Published online Jan 18, 2026. doi: 10.5312/wjo.v17.i1.112738
Revised: August 20, 2025
Accepted: November 18, 2025
Published online: January 18, 2026
Processing time: 157 Days and 22.4 Hours
Orthobiologics-biological substances like platelet-rich plasma (PRP), bone marrow aspirate concentrate, and stem cells-are increasingly used in musculoskeletal care to promote tissue repair and reduce reliance on invasive surgery. Despite global momentum, India's clinical adoption remains underexplored.
To inform education, policy, and resource allocation for the safe and effective adoption of orthobiologics in musculoskeletal care.
A cross-sectional electronic survey was conducted from January to March 2025 among orthopaedic surgeons, academicians, and trainees across India. The questionnaire assessed demographics, knowledge of orthobiologics, attitudes toward training and subspecialization, usage trends, regulatory awareness, and perceived barriers. Data were analyzed using descriptive statistics and χ2/Fisher’s exact tests, with P < 0.05 considered significant.
A total of 1280 valid responses were collected. Awareness of orthobiologics was high (97%), with PRP being the most familiar and widely used (80%). Formal training was reported by only 31%, though 85% showed interest in structured education, and 68% supported orthobiologics as a subspecialty. Satisfaction with clinical outcomes averaged 6.5 ± 2.3 out of 10 points. Barriers included high treatment cost (64%), poor patient awareness (90%), and limited access to biologics labs (18%). Regulatory understanding was moderate, with academic-affiliated clinicians more informed about stem cell guidelines.
Indian orthopaedic professionals demonstrate strong awareness and optimism toward orthobiologics, but widespread gaps in training, infrastructure, and regulation hinder broader adoption. Strategic investments in education, standardized protocols, and accessible facilities are essential to support safe and evidence-driven integration of regenerative therapies into clinical practice.
Core Tip: Indian orthopaedic professionals demonstrate strong awareness and growing interest in orthobiologic therapies, especially platelet-rich plasma. Despite this enthusiasm, the availability of formal training and understanding of regulatory frameworks remains limited. Systemic challenges-including treatment costs, infrastructure constraints, and patient unfamiliarity-continue to hinder broader application of advanced biologics. These findings underscore the need for targeted education, standardized protocols, and enhanced facilities to promote safe, evidence-based integration of regenerative therapies in routine musculoskeletal practice.
- Citation: Jeyaraman M, Jeyaraman N, Ramasubramanian S, Nallakumarasamy A, Devanand V, Muthu S. Mapping awareness and application of orthobiologics among orthopaedic professionals: A cross-sectional study. World J Orthop 2026; 17(1): 112738
- URL: https://www.wjgnet.com/2218-5836/full/v17/i1/112738.htm
- DOI: https://dx.doi.org/10.5312/wjo.v17.i1.112738
Orthobiologics, also known as regenerative orthopaedic therapies, encompass biological substances derived from cells, blood, or tissues that aid musculoskeletal healing. Examples include platelet-rich plasma (PRP), bone marrow aspirate concentrate (BMAC), mesenchymal stem cells, and other tissue-derived factors[1]. These interventions have progressed from experimental concepts to emerging treatment options for a variety of orthopaedic conditions (e.g., osteoarthritis, tendon injuries, fractures, spinal disorders)[2]. By harnessing the body’s natural healing pathways, orthobiologics can enhance tissue repair and potentially reduce the need for invasive surgeries or long-term pharmacotherapy[1].
Over the past decade, interest and investment in orthobiologics have surged worldwide. A growing number of orthopaedic practitioners are incorporating orthobiologic therapies into clinical practice, reflecting a shift toward biologically based treatments in sports medicine and joint preservation[3]. For example, a recent survey of sports medicine physicians in the United States found that two-thirds were using at least one orthobiologic modality, with PRP being the most commonly employed[2,4]. The global orthobiologics market is also expanding, driven by advances in biologic therapies and patient demand for minimally invasive options[5]. This rising adoption underscores the perceived value of orthobiologics as adjuncts or alternatives to traditional treatments.
Despite this enthusiasm, several challenges impede the full integration of orthobiologics into standard practice. The evidence base, while growing, remains mixed for many applications, leading to ongoing debates about efficacy and optimal indications[6]. Variations in product preparation and treatment protocols (for instance, different PRP for
A critical gap in the literature is understanding how these trends translate in the Indian context. While regenerative therapies are gaining popularity in India, there is little data on the awareness and practice patterns of orthobiologics among Indian orthopaedic professionals[9]. It remains unclear to what extent interest in orthobiologics has translated into clinical adoption, especially given challenges like limited formal training opportunities and uncertain guidelines. To address this gap, we conducted a cross-sectional survey to map the awareness and application of orthobiologic therapies among orthopaedic practitioners across India. The study aimed to assess clinicians’ familiarity with various orthobiologic modalities, evaluate their self-reported usage patterns and satisfaction, gauge their understanding of relevant regulations, and identify attitudes and perceived barriers to integrating regenerative therapies. By establishing baseline data on these parameters, our goal is to inform education, policy, and resource allocation for the safe and effective adoption of orthobiologics in musculoskeletal care.
This cross-sectional survey was conducted among orthopaedic professionals across India to assess their awareness, attitudes, and practices regarding orthobiologic therapies. Eligible participants included practising orthopaedic surgeons, academicians, and trainees actively involved in musculoskeletal care.
A structured, expert-reviewed questionnaire was deployed electronically. It comprised sections on:
Demographics: Age, gender, place of practice, years of experience.
Knowledge: Familiarity with orthobiologic modalities (e.g., PRP, BMAC, stem cells), understanding of preparation techniques and mechanisms.
Attitude: Interest in training, perceived value of orthobiologics, openness to subspecialty development.
Practice: Frequency of use, indications, satisfaction levels, perceived barriers.
Regulatory awareness: Understanding of national and international guidelines.
Items used binary (yes/no), multiple-choice, and Likert scale responses. The tool was piloted for clarity and content validity by a panel of orthobiologic experts. Formal training was defined as structured educational programs including workshops, certification courses, fellowship training, or continuing medical education specifically focused on orthobiologic preparation and application techniques[10].
Survey distribution occurred via professional orthopaedic networks, academic forums, and social media platforms between January and March 2025. Participation was voluntary and anonymous, with electronic informed consent embedded at survey initiation.
A total of 1450 questionnaires were distributed electronically across orthopaedic professional networks. After removing incomplete responses (< 80% completion) and duplicate entries, 1280 valid responses were included in the final analysis, yielding a response rate of 88.3%. Inclusion criteria comprised practicing orthopaedic surgeons, academicians, and trainees actively involved in musculoskeletal care within India. Exclusion criteria included incomplete questionnaires and responses from non-orthopaedic practitioners.
The study protocol was approved by the Institutional Ethics Committee of Sri Lalithambigai Medical College and Hospital, Dr MGR Educational and Research Institute, Chennai. Participant confidentiality and data protection were ensured throughout the conduction of the study.
Descriptive statistics summarised demographic profiles and response frequencies. Associations between variables were analysed using χ2 or Fisher’s exact tests. Mean satisfaction scores were calculated with standard deviation. Analysis was performed in SPSS v25, with significance set at P < 0.05.
The survey yielded 1280 valid responses from orthopaedic professionals across India, capturing a wide spectrum of practitioners from government medical colleges, private hospitals, and academic institutions. A majority of respondents (66%) were below the age of 40, reflecting a relatively younger demographic actively engaged in clinical care and medical education as shown in Table 1. Gender distribution was skewed toward male practitioners, comprising 96% of the cohort, which echoes established gender patterns in surgical specialities across India. Additionally, 52% of respondents were affiliated with teaching institutions, while 38% practised in private setups. Nearly half (48%) reported having less than five years of orthopaedic experience. These demographics suggest that emerging professionals in academic and early-career settings are at the forefront of orthobiologic exposure in India. This also implies an opportunity to integrate orthobiologic training earlier in surgical education, potentially shaping future practice norms and research interests. Usage patterns showed that PRP was predominantly employed across all practice settings, though practitioners with > 10 years experience were more likely to attempt advanced biologics compared to those with < 5 years experience (P < 0.001). Academic-affiliated practitioners demonstrated higher awareness of regulatory guidelines compared to private practice physicians (72% vs 58%, P < 0.05).
| Parameter | Category | Frequency (n) | Percentage (%) |
| Age | < 30 years | 539 | 42.1 |
| 30–39 years | 306 | 23.9 | |
| ≥ 40 years | 435 | 34.0 | |
| Gender | Male | 1230 | 96.1 |
| Female | 50 | 3.9 | |
| Years of orthopaedic experience | < 5 years | 617 | 48.2 |
| 5–10 years | 416 | 32.5 | |
| > 10 years | 247 | 19.3 | |
| Practice affiliation | Medical college | 667 | 52.1 |
| Private hospital | 485 | 37.9 | |
| Others | 128 | 10.0 |
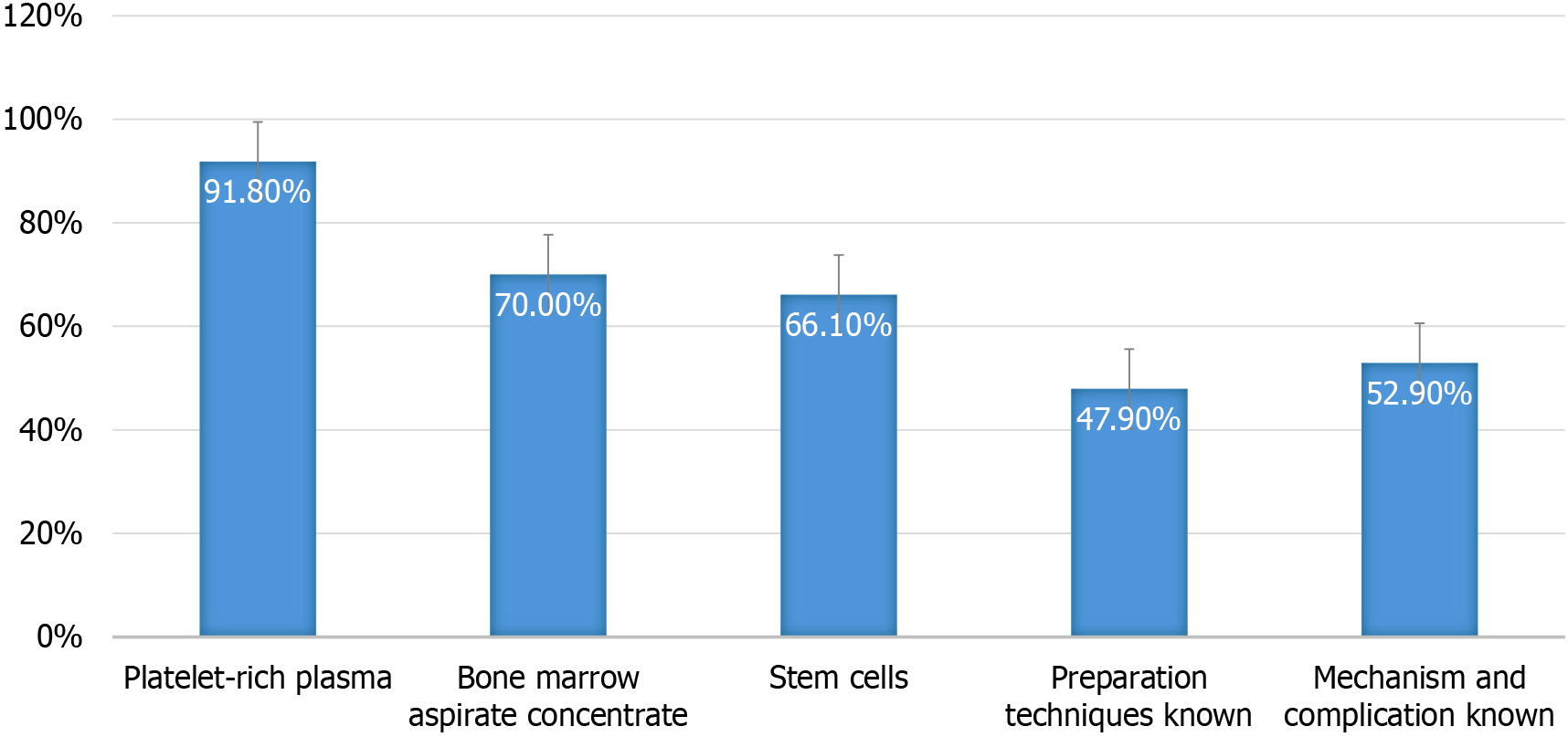
Knowledge regarding orthobiologics was remarkably high across the sample, with 97% of participants indicating they were familiar with regenerative therapies. PRP was the most widely known modality (92%), followed by BMAC (70%) and stem cells (66%) as shown in Figure 1. While foundational awareness appeared robust, knowledge about biologic mechanisms, preparation protocols, and clinical applications was more limited. Only 48% demonstrated familiarity with preparation techniques, and just 53% understood the underlying mechanisms and associated complications. This reveals a significant gap between surface-level recognition and deeper expertise, emphasising the urgent need for continuing medical education. Without comprehensive understanding, clinicians may adopt therapies without fully grasping patient selection, safety nuances, or biologic variability. Hence, structured training programs and detailed guidelines are essential to bridge this divide and ensure evidence-based application of orthobiologics.
Formal education in orthobiologics was received by only 31% of participants. Despite the low exposure to organised training, there was notable eagerness for professional development in this domain. Approximately 85% expressed a strong interest in structured workshops or certification programs. Moreover, 68% advocated for establishing orthobiologics as a dedicated subspecialty within orthopaedics. These findings illustrate a clear enthusiasm for expanding the field through academic and clinical formalisation. If such interest is harnessed by institutions and regulatory bodies, it could lead to the creation of fellowship programs, curriculum inclusion, and standard operating procedures tailored to regenerative therapies. The data imply that orthobiologics are not just viewed as supplementary tools, but as potential cornerstones of musculoskeletal care, especially in the management of chronic conditions and joint preservation strategies.
Usage trends were dominated by PRP, consistent with its global popularity and accessibility. Nearly 80% of respondents had employed PRP in their clinical practice. The most common indications included chronic tendinopathies (68%), early osteoarthritis (51%), avascular necrosis (55%), and delayed or non-union fractures (57%). Clinicians with formal orthobiologic education (defined as structured training programs) were significantly more likely to implement PRP therapies compared to those without such training (89% vs 76%, P < 0.01), underscoring the role of structured learning in bridging the knowledge-practice gap. These applications reflect both global trends and the evolving therapeutic paradigms in India, where minimally invasive interventions are increasingly embraced. Interestingly, satisfaction with PRP outcomes was generally high, with mean satisfaction scores reported at 6.5 ± 2.3 on a 10-point scale. Advanced biologics (BMAC and stem cells) showed similar satisfaction ratings among the limited number of users (6.2 ± 2.1, n = 89). However, such subjective ratings, while encouraging, must be interpreted with caution given the lack of standardised dosing, product variability, and methodological diversity. Positive outcomes reinforce the clinical utility of biologics when applied judiciously, yet they also necessitate further research to delineate optimal protocols and patient selection criteria. Patient selection criteria varied among practitioners. For PRP therapy, 73% of respondents considered disease severity as primary selection criteria, with 68% requiring failure of conservative treatment for at least 6 months. In osteoarthritis cases, 82% limited PRP use to Kellgren-Lawrence grades 1-2, while 15% extended application to grade 3 disease. Regional disparities were evident, with metropolitan practitioners having significantly higher access to biologics laboratories compared to non-metropolitan areas (28% vs 8%, P < 0.001).
While PRP use was prevalent, advanced biologic applications-such as BMAC and mesenchymal stem cell therapies-remained limited. High costs, lack of reimbursement, and inadequate lab infrastructure were cited as principal deterrents. Only 18% of clinicians reported access to a dedicated biologics lab, and 64% flagged cost as a significant barrier. These limitations are reflective of systemic challenges in scaling biologics in resource-limited settings. The absence of processing facilities and personnel trained in advanced techniques restricts the diffusion of novel therapies to tertiary centres and trial settings. This implies that for orthobiologics to evolve beyond PRP, significant investment in infrastructure, workforce development, and cost mitigation strategies is necessary.
Patient-related factors also posed challenges to clinical adoption. Approximately 90% of respondents observed that patients lacked awareness regarding orthobiologic therapies prior to counselling. Despite this, most patients were receptive after explanation, indicating that clinician-led education can play a transformative role in acceptance and compliance. This finding suggests that efforts to improve patient understanding-through brochures, multimedia education, or informed consent enhancements-could facilitate broader implementation. Furthermore, involving patients in shared decision-making becomes essential when therapies fall outside insurance coverage and depend on out-of-pocket expenditure. Barriers included high treatment costs, limited access to biologics labs (18%), and poor patient awareness (90%). These factors constrained the adoption of advanced biologics like BMAC and stem cells, which were significantly more common among senior clinicians and those with lab facilities (P < 0.001).
Regulatory awareness among clinicians was found to be moderate. Around two-thirds of respondents reported familiarity with Indian guidelines on stem cell use. However, understanding of rules surrounding allogeneic products and international regulatory frameworks was low. This knowledge gap may predispose practitioners to inadvertent breaches of regulatory boundaries, especially as biologics often fall into complex legal zones. The implication here is profound: Harmonised national guidelines, clinician-friendly summaries of permissible therapies, and compliance workshops are urgently needed to navigate this rapidly evolving terrain. Without clear direction, the adoption of orthobiologics may be uneven, and patient safety could be compromised. Regulatory familiarity was uneven—academic-affiliated participants were more informed about stem cell guidelines (P < 0.05) suggesting that institutional setting influences compliance awareness.
This survey of Indian orthopaedic practitioners reveals high enthusiasm for orthobiologics coupled with notable gaps in training and resources. Nearly all respondents were aware of orthobiologic therapies (about 97% awareness), yet only 31% had formal training, and under 25% could describe advanced preparation protocols. This gap between awareness and expertise underscores the need for education, echoing global observations that formal training in regenerative techniques has lagged behind clinical interest[1]. Recent literature corroborates this training deficit, with comprehensive reviews emphasizing that orthobiologic formulations remain largely heterogeneous in reporting, highlighting the urgent need for minimum reporting standards and higher-quality educational frameworks in orthopedic practice[10]. It is promising that 85% of respondents expressed interest in structured training programs, indicating a strong demand to build competency.
The limited laboratory infrastructure identified in our study (18% access) represents a critical bottleneck for advanced orthobiologic implementation. Closed-system point-of-care devices offer a promising solution by enabling bedside preparation of biological products, eliminating the need for off-site processing[11]. These systems could significantly expand access while maintaining quality control standards. Comprehensive training programs must extend beyond physician education to include multidisciplinary technical training for laboratory technicians, biomedical scientists, and quality control personnel. Such expanded expertise is essential for scaling orthobiologic capabilities, particularly given India's diverse healthcare landscape and varying resource availability across regions.
PRP was by far the most widely utilised orthobiologic in this cohort. Approximately 80% of surgeons had used PRP, primarily for chronic tendinopathies, early knee osteoarthritis, early-stage avascular necrosis, and aiding fracture healing. This finding aligns with global trends, where systematic reviews demonstrate that PRP injections have become increasingly prevalent in musculoskeletal conditions, with current literature exhibiting relative safety profiles and potential to accelerate soft tissue healing processes, particularly in tendinopathies and early osteoarthritis[12]. This mirrors global practice: PRP is the most popular orthobiologic modality internationally and is increasingly endorsed as a treatment option for knee osteoarthritis in mild to moderate stages[4,13]. PRP’s dominance is attributed to its relative ease of use, autologous safety, and a growing (though still mixed) evidence base supporting its efficacy in certain conditions.
The moderate regulatory awareness observed in our study (66%) contrasts sharply with established international guidelines. The European Society for Sports Traumatology, Knee Surgery and Arthroscopy has developed comprehensive consensus statements for orthobiologic applications, particularly for knee osteoarthritis[13]. India lacks similar standardized protocols, contributing to the variability in preparation techniques observed among practitioners. Table 2 illustrates the knowledge gaps between current Indian regulatory understanding and established international frameworks.
| Aspect | Indian clinician awareness (%) | International standard | Knowledge gap |
| PRP preparation protocols | 48 | ESSKA-ORBIT consensus available | High |
| Stem cell regulations | 66 | FDA/EMA guidelines established | Moderate |
| Quality control standards | 32 | ISO 13485 requirements | High |
| Patient selection criteria | 45 | Evidence-based algorithms | High |
In contrast, advanced orthobiologic therapies (e.g., BMAC or stem cell injections) were seldom used, mainly due to high cost and limited access to specialized facilities. Recent comprehensive reviews emphasize that orthobiologics represent a subset of regenerative therapies consisting of biologic substances that have grown in use and popularity, partly due to a growing evidence base, but implementation remains constrained by infrastructure and standardization challenges[14,15]. These obstacles are universal-such therapies require major infrastructure and are expensive (often not covered by insurance), confining their use to a few specialized centers or trials[1]. The low uptake of BMAC and similar modalities in our survey underscores how practical constraints restrict the translation of promising biologics into general practice. Expanding their use will require addressing cost barriers and investing in infrastructure.
The infrastructure limitations identified in our study-with only 18% having access to dedicated biologics laboratories-represent a critical bottleneck that aligns with recent evidence-based evaluations emphasizing that despite the promise of orthobiologic therapies, their clinical implementation requires standardized protocols and adequate facility infrastructure to ensure consistent outcomes[16]. Economic analysis reveals that while PRP therapy costs approximately ₹8000-₹15000 per session in India, surgical alternatives for early osteoarthritis range from ₹150000-₹300000. Cost-effectiveness studies from international literature suggest PRP may provide favorable economic outcomes compared to total knee replacement when applied appropriately in early-stage disease[17]. However, the absence of insurance coverage in India limits accessibility, particularly affecting patients in non-metropolitan areas where 64% reported cost as the primary barrier vs 58% in metropolitan regions.
Participants’ attitudes toward orthobiologics were overwhelmingly positive. More than 90% viewed regenerative therapies as a future pillar of orthopaedic care, and 68% supported developing a dedicated orthobiologics subspecialty. This optimism suggests that many clinicians are eager to integrate and formalize these treatments. Similar optimism is seen globally, though it must be tempered with scientific rigor. The strong interest in a subspecialty could encourage professional bodies to create focused fellowships or certification courses, helping to standardize practices and advance research in regenerative orthopaedics. This enthusiasm for subspecialty development is particularly relevant given that orthobiologic applications have expanded across multiple orthopedic subspecialties, with contemporary literature documenting their integration into sports medicine, joint preservation, and regenerative orthopedic practices through tiered treatment approaches[3].
The survey also highlighted gaps in regulatory knowledge. About two-thirds of respondents were aware of domestic regulations on stem cell use, but most were unfamiliar with guidelines for allogeneic products or international regulatory frameworks. Recent systematic analyses emphasize that regulatory and ethical aspects of orthobiologic therapies represent significant challenges, with considerable research efforts focused on developing strategies to modulate the biological environment for tissue regeneration while maintaining stringent safety standards and regulatory compliance[18]. Given the complex and evolving nature of orthobiologic regulation, this finding is understandable, yet it points to a need for better dissemination of guidelines. Clear national guidelines and education on what is permissible would help practitioners navigate legal boundaries and maintain patient safety.
Key barriers to broader orthobiologic adoption in practice were identified, mirroring those reported in other countries. Cost was a major issue: Without insurance coverage, these therapies are out-of-pocket, limiting access to wealthier patients. Only 18% had access to a dedicated biologics lab, meaning most lack facilities for advanced processing. This infrastructure gap (and few trained personnel) constrains use of therapies like BMAC. These barriers parallel findings from educational research in orthopedic surgery, where evolving surgical training dynamics necessitate continuous adaptation of educational content and training methodologies to match technological and therapeutic advances[19]. Investment in lab facilities and better reimbursement will be crucial to overcome these hurdles. Ethical concerns emerge from the regulatory knowledge gaps identified in our study. The proliferation of unproven stem cell therapies in India, despite regulatory restrictions, poses patient safety risks[20]. Our findings suggest that 34% of practitioners lack adequate understanding of permissible stem cell applications, potentially leading to inappropriate treatment recommendations. This knowledge deficit necessitates urgent implementation of evidence-based guidelines and ethical training programs to prevent exploitation of vulnerable patients seeking alternative treatments.
Despite the challenges, clinicians who used orthobiologics reported moderate to high satisfaction with their results, typically rating outcomes between 5 and 9 on a 10-point scale. This suggests that, in appropriate cases, orthobiologic interventions are perceived to provide tangible benefits. However, it is important to balance this enthusiasm with evidence-based caution. The medical literature shows variable results for many orthobiologic applications, and even expert panels have not reached consensus on optimal preparation or application protocols for PRP[6]. The evolving field of evidence-based orthobiologic practice continues to grapple with standardization challenges, as rapid utilization of these biologically derived therapies, including mesenchymal stem cells and BMAC, outpaces the development of robust clinical evidence and standardized protocols, necessitating ongoing research to establish optimal treatment algorithms[2]. Such inconsistencies in technique can lead to mixed outcomes. Therefore, continued research and standardized protocols are essential to validate these therapies and optimize outcomes.
This study has limitations. As a voluntary survey, there may be response bias (enthusiasts likely responded), and all data were self-reported. Nevertheless, the large, nationwide sample lends weight to the findings. Compared to global data, it appears India’s patterns in orthobiologic awareness and usage are broadly similar to international trends: PRP is widely adopted, more advanced biologics are approached cautiously, and common barriers (cost, training, evidence) prevail. Additional limitations include potential recall bias in self-reported satisfaction scores, as practitioners may overestimate positive outcomes due to confirmation bias. Regional disparities between metropolitan and non-metropolitan practitioners may have influenced results, with urban centers potentially overrepresented due to digital survey distribution methods. Long-term patient outcomes were not assessed, limiting our ability to correlate clinician satisfaction with actual therapeutic efficacy. Future studies should incorporate patient-reported outcome measures and standardized follow-up protocols to validate clinician perceptions.
Orthopaedic professionals in India exhibit widespread awareness and positive outlook toward orthobiologics, but face significant challenges in training, regulation, and infrastructure. Addressing these gaps requires specific interventions: Establishment of national orthobiologic workshops following international consensus protocols, subsidized point-of-care device initiatives to expand laboratory access, comprehensive regulatory education programs targeting the identified knowledge gaps, development of standardized patient selection criteria, and health insurance policy reforms to improve treatment accessibility. By implementing these targeted strategies, the orthopaedic community can ensure that enthusiasm for regenerative medicine translates into safe, effective, and equitable patient care across India's diverse healthcare landscape.
| 1. | Jeyaraman M, Muthu S, Amarnath SS. Barriers and Solutions Towards Integrating Orthobiologics into Clinical Orthopaedic Practice. Indian J Orthop. 2024;58:987-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Jeyaraman M, Jeyaraman N, Ramasubramanian S, Balaji S, Muthu S. Evidence-based orthobiologic practice: Current evidence review and future directions. World J Orthop. 2024;15:908-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Lattermann C, Leite CB, Frisbie DD, Schlegel TS, Bramlage LR, Koch T, Centeno C, Goodrich LR, Johnstone B, Trumper R, Watts A, Little C, Barry F, Guilak F, Mcilwraith CW. Orthobiologics in orthopedic applications: a report from the TMI Havemeyer meeting on orthobiologics. J Cartil Joint Preserv. 2022;2:100055. [DOI] [Full Text] |
| 4. | Noback PC, Donnelley CA, Yeatts NC, Parisien RL, Fleischli JE, Ahmad CS, Moorman CT, Trofa DP, Saltzman BM. Utilization of Orthobiologics by Sports Medicine Physicians: A Survey-based Study. J Am Acad Orthop Surg Glob Res Rev. 2021;5:e20.00185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Costa FR, Pires L, Martins RA, Santos M, Santos GS, Lana JV, Costa BR, Santos N, de Macedo AP, Kruel A, Lana JF. Orthobiologics Revisited: A Concise Perspective on Regenerative Orthopedics. Curr Issues Mol Biol. 2025;47:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Hohmann E. Editorial Commentary: Platelet-Rich Plasma for Musculoskeletal Conditions Is Supported by a Large Number of Clinical Studies, Particularly for Knee Osteoarthritis. Arthroscopy. 2024;40:478-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Pimenta C, Bettiol V, Alencar-Silva T, Franco OL, Pogue R, Carvalho JL, Felipe MSS. Advanced Therapies and Regulatory Framework in Different Areas of the Globe: Past, Present, and Future. Clin Ther. 2021;43:e103-e138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Carvalho E, Bettger JP, Goode AP. Insurance Coverage, Costs, and Barriers to Care for Outpatient Musculoskeletal Therapy and Rehabilitation Services. N C Med J. 2017;78:312-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Boopalan D, Pandian R, Kesavan G. Prospects for Stem Cell-Based Regenerative Therapies in India. Stem Cell Ther India. 2021;3:11-21. [DOI] [Full Text] |
| 10. | Obana KK, Schallmo MS, Hong IS, Ahmad CS, Moorman CT 3rd, Trofa DP, Saltzman BM. Current Trends in Orthobiologics: An 11-Year Review of the Orthopaedic Literature. Am J Sports Med. 2022;50:3121-3129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | McCarrel TM, Mall NA, Lee AS, Cole BJ, Butty DC, Fortier LA. Considerations for the use of platelet-rich plasma in orthopedics. Sports Med. 2014;44:1025-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Mlynarek RA, Kuhn AW, Bedi A. Platelet-Rich Plasma (PRP) in Orthopedic Sports Medicine. Am J Orthop (Belle Mead NJ). 2016;45:290-326. [PubMed] |
| 13. | Laver L, Filardo G, Sanchez M, Magalon J, Tischer T, Abat F, Bastos R, Cugat R, Iosifidis M, Kocaoglu B, Kon E, Marinescu R, Ostojic M, Beaufils P, de Girolamo L; ESSKA‐ORBIT Group. The use of injectable orthobiologics for knee osteoarthritis: A European ESSKA-ORBIT consensus. Part 1-Blood-derived products (platelet-rich plasma). Knee Surg Sports Traumatol Arthrosc. 2024;32:783-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 14. | Shapiro SA, Master Z, Arthurs JR, Mautner K. Tiered approach to considering orthobiologics for patients with musculoskeletal conditions. Br J Sports Med. 2023;57:179-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Bravo D, Jazrawi L, Cardone DA, Virk M, Passias PG, Einhorn TA, Leucht P. Orthobiologics A Comprehensive Review of the Current Evidence and Use in Orthopedic Subspecialties. Bull Hosp Jt Dis (2013). 2018;76:223-231. [PubMed] |
| 16. | Hussain N, Johal H, Bhandari M. An evidence-based evaluation on the use of platelet rich plasma in orthopedics - a review of the literature. SICOT J. 2017;3:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Raeissadat SA, Rahimi M, Rayegani SM, Moradi N. Cost-utility analysis and net monetary benefit of Platelet Rich Plasma (PRP), intra-articular injections in compared to Plasma Rich in Growth Factors (PRGF), Hyaluronic Acid (HA) and ozone in knee osteoarthritis in Iran. BMC Musculoskelet Disord. 2023;24:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 18. | Murray IR, Chahla J, Wordie SJ, Shapiro SA, Piuzzi NS, Frank RM, Halbrecht J, Okada K, Nakamura N, Mandelbaum B, Dragoo JL; Biologics Association, Borg-Stein J, Anz A, Gobbi A, Gomoll AH, Cole BJ, Lattermann C, Chu C, Grande DA, Saris DBF, Flanigan D, Kon E, Muschler GF, Malanga GA, Dummer G, Farr J, Tokish JM, Spindler KP, Horsch K, Zaslav K, McIntyre LF, Sgaglione NA, Sherman SL, Rodeo S, Awan TM, Vangsness CT. Regulatory and Ethical Aspects of Orthobiologic Therapies. Orthop J Sports Med. 2022;10:23259671221101626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Martinez VH, Zaheer A, McCarrell J, Checketts JX, Hanson CD. Education Research in Orthopaedic Surgery: A 6-Year Analysis. JB JS Open Access. 2023;8:e22.00090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Lohmor S, Verma R, Malik V, Kaushik D. Current status of Regulatory perspectives on Stem Cell Therapy in India. Chem Biol Lett. 2020;7:176-182. |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/