Published online Feb 18, 2026. doi: 10.5312/wjo.v17.i2.113405
Revised: September 21, 2025
Accepted: December 4, 2025
Published online: February 18, 2026
Processing time: 163 Days and 22.2 Hours
Osteoarthritis (OA) is a degenerative joint disease characterized by progressive articular cartilage destruction, subchondral bone alterations, and localized inflammation. With global population aging, the prevalence of OA continues to rise, imposing a substantial social and economic burden. In recent years, lipid me
Core Tip: This review summarizes the relationship between lipid metabolism disorders and osteoarthritis (OA), highlights the mechanisms by which representative phytochemicals (e.g., curcumin, green tea polyphenols, resveratrol) regulate lipid metabolism and attenuate OA progression, and further analyzes the endocrine role of adipose tissue, the impact of fatty acid metabolism on OA, and the therapeutic potential of phytochemicals through lipid metabolism modulation.
- Citation: Zhang T, Liu JL, Wang W, Ren K, Liu XM, Cao K, Li Z, Cheng XY, Zhang XY, Xu WS. Lipid metabolism disorders and osteoarthritis progression: Potential intervention with plant active ingredients. World J Orthop 2026; 17(2): 113405
- URL: https://www.wjgnet.com/2218-5836/full/v17/i2/113405.htm
- DOI: https://dx.doi.org/10.5312/wjo.v17.i2.113405
Osteoarthritis (OA) is a prevalent chronic degenerative joint disease, characterized by progressive articular cartilage degeneration, subchondral bone alterations, and localized chronic inflammation. OA primarily affects the knee, hip, and spine, particularly in older people, and its prevalence increases with age[1]. The clinical manifestations of OA include joint pain, functional impairment, and restricted mobility, which in severe cases, markedly reduce quality of life. Al
This review discusses the mechanisms of lipid metabolism in OA, examines how the endocrine functions of adipose tissue contribute to OA progression, evaluates the role of fatty acid metabolism, and analyzes how bioactive phytoche
Lipid metabolism encompasses the synthesis, catabolism, storage, transport, and utilization of fatty acids in the body. Disorders of lipid metabolism are strongly associated with multiple metabolic diseases (e.g., obesity, diabetes, and cardiovascular disease) and represent an important pathogenic mechanism in OA[7,8]. Adipose tissue functions not only as an energy reservoir but also as an endocrine organ that regulates immune responses, inflammation, and metabolic homeostasis through the secretion of adipokines (e.g., leptin, adiponectin, and TNF-α)[9]. Disorders of lipid metabolism, particularly imbalances in fatty acid metabolism, exacerbate inflammation, cartilage degradation, and osteophyte formation in OA. Adipokines play a pivotal role in OA pathogenesis, particularly in the context of obesity, diabetes, and other metabolic disorders, where their dysregulated secretion further accelerates disease progression[10].
Adipokines are bioactive molecules secreted by adipocytes, primarily including leptin, lipocalin, and TNF-α[11]. As a major proinflammatory adipokine, leptin primarily increases appetite, promotes fat storage, and plays a key role in chronic inflammation[12]. Elevated leptin levels are strongly associated with the onset and progression of OA. Studies have demonstrated that leptin enhances the secretion of inflammatory mediators and promotes cartilage degradation by activating signaling pathways such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK). Moreover, dysregulated adipokine secretion exacerbates chronic low-grade joint inflammation, leading to degenerative structural changes[13].
By contrast, lipocalin is an anti-inflammatory adipokine that regulates fatty acid metabolism and limits fat accumulation[14]. Lipocalin deficiency or dysfunction is strongly associated with OA development and progression. Clinical studies have shown that lipocalin levels are generally reduced in patients with OA[15]; therefore, enhancing lipocalin secretion may represent an effective strategy to alleviate OA symptoms.
Imbalance in fatty acid metabolism, particularly an altered omega-6/omega-3 fatty acid ratio, constitutes another important mechanism underlying OA progression. Omega-6 Fatty acids (e.g., arachidonic acid) are metabolized into prostaglandin E2 (PGE2) a potent proinflammatory mediator that promotes inflammation, cartilage degradation, and osteophyte formation[16]. By contrast omega-3 fatty acids (e.g. eicosapentaenoic acid and docosahexaenoic acid) exert anti-inflammatory effects and attenuate joint inflammation and damage in OA by inhibiting PGE2 synthesis[17]. Excessive ω-6 fatty acid intake in modern diets disrupts the omega-6/omega-3 balance thereby exacerbating OA pa
Adipose tissue serves not only as a site of energy storage but also as a crucial endocrine organ. It regulates systemic metabolism, immune responses, and inflammation through the secretion of various adipokines (e.g., leptin, lipocalin, and TNF-α). These adipokines play a pivotal role in the development and progression of OA, particularly in conditions such as obesity, metabolic syndrome, and diabetes mellitus, where their dysregulated secretion exacerbates OA pathogenesis[19].
The role of leptin as a key proinflammatory adipokine secreted by adipocytes has been extensively investigated. Elevated leptin levels not only increase appetite and fat storage but also accelerate OA progression by promoting inflammatory responses. Leptin aggravates joint inflammation by activating signaling pathways such as NF-κB and MAPK, thereby promoting cartilage degradation and joint destruction[20]. Therefore, targeting leptin secretion or receptor activity may represent an effective strategy to attenuate OA progression.
Lipocalin is an anti-inflammatory adipokine secreted by adipocytes that regulates fatty acid metabolism and limits fat accumulation. It attenuates chronic adipokine-driven inflammation and protects articular cartilage from degradation by activating signaling pathways such as AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor (PPAR)-γ. Lipocalin deficiency has been strongly associated with the onset and progression of OA[21]. Therefore, enhancing lipocalin expression may represent an effective therapeutic strategy for OA.
Fatty acid metabolism plays a critical role in OA development and progression, particularly when the omega-6/omega-3 fatty acid ratio is imbalanced. Excessive omega-6 fatty acids and their metabolites (e.g., PGE2) promote inflammation and accelerate articular cartilage degradation, thereby driving OA progression. By contrast omega-3 fatty acids exert anti-inflammatory effects and mitigate joint inflammation and cartilage damage in OA.
Modern diets are characterized by excessive omega-6 intake and insufficient omega-3 intake, resulting in an imbalanced omega-6/omega-3 ratio. Omega-6 fatty acid metabolites (e.g., PGE2) contribute to OA pathogenesis by promoting inflammation, accelerating cartilage degradation, and inducing osteophyte formation[22]. Therefore, restoring the omega-6/omega-3 balance particularly through increased omega-3 intake may represent an effective therapeutic strategy to alleviate OA symptoms.
Omega-3 fatty acids exert significant anti-inflammatory effects and can attenuate the inflammatory response induced by w-6 fatty acid metabolites through inhibition of PGE2 synthesis. Studies have demonstrated that increased omega-3 fatty acid intake slows OA progression and significantly improves joint function. Therefore, increasing omega-3 fatty acid intake or modulating fatty acid metabolism with plant-derived components (e.g., green tea polyphenols, and linolenic acid) is anticipated to be an effective therapeutic strategy for OA.
Overall, lipid metabolism disorders, particularly dysregulation of fatty acid metabolism, are closely associated with the onset and progression of OA. The endocrine effects of adipose tissue, abnormal adipokine secretion (e.g., leptin and lipocalin), and fatty acid metabolites exacerbate inflammation and articular cartilage degradation in OA. Plant-derived bioactive compounds slow OA progression by modulating lipid metabolism and alleviating inflammation induced by lipid metabolism disorders. Phytoconstituents such as curcumin, green tea polyphenols, resveratrol, and other phyto
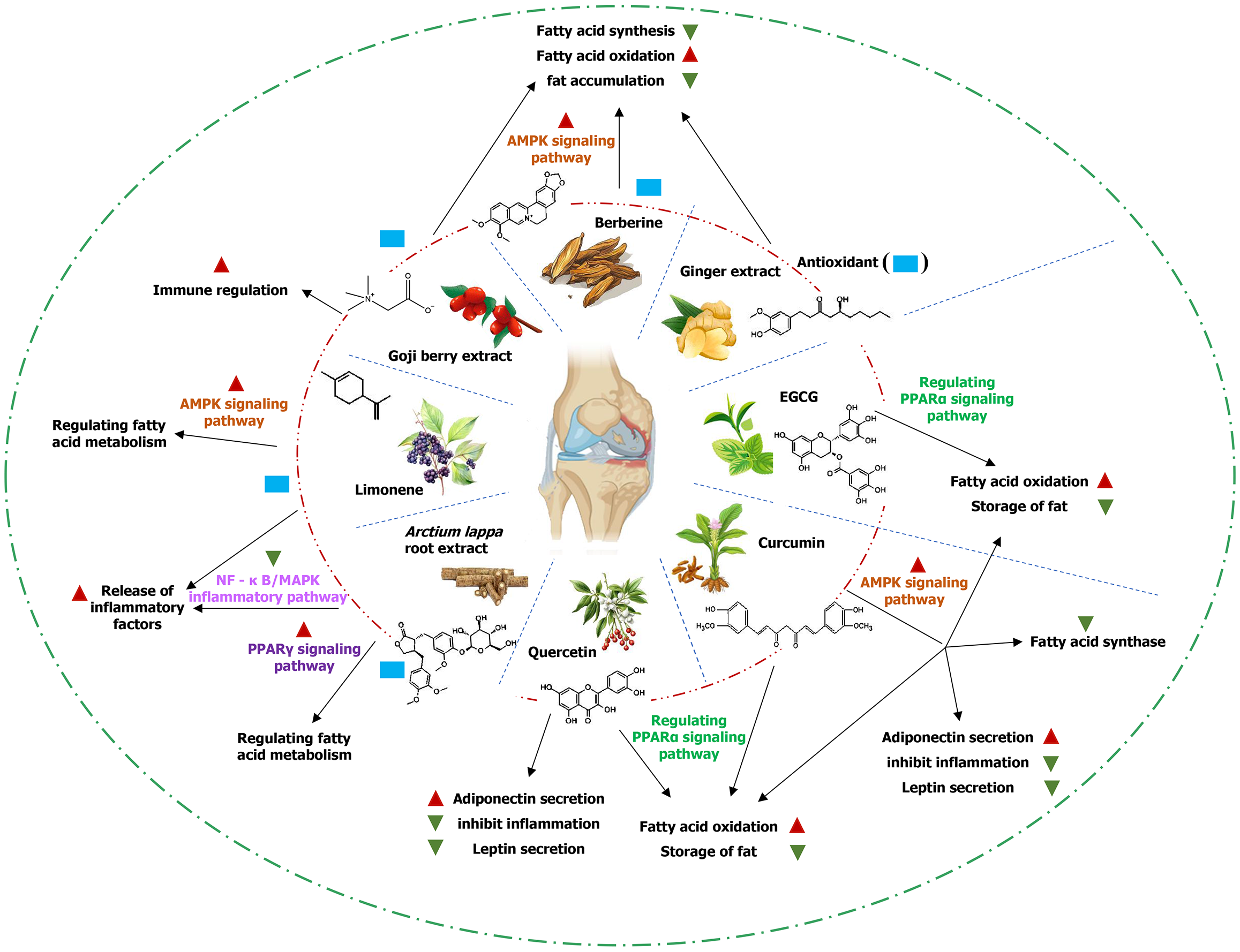
Owing to their diverse biological activities, plant-derived bioactive compounds represent effective natural therapeutics for modulating lipid metabolism and slowing the progression of OA. Regulation of lipid metabolism encompasses fatty acid synthesis and catabolism, adipokine secretion, adipocyte function, and related processes[23]. Numerous plant-derived bioactive compounds act through multiple pathways to ameliorate lipid metabolism disorders and mitigate inflammation and cartilage degradation induced by lipid metabolism dysregulation. The mechanisms by which common plant-derived bioactive compounds regulate lipid metabolism are detailed below (Figure 1).
Fatty acid synthesis represents a critical step in lipid metabolism with fatty acid synthase serving as a central regulatory enzyme. Excessive fatty acid synthesis results in lipid accumulation, which subsequently contributes to metabolic disorders and inflammatory responses particularly in obese individuals and patients with OA[24]. Numerous plant-derived bioactive compounds regulate lipid metabolism by suppressing the activity of fatty acid synthase and other key enzymes thereby reducing fatty acid synthesis.
Curcumin: Curcumin is a plant-derived bioactive compound with potent anti-inflammatory and antioxidant properties, widely investigated for the treatment of OA. Curcumin inhibits fatty acid synthesis through multiple mechanisms. Curcumin suppresses fatty acid synthesis by inhibiting fatty acid synthase activity, thereby preventing lipid accumulation. Studies have demonstrated that curcumin decreases the rate of fatty acid synthesis by downregulating fatty acid synthase expression thereby improving lipid metabolism[25]. Curcumin attenuates lipid synthesis by activating the AMPK signaling pathway and suppressing key enzymes involved in fatty acid biosynthesis.
Quercetin: Quercetin is a flavonoid abundantly present in fruits and vegetables, with diverse biological activities, particularly anti-inflammatory and antioxidant properties. Quercetin inhibits fatty acid synthesis through several mechanisms. Quercetin suppresses fatty acid synthesis by inhibiting fatty acid synthase activity[26]. Furthermore, by decreasing fatty acid synthesis, quercetin prevents lipid accumulation and attenuates the inflammatory response in OA[27]. Quercetin also reduces fatty acid synthesis and enhances lipid homeostasis by activating the PPARα signaling pathway.
Fatty acid oxidation is a critical process for maintaining lipid homeostasis and cellular function. Enhancement of fatty acid oxidation reduces lipid accumulation, corrects lipid metabolic disorders and slows the progression of OA. Numerous plant-derived active compounds enhance fatty acid catabolism and oxidation by activating key enzymes and signaling pathways[28].
Curcumin: Curcumin enhances fatty acid oxidation through the following mechanisms. Curcumin activates the AMPK signaling pathway a central regulator of fatty acid oxidation thereby enhancing lipid catabolism reducing lipid accumulation, and improving metabolic homeostasis. Curcumin enhances fatty acid oxidation and decreases lipid storage by stimulating the PPARα signaling pathway.
Green tea polyphenols: Epigallocatechin gallate (EGCG), a major catechin in green tea, possesses potent anti-inflammatory and antioxidant properties and is widely investigated in OA treatment. EGCG enhances fatty acid oxidation through the following mechanisms. EGCG activates the AMPK signaling pathway, thereby promoting fatty acid oxi
Adipokines including leptin and lipocalin play essential roles in lipid metabolism and OA pathogenesis. Leptin is a proinflammatory adipocyte-derived cytokine whereas lipocalin exerts anti-inflammatory effects. By modulating adi
Quercetin: Quercetin modulates adipokine secretion through the following mechanisms. Quercetin decreases leptin production in adipocytes, thereby attenuating leptin-induced inflammatory responses. As a proinflammatory adipokine, leptin plays a critical role in OA pathogenesis. By limiting leptin overproduction, quercetin helps mitigate chronic low-grade inflammation. Quercetin promotes lipocalin release, which exerts anti-inflammatory effects reduces adipokine-induced chronic inflammation and alleviates OA-related pathological changes.
Curcumin: Curcumin modulates adipokine balance through the following mechanisms. Curcumin suppresses adipokine-mediated inflammation by inhibiting leptin release. In addition, curcumin decreases the production of proinflammatory mediators thereby alleviating joint inflammation and contributing to the improvement of OA pathology. Curcumin upregulates lipocalin secretion via activation of the AMPK signaling pathway. Lipocalin reduces adipokine-driven chronic inflammation and protects chondrocytes against oxidative injury.
Oxidative stress is acritical contributor to the progression of OA, as excessive free radicals accelerate articular cartilage injury. Plant-derived bioactive compounds alleviate oxidative stress through antioxidant mechanisms and indirectly modulate lipid metabolism, thereby attenuating OA progression.
Curcumin (turmeric extract): Curcuminexerts antioxidant activity and improves lipid metabolism through the following mechanisms. Curcumin exhibits potent free-radical-scavenging activity thereby reducing oxidative-stress-induced cartilage damage and attenuating OA progression[30]. Curcumin reduces lipid deposition by inhibiting fatty acid synthesis and promoting fatty acid oxidation thereby mitigating inflammation induced by dysregulated lipid metabolism.
Berberine: Berberine a major bioactive alkaloid derived from traditional herbal medicine possesses marked antioxidant properties. Its effects on lipid metabolism involve the following mechanisms. Berberine reduces oxidative-stress-induced cartilage injury by scavenging free radicals and enhancing antioxidant enzyme activity. Berberine activates the AMPK signaling pathway suppresses lipid synthesis promotes fatty acid oxidation, and reduces lipid accumulation thereby attenuating inflammation associated with dysregulated lipid metabolism[31].
Limonene: Limonene is a naturally occurring monoterpenoid predominantly found in citrus fruits and various aromatic plants. It exhibits anti-inflammatory, antioxidant, and antimicrobial and antimicrobial properties, and has recently been investigated for its therapeutic potential in OA particularly through the regulation of lipid metabolism and attenuation of disease progression. Limonene inhibits inflammatory signaling pathways including NF-κB and MAPK thereby reducing their lease of proinflammatory mediators. In the context of lipid metabolism imbalance excessive cytokine production accelerates OA progression; limonene mitigates this process by suppressing these inflammatory pathways[32]. Limonene enhances AMPK activity, which promotes fatty acid oxidation and reduces lipid accumulation. By stimulating fatty acid oxidation while lipid synthesis, limonene helps restore lipid metabolic homeostasis[33]. Limonene demonstrates strong free-radical-scavenging capacity, alleviating oxidative-stress-induced cartilage damage. Considering the central role of oxidative stress in OA pathogenesis, limonene protects chondrocytes and slows OA progression through its antioxidant activity[34].
Arctium lappa root extract: Arctium lappa root commonly known as burdock root is a traditional herbal medicine widely used in traditional Chinese medicine. It exhibits diverse biological activities including anti-inflammatory, antioxidant, and antibacterial effects. Its main active constituents are polysaccharides, volatile oils, and flavonoids, which have been investigated for therapeutic applications in multiple diseases including OA. Burdock root extract is rich in polyphenols and flavonoids with potent anti-inflammatory activity. It reduces chronic low-grade inflammation associated with lipid metabolism disorders by inhibiting secretion of proinflammatory adipokines such as leptin from adipocytes. In addition, it suppresses inflammatory signaling pathways, including NF-κB and MAPK, thereby decreasing the release of inflammatory mediators in the joint microenvironment and alleviating OA symptoms. Burdock root extract modulates lipid metabolism by activating the PPARγ signaling pathway; acritical regulator of adipocyte differentiation and function. Activation of PPARγ enhances fatty acid oxidation reduces lipid accumulation and improves lipid homeostasis. By restoring adipocyte metabolic balance, burdock root extract attenuates inflammatory responses driven by excessive fat deposition[35]. The flavonoid and polysaccharide components in burdock root extract possess strong antioxidant capacity. They effectively scavenge free radical sand mitigate oxidative-stress-induced cartilage injury thereby slowing cartilage matrix degradation and OA progression. Given the central role of oxidative stress in OA pathogenesis, this antioxidant effect provides an additional protective mechanism[36].
Lycium barbarum extract: Goji berry (Lycium barbarum), a traditional Chinese herbal medicine contains abundant carotenoids, polysaccharides, flavonoids, and other bioactive compounds. In recent years, its extract has gained in
OA is a common degenerative joint disease in which lipid metabolism disorders play acritical role in disease onset and progression. Adipose tissue not only functions as an energy reservoir but also actively regulates immune responses, inflammation, and cartilage degradation through the secretion of bioactive molecules such as leptin and lipocalin. Dysregulation of lipid metabolism, particularly imbalances in fatty acid synthesis and catabolism promotes chronic low-grade inflammation in the joint microenvironment and accelerates OA progression. Consequently, targeting lipid me
Lipid metabolic alterations as potential biomarkers: Early diagnosis is essential for effective OA management, enabling timely intervention to slow disease progression. Lipid metabolic disturbances, particularly imbalances in adipokine secretion (e.g., leptin and lipocalin) and fatty acid metabolites (e.g., PGE2, omega-6/omega-3 fatty acid ratios), appear during the early stages of OA. These alterations hold promise as potential biomarkers offering opportunities for early disease detection and risk stratification.
Adipokines and fatty acid metabolites as early diagnostic biomarkers: Adipokines including leptin and lipocalin, as well as fatty acid metabolites such as PGE2 and other omega-6 derivatives exhibit measurable alterations during the early stages of OA and therefore, hold promise as diagnostic biomarkers. Clinical studies have demonstrated that patients with OA present elevated levels of leptin and reduced levels of lipocalin in synovial fluid, reflecting an imbalance in lipid metabolism and inflammatory homeostasis[40]. Monitoring these molecules provides a valuable basis for early detection of OA. Leptin is a proinflammatory adipokine secreted by adipocytes. Its elevated concentration in synovial fluid is closely associated with enhanced inflammatory responses and cartilage degradation in OA[41]. Detection of leptin level changes may therefore, serve as a sensitive indicator of early disease onset. In contrast lipocalin possesses anti-inflammatory activity, and decreased levels have been consistently observed in OA patients. Reduced lipocalin expression not only reflects impaired lipid metabolism but also correlates with disease progression. Metabolites derived from omega-6 fatty acids, particularly PGE2, are significantly elevated in the joint microenvironment of OA patients. These metabolites serve as markers of lipid metabolic imbalance and provide insights into the stage and severity of OA progression[42].
The value of plant-derived strategies for regulating lipid metabolism, as proposed in this review, must ultimately be confirmed by clinical efficacy and safety. In recent years, an increasing number of clinical studies have investigated and supported the potential of lipid-targeted interventions in OA treatment, providing preliminary yet promising clinical evidence for the arguments presented in this review. Multiple randomized controlled trials (RCTs) have demonstrated that direct supplementation with omega-3 polyunsaturated fatty acids significantly improves joint pain and function in patients with OA. In one RCT, daily supplementation with omega-3 fatty acids (including eicosapentaenoic acid and docosahexaenoic acid) for 24 weeks significantly reduced pain (visual analog scale scores) and improved Western Ontario and McMaster Universities Osteoarthritis Index functional indices compared with placebo. Serum levels of inflammatory markers, including C-reactive protein and interleukin-6, were significantly reduced[43]. These findings directly support the effectiveness of modulating fatty acid composition in alleviating OA symptoms and attenuating systemic inflammation.
In addition, several plant-derived bioactive compounds discussed in this review have progressed to clinical evaluation. For instance, a double-blind RCT in patients with knee OA demonstrated that daily intake of standardized curcumin extract (commonly coadministered with piperine to enhance bioavailability) significantly improved Western Ontario and McMaster Universities Osteoarthritis Index pain and function scores compared with placebo. The therapeutic effect was comparable to that of nonsteroidal anti-inflammatory drugs (NSAIDs), but with a significantly lower incidence of gastrointestinal adverse events[44]. Similarly, clinical trials of green tea extract (EGCG) have reported comparable outcomes, indicating its potential to alleviate joint discomfort and improve quality of life[45]. With regard to berberine, although preclinical studies suggest a cartilage-protective effect, most evidence derives from animal models and in vitro experiments. Rigorous clinical trials are urgently needed to verify its efficacy and long-term safety in human OA[46].
Although still preliminary, these clinical data from well-characterized patient cohorts strongly support the rationale and feasibility of targeting lipid metabolism through either direct fatty acid supplementation or plant-derived com
In the future, early diagnostic tools based on lipid metabolism disorders may become an important supplement to clinical management of OA. Establishing biomarker detection systems that integrate lipid-metabolism-related indicators with advanced technologies such as mass spectrometry, metabolomics, and genomics will enable accurate diagnosis at early disease stages. Such approaches not only provide a foundation for timely therapeutic intervention but also allow for dynamic monitoring of treatment efficacy. Moreover, evaluation of lipid metabolism profiles could guide clinical deci
Precision therapy: Individualized treatment strategies for lipid metabolism regulation: Precision therapy emphasizes the customization of treatment regimens based on individual variability, including genetic background, metabolic profiles, and pathological features. Lipid metabolism regulation offers significant potential within this framework for OA management. Advances in molecular biology, systems biology, and multiomics technologies are accelerating the de
Personalized lipid metabolism intervention: Personalized therapeutic strategies can be developed based on the lipid metabolism characteristics of OA patients, such as adipokine levels and fatty acid ratios. For instance, in patients with elevated leptin levels, plant-derived bioactive that predominantly inhibit leptin secretion, such as curcumin and quercetin, may be selected to attenuate inflammation. Conversely, in patients with disrupted fatty acid balance, particularly those exhibiting a high omega-6/omega-3 ratio, botanical compounds rich in omega-3 fatty acids (e.g., linolenic acid and green tea polyphenols) can be used to restore metabolic balance and mitigate the inflammatory response.
Combination therapy and targeted therapy: The integration of plant active ingredients with conventional pharmacological agents offers the potential for synergistic effects, enhanced therapeutic efficacy, and reduced adverse reactions. Botanical compounds can be combined with standard treatments, including NSAIDs, corticosteroids, or disease-mo
The clinical application of lipid metabolism regulation in OA holds significant promise, especially for early diagnosis and precision therapy. Future research directions are expected to focus on the following: (1) Innovation of early diagnostic tools: Development of biomarker-based detection systems linked to lipid metabolism, in combination with modern technologies such as mass spectrometry and metabolomics, will facilitate early diagnosis and timely intervention; (2) Personalized lipid metabolism intervention: Tailored therapies based on individual lipid metabolism profiles will re
Lipid metabolism regulation has significant clinical application prospects in the treatment of OA. Regulating lipid metabolism through plant active ingredients can alleviate chronic inflammation caused by lipid metabolism disorders, improve joint cartilage repair, and slow down the progression of OA. This study systematically reviewed the role of lipid metabolism disorders in the progression of OA. It explored in depth the potential mechanism of plant active ingredients regulating lipid metabolism through multitarget regulation to delay OA. Compared with previous studies, the innovation of this article mainly lies in the following aspects. Firstly, existing research mostly focuses on the role of a single fat factor or a certain type of fatty acid, while this review comprehensively analyzes the endocrine function of adipose tissue, the imbalance of fatty acid metabolism, and its complex network relationship with OA, providing a more comprehensive pathological mechanism perspective. Secondly, this review systematically classified for the first time the mechanisms of action of various plant components such as curcumin, quercetin, green tea polyphenols, naringenin, and burdock root extract in regulating lipid metabolism, and proposed their potential for combined application and personalized treat
| 1. | Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1458] [Cited by in RCA: 3060] [Article Influence: 437.1] [Reference Citation Analysis (1)] |
| 2. | Wei G, Lu K, Umar M, Zhu Z, Lu WW, Speakman JR, Chen Y, Tong L, Chen D. Risk of metabolic abnormalities in osteoarthritis: a new perspective to understand its pathological mechanisms. Bone Res. 2023;11:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 3. | Binvignat M, Sellam J, Berenbaum F, Felson DT. The role of obesity and adipose tissue dysfunction in osteoarthritis pain. Nat Rev Rheumatol. 2024;20:565-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 68] [Reference Citation Analysis (0)] |
| 4. | Zapata-Linares N, Loisay L, de Haro D, Berenbaum F, Hügle T, Geurts J, Houard X. Systemic and joint adipose tissue lipids and their role in osteoarthritis. Biochimie. 2024;227:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Maouche A, Boumediene K, Baugé C. Bioactive Compounds in Osteoarthritis: Molecular Mechanisms and Therapeutic Roles. Int J Mol Sci. 2024;25:11656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 6. | Zhang T, Deng W, Deng Y, Liu Y, Xiao S, Luo Y, Xiang W, He Q. Mechanisms of ferroptosis regulating oxidative stress and energy metabolism in myocardial ischemia-reperfusion injury and a novel perspective of natural plant active ingredients for its treatment. Biomed Pharmacother. 2023;165:114706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 7. | Collins KH, Lenz KL, Pollitt EN, Ferguson D, Hutson I, Springer LE, Oestreich AK, Tang R, Choi YR, Meyer GA, Teitelbaum SL, Pham CTN, Harris CA, Guilak F. Adipose tissue is a critical regulator of osteoarthritis. Proc Natl Acad Sci U S A. 2021;118:e2021096118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 142] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 8. | Bhattacharjee M, Escobar Ivirico JL, Kan HM, Shah S, Otsuka T, Bordett R, Barajaa M, Nagiah N, Pandey R, Nair LS, Laurencin CT. Injectable amnion hydrogel-mediated delivery of adipose-derived stem cells for osteoarthritis treatment. Proc Natl Acad Sci U S A. 2022;119:e2120968119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 9. | Toussirot E. Mini-Review: The Contribution of Adipokines to Joint Inflammation in Inflammatory Rheumatic Diseases. Front Endocrinol (Lausanne). 2020;11:606560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Dickson BM, Roelofs AJ, Rochford JJ, Wilson HM, De Bari C. The burden of metabolic syndrome on osteoarthritic joints. Arthritis Res Ther. 2019;21:289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Taylor EB. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin Sci (Lond). 2021;135:731-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 12. | Picó C, Palou M. Leptin and Metabolic Programming. Nutrients. 2021;14:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Apostu D, Lucaciu O, Mester A, Oltean-Dan D, Baciut M, Baciut G, Bran S, Onisor F, Piciu A, Pasca RD, Maxim A, Benea H. Systemic drugs with impact on osteoarthritis. Drug Metab Rev. 2019;51:498-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Fang H, Judd RL. Adiponectin Regulation and Function. Compr Physiol. 2018;8:1031-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 446] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 15. | Pereira Herrera B, Emanuel K, Emans PJ, van Griensven M, Cillero-Pastor B. Infrapatellar fat pad as a source of biomarkers and therapeutic target for knee osteoarthritis. Arthritis Res Ther. 2025;27:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Chen X, Liu J, Wang G, Sun Y, Ding X, Zhang X. Regulating lipid metabolism in osteoarthritis: a complex area with important future therapeutic potential. Ann Med. 2024;56:2420863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 17. | Cordingley DM, Cornish SM. Omega-3 Fatty Acids for the Management of Osteoarthritis: A Narrative Review. Nutrients. 2022;14:3362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 18. | Thomas S, Browne H, Mobasheri A, Rayman MP. What is the evidence for a role for diet and nutrition in osteoarthritis? Rheumatology (Oxford). 2018;57:iv61-iv74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 19. | Ait Eldjoudi D, Cordero Barreal A, Gonzalez-Rodríguez M, Ruiz-Fernández C, Farrag Y, Farrag M, Lago F, Capuozzo M, Gonzalez-Gay MA, Mera Varela A, Pino J, Gualillo O. Leptin in Osteoarthritis and Rheumatoid Arthritis: Player or Bystander? Int J Mol Sci. 2022;23:2859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 20. | Ilia I, Nitusca D, Marian C. Adiponectin in Osteoarthritis: Pathophysiology, Relationship with Obesity and Presumptive Diagnostic Biomarker Potential. Diagnostics (Basel). 2022;12:455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Economou A, Mallia I, Fioravanti A, Gentileschi S, Nacci F, Bellando Randone S, Lepri G, Guiducci S. The Role of Adipokines between Genders in the Pathogenesis of Osteoarthritis. Int J Mol Sci. 2024;25:10865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 22. | Petrenko V, Sinturel F, Riezman H, Dibner C. Lipid metabolism around the body clocks. Prog Lipid Res. 2023;91:101235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 23. | Wang C, Fang L, Shi M, Niu X, Li T, Li X, Cho K, He Y, Liu S, Lu A, Xing X, Lukowski J, Goo YA, Speakman JR, Chen D, O'Keefe RJ, Patti GJ, Zuscik MJ, Zhang B, Shen J. NFIA regulates articular chondrocyte fatty acid metabolism and joint homeostasis. Sci Transl Med. 2025;17:eadm9488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Moetlediwa MT, Jack BU, Mazibuko-Mbeje SE, Pheiffer C, Titinchi SJJ, Salifu EY, Ramharack P. Evaluating the Therapeutic Potential of Curcumin and Synthetic Derivatives: A Computational Approach to Anti-Obesity Treatments. Int J Mol Sci. 2024;25:2603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Peng H, Shahidi F. Quercetin Fatty Acid Monoesters (C2:0-C18:0): Enzymatic Preparation and Antioxidant Activity. J Agric Food Chem. 2022;70:14073-14083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Ruan H, Zhu T, Wang T, Guo Y, Liu Y, Zheng J. Quercetin Modulates Ferroptosis via the SIRT1/Nrf-2/HO-1 Pathway and Attenuates Cartilage Destruction in an Osteoarthritis Rat Model. Int J Mol Sci. 2024;25:7461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 27. | Tie F, Ding J, Hu N, Dong Q, Chen Z, Wang H. Kaempferol and Kaempferide Attenuate Oleic Acid-Induced Lipid Accumulation and Oxidative Stress in HepG2 Cells. Int J Mol Sci. 2021;22:8847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 28. | Wei H, Qin J, Huang Q, Jin Z, Zheng L, Zhao J, Qin Z. Epigallocatechin-3-gallate (EGCG) based metal-polyphenol nanoformulations alleviates chondrocytes inflammation by modulating synovial macrophages polarization. Biomed Pharmacother. 2023;161:114366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 29. | Baek HI, Shen L, Ha KC, Park YK, Kim CS, Kwon JE, Park SJ. Effectiveness and safety of steamed ginger extract on mild osteoarthritis: a randomized, double-blind, placebo-controlled clinical trial. Food Funct. 2024;15:9512-9523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 30. | Li J, Wang Y, Chen D, Liu-Bryan R. Oral administration of berberine limits post-traumatic osteoarthritis development and associated pain via AMP-activated protein kinase (AMPK) in mice. Osteoarthritis Cartilage. 2022;30:160-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 31. | Shaker DS, Ishak RAH, Elhuoni MA, Ghoneim AM. Boosting transdermal delivery of atorvastatin calcium via o/w nanoemulsifying system: Two-step optimization, ex vivo and in vivo evaluation. Int J Pharm. 2020;578:119073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | El-Dahmy RM, El-Nabarawi MA, Mostafa HG, Salama A, Sheta NM. Role of chitosan coated invasomes in enhancement of transdermal delivery of tenoxicam for management of osteoarthritis: In vitro characterization, statistical optimization, ex vivo, and in vivo assessments. Int J Pharm. 2025;678:125700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 33. | Melino VJ, Soole KL, Ford CM. Ascorbate metabolism and the developmental demand for tartaric and oxalic acids in ripening grape berries. BMC Plant Biol. 2009;9:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Anandakumar P, Kamaraj S, Vanitha MK. D-limonene: A multifunctional compound with potent therapeutic effects. J Food Biochem. 2021;45:e13566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 35. | Liu Y, Hou M, Pan Z, Tian X, Zhao Z, Liu T, Yang H, Shi Q, Chen X, Zhang Y, He F, Zhu X. Arctiin-reinforced antioxidant microcarrier antagonizes osteoarthritis progression. J Nanobiotechnology. 2022;20:303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 36. | Maeta A, Okamoto Y, Ishikawa H, Matsunaga T, Takahashi K. Japanese Leaf Burdock Extract Inhibits Adipocyte Differentiation in 3T3-L1 Cells. Plant Foods Hum Nutr. 2025;80:32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Ren Y, Wang K, Wu Y, Li J, Ma J, Wang L, Zhang C, Li J, Wei Y, Yang Y. Lycium barbarum polysaccharide mitigates high-fat-diet-induced skeletal muscle atrophy by promoting AMPK/PINK1/Parkin-mediated mitophagy. Int J Biol Macromol. 2025;301:140488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 38. | Ni J, Au M, Kong H, Wang X, Wen C. Lycium barbarum polysaccharides in ageing and its potential use for prevention and treatment of osteoarthritis: a systematic review. BMC Complement Med Ther. 2021;21:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Zhu J, Zhen G, An S, Wang X, Wan M, Li Y, Chen Z, Guan Y, Dong X, Hu Y, Cao X. Aberrant subchondral osteoblastic metabolism modifies Na(V)1.8 for osteoarthritis. Elife. 2020;9:e57656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Collins KH, Lenz KL, Welhaven HD, Ely E, Springer LE, Paradi S, Tang R, Braxton L, Akk A, Yan H, Zhang B, Wu X, Atkinson JP, Oestreich AK, June RK, Pham CTN, Guilak F. Adipose-derived leptin and complement factor D mediate osteoarthritis severity and pain. Sci Adv. 2025;11:eadt5915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 41. | Jiang W, Jin Y, Zhang S, Ding Y, Huo K, Yang J, Zhao L, Nian B, Zhong TP, Lu W, Zhang H, Cao X, Shah KM, Wang N, Liu M, Luo J. PGE2 activates EP4 in subchondral bone osteoclasts to regulate osteoarthritis. Bone Res. 2022;10:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 42. | Henrotin Y, Malaise M, Wittoek R, de Vlam K, Brasseur JP, Luyten FP, Jiangang Q, Van den Berghe M, Uhoda R, Bentin J, De Vroey T, Erpicum L, Donneau AF, Dierckxsens Y. Bio-optimized Curcuma longa extract is efficient on knee osteoarthritis pain: a double-blind multicenter randomized placebo controlled three-arm study. Arthritis Res Ther. 2019;21:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 43. | Li M, Tian F, Guo J, Li X, Ma L, Jiang M, Zhao J. Therapeutic potential of Coptis chinensis for arthritis with underlying mechanisms. Front Pharmacol. 2023;14:1243820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 44. | Richette P, Latourte A, Frazier A. Safety and efficacy of paracetamol and NSAIDs in osteoarthritis: which drug to recommend? Expert Opin Drug Saf. 2015;14:1259-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 45. | Zhu W, Tang H, Cao L, Zhang J, Li J, Ma D, Guo C. Epigallocatechin-3-O-gallate ameliorates oxidative stress-induced chondrocyte dysfunction and exerts chondroprotective effects via the Keap1/Nrf2/ARE signaling pathway. Chem Biol Drug Des. 2022;100:108-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 46. | Li N, Chen QW, Gong XL, Liu F, Zhang B, Wang QS, Zhang H, Han JJ. Metformin and berberine synergistically improve NAFLD via the AMPK-SREBP1-FASN signaling pathway. Sci Rep. 2025;15:29400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/