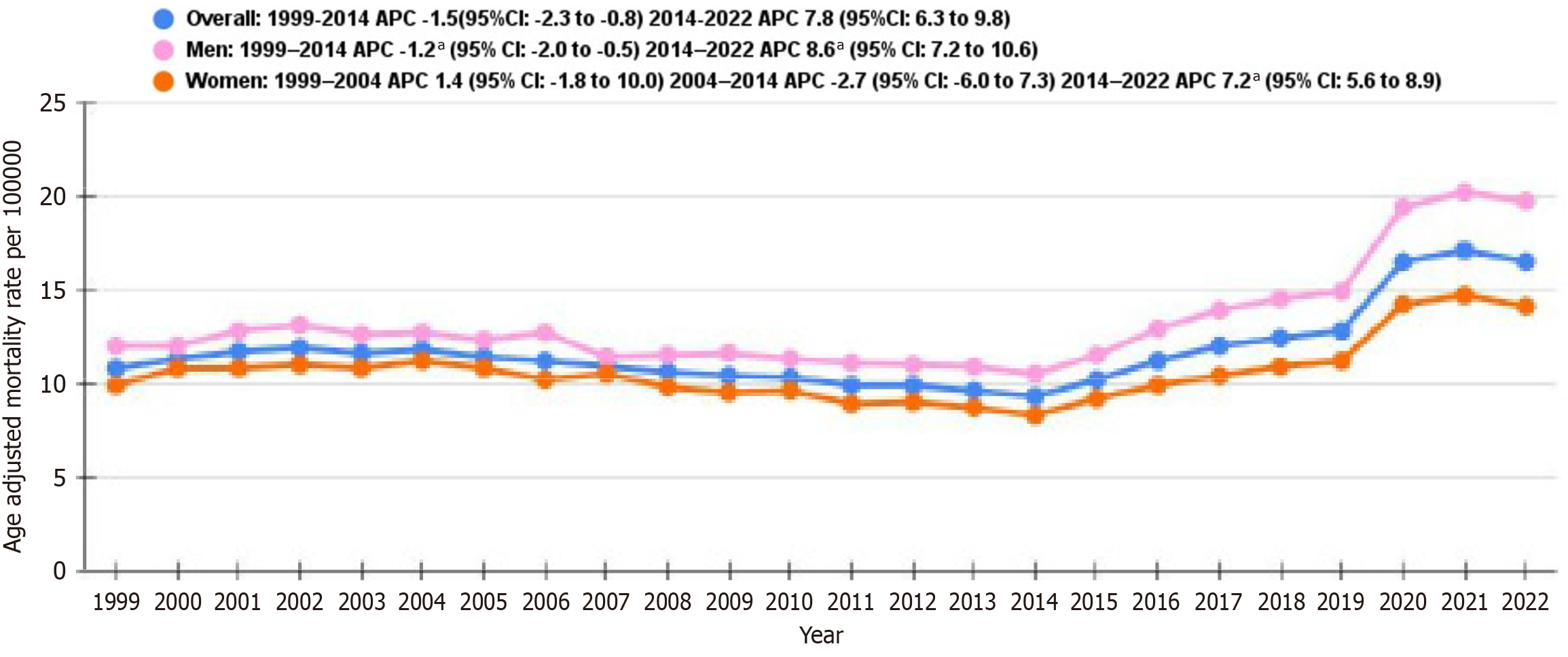
From 1999 to 2014, the overall AAMR decreased with an APC of -1.5 (95%CI: -2.3 to -0.8) but increased significantly from 2014 to 2022 with an APC of 7.8 (95%CI: 6.3-9.8) (Figure 1 and Supplementary Table 4).
For men, the rates saw a decline from 1999 to 2014 with an APC of -1.2 (95%CI: -2.0 to -0.5), accompanied by a marked increase from 2014 to 2022 with an APC of 8.6 (95%CI: 7.2-10.6). In women, the rates initially decreased from 1999 to 2004 with an APC of -2.7 (95%CI: -6.0 to -0.7) but then rose prominently from 2014 to 2022 with an APC of 7.2 (95%CI: 5.6-8.9) (Figure 1 and Supplementary Table 4).
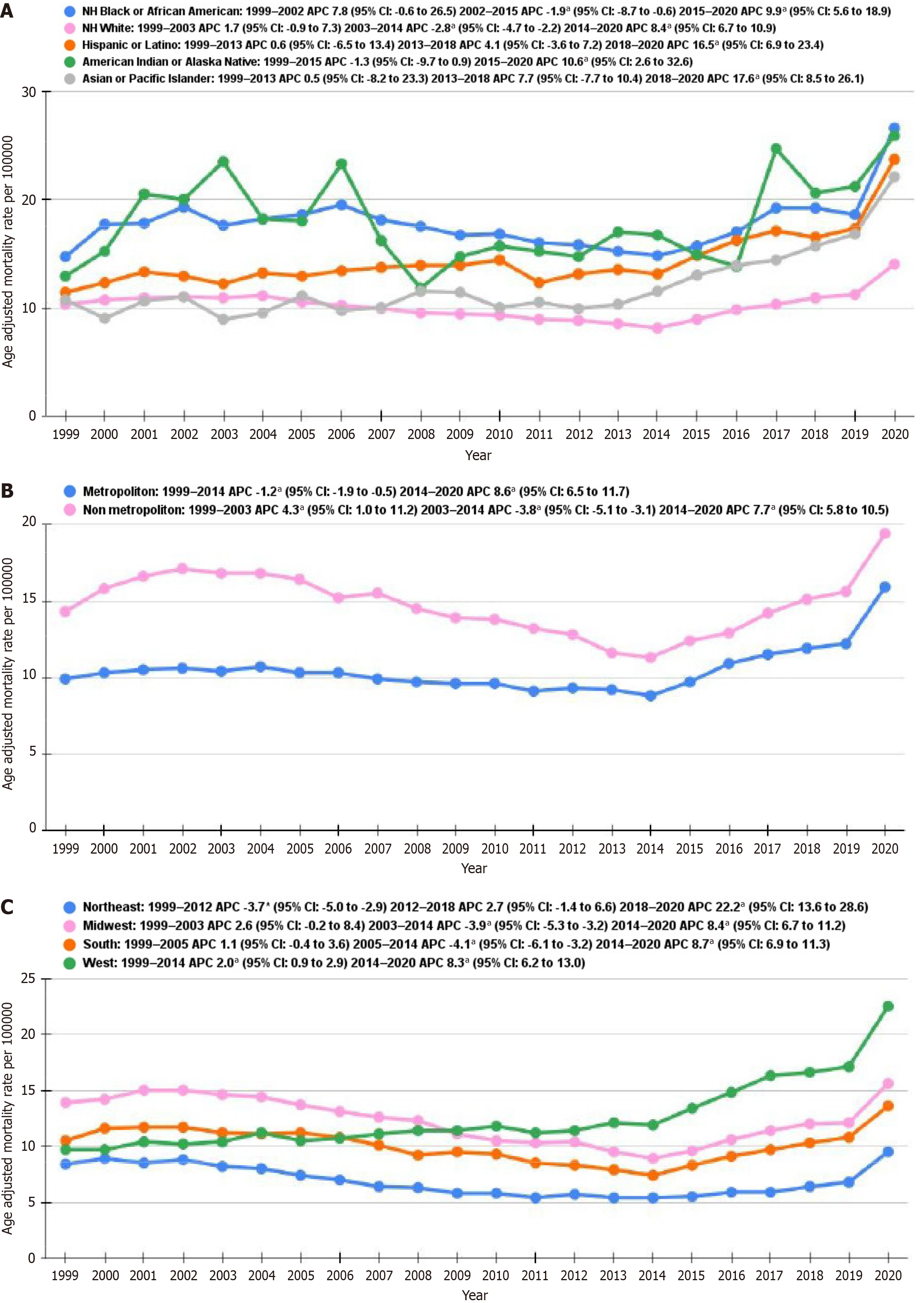
NH Black or African Americans at first showed a notable increase in AAMR from 1999 to 2002 with an APC of 7.8 (95%CI: 0.6-26.5) (Figure 2A and Supplementary Table 5). Subsequently, a decrease from 2002 to 2015 with an APC of -1.9 (95%CI: -8.7 to -0.6), accompanied by a rise from 2015 to 2020 with an APC of 9.3 (95%CI: 5.6-18.9) were recorded. NH White populations indicated an initial increase from 1999 to 2003 with an APC of 1.7 (95%CI: 0.9-7.3), with a subsequent decline from 2003 to 2014 with an APC of -2.8 (95%CI: -4.7 to -2.2), and an increase from 2014 to 2020 with an APC of 8.4 (95%CI: 6.7-10.9). Hispanic or Latino populations demonstrated a rise from 2013 to 2018 with an APC of 4.1 (95%CI: 3.6-7.2) and a marked increase from 2018 to 2020 with an APC of 16.5 (95%CI: 6.9-23.4). The American Indian or Alaska Native group exhibited a steady increase from 2015 to 2020 with an APC of 10.6 (95%CI: 2.6-32.6), whereas the Asian or Pacific Islander group displayed a steep increase from 2018 to 2020 with an APC of 17.6 (95%CI: 8.5-26.1).
The trends in AAMR per 100000 for metropolitan and non-metropolitan areas displayed distinct patterns. From 1999 to 2014, urban areas exhibited a minor decline in AAMR with an APC of -1.2 (95%CI: -1.9 to -0.5) (Figure 2B and Supplementary Table 6). From 2014 to 2020, there was a significant increase with an APC of 8.6 (95%CI: 6.5-11.7). On the other hand, non-metropolitan areas initially showed an increase from 1999 to 2003 with an APC of 4.3 (95%CI: 1.0-11.2). This was followed by a decline from 2003 to 2014 with an APC of -3.8 (95%CI: -5.1 to -3.11). Like metropolitan areas, non-metropolitan areas demonstrated an upward trend from 2014 to 2020, with an APC of 7.7 (95%CI: 5.8-10.5).
The analysis of AAMR by census region indicated that the West region had the highest AAMR noted at 14.4 (95%CI: 14.3-14.5), followed by the Midwest at 12.8 (95%CI: 12.7-12.9), and the South at 11.0 (95%CI: 10.9-11.1). The Northeast region had the lowest AAMR at 7.0 (95%CI: 6.9-7.1). These results illustrate significant regional differences, with the West having the highest AAMR and the Northeast the lowest.
For the Northeast region, the AAMR reduced significantly from 1999 to 2012, with an APC of -3.7 (95%CI: -5.0 to -2.9) (Figure 2C and Supplementary Table 7). From 2012 to 2018, the rate indicated a slight upward trend with an APC of 2.7 (95%CI: -1.4-6.6), with a subsequent sharp rise from 2018 to 2020, with an APC of 22.2 (95%CI: 13.6-28.6). In the Midwest, the rate displayed stability between 1999 and 2003 with an APC of 2.6 (95%CI: -0.2-8.4) but declined from 2003 to 2014 with an APC of -3.9 (95%CI: -5.3 to -3.2). This was accompanied by a significant increase from 2014 to 2020, with an APC of 8.4 (95%CI: 6.7-11.2). The South showed a slight rise from 1999 to 2005, with an APC of 1.1 (95%CI: -0.4-3.6), followed a notable decline occurring from 2005 to 2014, with an APC of -4.1 (95%CI: -6.1 to -3.2). From 2014 to 2020, the rates surged with an APC of 8.7 (95%CI: 6.9-11.3). The West region had a moderate decline from 1999 to 2014, with an APC of -2.0 (95%CI: -0.9-2.9), and an increase from 2014 to 2020 with an APC of 8.3 (95%CI: 6.2-13.0).
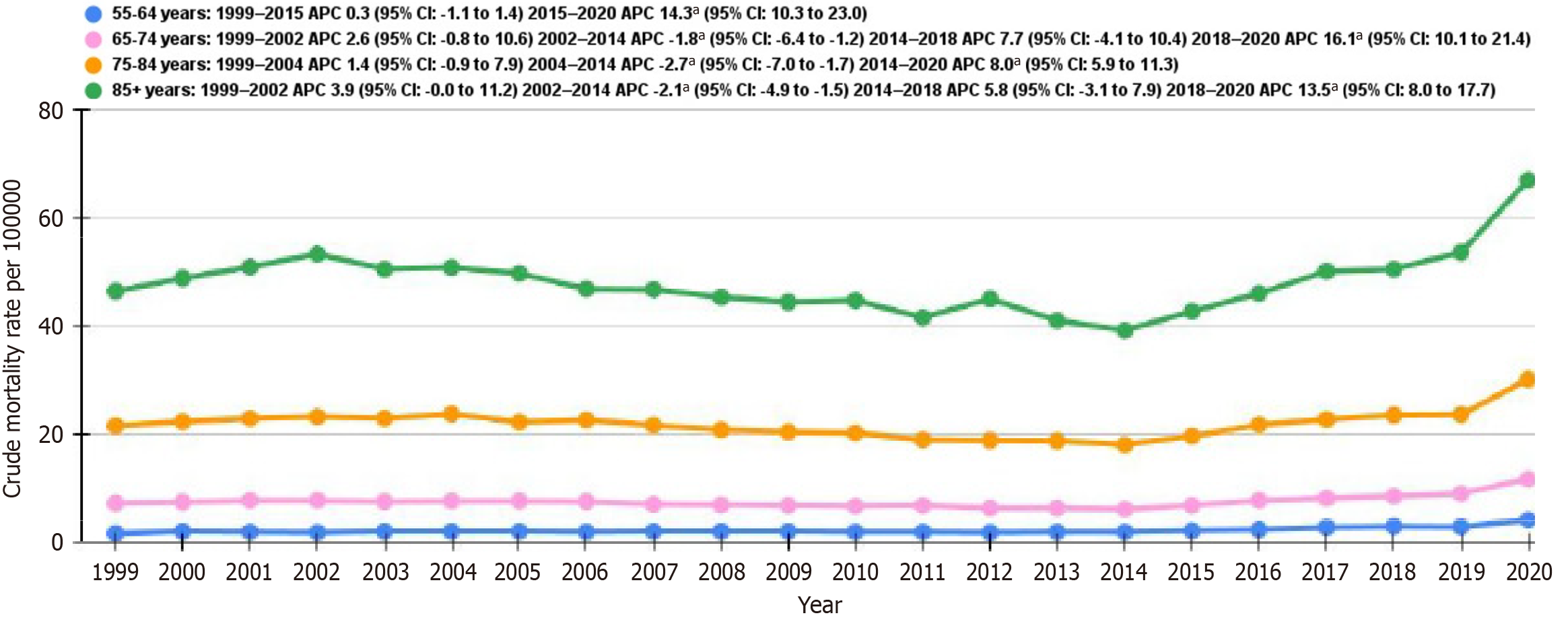
CMR for individuals aged 55-64 years mainly remained constant from 1999 to 2015, with an APC of 0.3 (95%CI: -1.1-1.4), with a subsequent sharp rise between 2015 and 2020, with an APC of 14.3 (95%CI: 10.3-23.0) (Figure 3 and Supplementary Table 8). For those aged 65-74 years, the rates initially increased from 1999 to 2002 with an APC of 2.6 (95%CI: -0.8-10.6), then decreased from 2002 to 2014 with an APC of -1.8 (95%CI: -5.4 to -1.2), and subsequently increased between 2014 and 2018 with an APC of 7.7 (95%CI: 4.1-10.4) and from 2018 to 2020 with an APC of 16.1 (95%CI: 10.1-21.4). Among individuals aged 75-84 years, the rates displayed a minor increase from 1999 to 2004 with an APC of 1.4 (95%CI: -0.9-7.9), followed by a decrease from 2004 to 2014 with an APC of -2.7 (95%CI: -7.0 to -1.7). A marked increase was noted from 2014 to 2020, with an APC of 8.0 (95%CI: 5.9-11.3). For individuals aged 85 years and older, the CMR rose at first from 1999 to 2002 with an APC of 3.9 (95%CI: 0.0-11.2), with a subsequent decline from 2002 to 2014 with an APC of -2.1 (95%CI: -4.9 to -1.5), accompanied by an increase from 2014 to 2018 with an APC of 5.8 (95%CI: -3.1-7.9) and from 2018 to 2020 with an APC of 13.5 (95%CI: 8.0-17.7).
The analysis of AAMR across states revealed that West Virginia had the highest AAMR recorded at 18.7 (95%CI: 17.9-19.5), while Nevada had the lowest at 3.8 (95%CI: 3.5-4.2) (Supplementary Table 9). States in the top 90th percentile of AAMR, with rates above 16.7, included Nebraska (17.0, 95%CI: 16.2-17.8), Iowa (16.8, 95%CI: 16.2-17.4), Ohio (16.7, 95%CI: 16.4-17.1), Oklahoma (16.6, 95%CI: 16.1-17.2), Minnesota (15.8, 95%CI: 15.3-16.2), Oregon (18.1, 95%CI: 17.6-18.7), Tennessee (18.3, 95%CI: 17.8-18.8), and West Virginia. On the other hand, states in the lowest 10th percentile, with rates below 6.0, included Nevada, Massachusetts (4.3, 95%CI: 4.1-4.5), New York (5.1, 95%CI: 4.9-5.2), New Jersey (5.8, 95%CI: 5.6-6.0), Louisiana (5.8, 95%CI: 5.5-6.1), Florida (5.9, 95%CI: 5.8-6.0), and Connecticut (5.9, 95%CI: 5.6-6.3). This distribution highlights significant variation in AAMR across the states, with the highest rates being recorded in West Virginia and the lowest in Nevada.
This study provides a novel and comprehensive analysis of NIDDM and stroke-related mortality trends in the United States over twenty-three years, from 1999 to 2022. Our analysis revealed that AAMR varied not only by sex, race/ethnicity, and age, but also by region of the patient population, including state-to-state differences, census data, and urbanization status across the United States.
We found that men showed consistently slightly higher AAMRs compared to women throughout the study. The rates showed a decline from 1999 to 2014 for both men and women, but this decline was more pronounced for women with an APC of -2.7 (95%CI: -6.0 to -0.7) vs -1.7 (95%CI: Compared to 2.0 to -0.5) for men. There was a steady incline in AAMRs for both men (APC 8.6, 95%CI: 7.2-10.6) and women (APC 7.2, 95%CI: 5.6-8.9) from 2014 to 2022. The cause of this decline is not established yet, but it may be due to the prevalence of diabetes mellitus in recent years. According to the World Health Organization, the prevalence of diabetes mellitus has increased from 200 million in 1990 to 830 million in 2022 worldwide which includes NIDDM in addition to other subtypes. Mulnier et al[14] reported the adjusted hazard-adjusted stroke in people with diabetes, comparing people with diabetes to diabetic individuals, and found it to be slightly higher in women [hazard ratio (HR) = 2.32, 95%CI: 2.16-2.49] than men (HR = 2.08, 95%CI: 1.94-2.24)[14]. Although these findings suggest that women with diabetes have an increased risk of stroke, age-adjusted mortality data have not been reported for all populations with similar results. The outcomes were comparable to those in similar results, and were identical in 2017-2018 vs 1999-2000. Mortality rate was significantly higher in rural men (+18.2) but lower in rural women (-14.0). However, since this study was conducted in 2018, it does not reflect the mortality changes that occurred during the coronavirus disease 2019 (COVID-19) pandemic[5].
Race-stratified mortality rates highlight the continued existence of racial differences in NIDDM and stroke-related health consequences. NH black or African American people showed the highest AAMRs (APC 7.8, 95%CI: 0.6-26.5 from 1999 to 2002) among other ethnicities throughout the study period. Although it was surpassed by the American Indian or Alaskan Native group intermittently throughout the study period (APC 10.6, 95%CI: 2.6-32.6 from 2015 to 2020), the African American population consistently showed the highest AAMR. Although there are limited specific causes available among various races/ethnicities affected with diabetes mellitus, an indirect comparison can be drawn. African Americans are at substantially higher risk of developing diabetes mellitus, and national AAMR are twice as high for African Americans as compared to whites[15]. Similarly, on-Hispanic white populations showed an initial increase from 1999 to 2003, followed by a decline from 2003 to 2014, and then an increase from 2014 to 2022 in AAMR. Hispanic or Latino and Asian or Pacific Islander groups displayed an increase in AAMR from 2018 to 2020. The rise in mortality trends in Asian or Pacific island groups might also be due to cultural dietary patterns as well as language barriers. Lee et al[16] studied that many Asian American immigrants face difficulties navigating the healthcare system, receive minimal counseling on diet and medications, and often lack access to linguistically appropriate care. This increase in diabetes among Asians after 2018 is likely multifactorial, involving the aging immigrant population, persistent cultural and linguistic barriers, insurance and access disparities, and the additional burden of rural healthcare inequities[17,18].
Our study showed significant geographical disparities with higher AAMR in the western states and Alaska compared to other states. The Midwest and the southern region followed this. Northeast had the lowest AAMR, which significantly reduced from 1999 to 2012, followed by a steady rise until 2018, when the COVID-19 pandemic hit. There was a sharp increase in AAMR from 2018 to 2020 (APC 22.2, 95%CI: 13.6-28.6). These disparities are most likely due to limited healthcare access. In addition, state-specific factors such as Medicaid expansion status, healthcare infrastructure, and demographic distribution might also explain these disparities. For example, California's large Asian/Pacific Islander population—among whom we observed a steep post-2018 mortality rise—may have contributed to elevated Western region rates. Conversely, states like Nevada may benefit from younger populations or better access to stroke care. These findings underscore the importance of socioeconomic-level policy, socioeconomic conditions, and population com
From 1990 to 2010, the annual prevalence of diabetes for adults aged 65 to 79 years increased annually and then plateaued. Since 2011, the annual incidence of diabetes has decreased by 8.1%[20]. Dugani et al[5] showed that, along with this decrease in incidence, the annual diabetes mortality rate decreased primarily among individuals aged 75 years or older, accounting for the plateau, with a slight improvement inrural in rural countiescross sectional study by Lutfiyya et al[21] in United States adults of 65 years and above with diabetes living in rural vs non-rural areas had higher odds of not having healthcare practitioner, deferring care because of medical cost, and having an annual household income of less than $35000, placing them at greater risk of not receiving adequate diabetes care[21]. Additionally, rural counties often have longer travel times to stroke centers, which can delay critical acute care. Rural areas experience significantly poorer diabetes care than urban regions due to limited healthcare access, food insecurity, and technological barriers to telemedicine. These disparities result in clinical outcomes and highlight the need for targeted, place-based interventions such as digital literacy, hybrid models, and community-based interventions (e.g., community health workers, mobile telehealth units, etc.)[22-25]. Our study highlights persistent rural disparities, with higher baseline mortality rates in non-metropolitan areas compared to metropolitan areas. From 1999 to 2014, urban areas showed a minor decline (APC -1.2, 95%CI: -1.9 to -0.5) in AAMR, whereas non-metropolitan areas showed a minor increase in AAMR from 1999 to 2003 and a subsequent decline following the downward trend until 2014. Both rural and non-remote counties showed an upward trend in AAMR from 2014 to 2022.
The CMR stratified by age in comorbid NIDDM and stroke showed a marked rise in mortality for ages 55-65 and 85+ from 2015 onwards (APC 14.3 and 13.5, respectively). Almost all age groups show a constant or downward trend in AAMR between 2002 and 2014. A similar study by Tabbalat et al[26] was conducted in adults aged 18 and above, examining socioeconomic trends and the socioeconomic burden among hospitalized patients with diabetes mellitus from 2005 to 2014. Although the incidence of stroke amongst diabetes patients increased, age-adjusted mortality decreased in both hemorrhagic and ischemic stroke, but remained unchanged for Transient Ischemic Attack patients. They report this decline because of higher stroke-related hospitalization costs[26]. While previous studies[8], have reported increasing stroke mortality among younger adults under 55, our findings highlight the disproportionate burden of comorbid NIDDM and stroke among the younger elderly population, particularly those aged 55-64. These groups, which are diagnosed with diabetes and have early vascular damage, have a higher risk of developing complications. This shows state disparities and significant differences in AAMR between various states. West Virginia had the highest AAMR, while Nevada had the lowest. States with the lowest 10th percentile AAMR included those with rich healthcare resources, e.g., New York and New Jersey. On the other hand, states with AAMR in the top 90th percentile included Nebraska, Iowa, Oklahoma, Minnesota, Oregon, Tennessee, and West Virginia.
The sharp increases in mortality observed between 2018 and 2020 across several subgroups may be partially attributable to the COVID-19 pandemic. The pandemic might have influenced the results either directly as a cause of death or indirectly, such as through health strain. However, causality cannot be confirmed from death certificate data alone.
A key limitation of this study is the reliance on death certificate data, which may underreport chronic conditions like NIDDM and minor stroke events. Validation studies have shown variable accuracy, particularly for contributing causes of death. This may lead to an underestimation of mortality trends, although the use of multiple cause-of-death coding likely improves capture compared to relying solely on underlying cause. Additionally, the CDC WONDER database provides racial/ethnic data under broad categories, which may limit the identification of important subgroup differences.
Diabetes (including NIDDM) and stroke are major public health problems, and higher rural prevalence has prompted further actions such as rural-specific technology interventions[27], community programs[28,29], and redesigned primary care models[30]. Additional public health interventions focused on these disparities, increasing healthcare access and diabetes care to underserved and rural communities, would improve outcomes. Enhanced screening for identifying factors, improved access to healthy food and lifestyle interventions, increased availability of stroke care, and access to specialized and overall health care should be a priority. Communities have a socioeconomic foundation to address the socioeconomic determinants of the disparities. The American Heart Association and American Stroke Association collaborated with rural health experts to release a 2020 call to action advisory, aiming to improve rural health (Harrington et al[31], 2020).
In conclusion, our study underscores persistent and widening disparities in NIDDM and stroke-related mortality across sex, race, geography, and rurality in the United States Addressing these gaps requires urgent investment in rural he
| 1. | National Institutes of Health. National Institute of Diabetes and Digestive and Kidney Diseases. [cited 2025 Jan 30]. Available from: https://www.niddk.nih.gov. |
| 2. | Parker ED, Lin J, Mahoney T, Ume N, Yang G, Gabbay RA, ElSayed NA, Bannuru RR. Economic Costs of Diabetes in the U.S. in 2022. Diabetes Care. 2024;47:26-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 391] [Article Influence: 195.5] [Reference Citation Analysis (0)] |
| 3. | Correction to: Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation. 2023;147:e622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 91] [Reference Citation Analysis (1)] |
| 4. | Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: Insights from Yesterday, Today, and Future Trends. Popul Health Manag. 2017;20:6-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 465] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 5. | Dugani SB, Wood-Wentz CM, Mielke MM, Bailey KR, Vella A. Assessment of Disparities in Diabetes Mortality in Adults in US Rural vs Nonrural Counties, 1999-2018. JAMA Netw Open. 2022;5:e2232318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 6. | Siddiqui ZS, Xiao Y, Ansong PO, Muthu SS, Sony A, Doghouz S, Godavarthi A. A 22-Year Study to Assess Disparities in Place of Death Among Patients With Diabetes. Cureus. 2023;15:e49929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | McCandless MG, Powers AY, Baker KE, Strickland AE. Trends in Demographic and Geographic Disparities in Stroke Mortality Among Older Adults in the United States. World Neurosurg. 2024;185:e620-e630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 8. | Mercy UC, Farhadi K, Ogunsola AS, Karaye RM, Baguda US, Eniola OA, Yunusa I, Karaye IM. Revisiting recent trends in stroke death rates, United States, 1999-2020. J Neurol Sci. 2023;451:120724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 9. | United States Centers for Disease Control and Prevention. CDC WONDER. [cited 2025 Jan 30]. Available from: https://wonder.cdc.gov/. |
| 10. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5754] [Cited by in RCA: 11532] [Article Influence: 640.7] [Reference Citation Analysis (0)] |
| 11. | Ingram DD, Franco SJ. 2013 NCHS Urban-Rural Classification Scheme for Counties. Vital Health Stat 2. 2014;1-73. [PubMed] |
| 12. | Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep. 1998;47:1-16, 20. [PubMed] |
| 13. | Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 125] [Reference Citation Analysis (0)] |
| 14. | Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA, De Vries CS. Risk of stroke in people with type 2 diabetes in the UK: a study using the General Practice Research Database. Diabetologia. 2006;49:2859-2865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung HC. Deaths: final data for 2009. Natl Vital Stat Rep. 2011;60:1-116. [PubMed] |
| 16. | Lee S, Martinez G, Ma GX, Hsu CE, Robinson ES, Bawa J, Juon HS. Barriers to health care access in 13 Asian American communities. Am J Health Behav. 2010;34:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Shah NS, Kandula NR, Commodore-Mensah Y, Morey BN, Patel SA, Wong S, Yang E, Yi S; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing; Council on Hypertension; Council on Lifestyle and Cardiometabolic Health; Council on Basic Cardiovascular Sciences; Council on Clinical Cardiology; Council on Peripheral Vascular Disease; and Council on Quality of Care and Outcomes Research. Social Determinants of Cardiovascular Health in Asian Americans: A Scientific Statement From the American Heart Association. Circulation. 2024;150:e296-e315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 18. | Probst JC, Zahnd WE, Hung P, Eberth JM, Crouch EL, Merrell MA. Rural-Urban Mortality Disparities: Variations Across Causes of Death and Race/Ethnicity, 2013-2017. Am J Public Health. 2020;110:1325-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Ariss RW, Minhas AMK, Lang J, Ramanathan PK, Khan SU, Kassi M, Warraich HJ, Kolte D, Alkhouli M, Nazir S. Demographic and Regional Trends in Stroke-Related Mortality in Young Adults in the United States, 1999 to 2019. J Am Heart Assoc. 2022;11:e025903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 20. | Benoit SR, Hora I, Albright AL, Gregg EW. New directions in incidence and prevalence of diagnosed diabetes in the USA. BMJ Open Diabetes Res Care. 2019;7:e000657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 21. | Lutfiyya MN, McCullough JE, Mitchell L, Dean LS, Lipsky MS. Adequacy of diabetes care for older U.S. rural adults: a cross-sectional population based study using 2009 BRFSS data. BMC Public Health. 2011;11:940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Kurani SS, Lampman MA, Funni SA, Giblon RE, Inselman JW, Shah ND, Allen S, Rushlow D, McCoy RG. Association Between Area-Level Socioeconomic Deprivation and Diabetes Care Quality in US Primary Care Practices. JAMA Netw Open. 2021;4:e2138438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 23. | Foss R, Fischer K, Lampman MA, Laabs S, Halasy M, Allen SV, Garrison GM, Sobolik G, Bernard M, Sosso J, Thacher TD. Disparities in Diabetes Care: Differences Between Rural and Urban Patients Within a Large Health System. Ann Fam Med. 2023;21:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 24. | American Diabetes Association Professional Practice Committee. 1. Improving Care and Promoting Health in Populations: Standards of Care in Diabetes-2025. Diabetes Care. 2025;48:S14-S26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 25. | Dhediya R, Chadha M, Bhattacharya AD, Godbole S, Godbole S. Role of Telemedicine in Diabetes Management. J Diabetes Sci Technol. 2023;17:775-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Tabbalat A, Dargham S, Al Suwaidi J, Aboulsoud S, Al Jerdi S, Abi Khalil C. Mortality and socio-economic outcomes among patients hospitalized for stroke and diabetes in the US: a recent analysis from the National Inpatient Sample. Sci Rep. 2021;11:8204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Egede LE, Dismuke CE, Walker RJ, Williams JS, Eiler C. Cost-Effectiveness of Technology-Assisted Case Management in Low-Income, Rural Adults with Type 2 Diabetes. Health Equity. 2021;5:503-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Rafie C, Hosig K, Wenzel SG, Borowski S, Jiles KA, Schlenker E. Implementation and outcomes of the Balanced Living with Diabetes program conducted by Cooperative Extension in rural communities in Virginia. Rural Remote Health. 2021;21:6620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Higa C, Davidson EJ, Loos JR. Integrating family and friend support, information technology, and diabetes education in community-centric diabetes self-management. J Am Med Inform Assoc. 2021;28:261-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Bray P, Cummings DM, Morrissey S, Thompson D, Holbert D, Wilson K, Lukosius E, Tanenberg R. Improved outcomes in diabetes care for rural African Americans. Ann Fam Med. 2013;11:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Harrington RA, Califf RM, Balamurugan A, Brown N, Benjamin RM, Braund WE, Hipp J, Konig M, Sanchez E, Joynt Maddox KE. Call to Action: Rural Health: A Presidential Advisory From the American Heart Association and American Stroke Association. Circulation. 2020;141:e615-e644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 270] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/