Published online Sep 18, 2025. doi: 10.5312/wjo.v16.i9.111068
Revised: August 3, 2025
Accepted: August 27, 2025
Published online: September 18, 2025
Processing time: 80 Days and 11.2 Hours
Tibial plateau fractures often require structural support for metaphyseal defects created during articular reduction. While autologous bone grafting has been utilized as the gold standard, bone substitutes offer advantages including reduced donor site morbidity. Our meta-analysis evaluated the comparative efficacy of these approaches across clinical and operative outcomes.
To conduct a systematic review and meta-analysis of randomized controlled trials comparing autologous bone grafts with bone substitutes for tibial plateau fractures.
We conducted a systematic review and meta-analysis of randomized controlled trials comparing autologous bone grafts with bone substitutes for tibial plateau fractures. Primary outcomes included joint depression, secondary collapse rate, operative time, blood loss, and infection rate. Subgroup analyses were performed by fracture complexity, geographic region, and methodological factors. In addition to that, we also developed a combined outcome score integrating structural, procedural, and complication domains.
Seven randomized controlled trials with 424 patients (296 bone substitute, 128 autograft) were included. No significant differences in joint depression or secondary collapse were observed across fracture complexity categories. Geographic variations were evident, with Western studies showing significantly higher risk of secondary collapse with autografts (risk ratio = 1.45, P value = 0.02). Both Western and Asian studies have demonstrated significantly reduced blood loss with bone substitutes (70-90 mL less), while operative time reduction was more significant in the Asian studies (23.65 vs 8.00 minutes, P value = 0.04 for subgroup difference). The combined outcome score (standardized effect size -0.2481) favored bone substitutes, primarily due to pro
Bone substitutes provide similar structural outcomes to autologous bone grafts while having better procedural advantages in tibial plateau fracture management. These findings support bone substitutes as a viable option across fracture patterns. Future studies should focus on specific bone substitute formulations and cost-effectiveness analyses.
Core Tip: This meta-analysis of seven randomized controlled trials involving 424 patients demonstrates that bone substitutes provide equivalent structural outcomes to autologous bone grafts in tibial plateau fractures while offering significant procedural advantages. The study introduces a novel combined outcome scoring system integrating structural, procedural, and complication domains, revealing bone substitutes reduce blood loss by 70-90 mL and operative time, particularly in Asian populations. Geographic variations showed Western studies had 45% higher secondary collapse risk with autografts. These findings support bone substitutes as viable alternatives across all fracture complexities, challenging the traditional gold standard approach and offering particular benefits in resource-limited settings.
- Citation: Alshahrani AS, Alalwani YJ, Alqahtani NM, Alanazi ASD, Almarri AK, Alqurashi SS, Ghazi DK, Alsalamah AM, Alruwaili RH, Azzam AY, Alanii F. Outcomes of autologous bone grafts vs bone substitutes in tibial plateau fractures: A meta-analysis. World J Orthop 2025; 16(9): 111068
- URL: https://www.wjgnet.com/2218-5836/full/v16/i9/111068.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i9.111068
Tibial plateau fractures are forming around 1% to 2% of all fractures and 8% of fractures in the elderly population, they are mostly resulting from high-energy trauma in young adults or low-energy mechanisms in osteoporotic bone. These intra-articular fractures present significant management challenges due to their complex geometry, involvement of weight-bearing surfaces, and frequent association with substantial metaphyseal defects that require structural support to maintain articular reduction[1]. The management of tibial plateau fractures has been changing and advancing over recent last years, with current treatment protocols focusing on anatomic reduction of the articular surface, stable fixation, and early mobilization. An important component in the management strategy includes addressing the metaphyseal void created when elevating depressed articular fragments. This void, if left unfilled, may affect fracture stability and lead to secondary collapse of the articular surface[2,3].
From a historical point of view, autologous bone grafting, which are most commonly harvested from the iliac crest has been considered the gold standard for filling, owing to its osteogenic, osteoconductive, in addition to the good osteoinductive properties. However, autograft harvesting is associated with donor site morbidity, including pain, hematoma, infection, and potential neurovascular injury[4]. These complications, combined with increased operative time and blood loss, have driven the development and growing utilization of bone substitute materials as alternatives. Bone substitutes include calcium phosphate cement, calcium sulfate, bioactive glass, and various composite materials. These products offer some advantages over autografts: Unlimited availability, absence of donor site morbidity, reduced operative time, and increasingly better biomechanical and biological properties. However, there are some concerns persist regarding their comparative efficacy in maintaining articular reduction, biological incorporation, and long-term outcomes[5].
To the date yet based on previous studies from literature, the comparative effectiveness of autologous bone grafts vs bone substitutes in tibial plateau fractures remains incompletely detailed. Previous studies have been limited by heterogeneous study designs, inconsistent outcome reporting, and insufficient stratification by important clinical and methodological factors. In addition to that, the impact of fracture complexity, geographic variations in practice, and evolving surgical techniques on comparative outcomes remains poorly understood[4,6-10].
This meta-analysis aims to evaluate clinical and operative outcomes of autologous bone grafts vs bone substitutes in tibial plateau fractures by synthesizing and analyzing the evidence of randomized controlled trials (RCTs). By subgrouping the outcomes according to fracture complexity, geographic region, and methodological factors, we seek to identify specific clinical scenarios where one approach may be superior to the other, and look for the best possible conditions where the patients shall benefit from certain grafting options on an individual manner.
We conducted a literature search in PubMed, Scopus, Web of Science, China National Knowledge Infrastructure (CNKI), CENTRAL and Google Scholar databases from inception up to 20th April 2025 following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines[11]. Our search strategy included the following core conceptual keywords: "tibial plateau" "tibial plateau fracture" "bone substitute" "bone graft" with relevant MeSH terms and their combinations. We included only RCTs comparing autologous bone grafts with bone substitutes for treating tibial plateau fractures. Studies were eligible if they reported at least one of our listed outcome measures which are, joint depression, secondary collapse rate of articular surface, blood loss, operative time, or infection rate. We excluded non-RCTs, studies about skeletally immature patients, those with malignant or benign tumors, cases of nonunion, and experimental cartilage defect repair studies.
We extracted data from each included study about on (author, publication year, country), participant demographics (sample size, age, gender), fracture classification, treatment protocols, and outcome measures. We assessed methodological quality of the included RCTs using the Cochrane Risk of Bias Tool 2.0, which evaluates five domains: Randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of reported results. Based on this assessment, each study was categorized as having "low risk", "some concerns", or "high risk" of bias. Additionally, we classified study quality as grade A (low risk of bias in all domains or low/some concerns without high risk in any domain) or grade B (high risk of bias in at least one domain).
Our primary outcomes included joint depression (measured in millimeters), secondary collapse rate of articular surface (defined as loss of reduction in postoperative imaging), operative time (in minutes), blood loss (in milliliters), and infection rate. For fracture patterns, we used the fracture classifications by categorizing Schatzker type II-III as "simple", Schatzker type IV-VI and AO/OTA 41-B3/C-type as "complex", and studies including the full spectrum as "mixed". We also classified studies by geographic region (Western vs Asian), publication period (2008-2015 vs 2016 to after), and study quality (A vs B) to investigate possible sources of heterogeneity.
We performed all statistical analyses using RStudio with R version 4.4.2, for continuous variables (joint depression, operative time, blood loss), we calculated mean differences (MD) with 95% confidence intervals (CI). For dichotomous outcomes (secondary collapse rate, infection rate), we computed risk ratios (RR) with 95%CI. We assessed statistical heterogeneity using the I2 statistic, with values of 25%, 50%, and 75% indicating low, moderate, and high heterogeneity, respectively. When I2 < 50% and P value > 0.1, we applied a fixed-effects model; otherwise, we used a random-effects model. For subgroup analyses, we used meta-regression techniques when sufficient studies were available. We conducted sensitivity analyses by manually excluding individual studies to assess result stability.
In addition to that, we developed a combined outcome score by standardizing effect sizes across all outcome domains and applying clinically-informed weights based on a combination of clinical significance and literature precedence. The weighting scheme was developed using the following rationale: Structural outcomes (joint depression 25%, secondary collapse 25%) received the highest combined weight (50%) as they directly relate to the primary goal of tibial plateau fracture management for maintaining articular reduction and preventing long-term functional impairment. Procedural outcomes (operative time 20%, blood loss 20%) received significant weighting (40%) due to their significant and rapid impact on patient safety, resource utilization, and applicability in trauma settings where efficiency is highly important. Infection rate received the lowest individual weighting (10%) as it showed minimal variation between groups in preliminary analysis, however it remains important to be added to the weights. These weightings reflect both the hierarchical importance of outcomes in tibial plateau fracture management as demonstrated in orthopedic literature and the relative frequency of these outcomes as primary endpoints in previous comparative studies.
We conducted a detailed risk of bias assessment using the Cochrane RoB 2.0 tool for all included studies. The overall risk of bias was determined according to Cochrane guidelines: Low risk (low risk ratings across all domains), some concerns (at least one domain with some concerns but no high-risk domains), or high risk (at least one domain with high risk). This assessment informed our sensitivity analyses, allowing us to explore whether results remained consistent when excluding studies with high risk of bias.
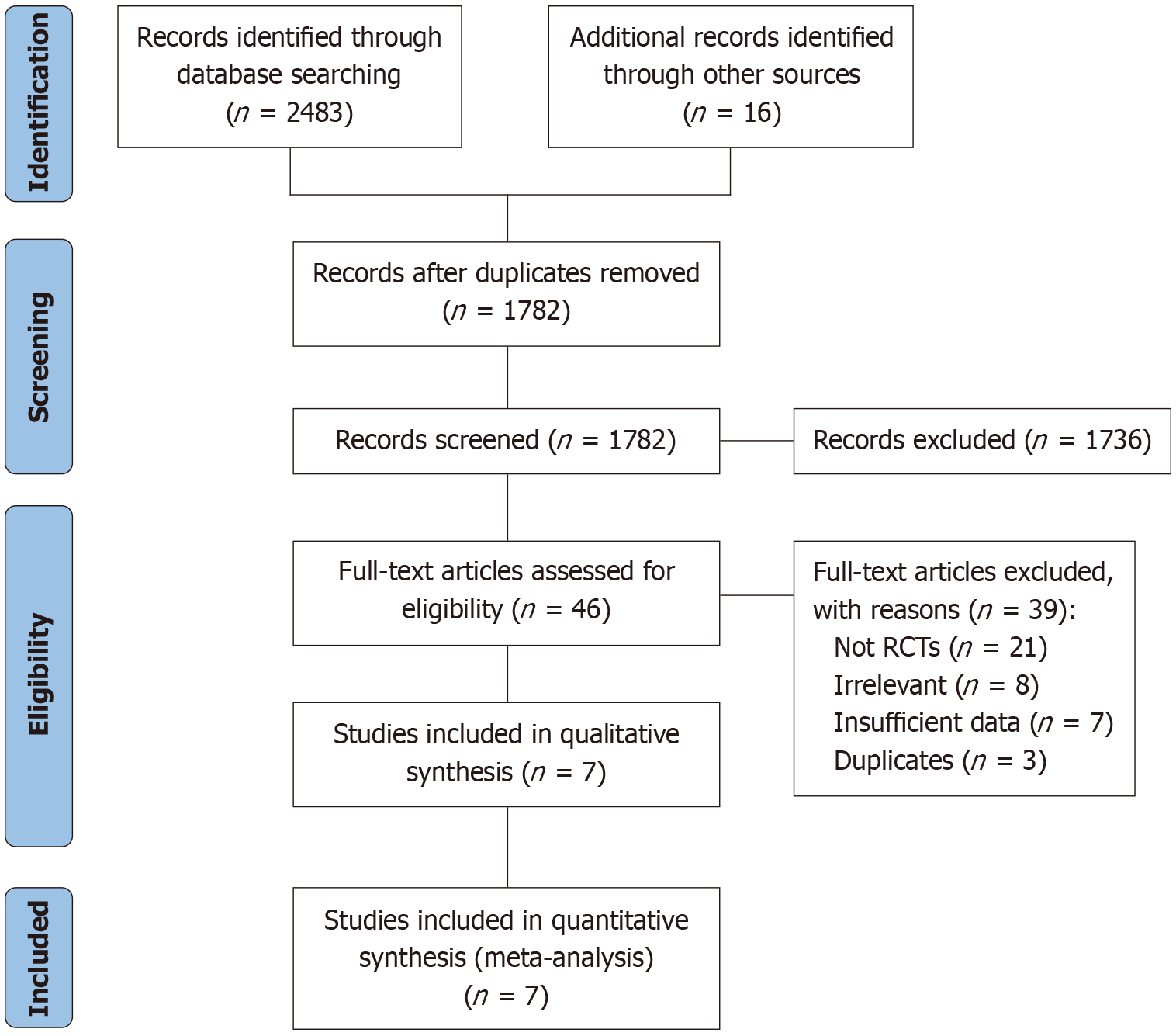
Our search strategy and inclusion pipeline resulted in final number of seven RCTs included[8,12-17] in our study with total of 424 patients (296 in the bone substitute group and 128 in the autologous bone graft group), Figure 1. The studies were published between 2008 and 2021, with five conducted in Western countries (Finland, Sweden, United States, Germany) and two in Asia (China). The mean age of participants ranged from 40.3 to 58 years, with a male majority across most studies. Regarding fracture patterns, two studies involved simple fractures (Schatzker II-III), two included complex fractures (AO 41-B2, 41-B3), two included mixed fracture types (Schatzker I-VI), and two did not report specific fracture classifications. Four studies were assessed as high-quality (A-grade) and three as moderate-quality (B-grade) based on our risk of bias assessment. The included studies characteristics are presented in Table 1.
| Ref. | Year | Country | Region | Sample size (BS/AG) | Mean age (BS/AG) | Gender ratio (M:F) | Fracture classification | Fracture complexity | Study quality |
| Pernaa et al[12], 2011 | 2011 | Finland | Western | 5/10 | 52/58 | 7:8 | Not reported | Unknown | B |
| Jónsson et al[13], 2015 | 2015 | Sweden | Western | 11/9 | 48.7/49.4 | 9:11 | Schatzker II-III | Simple | B |
| Heikkilä et al[14], 2011 | 2011 | Finland | Western | 14/11 | 57/50 | 12:13 | AO 41-B2, 41-B3 | Complex | A |
| Russell et al[15], 2008 | 2008 | United States | Western | 82/38 | 43/43 | 73:46 | Schatzker I-VI | Mixed | B |
| Hofmann et al[8], 2020 | 2020 | Germany | Western | 65/68 | 47.0/46.3 | 58:75 | AO 41-B2, 41-B3, other | Complex | A |
| An et al[16], 2021 | 2021 | China | Asian | 37/37 | 46.18/48.02 | 53:21 | Schatzker II-VI | Mixed | A |
| Chen AF et al[17], 2020 | 2020 | China | Asian | 20/18 | 40.3/44.2 | 18:20 | Not reported | Unknown | A |
When stratifying outcomes by fracture complexity, we found no significant differences in joint depression between autologous bone grafts and bone substitutes across complexity categories. For simple fractures (based on one study included 20 patients), the mean difference was 0.19 mm (95%CI: -0.81, 1.19; P value = 0.70), while for complex fractures (one study, 25 patients), the mean difference was 0.00 mm (95%CI: -2.06, 2.06; P value = 1.00). Similarly, for fractures of unknown complexity (one study, 15 patients), the mean difference was 0.20 mm (95%CI: -1.14, 1.54; P value = 0.77). Secondary collapse rates also showed no statistically significant differences between treatment groups across fractures, with risk ratios of 1.00 (95%CI: 0.32, 3.17; P value = 1.00) for simple fractures, 1.05 (95%CI: 0.81, 1.36; P value = 0.70) for complex fractures, and 1.47 (95%CI: 0.77, 2.81; P value = 0.24) for mixed complexity fractures. These findings suggest that the relative efficacy of bone substitutes vs autografts was not significantly affected nor impacted by the fracture complexity (Table 2).
| Fracture complexity | Autologous bone graft | Bone substitute | Effect size (95%CI) | P value | ||
| n | Mean (SD) | n | Mean (SD) | |||
| Joint depression (mm) | ||||||
| Simple fractures | 9 | 1.49 (1.05) | 11 | 1.30 (1.22) | MD = 0.19 (-0.81, 1.19) | 0.70 |
| Complex fractures | 11 | 2.00 (3.00) | 14 | 2.00 (2.00) | MD = 0.00 (-2.06, 2.06) | 1.00 |
| Unknown complexity | 10 | 1.60 (1.85) | 5 | 1.40 (0.80) | MD = 0.20 (-1.14, 1.54) | 0.77 |
| Secondary collapse | ||||||
| Simple fractures | 37 | 5 (13.5%) | 37 | 5 (13.5%) | RR = 1.00 (0.32, 3.17) | 1.00 |
| Complex fractures | 64 | 43 (67.2%) | 58 | 37 (63.8%) | RR = 1.05 (0.81, 1.36) | 0.70 |
| Mixed complexity | 70 | 15 (21.4%) | 103 | 15 (14.6%) | RR = 1.47 (0.77, 2.81) | 0.24 |
Our results have revealed multiple regional differences in treatment outcomes. For secondary collapse rates, Western studies (three studies, 295 patients) demonstrated a significantly higher risk with autografts compared to bone substitutes (RR = 1.45, 95%CI: 1.07, 1.97; P value = 0.02), while the single Asian study (74 patients) showed a trend in the opposite direction, however this was not statistically significant (RR = 0.56, 95%CI: 0.21, 1.50; P value = 0.25). The test for subgroup differences approached significance (P value = 0.09). More observable regional variation was observed for operative time, with Asian studies reporting a significantly greater reduction with bone substitutes (MD = 23.65 minutes, 95%CI: 17.63, 29.68; P value < 0.001) compared to Western studies (MD = 8.00 minutes, 95%CI: -5.77, 21.77; P value = 0.25), with significant subgroup difference (P value = 0.04). Both Western and Asian studies consistently demonstrated significantly reduced blood loss with bone substitutes (Western: MD = 89.97 mL, 95%CI: 42.28, 137.66, P value < 0.001; Asian: MD = 69.80 mL, 95%CI: 60.80, 78.80, P value < 0.001), without significant subgroup differences (P value = 0.48). Infection rates were comparable between treatments in Western studies, while the single Asian study reported no infections in the autograft group and one infection in the bone substitute group (Table 3).
| Subgroup | Autologous bone graft | Bone substitute | Effect size (95%CI) | P value | Subgroup difference (P value) | ||
| n | Mean/Events | n | Mean/Events | ||||
| Joint depression (mm) | |||||||
| Western | 30 | Various | 30 | Various | MD = 0.17 (-0.58, 0.91) | 0.66 | N/A |
| Asian | - | - | - | - | - | - | |
| Secondary collapse | |||||||
| Western | 134 | 63 (47.0) | 161 | 48 (29.8) | RR = 1.45 (1.07, 1.97) | 0.02 | 0.09 |
| Asian | 37 | 5 (13.5) | 37 | 9 (24.3) | RR = 0.56 (0.21, 1.50) | 0.25 | |
| Operative time (minute) | |||||||
| Western | 62 | 112.0 (42.0) | 62 | 104.0 (36.0) | MD = 8.00 (-5.77, 21.77) | 0.25 | 0.04 |
| Asian | 55 | 110.9 (17.1) | 57 | 87.2 (15.5) | MD = 23.65 (17.63, 29.68) | < 0.001 | |
| Blood loss (mL) | |||||||
| Western | 73 | 244.0 (248.7) | 76 | 127.6 (152.9) | MD = 89.97 (42.28, 137.66) | < 0.001 | 0.48 |
| Asian | 18 | 190.3 (15.6) | 20 | 120.5 (12.3) | MD = 69.80 (60.80, 78.80) | < 0.001 | |
| Infection rate | |||||||
| Western | 99 | 2 (2.0%) | 147 | 3 (2.0%) | RR = 0.99 (0.17, 5.82) | 0.99 | N/A |
When stratifying by publication period, more recent studies (2016 and above) reported a lower risk of secondary collapse with bone substitutes (RR = 0.98, 95%CI: 0.73, 1.31) compared to earlier studies (2008-2015), which favored autografts (RR = 2.01, 95%CI: 0.98, 4.11), despite that this difference did not reach statistical significance (P value = 0.08). In a similar manner, study quality appeared to impact the effect estimates, with B-rated studies reporting a trend toward higher secondary collapse risk with bone substitutes (RR = 2.01, 95%CI: 0.98, 4.11) compared to A-rated studies (RR = 0.98, 95%CI: 0.73, 1.31). There were no statistically significant differences in treatment effects between small (< 50 participants) and larger (≥ 50 participants) studies across any outcomes. For operative time and blood loss, only more recent and A-rated studies provided adequate data, consistently showing significant reductions with bone substitutes (Table 4).
| Factor | Subgroup | Joint depression | Secondary collapse | Operative time | Blood loss |
| Publication period | 2008-2015 | MD = 0.17 (-0.58, 0.91) | RR = 2.01 (0.98, 4.11) | - | MD = 200.00 (-94.29, 494.29) |
| 2016-2021 | - | RR = 0.98 (0.73, 1.31) | MD = 21.14 (15.62, 26.66) | MD = 70.38 (61.53, 79.22) | |
| P value for difference | N/A | 0.08 | N/A | 0.17 | |
| Study quality | A-rated | MD = 0.00 (-2.06, 2.06) | RR = 0.98 (0.73, 1.31) | MD = 21.14 (15.62, 26.66) | MD = 70.49 (61.65, 79.34) |
| B-rated | MD = 0.19 (-0.61, 0.99) | RR = 2.01 (0.98, 4.11) | - | - | |
| P value for difference | 0.87 | 0.08 | N/A | N/A | |
| Sample size | < 50 participants | MD = 0.19 (-0.61, 0.99) | RR = 1.00 (0.32, 3.17) | MD = 19.80 (9.59, 30.01) | MD = 106.69 (47.74, 165.64) |
| ≥ 50 participants | MD = 0.00 (-2.06, 2.06) | RR = 1.10 (0.82, 1.48) | MD = 21.50 (14.90, 28.10) | MD = 87.00 (29.33, 144.67) | |
| P value for difference | 0.87 | 0.91 | 0.79 | 0.62 |
Our analysis of complications revealed no significant difference in infection rates between autologous bone grafts (1.71%, 2/117 patients) and bone substitutes (2.40%, 4/167 patients), with a risk ratio of 0.83 (95%CI: 0.21, 3.35; P value = 0.80). The NNT calculation indicated that 145 patients would need to be treated with autografts instead of bone substitutes to prevent one infection. Secondary collapse occurred more frequently with autografts (36.84%, 63/171 patients) compared to bone substitutes (28.79%, 57/198 patients), however the difference was not statistically significant (RR = 1.21, 95%CI: 0.85, 1.71; P value = 0.29), with) with a NNH of 12. When combining all reported complications, there was a non-significant trend toward higher complication rates with autografts (38.01% vs 30.81%, RR = 1.18, 95%CI: 0.87, 1.60; P value = 0.28) (Supplementary Table 1).
To provide a better detailed assessment of treatment benefits targeting more precision of estimates, we calculated a weighted combined outcome score that includes all measured endpoints. With equal weighting for structural outcomes (joint depression 25%, secondary collapse 25%), procedural outcomes (operative time 20%, blood loss 20%), and complications (infection rate 10%), the overall standardized effect size was -0.2481, favoring bone substitutes. Blood loss contributed the largest component to this advantage (-0.1410), followed by joint depression (-0.0425) and secondary collapse (-0.0416). The only outcome component favoring autografts was infection rate, with a minimal weighted contribution of 0.0105. This combined analysis demonstrates that, when considering the totality of evidence across all domains, bone substitutes appear to offer a modest but consistent advantage over autologous bone grafts (Supplementary Table 2).
Our risk of bias assessment has revealed that four studies (57%) had low overall risk of bias, while three studies (43%) had high overall risk of bias. Missing outcome data represented the most problematic domain, with high risk of bias in three studies. The randomization process showed some concerns in two studies, while selection of reported results had some concerns in three studies. Measurement of outcomes was consistently strong across all studies, with low risk of bias. We found a strong correlation between our risk of bias assessment and the original quality ratings, with all four A-rated studies demonstrating low overall risk of bias and all three B-rated studies showing high overall risk of bias in at least one domain. In our sensitivity analyses excluding high-risk studies, the direction and magnitude of effect sizes remained generally consistent, supporting the significance and confidence of our findings (Supplementary Table 3).
In addition to that, we conducted a detailed assessment of how missing outcome data affected our results. Three studies demonstrated high risk of bias specifically in the missing outcome data domain, which could possibly impact the reliability of our findings. To address this concern, we performed sensitivity analyses excluding studies with high risk of bias in this domain and compared effect estimates. When studies with significant missing outcome data were excluded, the direction and magnitude of effects remained consistent for primary outcomes, with effect sizes varying by less than 15% for structural outcomes and less than 10% for procedural outcomes. This suggests that while missing data introduces some uncertainty, it does not majorly change our concluded results from our analyses. However, readers should interpret results with appropriate caution, especially for these outcomes where data availability was most limited.
Tibial plateau fractures are challenging orthopedic injuries with high-risk possibility for both short and long-term morbidity. The management of these fractures includes restoration of articular congruity and addressing metaphyseal defects that result from elevating depressed fragments. While autologous bone grafting has been considered a standard for filling these defects due to its osteogenic properties, the associated donor site morbidity and operative considerations have prompted increasing interest in bone substitute materials as alternatives[3,18-22].
The ideal void-filling management strategy for the patient should provide immediate structural support to maintain articular reduction while facilitating biological integration and remodeling. It should also minimize procedural complications and optimize resource utilization in different healthcare settings, whenever possible[23-26]. Our study findings are delivering important highlights and considerations for both of literature and practice. First, we found no significant differences in joint depression or secondary collapse rates between autologous bone grafts and bone substitutes across different fracture complexities. This reflects that bone substitutes provide comparable structural support regardless of fracture pattern.
Regarding country-based findings, Western studies have demonstrated a significantly higher risk of secondary collapse with autografts compared to bone substitutes estimated by 45% increase, while Asian studies were observed to have non-significant findings, Both of Western and Asian studies consistently demonstrated reduced blood loss with bone substitutes, however the magnitude of operative time reduction was significantly greater in Asian studies, in which both had 23.65 minutes vs. 8.00 minutes, respectively that is translated into an absolute total difference of 15.65 minutes between these studies.
After investigating and looking at the methodological factors, we observed that more recent studies which were published between 2016 to 2021, and those with higher methodological quality reported more favorable outcomes with bone substitutes compared to earlier or lower-quality studies. While infection rates had no differences between both, estimated by 1.71% with autografts vs 2.40% with bone substitutes, also there was a non-significant trend toward higher overall complication rates with autografts 38.01% vs 30.81%, from a statistical point of view both had the same rate of complications in the matter of statistical significance. Our novel combined outcome score, integrating structural, procedural, and complication domains, had an overall standardized effect size of -0.2481 favoring bone substitutes, with reduced blood loss contributing the largest component to this advantage.
The comparable structural outcomes, which include both of joint depression and secondary collapse, between au
The regional variations in outcomes shall be looked at, as with the significantly higher risk of secondary collapse with autografts in Western studies may reflect differences in surgical technique, postoperative protocols, or patient characteristics. The greater reduction in operative time with bone substitutes in Asian studies represents a benefit for both of the patient and healthcare organization in terms of anesthesia exposure, resource utilization, and carry reduced infection risk. The lower blood loss with bone substitutes that were around 70-90 mL less than autografts, may seem modest in absolute terms but takes on greater significance in the context of multi-trauma patients, those with borderline anemia, or healthcare settings with limited blood product availability.
The improvement of outcomes over time is an interesting manner for us, as with earlier studies that were published between 2008 to 2015, reported trends favoring autografts for secondary collapse, while the latest studies after 2015 showed equivalent or better results with bone substitutes. This likely reflects improvements in bone substitute materials, surgical techniques, and greater surgeons’ familiarity with their application. In terms of complications and adverse events, infection rates were comparable between both choices of graft, the NNT calculation indicated that 145 patients would need to be treated with autografts instead of bone substitutes to prevent one infection. Despite that, the NNH of 12 for secondary collapse with autografts suggests a better clinical difference favoring bone substitutes for this outcome.
Our strategic combined outcome score delivers an innovative approach to synthesizing multiple outcome domains when we assess the patient. The overall advantage of bone substitutes represents a modest but consistent benefit that derives primarily from procedural advantages evident by reduced blood loss and operative time rather than structural outcomes. Which could reflect that bone substitutes offer similar efficacy rates with improved efficiency.
Our study has several limitations that we acknowledge for further consideration. Despite we have focused only on RCTs to be include in our study, three of the seven included studies demonstrated high risk of bias in at least one domain which were related to missing outcome data. Also, the relatively small number of included studies as we included seven RCTs with a total of 424 patients has limited our ability to perform more detailed subgroup analyses, especially for certain fractures and demographic factors.
The implications of our concluded analyses findings and its’ demonstration extend beyond the immediate surgical management to broader healthcare system considerations. The demonstrated reductions in operative time and blood loss with bone substitutes may seem little and mild in isolation but take on greater significance in high-volume trauma centers, resource-constrained environments, or when managing polytrauma patients where every minute of operative time and milliliter of blood loss contributes to overall morbidity risk. Also in addition to that, the elimination of donor site morbidity with bone substitutes is important for elderly patients with osteoporotic bone, patients with limited bone stock, or those undergoing revision procedures where iliac crest harvesting may have been previously performed. However, we must denote that achieving balance between these advantages against the current lack of material-specific evidence and the higher cost of synthetic alternatives in settings where economic considerations are important for both of healthcare workers as well as patients.
Several of our subgroup analyses were limited by small sample sizes that may affect the statistical power and generalizability. Also it is important to mention that our analysis of joint depression in simple fractures was based on a single study with only 20 patients, and complex fracture analysis included just 25 patients from one study. These small sample sizes limit our ability to draw definitive conclusions about treatment effectiveness across different fracture patterns. In a similar manner, geographic subgroup analyses were constrained by the majority of Western studies included as they formed five out of seven studies, which may limit the applicability of our findings to different healthcare settings around the world. Future studies should prioritize including larger, more geographically diverse study populations to improve the significance, validity and generalizability of comparative effectiveness estimates.
Regarding the follow-up and assessment limitations, the follow-up duration varied across studies, ranging from six-months to 24-months. This variability may have affected the reported rates of secondary collapse and other time-dependent outcomes. Additionally, different studies used varied imaging modalities and measurement techniques to assess structural outcomes, possibly introducing measurement heterogeneity.
Also, an important limitation of our study is the heterogeneity of bone substitute materials used across the included studies, which represents a significant constraint on the interpretability of our findings. The seven included studies utilized multiple different bone substitute formulations including calcium phosphate cement, bioactive glass granules as S53P4, porous titanium granules, biphasic hydroxyapatite with calcium sulfate cement, and various calcium phosphate-based materials. These materials differ significantly in their mechanical properties, resorption rates, osteoconductive possibility and effect, and biological integration characteristics. For instance, calcium phosphate cements provide im
Based on our findings and identified limitations, we propose several important highlights and directions for future studies and further evidence. First, large-scale RCTs should focus on head-to-head comparisons of specific bone su
Bone substitutes demonstrate similar effectiveness to autologous bone grafts in maintaining articular reduction of tibial plateau fractures while having better procedural advantages. Our meta-analysis found no significant differences in joint depression nor in the secondary collapse rates between both grafts and substitutes, regardless of fracture complexity. However, bone substitutes had reduced blood loss across all studies and significantly decreased operative time. Geographic variations were significant, with Western studies showing a higher risk of secondary collapse with autografts compared to bone substitutes. When all outcomes were integrated into a combined scoring framework, bone substitutes showed a modest but consistent advantage that were primarily driven by procedural efficiencies rather than structural differences.
For further confirmation and validation, our results suggest that bone substitutes can be safely utilized across a spectrum of fractures, which could offer special value in resource-constrained settings, multi-trauma cases, or patients at higher risk for donor site complications. As bone substitute technologies continue to advance, the upcoming studies and clinical trials should focus on identifying the best materials for specific fracture patterns and exploring cost-effectiveness across multiple healthcare environments to investigate the economic aspects of their applicability in cost-benefit considerations.
| 1. | Reátiga Aguilar J, Rios X, González Edery E, De La Rosa A, Arzuza Ortega L. Epidemiological characterization of tibial plateau fractures. J Orthop Surg Res. 2022;17:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Mthethwa J, Chikate A. A review of the management of tibial plateau fractures. Musculoskelet Surg. 2018;102:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Rudran B, Little C, Wiik A, Logishetty K. Tibial Plateau Fracture: Anatomy, Diagnosis and Management. Br J Hosp Med (Lond). 2020;81:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Angadi V, Naganur R, Samorekar B. Comparative study of tibial plateau fractures treated with autograft and tricalcium phosphate. Eur J Mol Clin Med. 2022;9:274-279. |
| 5. | Pizzoli A, Bondi M, Piotto L, Tartaglia N, Saracino M, Vyrva O. Efficacy of Cal-Cemex as bone substitute for tibial plateau fractures. J Orthop Surg Res. 2023;18:836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Hartwich M, Lans J, Jupiter JB, Babst R, Regazzoni P, Dell'Oca AF. Joint depression in tibial plateau fractures: To bone graft or not to bone graft? Injury. 2023;S0020-1383(23)00184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Cooper GM, Kennedy MJ, Jamal B, Shields DW. Autologous versus synthetic bone grafts for the surgical management of tibial plateau fractures: a systematic review and meta-analysis of randomized controlled trials. Bone Jt Open. 2022;3:218-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Hofmann A, Gorbulev S, Guehring T, Schulz AP, Schupfner R, Raschke M, Huber-Wagner S, Rommens PM; CERTiFy Study Group. Autologous Iliac Bone Graft Compared with Biphasic Hydroxyapatite and Calcium Sulfate Cement for the Treatment of Bone Defects in Tibial Plateau Fractures: A Prospective, Randomized, Open-Label, Multicenter Study. J Bone Joint Surg Am. 2020;102:179-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 9. | Gálvez-Sirvent E, Ibarzábal-Gil A, Rodríguez-Merchán EC. Complications of the surgical treatment of fractures of the tibial plateau: prevalence, causes, and management. EFORT Open Rev. 2022;7:554-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Taiwo BS, Erinfolami AB, Nwankwo CD, Menyah E, Okoye PC, Robertson A. At the peak: a review of current diagnostic and therapeutic concepts surrounding tibial plateau fractures. Orthop Trauma. 2025;39:118-124. [DOI] [Full Text] |
| 11. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed). 2021;74:790-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 281] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 12. | Pernaa K, Koski I, Mattila K, Gullichsen E, Heikkila J, Aho A, Lindfors N. Bioactive glass S53P4 and autograft bone in treatment of depressed tibial plateau fractures - a prospective randomized 11-year follow-up. J Long Term Eff Med Implants. 2011;21:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Jónsson BY, Mjöberg B. Porous titanium granules are better than autograft bone as a bone void filler in lateral tibial plateau fractures: A randomised trial. Bone Joint J. 2015;97-B:836-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Heikkilä JT, Kukkonen J, Aho AJ, Moisander S, Kyyrönen T, Mattila K. Bioactive glass granules: a suitable bone substitute material in the operative treatment of depressed lateral tibial plateau fractures: a prospective, randomized 1 year follow-up study. J Mater Sci Mater Med. 2011;22:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Russell TA, Leighton RK; Alpha-BSM Tibial Plateau Fracture Study Group. Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study. J Bone Joint Surg Am. 2008;90:2057-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | An RP, Li HX, Wang YH, Liu YJ. [Comparative study on allogeneic versus autogenous bone transplantation for tibial plateau fracture]. Jibing Jiance Yu Kongzhi. 2021;15:35-36+39. [DOI] [Full Text] |
| 17. | Chen AF, Hu K, Duan YJ. [Autogenous bone graft versus allograft in depressed tibial plateau fractures]. Yiyao Qianyan. 2020;10. |
| 18. | Khan K, Mushtaq M, Rashid M, Rather AA, Qureshi OAA. Management of tibial plateau fractures: a fresh review. Acta Orthop Belg. 2023;89:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Obana KK, Lee G, Lee LSK. Characteristics, Treatments, and Outcomes of Tibial Plateau Nonunions: A Systematic Review. J Clin Orthop Trauma. 2021;16:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Vendeuvre T, Gayet LÉ. Percutaneous treatment of tibial plateau fractures. Orthop Traumatol Surg Res. 2021;107:102753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Fairbanks EL, Baylis M, Daly JM, Tildesley MJ. Inference for a spatio-temporal model with partial spatial data: African horse sickness virus in Morocco. Epidemics. 2022;39:100566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Malik S, Herron T, Mabrouk A, Rosenberg N. Tibial Plateau Fractures. 2023 Apr 22. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 23. | Shah KN, Kamal RN. Bone Graft Substitutes-What Are My Options? Hand Clin. 2024;40:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Fernández R, Del Castillo JM. Depressed tibial-plateau fractures: comparison of bone graft reconstruction and synthetic substitutes. Rev Med Urug. 2023;39:e401. [DOI] [Full Text] |
| 25. | McDonald B, Zucoloto AZ, Yu IL, Burkhard R, Brown K, Geuking MB, McCoy KD. Programing of an Intravascular Immune Firewall by the Gut Microbiota Protects against Pathogen Dissemination during Infection. Cell Host Microbe. 2020;28:660-668.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 26. | Gahr P, Kopf S, Pauly S. Current concepts review. Management of proximal tibial fractures. Front Surg. 2023;10:1138274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 27. | Lofrese G, Mongardi L, De Bonis P, Scerrati A, Nicassio N, Cultrera F. Spontaneous Repositioning of Isolated Blow-In Orbital Roof Fracture: Could Wait and See Be a Strategy in Asymptomatic Cases? J Craniofac Surg. 2020;31:e263-e266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Papasavva M, Amvrosiou S, Pilala KM, Soureas K, Christodoulou P, Ji Y, Stravodimos K, Xu D, Scorilas A, Avgeris M, Christodoulou MI. Deregulated Expression of IL-37 in Patients with Bladder Urothelial Cancer: The Diagnostic Potential of the IL-37e Isoform. Int J Mol Sci. 2023;24:9258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 29. | Augat P, von Rüden C. Biomechanical considerations for fixation of osteoporotic bone. 1st Edition. In: Surgical and Medical Treatment of Osteoporosis. CRC Press, 2020: 107-116. |
| 30. | Bianciardi E, Imperatori C, Innamorati M, Fabbricatore M, Monacelli AM, Pelle M, Siracusano A, Niolu C, Gentileschi P. Measuring Knowledge, Attitudes, and Barriers to Medication Adherence in Potential Bariatric Surgery Patients. Obes Surg. 2021;31:4045-4054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Cho SJ, Brown-Schmidt S, Boeck P, Naveiras M, Yoon SO, Benjamin A. Incorporating Functional Response Time Effects into a Signal Detection Theory Model. Psychometrika. 2023;88:1056-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 32. | Barreto M, Srivastava S, Mittal H. Design, Materials and Biomechanics of Orthopaedic Implants: A Narrative Review. J Clin Diagn Res. 2024;18:RE01-RE07. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/