Published online Sep 18, 2015. doi: 10.5312/wjo.v6.i8.641
Peer-review started: February 6, 2015
First decision: June 3, 2015
Revised: June 17, 2015
Accepted: July 29, 2015
Article in press: August 3, 2015
Published online: September 18, 2015
Processing time: 225 Days and 18.4 Hours
AIM: To quantify the wrist cartilage cross-sectional area in humans from a 3D magnetic resonance imaging (MRI) dataset and to assess the corresponding reproducibility.
METHODS: The study was conducted in 14 healthy volunteers (6 females and 8 males) between 30 and 58 years old and devoid of articular pain. Subjects were asked to lie down in the supine position with the right hand positioned above the pelvic region on top of a home-built rigid platform attached to the scanner bed. The wrist was wrapped with a flexible surface coil. MRI investigations were performed at 3T (Verio-Siemens) using volume interpolated breath hold examination (VIBE) and dual echo steady state (DESS) MRI sequences. Cartilage cross sectional area (CSA) was measured on a slice of interest selected from a 3D dataset of the entire carpus and metacarpal-phalangeal areas on the basis of anatomical criteria using conventional image processing radiology software. Cartilage cross-sectional areas between opposite bones in the carpal region were manually selected and quantified using a thresholding method.
RESULTS: Cartilage CSA measurements performed on a selected predefined slice were 292.4 ± 39 mm2 using the VIBE sequence and slightly lower, 270.4 ± 50.6 mm2, with the DESS sequence. The inter (14.1%) and intra (2.4%) subject variability was similar for both MRI methods. The coefficients of variation computed for the repeated measurements were also comparable for the VIBE (2.4%) and the DESS (4.8%) sequences. The carpus length averaged over the group was 37.5 ± 2.8 mm with a 7.45% between-subjects coefficient of variation. Of note, wrist cartilage CSA measured with either the VIBE or the DESS sequences was linearly related to the carpal bone length. The variability between subjects was significantly reduced to 8.4% when the CSA was normalized with respect to the carpal bone length.
CONCLUSION: The ratio between wrist cartilage CSA and carpal bone length is a highly reproducible standardized measurement which normalizes the natural diversity between individuals.
Core tip: Wrist cartilage cross-sectional area has been quantified in wrists of healthy subjects using 3T magnetic resonance imaging. Based on a semi-automatic segmentation method, the reproducibility of the measurements is high as compared to previous studies. A standardized quantitative index has been proposed. This standardized index can be used for future follow-up studies. The measurements performed in a small group of subjects should be further confirmed in a larger group.
- Citation: Zink JV, Souteyrand P, Guis S, Chagnaud C, Fur YL, Militianu D, Mattei JP, Rozenbaum M, Rosner I, Guye M, Bernard M, Bendahan D. Standardized quantitative measurements of wrist cartilage in healthy humans using 3T magnetic resonance imaging. World J Orthop 2015; 6(8): 641-648
- URL: https://www.wjgnet.com/2218-5836/full/v6/i8/641.htm
- DOI: https://dx.doi.org/10.5312/wjo.v6.i8.641
Progressive damage to articular cartilage has been appreciated in a variety of pathological situations including rheumatoid arthritis (RA)[1] and osteoarthritis (OA)[2]. In addition, early detection of such damage has important implications for both grading disease severity and for the assessment of therapeutic efficacy of interventions, underlying the need for reliable and accurate imaging methods. Magnetic resonance imaging (MRI) is well suited to this application as it can provide a 3D dataset of joint anatomy as a whole and not indirectly as a space between opposite cartilage cortices as with conventional radiography. Considering the relatively large amount of cartilage at the knee, MRI investigations have provided interesting information relating to cartilage loss in OA[2-4]. In contrast, for the wrist, a site commonly affected in RA, the obvious limitation related to the corresponding cartilage size has made the investigation much more challenging. Initial investigations performed at a conventional magnetic field of 1.5T have been inconclusive in measuring early degenerative changes[5]. Accordingly, the initial evaluation score of RA using MRI (RA-MRIS), proposed by the Outcome Measures in Rheumatology (OMERACT) group, has been mainly based on the detection of bone erosions, bone edema and synovial thickening[6,7] whereas cartilage lesion criteria were ignored. Due to technological advances in MRI and corresponding improvement in image quality, the last OMERACT conference suggested taking cartilage criteria into account for the RA-MRIS score[8]. On the basis of a comparative analysis of joint space narrowing (JSN) using conventional radiography and MRI, the OMERACT group proposed to include the criterion of cartilage lesion in the new RA-MRI score using grading of JSN[8]. Using the same kind of approach, Peterfy et al[9] further confirmed that MRI JSN scoring may offer a viable alternative to conventional JSN radiographic scoring. However, the qualitative approach described requires highly-trained observers and provides a gross score which may not be sensitive enough to detect early cartilage alteration.
Besides this qualitative approach, other MRI techniques allowing for the indirect investigation of the chemical composition of cartilage (collagen and proteoglycans) have been developed. They are mainly related to T1 and T2 relaxation times mapping, magnetisation transfer, diffusion MRI or sodium MRI[10,11]. Among those techniques, the delayed gadolinium (Gd) enhanced MRI of cartilage (dGEMRIC) has provided promising results[12]. The potential drawbacks of this technique are that it is time consuming and requires repeated investigations prior to, and then an hour after, contrast agent injection.
Again and likely for the above reasons, this kind of MRI investigation has been mainly performed in knees of OA patients[13-15] with no data reported for wrist cartilage.
It is noteworthy that most of the MRI studies reported so far have been conducted using a conventional magnetic field, i.e., 1.5T but a few studies have clearly indicated that MRI investigations at higher field, e.g., 3T could provide a better signal/noise ratio and a more accurate identification of certain anatomical structures[16-19].
In the present study, taking advantage of high-field MRI, we sought to determine whether cartilage cross-sectional area could be quantified at the wrist level in healthy volunteers. Additionally, we aimed to define the normal range for this quantitative index and the reproducibility of the corresponding measurements.
The fourteen healthy subjects (6 females and 8 males) included in the study provided written informed consent for the protocol which had received the approval of the local ethics committee (Aix-Marseille University ethics committee). Their ages ranged between 30 and 58 years (mean ± SD = 47.4 ± 8.9). None of them reported any joint pain, past wrist trauma or other rheumatologic pathologies.
MRI investigations were performed at 3 T using a Siemens VERIO 3 T scanner (Siemens, Erlangen, Germany). Subjects were asked to lie down in a supine position inside the scanner. To ensure a comfortable position, a home-built rigid platform was attached to the scanner bed so that the right hand was positioned above the pelvic region. The wrist was then wrapped with a flexible surface coil. This position was not only comfortable for the subjects but also allowed us to localize the wrist at the magnet center, a place where magnetic field homogeneity is optimal.
After a localization procedure using scout images, two 3D MRI sequences of the entire carpus and metacarpal-phalangeal area were performed, i.e., the volumetric interpolated breath-hold examination (VIBE) sequence, mainly used for abdominal investigations[20,21] and the double-echo steady-state (DESS) sequence previously used for knee cartilage imaging[22-25]. The corresponding sequence parameters are summarized in Table 1.
| Sequence | FOV (mm) | ST (mm) | RT (ms) | ET (ms) | AT (S) | Matrix size | Resolution (mm) |
| 3D DESS | 130 × 130 | 0.5 | 14 | 5.2 | 330 | 256 × 256 | 0.5 × 0.5 |
| 3D VIBE | 130 × 130 | 0.5 | 10 | 3.38 | 290 | 256 × 256 | 0.5 × 0.5 |
The entire segmentation protocol was performed on a slice of interest selected from each 3D dataset on the basis of anatomical criteria (described below). Cartilage cross-sectional areas (CSA) between opposite bones in the carpal region were manually selected. Then a thresholding process was applied on the corresponding region so that only the voxels within the proper signal intensity setting[26,27] were kept and counted. The cartilage CSA was automatically computed considering the image resolution, the slice thickness and the number of voxels. This strategy was similar to what has been previously described for knee and metacarpophalangeal joints[2-4].
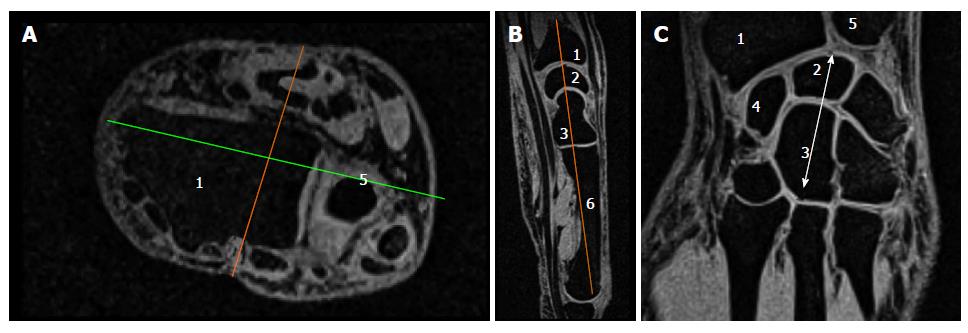
The different steps leading to the selection of the slice of interest are illustrated in Figure 1.
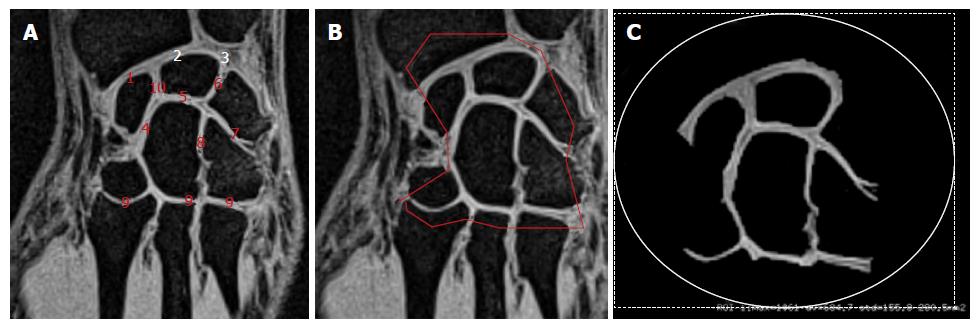
We initially looked at the anterior flat border of the radius in the axial plane and drew a line parallel to it through the middle part of the distal forearm. In the corresponding perpendicular sagittal view, we then selected the slice passing through the distal radius, lunate, capitate and the third metacarpal base. The corresponding coronal section was located through the middle of the bones mentioned above. As illustrated in Figure 1A, various joint spaces were visible on this slice and we manually selected the area of interest (Figure 1B), including the cartilage in the radio-carpal joint, the intercarpal joints and the carpo-metarcarpal joints (joints # 2; 3 and 4 in Figure 2A). At this stage, we used a thresholding process allowing to maintain the signal related to the cartilage alone. The same threshold was applied for each subject. In addition to those measurements, the carpus length was measured as indicated in Figure 1C from the proximal aspect of the lunate to the distal aspect of the capitate.
This segmentation process and the corresponding measurements were performed twice by the same experienced radiologist (JVZ, 3-mo interval) and once by two senior radiologists (JVZ and PS). The measurements’ reliability was evaluated, comparing between the two operators’ measurements. Measurement reproducibility was assessed via repeated measurements performed by the same operator.
Comparisons of measurements were performed using Wilcoxon tests. Test-retest reliability was analyzed using coefficient of variation (CV) and intra-class correlation coefficients (ICC). Relative reliability is related to an individual maintaining his/her position within a sample with repeated measurements[28]. We assessed this type of reliability with the ICC, a two-way random effects model with single measurement reliability in which variance over the repeated sessions is considered. The ICC indicates the error in measurements as a proportion of the total variance in scores. Absolute reliability is the degree to which repeated measurements vary for individuals[28]. This was performed by calculating the CV for each subject, and then reporting the mean CV for the respective dependent variables. Accordingly, the CV was initially calculated as the SD of measurements recorded for repeated measures and was then divided by the mean of both measurements. Spearman’s rank correlation coefficient was calculated to investigate the relationship between measurements obtained with both MR sequences. Statistical analyses were performed using Statistical software for Windows (Statsoft, version 5.5; Statistica, Tulsa, OK, United States). ICC analysis was done using a downloadable excel spreadsheet[29]. A P value of 0.05 was chosen as the significant threshold.
As illustrated in Figures 1 and 2, the MR sequences we used generated images of the carpal joint in which articular cartilage appeared as a high signal intensity contrasting with the low signal-intensity subchondral bone.
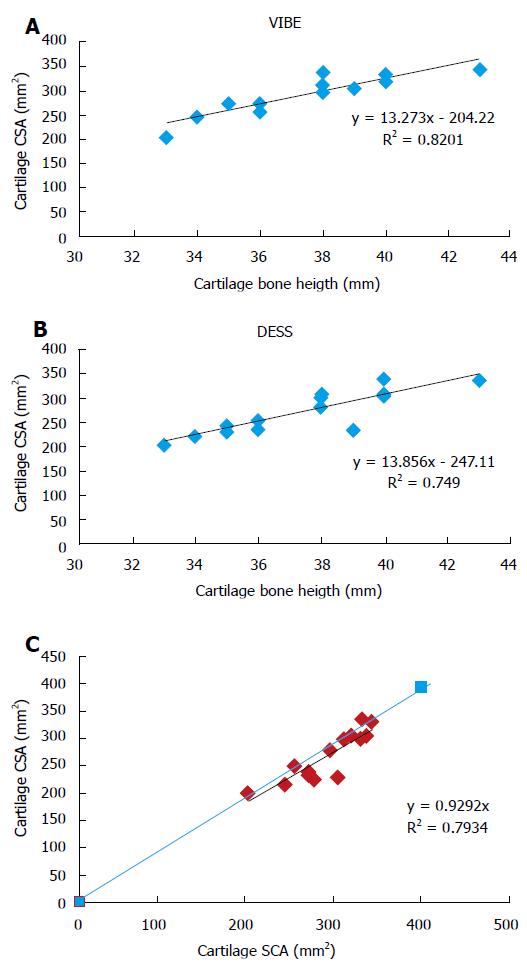
The carpus length averaged over the group was 37.5 ± 2.8 mm with a 7.45% between-subjects coefficient of variation. Cartilage cross-sectional area (in mm2) measured on the selected slices from images recorded with both VIBE and DESS sequences are summarized in Table 2.
The averaged cartilage cross-sectional area obtained with the VIBE sequence was 292.4 ± 39 mm2 whereas the corresponding value with the DESS sequence was significantly lower (270.4 ± 50.6 mm2, P = 5.6 × 10-7). Interestingly, the between-subjects coefficients of variation were similar for both MRI sequences (14.1% and 16.8% for the VIBE and DESS sequences respectively). The coefficients of variation computed for the repeated measurements were also comparable for the VIBE (2.4%) and the DESS (4.8%) sequences (Table 2). As illustrated in Table 2, measurements performed twice by the same operator and by an additional operator were very similar. The corresponding intra-class coefficients were higher than 0.9 in each case (Table 2). Interestingly, cartilage cross-sectional area measurements were significantly and linearly related to carpal bone length measurements (Figures 3A and B). As compared to the CSA values, the corresponding standardized measurements, i.e., the cartilage cross-sectional area scaled to the carpal bone length displayed smaller coefficients of variation, i.e., 8.4% and 10.6% for the measurements obtained from the VIBE and the DESS sequences respectively.
This positive linear relationship was found for measurements performed from MR images obtained with both MRI sequences, i.e., VIBE (R2 = 0.82) and DESS (R2 = 0.75).
In addition, as illustrated in Figure 3C, these measurements were linearly and significantly related (R2 = 0.79) to each other. However, the cartilage CSA values quantified on the basis of the VIBE sequence were consistently larger than those obtained with the DESS sequence.
In the present study, we demonstrated that use of high-field MRI combined with a semi-automatic segmentation process can be utilized to measure cartilage cross-sectional area at the wrist, a site commonly affected in RA, accurately. On that basis, we investigated the normal range of the carpal cartilage cross-sectional area of healthy adults, the physiological inter-individual heterogeneity and a potential way of normalizing the corresponding measurements.
It is noteworthy that the initial scoring system devised in the field of MRI investigations of rheumatoid arthritis (RAMRIS) disregarded cartilage MRI quantification due to its low sensitivity[6,7]. On the basis of MRI investigations performed at higher-field, i.e., 3T, cartilage thickness has been recently introduced as a potential surrogate marker for RA[8] severity. In the current situation, however, we are still lacking quantitative information related to the normal range of cartilage cross-sectional area in healthy human wrists and the reproducibility of the corresponding measurements has never been assessed.
From a methodological point of view, we showed that high-field 3D MR imaging using both VIBE and DESS sequences provides adequate spatial resolution and signal to noise ratio allowing accurate and reproducible quantification of the articular cartilage area in the carpal joints. The results of the present study clearly indicate that measurements obtained from both MRI sequences are largely reproducible. On the basis of repeated measurements performed by the same and by two different operators, we calculated intra-class coefficients which were higher than 0.9. These values can be compared to the ICC of 0.18 initially reported by the OMERACT initiative[30]. Significantly, they are similar to those reported using the recent MRI scoring system related to cartilage narrowing in the wrist of patients with RA[1], indicating that the reproducibility of our quantitative approach is similar to what has been obtained for a scoring system, a qualitative approach by definition[1]. The corresponding coefficients of variation were also similar using both techniques.
Of importance, the natural variability of wrist cartilage cross-sectional area was related to the measured carpal bone length. The highly significant relationships depicted in Figures 3A and B suggested that cartilage CSA would vary in the same way as the carpal bone length and that the corresponding ratio might be used as a normalized index. This normalization procedure is comparable to what has been previously reported for the knee cartilage volume using tibial head diameter[31] or the tibial plateau area[32] as normalization indices. In both cases, the normalization procedure led to a reduction of the coefficient of variation from 19% to 13% which is comparable to the reduction we reported for our normalized measurements, i.e., from 14% to 8% and from 16% to 10% for the VIBE and the DESS sequences respectively. In addition, these results indicate that cartilage cross-sectional area or volume measurements cannot stand alone as a diagnostic criterion of cartilage loss. On the other hand, the highly linear relationship between cartilage area and carpal bone length provides a standardized measurement which might be helpful in future studies conducted in wrist of patients with rheumatologic diseases.
With an eye toward practicality, and in consideration of protocol duration constraints imposed on MRI with Gd injection[33], we deliberately chose in the present study to avoid gadolinium injection and used conventional MRI sequences, i.e., two ultra-fast gradient echo pulse MRI sequences. These sequences provide high spatial resolution within an acceptable acquisition time, i.e., about 5 min. This type of sequence has already proved its utility for the detection of focal cartilage abnormality[11]. Contrary to most of the fast gradient echo pulse sequences providing a T1-based contrast, the DESS sequence offered a T2-weighted contrast. This sequence is actually a combination of fast imaging in the steady precession (FISP) and PSIF (backward-running FISP) and allows T2 weighted imaging with a fast gradient echo technique which has proven its usefulness for the detection of cartilage abnormalities[22-25]. In contrast, the VIBE sequence has not been used very much in the field of musculo-skeletal investigations but rather for head and neck or abdominal imaging[21]. It provides a T1-based contrast. While current guidelines in RA recommend the use of T1 weighted imaging, we demonstrated in the present study that images with both T1 and T2 contrast can be used for quantitative investigation of cartilage. In addition, the three-dimensional nature of the MRI sequences used herein, permit investigation not only of cartilage but also of bone erosions, an index in the RAMRIS score. Further, in that configuration, one is able to choose a well-defined segment in 3 dimensional space and investigate parallel joint spaces for each subject. Such an opportunity would not have been possible using a 2D scheme, given the slightly different orientations of wrist within the flexible coil. The different contrasts related to the VIBE and the DESS sequences may explain the slightly different cartilage area measurements we obtained. However, the highly significant linear relationship between measurements obtained with both methods on the one hand, and with the carpal bone length measurements on the other hand strongly suggest that both methods can be used.
Both operators in the present study were experienced radiologists and processed the MR images after a training period. Considering the multiple steps of the semi-automatic procedure, one may have expected a large operator dependency of the results. And yet, the corresponding ICC were not appreciably different when data were examined by both readers or repeatedly by the same reader, indicating the high reliability of this approach and suggesting that such a reliability can be achieved by any trained reader using similar MRI scans. Automatic segmentation tools have been developed for the measurement of cartilage volume and thickness in the knee[34,35]. Considering the low contrast between cartilage and adjacent structures in the wrist, with its far smaller cartilage thickness, it seems unlikely that such automatic tools could be used for the quantitative investigation of cartilage in the wrist.
The limitations of the current study arise from the modest sample size and the limited age range of the subjects. Of relevance, no marked changes in knee cartilage volume have been identified in healthy subjects with ages ranging from 24 to 82 years[31]. In contrast, a linear decrease of cartilage thickness has been reported in the weight-bearing areas of the femur in healthy subjects[32]. Further studies should be performed in a larger group of subjects in order to determine whether carpal cartilage cross-sectional area remains stable with respect to age.
In conclusion, we report herein for the first time the normal range of wrist cartilage cross-sectional area in healthy subjects. We demonstrated that reproducible measurements of carpal cartilage cross-sectional area can be performed using high-field MRI and that there exists a wide variation in cartilage cross-sectional area in the normal human wrist. We also showed that a standardized index of cartilage cross-sectional area can be calculated taking into account carpal bone length. The corresponding normalized measurement may be helpful for future studies aiming at investigating wrist cartilage in patients with rheumatologic diseases. Technical issues related to the occurrence of joint effusion might be problematic in differentiating fluid from cartilage and likely be a substantial source of error in inflammatory arthritis. Future studies addressing these questions will possibly benefit from technical evolutions allowing the suppression of MRI signal from fluid[36]. These findings encourage further efforts towards quantitative standardized MRI of cartilage at high field. Additional studies are warranted in order to determine the sensitivity of such normalized indices with respect to the RAMRIS index[37].
We would like to thank Dr. Badih Ghattas (Department of Mathematics, Luminy Sciences University - Marseille-France) for the thorough review of the statistical methods.
Although magnetic resonance imaging (MRI) has been recently recognized as a potentially useful tool for appreciating and characterizing cartilage loss at the wrist, the corresponding data are few and qualitative in nature.
There is a need for reproducible quantitative measurements of cartilage cross sectional area (CSA) at the wrist which could be used as surrogate marker of disease progression in rheumatoid arthritis (RA) and other rheumatologic diseases.
In the present study the authors used a semi-automatic method in order to quantify CSA at the wrist level from a 3D MRI dataset. The corresponding measurement was highly reproducible and linearly linked to the carpal bone length. The corresponding standardized ratio, i.e., CSA/carpal bone length captures the natural diversity between subjects and allows a substantial reduction of the coefficient of variation calculated between the subjects.
The standardized ratio between cartilage CSA and the carpal bone length captures the natural diversity between subjects and might be a helpful surrogate marker of disease progression in RA and other rheumatologic diseases.
The authors have performed a good study, the manuscript is interesting.
| 1. | McQueen F, Clarke A, McHaffie A, Reeves Q, Williams M, Robinson E, Dong J, Chand A, Mulders D, Dalbeth N. Assessment of cartilage loss at the wrist in rheumatoid arthritis using a new MRI scoring system. Ann Rheum Dis. 2010;69:1971-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Burgkart R, Glaser C, Hyhlik-Dürr A, Englmeier KH, Reiser M, Eckstein F. Magnetic resonance imaging-based assessment of cartilage loss in severe osteoarthritis: accuracy, precision, and diagnostic value. Arthritis Rheum. 2001;44:2072-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Gandy SJ, Dieppe PA, Keen MC, Maciewicz RA, Watt I, Waterton JC. No loss of cartilage volume over three years in patients with knee osteoarthritis as assessed by magnetic resonance imaging. Osteoarthritis Cartilage. 2002;10:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Eckstein F, Charles HC, Buck RJ, Kraus VB, Remmers AE, Hudelmaier M, Wirth W, Evelhoch JL. Accuracy and precision of quantitative assessment of cartilage morphology by magnetic resonance imaging at 3.0T. Arthritis Rheum. 2005;52:3132-3136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Recht MP, Resnick D. Magnetic resonance imaging of articular cartilage: an overview. Top Magn Reson Imaging. 1998;9:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | McQueen F, Lassere M, Edmonds J, Conaghan P, Peterfy C, Bird P, O’Connor P, Ejbjerg B, Klarlund M, Stewart N. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Summary of OMERACT 6 MR Imaging Module. J Rheumatol. 2003;30:1387-1392. [PubMed] |
| 7. | Østergaard M, Edmonds J, McQueen F, Peterfy C, Lassere M, Ejbjerg B, Bird P, Emery P, Genant H, Conaghan P. An introduction to the EULAR-OMERACT rheumatoid arthritis MRI reference image atlas. Ann Rheum Dis. 2005;64 Suppl 1:i3-i7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Ostergaard M, Bøyesen P, Eshed I, Gandjbakhch F, Lillegraven S, Bird P, Foltz V, Boonen A, Lassere M, Hermann KG. Development and preliminary validation of a magnetic resonance imaging joint space narrowing score for use in rheumatoid arthritis: potential adjunct to the OMERACT RA MRI scoring system. J Rheumatol. 2011;38:2045-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Peterfy CG, DiCarlo JC, Olech E, Bagnard MA, Gabriele A, Gaylis N. Evaluating joint-space narrowing and cartilage loss in rheumatoid arthritis by using MRI. Arthritis Res Ther. 2012;14:R131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Chen CA, Kijowski R, Shapiro LM, Tuite MJ, Davis KW, Klaers JL, Block WF, Reeder SB, Gold GE. Cartilage morphology at 3.0T: assessment of three-dimensional magnetic resonance imaging techniques. J Magn Reson Imaging. 2010;32:173-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Gold GE, McCauley TR, Gray ML, Disler DG. What’s new in cartilage? Radiographics. 2003;23:1227-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Trattnig S, Marlovits S, Gebetsroither S, Szomolanyi P, Welsch GH, Salomonowitz E, Watanabe A, Deimling M, Mamisch TC. Three-dimensional delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) for in vivo evaluation of reparative cartilage after matrix-associated autologous chondrocyte transplantation at 3.0T: Preliminary results. J Magn Reson Imaging. 2007;26:974-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Burstein D, Bashir A, Gray ML. MRI techniques in early stages of cartilage disease. Invest Radiol. 2000;35:622-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 168] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Sittek H, Eckstein F, Gavazzeni A, Milz S, Kiefer B, Schulte E, Reiser M. Assessment of normal patellar cartilage volume and thickness using MRI: an analysis of currently available pulse sequences. Skeletal Radiol. 1996;25:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Maataoui A, Graichen H, Abolmaali ND, Khan MF, Gurung J, Straub R, Qian J, Hinterwimmer S, Ackermann H, Vogl TJ. Quantitative cartilage volume measurement using MRI: comparison of different evaluation techniques. Eur Radiol. 2005;15:1550-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Bolog N, Nanz D, Weishaupt D. Muskuloskeletal MR imaging at 3.0 T: current status and future perspectives. Eur Radiol. 2006;16:1298-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Saupe N, Prüssmann KP, Luechinger R, Bösiger P, Marincek B, Weishaupt D. MR imaging of the wrist: comparison between 1.5- and 3-T MR imaging--preliminary experience. Radiology. 2005;234:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Weber MA, von Stillfried F, Kloth JK, Rehnitz C. Cartilage imaging of the hand and wrist using 3-T MRI. Semin Musculoskelet Radiol. 2012;16:71-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Wieners G, Detert J, Streitparth F, Pech M, Fischbach F, Burmester G, Ricke J, Backhaus M, Bruhn H. High-resolution MRI of the wrist and finger joints in patients with rheumatoid arthritis: comparison of 1.5 Tesla and 3.0 Tesla. Eur Radiol. 2007;17:2176-2182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Hudelmaier M, Glaser C, Pfau C, Eckstein F. Comparison between different implementations of the 3D FLASH sequence for knee cartilage quantification. MAGMA. 2012;25:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Lee VS, Lavelle MT, Rofsky NM, Laub G, Thomasson DM, Krinsky GA, Weinreb JC. Hepatic MR imaging with a dynamic contrast-enhanced isotropic volumetric interpolated breath-hold examination: feasibility, reproducibility, and technical quality. Radiology. 2000;215:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 136] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Duc SR, Koch P, Schmid MR, Horger W, Hodler J, Pfirrmann CW. Diagnosis of articular cartilage abnormalities of the knee: prospective clinical evaluation of a 3D water-excitation true FISP sequence. Radiology. 2007;243:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Hardy PA, Recht MP, Piraino D, Thomasson D. Optimization of a dual echo in the steady state (DESS) free-precession sequence for imaging cartilage. J Magn Reson Imaging. 1996;6:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 112] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Eckstein F, Hudelmaier M, Wirth W, Kiefer B, Jackson R, Yu J, Eaton CB, Schneider E. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis. 2006;65:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 224] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 25. | Ruehm S, Zanetti M, Romero J, Hodler J. MRI of patellar articular cartilage: evaluation of an optimized gradient echo sequence (3D-DESS). J Magn Reson Imaging. 1998;8:1246-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Peterfy CG, van Dijke CF, Janzen DL, Glüer CC, Namba R, Majumdar S, Lang P, Genant HK. Quantification of articular cartilage in the knee with pulsed saturation transfer subtraction and fat-suppressed MR imaging: optimization and validation. Radiology. 1994;192:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 281] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Peterfy CG, van Dijke CF, Lu Y, Nguyen A, Connick TJ, Kneeland JB, Tirman PF, Lang P, Dent S, Genant HK. Quantification of the volume of articular cartilage in the metacarpophalangeal joints of the hand: accuracy and precision of three-dimensional MR imaging. AJR Am J Roentgenol. 1995;165:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26:217-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2134] [Cited by in RCA: 2413] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 29. | Hopkins WG, Schabort EJ, Hawley JA. Reliability of power in physical performance tests. Sports Med. 2001;31:211-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 497] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 30. | Lassere M, McQueen F, Østergaard M, Conaghan P, Shnier R, Peterfy C, Klarlund M, Bird P, O’Connor P, Stewart N. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Exercise 3: an international multicenter reliability study using the RA-MRI Score. J Rheumatol. 2003;30:1366-1375. [PubMed] |
| 31. | Eckstein F, Winzheimer M, Westhoff J, Schnier M, Haubner M, Englmeier KH, Reiser M, Putz R. Quantitative relationships of normal cartilage volumes of the human knee joint--assessment by magnetic resonance imaging. Anat Embryol (Berl). 1998;197:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Karvonen RL, Negendank WG, Teitge RA, Reed AH, Miller PR, Fernandez-Madrid F. Factors affecting articular cartilage thickness in osteoarthritis and aging. J Rheumatol. 1994;21:1310-1318. [PubMed] |
| 33. | Tiderius CJ, Olsson LE, Leander P, Ekberg O, Dahlberg L. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn Reson Med. 2003;49:488-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 248] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 34. | Pelletier JP, Raynauld JP, Abram F, Haraoui B, Choquette D, Martel-Pelletier J. A new non-invasive method to assess synovitis severity in relation to symptoms and cartilage volume loss in knee osteoarthritis patients using MRI. Osteoarthritis Cartilage. 2008;16 Suppl 3:S8-13. [PubMed] |
| 35. | Chen CA, Lu W, John CT, Hargreaves BA, Reeder SB, Delp SL, Siston RA, Gold GE. Multiecho IDEAL gradient-echo water-fat separation for rapid assessment of cartilage volume at 1.5 T: initial experience. Radiology. 2009;252:561-567. [PubMed] |
| 36. | Madelin G, Babb J, Xia D, Chang G, Krasnokutsky S, Abramson SB, Jerschow A, Regatte RR. Articular cartilage: evaluation with fluid-suppressed 7.0-T sodium MR imaging in subjects with and subjects without osteoarthritis. Radiology. 2013;268:481-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 37. | McQueen FM, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan PL, McLean L. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals a high prevalence of erosions at four months after symptom onset. Ann Rheum Dis. 1998;57:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 298] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Cai W, Franklyn MJ, Maataoui A S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK