INTRODUCTION
Inflammatory bone diseases constitute a group of chronic inflammatory disorders predominantly affecting the spine, osteoarticular structures, and periarticular tissues[1,2]. Clinically prevalent subtypes include inflammatory arthritis (IA) and osteoarthritis (OA), with rheumatoid arthritis (RA) and ankylosing spondylitis (AS) being the most incident forms of IA[3,4]. Characterized by persistent pain, progressive joint bone destruction, structural deformities and eventual severe disability, these disorders collectively impose substantial socioeconomic burdens and profoundly compromise patients’ physical and psychological well-being[5-7]. Although characterized by distinct yet interconnected pathogenic mechanisms, chronic inflammation acts as the central driver underlying the initiation and progression of inflammatory bone diseases[7,8]. The pathogenesis of IA is primarily characterized by autoimmune dysregulation, wherein disrupted immune homeostasis activates the production of autoantibodies, leading to excessive release of pro-inflammatory cytokines by an aberrantly activated immune system. Sustained inflammatory microenvironments result in severe disruption of physiological bone metabolism, predominantly manifesting as abnormal proliferation of osteoclasts and irreversible chondral damage. Furthermore, dysregulated cytokine networks have been extensively implicated in the pathogenesis of IA. Notably, interleukin (IL)-13 and IL-4, serving as pivotal mediators of T helper (Th)-2-driven immune responses, exert potent immunomodulatory effects by orchestrating the functional modulation of diverse immune cell populations, including B lymphocytes, eosinophils, basophils, and monocytes[9-13]. This regulatory cascade ultimately potentiates the expression of downstream inflammatory mediators through transcriptional activation of nuclear factor kappa B (NF-κB), signal transducer and activator of transcription (STAT) and other signaling pathways. In contrast to IA, OA was historically conceptualized as a predominantly degenerative disorder affecting weight-bearing joints such as the knee, with its pathological hallmarks centered on articular cartilage degradation leading to mechanical pain and structural deformity[14,15]. However, emerging evidence underscores the pivotal role of chronic low-grade inflammation in OA progression, wherein synovitis has been identified as a critical contributor to OA pathogenesis[7,16]. Biomechanical overloading induced by trauma or obesity triggers excessive joint stress, thereby activating pro-inflammatory signaling cascades that drive the release of inflammatory mediators. This perpetuates a self-amplifying synovial inflammatory microenvironment, ultimately exacerbating cartilage catabolism and subchondral bone remodeling through extracellular matrix destruction[17,18]. While OA has complex mechanisms including chondrocyte apoptosis and biomechanical imbalance, it shares deep pathological links with IA. Both exhibit widespread inflammation causing chronic damage to subchondral bone, synovium, and periarticular tissues, thus being classified as inflammatory bone diseases[1-3]. Current pharmacotherapeutic regimens for IA primarily comprise disease-modifying antirheumatic drugs and nonsteroidal anti-inflammatory drugs[19,20]. While OA management employs diverse therapeutic modalities, its fundamental therapeutic strategies mainly target inflammation mitigation to alleviate arthralgia and decelerate disease progression[21]. Notably, these pharmacotherapies are invariably associated with dose-dependent adverse effects, including but not limited to gastrointestinal mucosal injury, cardiovascular complications and hepatorenal toxicities, which collectively pose significant challenges to the clinical management of inflammatory bone diseases[22,23]. Traditional Chinese medicine (TCM) boasts a time-honored historical lineage and extensive clinical application in the management of inflammatory bone diseases[24-26]. According to TCM theory, these conditions are categorized under the classical concept of “Bi syndrome”, a pathophysiological state arising from the synergistic invasion of three exogenous pathogens - pathogenic wind, cold and dampness - which obstruct the circulation of Qi and blood within the meridians and joint spaces. This obstruction manifests as localized stagnation, ultimately progressing to pain, swelling and functional impairment through mechanisms involving synovial and collateral vessel lesions. Notably, while TCM and Western medicine diverge in their conceptual frameworks regarding inflammatory bone diseases, they exhibit convergent therapeutic approaches. Importantly, numerous anti-inflammatory herbal agents within TCM have been validated to delay disease progression[26-28]. Previous studies have demonstrated that TCM monotherapy or integrated TCM-Western medicine regimens exhibited superior therapeutic efficacy compared to Western medicine alone in managing inflammatory bone diseases, alongside more favorable safety profiles[29,30]. Therefore, delineating the precise molecular targets of TCM-derived anti-inflammatory agents holds critical significance for expanding the therapeutic strategies against inflammatory bone diseases. However, existing studies suffers from limited systematic summary of the anti-inflammatory mechanisms by TCM. This review aims to provide a comprehensive review of molecular mechanisms underlying TCM interventions in IA and OA, thereby providing a new perspective for the clinical management of inflammatory bone diseases (Figure 1).
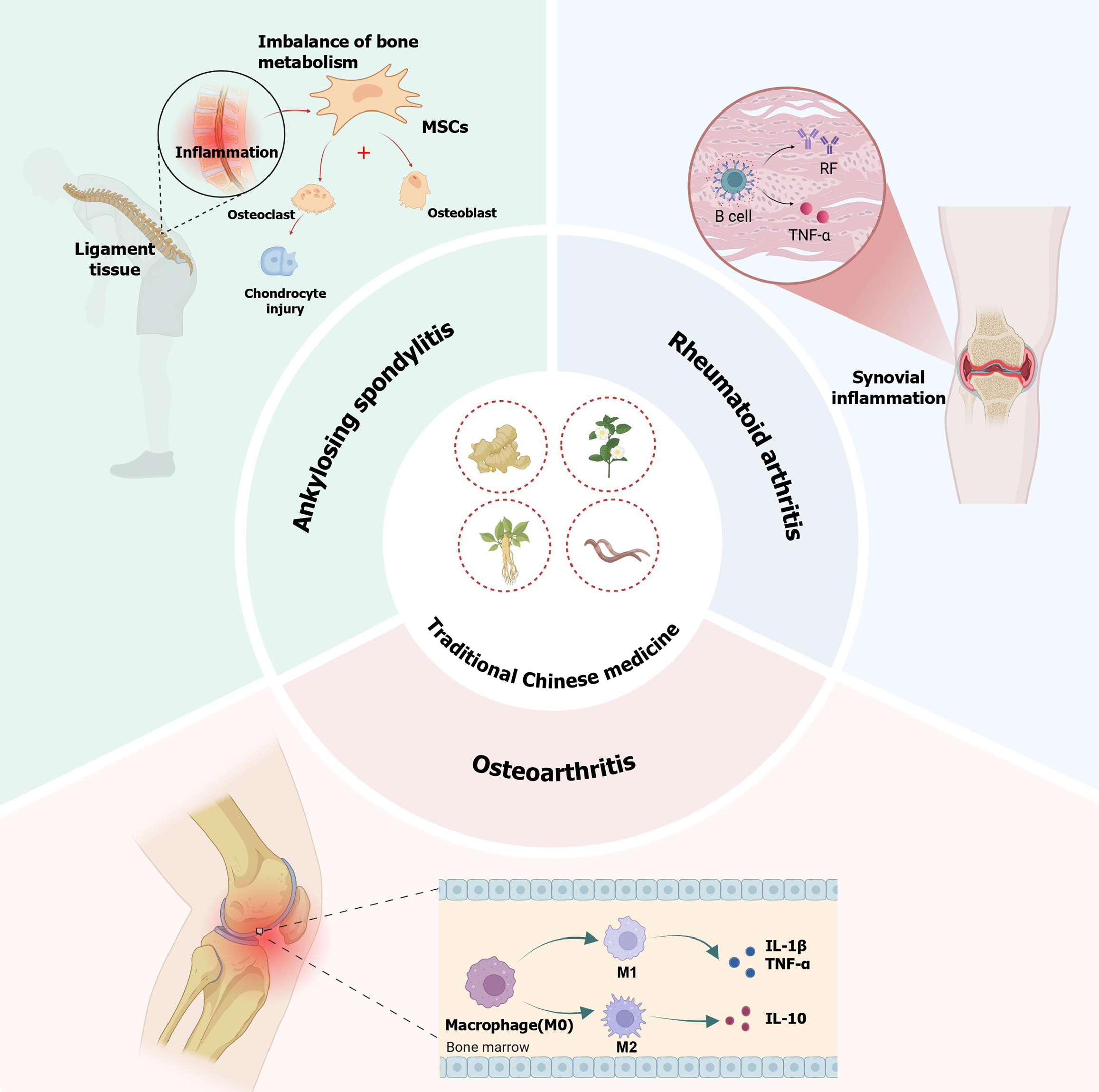
Figure 1 A summary of the important pathogenesis and pathological characteristics of rheumatoid arthritis, ankylosing spondylitis and osteoarthritis and the therapeutic effect of traditional Chinese medicine.
MSCs: Mesenchymal stem cells; RF: Rheumatoid factor; TNF-α: Tumor necrosis factor α; IL: Interleukin.
TCM CAN EFFECTIVELY RELIEVE THE SYMPTOMS OF RA BY REDUCING SYNOVIAL INFLAMMATION
RA, a high-prevalence chronic autoimmune disorder, is pathologically defined by synovial hyperplasia with neoangiogenesis, progressive erosion of articular cartilage, and subchondral bone destruction[31,32]. The advanced stage of RA often leads to severe joint deformities and irreversible functional disability, which seriously reduces the quality of life of patients[33,34]. Current Western pharmacotherapeutic strategies for RA remain constrained, primarily comprising disease-modifying antirheumatic drugs and glucocorticoids[35]. Unfortunately, although these agents ameliorate clinical manifestations such as joint swelling and morning stiffness, the degree of improvement is incomplete, and it is accompanied by intolerable adverse drug reactions including gastrointestinal ulceration, cardiovascular complications and Glucocorticoid-induced metabolic disturbances[36].
Several previous studies have confirmed that TCM demonstrate significant clinical efficacy in treating RA. Pan et al[37] demonstrated that Guizhi Shaoyao Zhimu decoction significantly decreased the arthritis score, mitigated joint swelling and bone damage and lowered the pathological score. Additionally, it effectively reduced the serum levels of matrix metalloproteinases in mice with collagen-induced arthritis[37]. The underlying mechanism of this therapeutic effect may be attributed to the fact that this herbal remedy reduces the level of synovial inflammation by significantly inhibiting the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/NF-κB signaling pathway regulated by secretory leukocyte protease inhibitor. Another study found that zingerone, a small molecule derived from ginger, significantly decreased the expression of inflammatory factors such as NF-κB transforming growth factor-β, tumor necrosis factor α (TNF-α), IL-1β, and IL-6 in the RA animal model[38]. Notably, zingerone also effectively restored antioxidant enzyme levels and further attenuated inflammatory responses[38]. Curcuma longa, a member of the Zingiberaceae family, is an important natural compound known for its anti-inflammatory and antioxidant properties. The active component of Curcuma longa is curcumin[39,40]. Previous studies have demonstrated that curcuminoids effectively suppress the production of proinflammatory cytokines, including TNF-α, IL-1β, IL-6, IL-12 and IL-8, as well as chemokines, thereby alleviating inflammation associated with RA[41]. Zeng et al[42] conducted a meta-analysis to comprehensively assess the clinical efficacy of curcumin in RA treatment and revealed that curcumin inhibits osteoclast differentiation by downregulating the expression of the RANK/RANKL gene and associated proteins. Additionally, curcumin balances bone metabolism in RA patients through the inhibition of NF-κB signaling pathway activation[42]. Sinomenine (SIN), an isoquinoline alkaloid derived from Sinomenium acutum, has been utilized for the treatment of RA[43]. It has garnered significant attention due to its comparable efficacy to methotrexate in treating RA, while exhibiting fewer adverse effects[44]. The influence of SIN on the progression of RA inflammation is primarily mediated through the regulation of immune cells and the suppression of inflammation-related signaling pathways[45]. Adenosine monophosphate-activated protein kinase (AMPK) has been identified as a pivotal factor in maintaining intracellular adenosine monophosphate/adenosine triphosphate balance. It interacts with other key enzymes involved in cell growth and metabolism, such as acetyl-CoA carboxylase, 3-hydroxy-3-methylglutaryl-CoA reductase and glucose-6-phosphate dehydrogenase, thereby modulating energy metabolism[46]. SIN has been shown to inhibit the differentiation of RA-fibroblast-like synoviocytes by reducing the phosphorylation level of AMPK. Additionally, SIN suppresses the production of pro-inflammatory cytokines, including TNF-α, IL-1β and IL-6, ultimately exerting a therapeutic effect in preventing the progression of RA[47]. In addition, the gut-joint axis also appears to be an underlying pathological mechanism in RA, and SIN has been shown to significantly increase the abundance of Lactobacillus in the gut microbiota. This change reactively increases the levels of tryptophan metabolites and they can effectively activate aryl hydrocarbon receptor level to affect the tissue selective migration of Th17 and Treg cells to joints and synovium. This new pharmacological mechanism provides an extremely feasible idea for the treatment of RA[48]. In addition, previous studies have indicated that neutrophil extracellular traps (NETs) play a significant role in the activation of RA-fibroblast-like synoviocytes[49]. NETs are able to potentially compromise the cartilage matrix and trigger the release of membrane-bound peptidylarginine deiminase-2 through elastase-mediated mechanisms, thereby contributing to extensive articular cartilage damage[50]. Huangqin Qingre Chubi Capsule is a classical TCM compound preparation utilized for the treatment of RA. Li et al[51] demonstrated that Huangqin Qingre Chubi Capsule can block the activation of the p38/mitogen-activated protein kinases (MAPK) signaling pathway and inhibit NETs formation, thereby ameliorating the inflammatory microenvironment in RA[51]. The Janus kinase (JAK)/STAT signaling pathway plays a crucial role in RA inflammation and joint destruction by mediating the signal transduction of various inflammatory factors, including IL-2, IL-4, IL-7, IL-9, IL-15, and TNF-α[52]. Genkwanin, a small molecule derived from Daphne genkwa, has been shown to effectively suppress the activation of both the JAK/STAT and NF-κB signaling pathways, leading to a reduction in the levels of pro-inflammatory cytokines such as TNF-α and IL-6[53]. Another study suggested that Yishen Tongbi Decoction, a traditional Chinese prescription, may exert anti-inflammatory effects and prevent cartilage destruction through the regulation of the JAK/STAT3/suppressor of cytokine signaling 3 signaling pathway[54].
From the aforementioned description, it is evident that TCM hold significant potential in the treatment of RA (Figure 2). TCM can modulate the release of inflammatory factors from immune cells by targeting multiple inflammatory signaling pathways, such as PI3K/AKT/NF-κB, JAK/STAT, and AMPK. Additionally, it influences the directional migration of Th17 and Treg cells to inflamed joints and synovitis via its action on the gut-joint axis. Furthermore, TCM inhibit the activation of NETs and other mechanisms, thereby mitigating the progression of RA-associated inflammation. Consequently, it is logical to propose that future research should focus on exploring the anti-inflammatory capabilities of TCM in greater depth. Particular attention should be directed toward identifying effective active compounds within TCM formulations and evaluating the potential of novel combinations of these compounds.
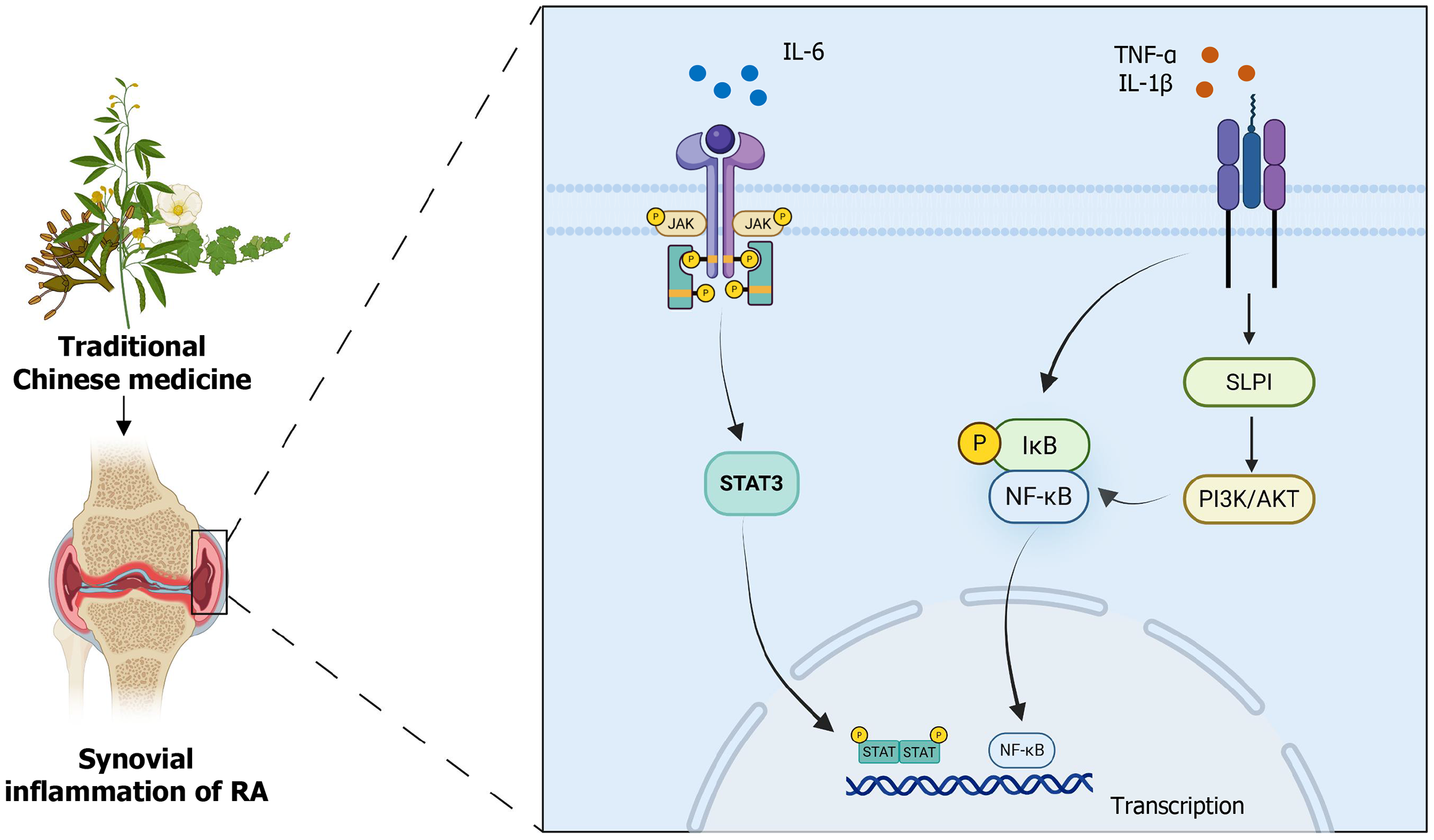
Figure 2 Traditional Chinese medicine reduce the synovial inflammation of rheumatoid arthritis through phosphatidylinositol 3-kinase/protein kinase B, Janus kinase/signal transducer and activator of transcription 3 and nuclear factor kappa B signaling pathways.
RA: Rheumatoid arthritis; TNF-α: Tumor necrosis factor α; IL: Interleukin; NF-κB: Nuclear factor kappa B; IκB: IkappaB; SLPI: Secretory leukocyte protease inhibitor; PI3K/AKT: Phosphatidylinositol 3-kinase/protein kinase B; STAT3: Signal transducer and activator of transcription 3.
TCM INHIBIT THE INFLAMMATORY BONE DESTRUCTION TO DELAY THE PROGRESSION OF AS
Inflammatory bone destruction represents a critical component in the pathogenesis of AS, encompassing enthesitis, systemic osteoporosis, and spinal and joint bone erosion[55,56]. The disorder of immune system is an important persistent factor of chronic inflammation in AS. The balance of Th1 and Th17 cells is disrupted, leading to abnormal secretion of large quantities of IL-17, IL-22, and TNF-α, which cause enthesitis and periosteal tissue inflammation[57,58]. Similar to the management of RA, the medical treatment options for AS are currently limited. Unfortunately, in the advanced stages of AS, many patients often require surgical intervention to address severe thoracolumbar kyphosis[59,60]. Therefore, it is highly significant to investigate the feasibility of early pharmacological intervention for AS. In recent years, an increasing number of studies have focused on examining the potential efficacy and reliability of TCM for AS.
Our previous research has demonstrated that extracellular vesicles in the AS ligament tissue were capable of transferring IL-17A and activating the JAK-STAT3 signaling pathway in mesenchymal stem cells. This process subsequently contributes to pathological inflammatory bone destruction and new bone formation in AS[61]. Therefore, the inhibition of the JAK-STAT3 and IL-17 inflammatory pathway may be a key approach for the treatment of AS[62]. Ding et al[63] found that triptolide, a major bioactive compound derived from Tripterygium wilfordii, inhibits the JAK/STAT3 signaling pathway by interacting with the long non-coding RNA NONHSAT227927.1, thereby alleviating the inflammatory response associated with AS[63]. Chen et al[64] also demonstrated that Tripterygium glycosides could significantly reduce the level of inflammation in AS patients by inhibiting the concentration of IL-17 in peripheral blood. Xinfeng capsule, a compound preparation of TCM, is widely used in the treatment of AS. The active components quercetin and kaempferol contained in Xinfeng capsule have been confirmed to effectively improve the hypercoagulable state of blood and inflammatory markers in AS by downregulating the NF-κB and IL-17 signaling pathways[65]. The abnormal activation of the toll-like receptor (TLR) 4/NF-κB/NOD-like receptor 3 (NLRP3) inflammasome pathway serves as an important inflammatory initiating factor in AS. Previous study has found that resveratrol could restore intestinal mucosal barrier function and modulated the composition of the intestinal microbiota by downregulating the toll-like receptor 4/NF-κB/NLRP3 pathway, thereby exerting an anti-AS effect[66]. Feng et al[67] confirmed through the construction of the AS animal model that punicalagin can effectively enhance the antioxidant stress capacity and modulate the NF-κB/Th17/JAK2/STAT3 signaling pathway, consequently alleviating the inflammatory response in AS. Dong et al[68] found that Chrysanthemum indicum significantly down-regulates the protein expression levels of NF-κB, Dickkopf-1 and sclerostin, consequently inhibiting the production of downstream pro-inflammatory factors such as TNF-α, IL-1β, and IL-6. All of the aforementioned studies emphasize a seemingly potential connection between the NF-κB pathway and oxidative stress in the progression of AS. Consequently, we investigated this hypothesis and discovered that the NF-κB pathway and reactive oxygen species are interconnected in the development of AS-associated inflammation. Furthermore, cynarin can exhibit its anti-inflammatory effects by disrupting the coupling between the NF-κB pathway and oxidative stress[69].
Inflammation and heterotopic ossification are interconnected processes that play complementary roles in the pathogenesis of AS[70]. Previous studies have found that TCM can interfere with abnormal bone formation through its anti-inflammatory properties. Li et al[71] suggested that Danshensu, an active compound derived from TCM, can suppress AS-related inflammatory bone formation by reducing the phosphorylation of JNK and ERK in AS fibroblasts. Another study showed that triptolide decreases bone morphogenetic protein receptor type II, small mothers against decapentaplegic (Smad) 1 and Smad4 in rats with AS. This reduction contributes to the inhibition of inflammation and osteogenic differentiation associated with AS[72]. Bushen Qiangji Granule is a TCM compound with good clinical efficacy. Liu et al[73] found that Bushen Qiangji Granule could reduce the osteogenic differentiation of AS fibroblasts by inhibiting the abnormal activation of BMP/Smads signaling pathway.
The etiology of AS is highly complex, and inflammatory bone destruction remains a hallmark throughout the disease progression. TCM, with its multi-target and multi-component nature, exhibits considerable therapeutic potential for AS (Figure 3). Nevertheless, compared to RA, the application of TCM in AS remains underexplored. Current research predominantly focuses on limited therapeutic targets and signaling pathways, while there is a paucity of efficacy validation through human studies.
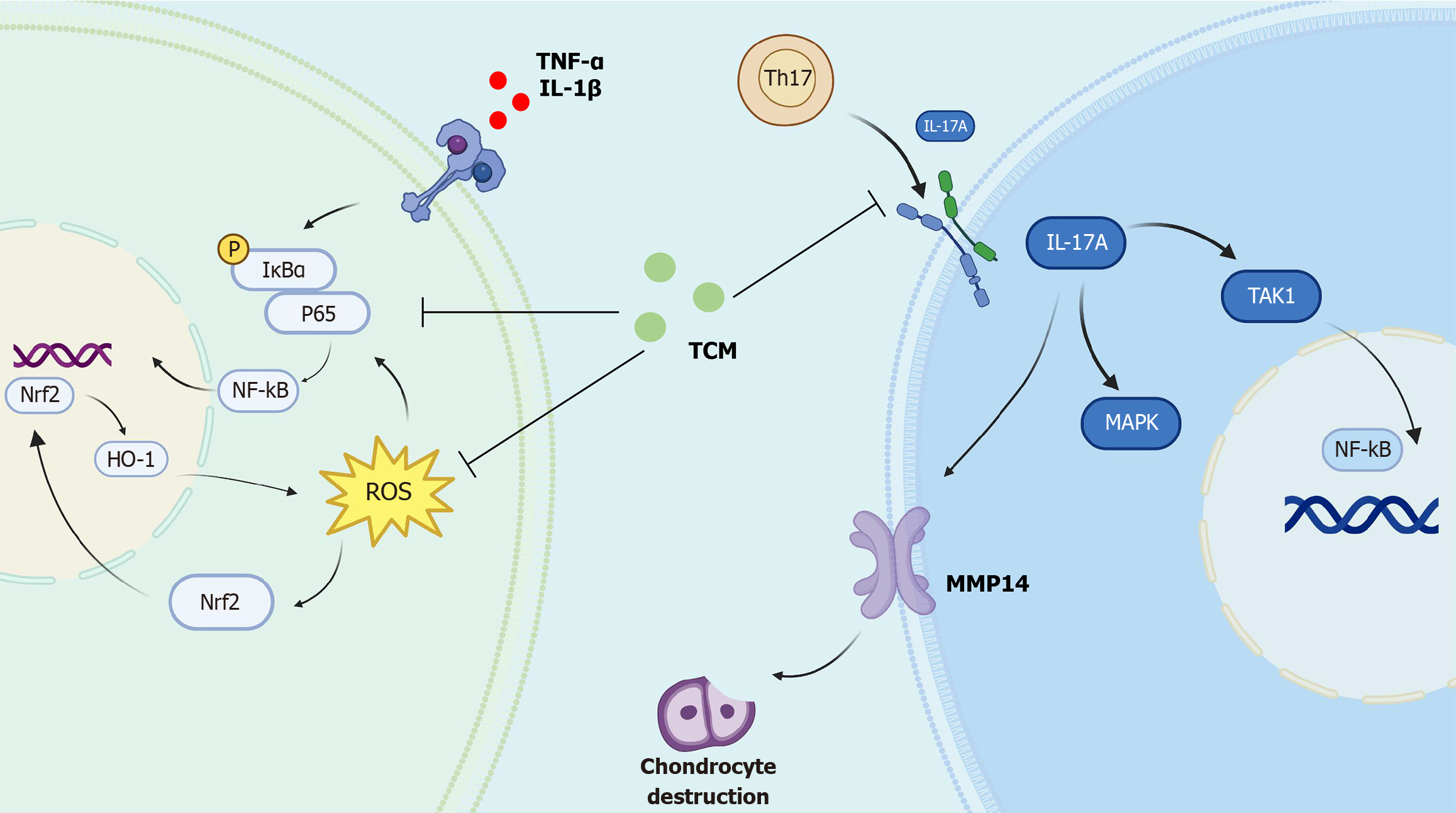
Figure 3 Traditional Chinese medicine alleviate inflammatory bone destruction in ankylosing spondylitis through nuclear factor erythroid/reactive oxygen species/nuclear factor kappa B axis and interleukin-17A signaling pathway.
TH17: T helper-17; TNF-α: Tumor necrosis factor α; IL: Interleukin; IκBα: IkappaB α; NF-κB: Nuclear factor kappa B; Nrf2: Nuclear factor erythroid 2-related factor 2; ROS: Reactive oxygen species; TCM: Traditional Chinese medicine; MMP14: Matrix metalloproteinase 14; TAK1: Transforming growth factor beta-activated kinase 1; MAPK: Mitogen-activated protein kinase.
TCM EXERTS AN ANTI-OA EFFECT BY INHIBITING MULTIPLE INFLAMMATORY SIGNALING PATHWAYS
OA was historically regarded as a degenerative disease primarily characterized by cartilage destruction[74,75]. However, as research has advanced, an increasing number of studies suggest that synovial inflammation plays a critical role in the pathogenesis of OA[76-78]. Long-term chronic inflammation contributes to the degradation of the cartilage extracellular matrix and induces chondrocyte apoptosis, ultimately resulting in subchondral bone deformation, joint deformity and mobility impairments. Therefore, the clinical management of OA frequently relies on the anti-inflammatory strategy. TCM possesses extensive practical experience and a well-established theoretical foundation in treatment of OA. However, elucidating the molecular mechanisms of TCM in OA treatment through the lens of modern medical theory remains a great challenge that requires urgent attention. Consequently, focusing on the anti-inflammatory effects of active TCM components targeting specific signaling pathways may pave the way for novel therapeutic approaches in OA.
Curcumin, as previously mentioned, has been extensively documented for its potential to delay the progression of OA. Qiu et al[79] reported that curcumin exerts its therapeutic effects in OA treatment by modulating the exosomes derived from mesenchymal stem cells and subsequently regulating the miR-124/NF-κB and miR-143/Rho-associated coiled-coil containing protein kinase 1/TLR9 signaling pathways. Wang et al[80] demonstrated that curcumin can suppress the activation of NF-κB and hypoxia-inducible factor-2α in chondrocytes, thereby markedly attenuating IL-1β-induced inflammatory damage to chondrocytes[80]. Dysregulation of the p38/MAPK pathway has been shown to induce a broad spectrum of inflammatory responses and exacerbate the degradation of the cartilage matrix. Another study combined with network pharmacological analyses and biological experiments, suggested that the p38/MAPK pathway may serve as a key target for curcumin in the treatment of OA[81]. Curcumin is capable of downregulating downstream inflammatory factors by inhibiting the phosphorylation of the p38/MAPK pathway. In addition, Jiang et al[82] found that curcumin protects cartilage by inhibiting oxidative stress, inflammation and degradation of the cartilage extracellular matrix through activation of the nuclear factor erythroid 2-related factor 2 (NRF2)/antioxidant response element signaling pathway. Similar to curcumin, Salvianolic Acid A, a bioactive compound extracted from the Danshen, has suggested significant anti-inflammatory effects through the inhibition of the NF-κB and p38/MAPK signaling pathways[83]. Lu et al[84] confirmed that Oroxin B, a flavonoid compound, inhibits the activation of the PI3K/AKT/mammalian target of the rapamycin signaling pathway, thereby reducing inflammation and providing protective effects on cartilage. The NLRP3 inflammasome plays an important role in the process of chondrocyte inflammatory pyroptosis[85,86]. Abnormally elevated levels of the NLRP3 inflammasome and its downstream proinflammatory cytokines IL-1β and IL-18 have been detected in the synovium of OA animal models, suggesting that the activation of the NLRP3 inflammasome is closely associated with the pathogenesis of OA[87,88]. TCM has been extensively applied to inhibit the NLRP3 inflammasome. For instance, Zu et al[89] demonstrated that Icariin significantly downregulated the expression of NLRP3, IL-1β and IL-18, thereby effectively modulating the inflammatory pyroptosis of chondrocytes. Another study found that Cucurbitacin B can alleviate inflammation and retard the degradation of the extracellular matrix in OA animal model by activating the NRF2 pathway and suppressing the NF-κB/NLRP3 inflammasome signaling pathway[90]. Similarly, Li et al[91] demonstrated that Forsythoside A, a phenethyl alcohol glycoside extracted from Forsythia fruit, activates the NRF2 pathway and suppresses NLRP3 inflammasome activation, consequently inhibiting chondrocyte senescence and mitigating the progression of OA. These studies show that the oxidative stress-protective pathway NRF2 and the NLRP3 inflammasome play a crucial role in the pathogenesis of OA. Additionally, small molecules derived from TCM can suppress OA by interfering with the NRF2/NLRP3 inflammatory signaling pathway axis.
Radix Achyranthis Bidentatae (AB) is a representative herbal medicine used in the treatment of OA within TCM. Previous researches have suggested that AB can target multiple pathogenic factors associated with OA[92,93]. AB effectively reduces the expression levels of inflammatory cytokines in synovial tissue, such as TNF-α, IL-2 and IL-6, by modulating the balance between Th17 and Treg cells. Furthermore, AB enhances the expression of type II collagen, thereby promoting the restoration of the cartilage matrix. Additionally, AB exerts anti-inflammatory effects by acting on the IL-17 signaling pathway[94].
The advantage of TCM in the treatment of OA is attributed to its multi-component and multi-target characteristics. Xia et al[95] demonstrated that Jiawei Yanghe decoction could reduce the expression of inflammatory apoptosis-related genes, such as Caspase-3 and Caspase-9, in cartilage by inhibiting the Wnt/β-catenin signaling pathway, thereby exerting protective effects on cartilage and providing anti-inflammatory benefits. Zheng et al[96] found that Duhuo Jisheng decoction reduces the expression of IL-1β, TNF-α and IL-6 by modulating the IL-6/STAT3 signaling pathway, thereby alleviating inflammation in OA. Yan et al[97] reported that quercetin and kaempferol, the active compounds in Wutou Decoction, exert their effects on key targets such as TNF, IL-6 and IL-1β, which are associated with conditional OA immune inflammation. Yang et al[98] found that the Gubi Zhitong formula could alleviate OA-related inflammation and cartilage damage by modulating B-cell lymphoma 2 interacting protein 3-like-mediated mitophagy. Fan et al[99] shown that Soufeng sanjie formula could inhibit the M1 polarization of synovial macrophages and inhibit the levels of intestinal metabolite 18-hydroxyoleic acid. Furthermore, the expression of IL-6, IL-1β and TNF-α was decreased. Xiong et al[100] demonstrated that the BuShen HuoXue Formula can inhibit IL-1β-mediated chondrocyte apoptosis, thereby exerting an anti-OA effect. Sun et al[101] used network pharmacology and inflammation-related analyses to demonstrate that Zhang’s Xibi formula can modulate the expression of immune-related proteins in the joints of OA mice, including IL-17 and extracellular signal-regulated kinase 1, thereby alleviating OA symptoms[101].
In summary, a large number of studies have validated the efficacy of TCM in suppressing immune-related inflammatory responses in OA. The ongoing identification of effective bioactive molecules from TCM can offer novel strategies for OA treatment (Figure 4). However, current studies are characterized by relatively single mechanisms and a lack of interconnectedness. In the future, it will be essential to use multi-omics technologies to comprehensively and profoundly investigate the underlying mechanisms of TCM in the treatment of OA.
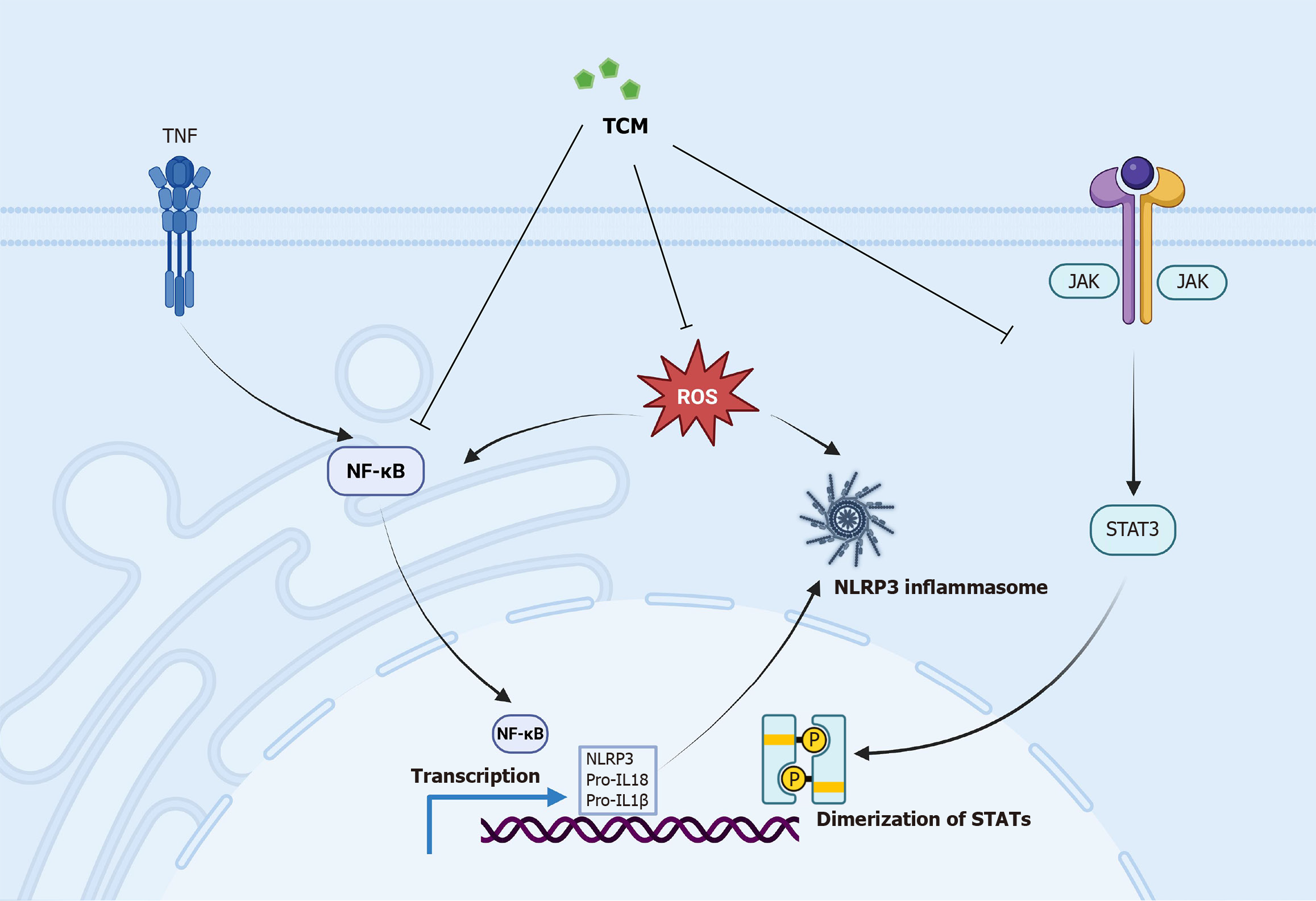
Figure 4 Traditional Chinese medicine suppress osteoarthritis-related inflammation by inhibiting the Janus kinase/signal transducer and activator of transcription 3, nuclear factor kappa B, reactive oxygen speciesand NOD-like receptor 3 inflammatory signaling pathways.
TNF: Tumor necrosis factor; NF-κB: Nuclear factor kappa B; ROS: Reactive oxygen species; TCM: Traditional Chinese medicine; IL: Interleukin; NLRP3: NOD-like receptor 3; STAT: Signal transducer and activator of transcription; JAK: Janus kinase.
CONCLUSION
This article provides a comprehensive analysis of the therapeutic effects of monomeric and compound formulations of TCM on inflammatory bone diseases. Focusing on the inflammatory response, the core pathological mechanism of inflammatory bone diseases, we systematically elucidate how TCM can mitigate immune-related inflammation by modulating various molecular mechanisms, thereby slowing the progression of RA, AS and OA. We also found that common factors such as IL-17, NF-κB, JAK/STAT, NLRP3 and reactive oxygen species play key roles in initiating inflammatory processes in inflammatory bone diseases. We anticipate that this review will offer novel insights into TCM-based research for treating inflammatory bone diseases and inspire further exploration of TCM active components targeting common inflammatory pathways.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade C
Creativity or Innovation: Grade D
Scientific Significance: Grade C
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Almahasneh F, PhD, Assistant Professor, Jordan S-Editor: Bai Y L-Editor: A P-Editor: Zhao YQ