INTRODUCTION
Hip pain in young patients is a common complaint that can pose diagnostic challenges due to its non-specific symptoms and referred pain arising from structures nearby such as the lumbosacral spine, pelvis, and perineum. Clinical evaluation of hip pain requires strong foundational knowledge of relevant anatomy, careful history-taking, focused physical examination, and appropriate imaging techniques. Hip arthroscopy allows for excellent visualization of the articular surfaces of the hip joint, as well as the extra-articular and peri-trochanteric spaces. With the rapid advancements in hip arthroscopy techniques over the past decade, it has become an essential tool in the assessment and management of non-arthritic hip pain in young patients. This article aims to provide an evidence-based and contemporary update on ten common causes of non-arthritic hip pain. Each condition is discussed with an overview of relevant anatomy, clinical features and evaluation methods, as well as treatment and management strategies, emphasizing state-of-the-art arthroscopic techniques where applicable. These causes are categorized into three groups based on predominant symptom location: (1) Anterior hip symptoms, including femoroacetabular impingement (FAI) syndrome, labral tears, chondral injuries, hip instability, and internal snapping hip syndrome; (2) Lateral hip symptoms, such as greater trochanteric pain syndrome and external snapping hip syndrome; and (3) Posterior hip symptoms, including deep gluteal syndrome (DGS), ischio-femoral impingement (IFI), and proximal hamstring injuries. This categorisation facilitates the readers’ understanding and enhance evaluation of hip pain in the clinical setting. By employing a systematic evaluation approach, clinicians can enhance their ability to diagnose and effectively treat patients with non-arthritic hip pain.
ANTERIOR HIP SYMPTOMS
FAI syndrome
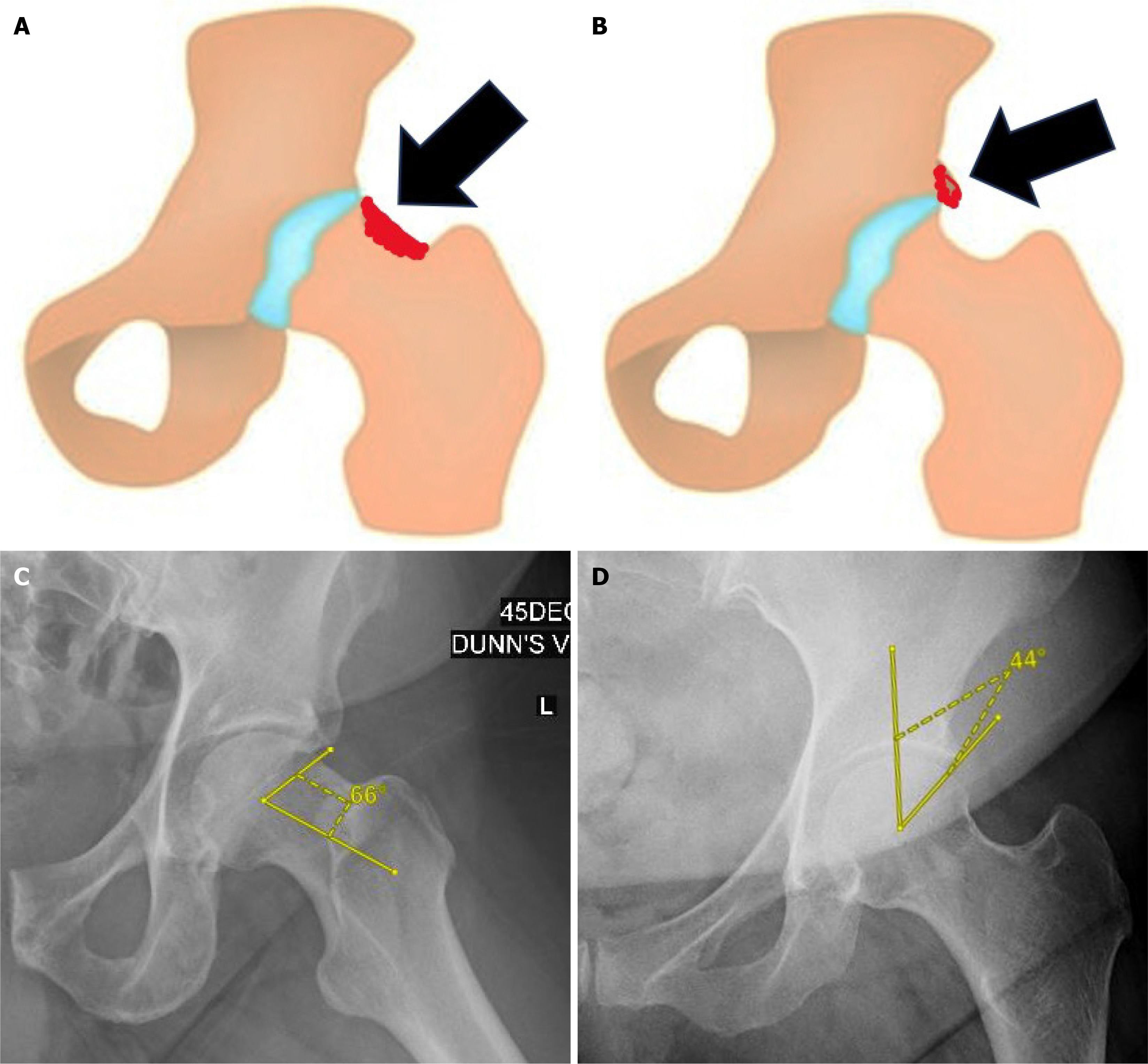
Relevant anatomy: FAI arises from abnormal contact between the proximal femur and acetabular rim during terminal motion of the hip as a result of aberrant femoral and/or acetabular morphology[1]. Cam morphology is characterised by a loss of sphericity at the femoral head-neck junction (Figure 1A). The aspherical femoral head creates shearing forces along the acetabular cartilage resulting in chondral delamination, as well as labral avulsion, in the anterosuperior acetabular rim[1]. Pincer morphology involves global or focal (usually anterosuperior) over-coverage of the femoral head by the acetabulum (Figure 1B). Intrasubstance tears of the anterosuperior labrum and ‘contre-coup’ posteroinferior chondral lesions occur as a result of the over coverage and leverage of the femoral head in the acetabulum[1]. These labral and chondral injuries predispose to early development of hip osteoarthritis[1-3].
Figure 1 Femoroacetabular impingement syndrome.
A: Cam deformity; B: Pincer deformity; C: Increased alpha angle > 60 degrees; D: Increased lateral center-edge angle > 40 degrees.
Clinical features and evaluation: The 2016 Warwick Agreement on FAI syndrome emphasised the need for relevant symptoms and signs to be present, in addition to the abovementioned morphological features, for the diagnosis of FAI syndrome[4]. The main symptom in FAI syndrome is motion- or position-related hip and/or groin pain, which may also extend to the lower back, thigh, and knee. Associated symptoms include clicking, catching, locking, and stiffness. These manifestations are commonly observed in active young to middle-aged adults and may significantly limit daily activities and overall function. A key clinical sign of FAI syndrome is pain reproduction upon flexion, adduction, and internal rotation of the hip. Posteroinferior impingement may also occur and is elicited by passive hip extension and external rotation.
Radiological evaluation of FAI syndrome includes an anteroposterior (AP) radiograph of the pelvis and Dunn view radiograph of the hip(s). The Dunn view radiograph is especially important in the evaluation of FAI syndrome due to its increased sensitivity for detecting femoral head-neck asphericity. An alpha angle > 60 degrees[5] suggests cam morphology (Figure 1C). The lateral center-edge angle, as measured on the AP pelvis radiograph, evaluates for acetabular over coverage. A value of > 40 degrees[6] is indicative of pincer morphology (Figure 1D). The AP pelvis radiograph may also indicate acetabular retroversion when the ‘crossover’ sign is present[7]. Cross-sectional imaging with computed tomography, magnetic resonance imaging (MRI) and magnetic resonance arthrogram (MRA) allow for better visualisation of morphology such as acetabular and femoral version; as well as associated soft tissue injuries.
Management: Patients presenting with FAI syndrome may first undergo a trial of conservative management. This includes patient education, activity modification, physiotherapy and analgesia. Analgesia options include oral medications and intra-articular anaesthesia and steroid injection. Image-guided intra-articular injections provide diagnostic and therapeutic value in the initial evaluation of a patient with suspected FAI syndrome[8]. A prospective cohort study by Zogby et al[9] found that conservative management of FAI syndrome led to significant improvements in patient reported outcomes at a mean 5-year follow-up. Surgical intervention may be necessary for patients presenting with significant intra-articular pathology, those who have not responded to conservative treatment, or individuals whose initial symptoms are severely debilitating. Hip arthroscopy results in significant improvements of patient-reported outcomes[10,11], and has been shown to be superior to open surgery with better outcomes and fewer complications[12]. Surgical techniques include femoral osteoplasty to correct cam deformities and acetabuloplasty to address pincer deformities. Two separate multi-center, assessor-blinded, randomized controlled trials, namely the United Kingdom FASHIoN[13] and FAIT[14] trials, concluded that while both hip arthroscopy and physiotherapy improved hip-related quality of life, arthroscopy resulted in a significantly greater degree of improvement.
Labral tears
Relevant anatomy: The acetabular labrum is a horseshoe-shaped structure that is attached to the acetabular rim and comprises both capsular and articular components[15]. The capsular component consists of dense connective tissue while the articular component is composed of fibrocartilage. The labrum is intrinsically avascular and receives blood supply from the surrounding capsule and synovium[16]. Labral innervation arises from branches of the nerve to the quadratus femoris and the obturator nerve, with multiple studies consistently demonstrating the presence of free nerve endings and nerve end organs in the acetabular labrum[17]. The labrum creates a seal which results in negative pressure within the central compartment of the hip joint. This negative pressure serves multiple functions including joint lubrication, cartilage nutrition, joint stability, as well as load distribution[2]. Additionally, the labrum also contributes to proprioception through nerve end organs[17]. The Multicentre Arthroscopy of the Hip Outcomes Research Network classifies labral injuries of the hip based on size, tear pattern and intrasubstance changes[18]. The labrum may be normal in size, hypoplastic or hyperplastic. Tear patterns range from chondro-labral separation to complete undersurface/intra-substance tears as well as complex degenerative tears. Intrasubstance changes include mucoid degeneration, calcification, ossification and ganglion cyst formation.
Clinical features and evaluation: Labral tears can be a primary source of significant hip pain. Moreover, labral tears are commonly associated with other hip pathologies such as FAI and hip dysplasia[19], further contributing to symptoms. Groin pain is the primary symptom in labral tears but pain may also radiate to the lateral hip, buttock, thigh and knee. The onset of pain may be post-traumatic but is more often insidious. Pain is usually reproduced with the flexion, adduction, and internal rotation impingement test. MRA is superior to MRI in the evaluation of suspected labral tears due to the use of intra-articular gadolinium contrast. Indicative MRA features of a labral tear include increased signal intensity, absence of the labral recess, labral thickening, displacement from the acetabular rim, and visualization of contrast within the labral substance or at the labral-acetabular junction. The presence of a paralabral cyst may suggest an undisplaced labral tear. However, due to its limited sensitivity[20], a negative MRA does not rule out labral tears and surgery should be considered especially if symptoms are persistent.
Management: Surgical management of labral tears depends on factors such as viability of the labrum, the stability of the tear, and any associated hip pathologies. For small, stable, isolated tears with viable labral tissue, debridement and partial labrectomy may be appropriate[21]. Labral repair is recommended for unstable tears with viable tissue[22] and has demonstrated excellent outcomes and a high rate of survivorship at long-term follow-up[23]. It is also superior to debridement in patients with concomitant FAI[24]. For irreparable labral tears, labral reconstruction has emerged as a viable technique in the past decade, demonstrating good outcomes and survivorship at mid- to long-term follow-up[25].
Chondral injuries
Relevant anatomy: The articular surfaces of the acetabulum and femoral head are covered by varying thicknesses of hyaline cartilage composed of type II collagen. Cartilage nutrition is derived from diffusion through synovial fluid in the superficial layers and direct vascularity in the deeper calcified zone[26]. Chondral lesions in the hip can occur on the femoral head and/or acetabular fossa. Femoral sided lesions often arise from trauma such as dislocation or impaction; and can be classified based on the extent of chondral damage - ranging from thinning and softening to fibrillation and delamination[27]. Acetabular chondral lesions typically result from aberrant morphology, as seen in FAI syndrome and hip dysplasia, with the anterosuperior acetabulum being the most commonly affected area[28]. These lesions are classified based on geographic zones as defined by Ilizaliturri et al[29]; as well as thickness and size of the lesion[30].
Clinical features and evaluation: Chondral lesions are frequently associated with other hip pathologies such as FAI syndrome and labral tears, and present in a similar fashion. Activity-related anterior hip and groin pain predominate while secondary symptoms such as clicking, catching or locking may also be present in patients with full thickness delamination[27]. Passive log roll and impingement tests may reproduce symptoms of pain and clicking. MRA and delayed gadolinium enhanced MRI of cartilage are preferred imaging modalities for evaluating chondral lesions due to their improved visualisation and good correlation with arthroscopy findings[30].
Management: Chondral lesions have a limited capacity for complete healing[27,30]. While full thickness injuries that penetrate into subchondral bone allow for migration of mesenchymal cells and formation of an inflammatory ‘super clot’ to promote healing, the newly formed fibrocartilage differs in structure from hyaline cartilage in its collagen composition[31]. Biological treatment methods play a role in the conservative management of chondral lesions by promoting the regeneration of damaged cartilage. These include hyaluronic acid, platelet-rich plasma, mesenchymal stem cell and bone marrow aspirate concentrate injections[26]. Surgical options for managing chondral lesions in the hip include chondroplasty, cartilage repair, microfracture, and cartilage transplant. Chondroplasty is a suitable option for low-grade partial thickness lesions, aiming to debride unstable flaps and remove potential mechanical blocks in the hip joint[32]. For small full thickness chondral lesions, microfracture is a simple and cost-effective option to promote the formation of fibrocartilage[33]. Enhanced microfracture incorporates the use of adjuncts such as implantable scaffolds, growth factors and stem cells to promote chondrogenesis and improve the quality of repair tissue[26]. Larger chondral lesions may be treated with various cartilage transplant techniques ranging from autologous chondrocyte implantation to osteochondral autograft transplantation and osteochondral allograft transplantation[26]. Associated pathologies, such as FAI syndrome and labral tears, must also be addressed during surgery.
Hip instability
Relevant anatomy: Hip joint stability is contributed largely by bony anatomy, with the acetabulum morphology providing inherent static stability of the femoral head preventing extreme ranges of motion[34]. The labrum, joint capsule, surrounding ligaments and muscles each play separate but crucial roles to confer additional stability to the hip joint[35]. Hip instability is an emerging phenomenon and a real cause of hip pain and loss of function in the young active individual. For the purposes of this article, the term refers to instability that is not hip dislocation. It is recognized as “extra physiological hip movement causing pain”[36]. Causes of the condition can be multifactorial and include: (1) Bony factors such as acetabular dysplasia; (2) Soft tissue factors such as collagen disorders or labrum and capsular injury; and (3) The hip joint being subject to supra-physiological activity stresses[37].
Clinical features and evaluation: Hip instability can cause pain in the groin, thigh, or buttock. The patient may also report a sensation of giving way and instability, followed by apprehension[37]. The past medical history must be examined for connective tissue disorders that cause generalized laxity, prior significant hip trauma or prior surgical history which may point towards an iatrogenic cause of instability. Physical examination of the patient would be aimed at establishing symptomatic hip hypermobility. Some of the provocative tests that have been described include the prone external rotation test and anterior apprehension test, where a positive test indicates discomfort in the groin when the examiner passively tries to “destabilize” the hip joint[38]. A positive log roll test can also help to indicate underlying anterior capsular laxity or deficiency. Thorough examination of the deep hip muscles is of paramount importance - the iliopsoas or gluteus muscles can often be irritable as they tighten to compensate for hip joint laxity, and weakness of muscles surrounding the hip joint will also lead to a degree of instability.
Radiographs should be obtained to rule out any underlying acetabular dysplasia. A lateral centre-edge angle of less than 20 degrees, or an upsloping acetabulum with a Tonnis angle of greater than 10 degrees both point toward acetabular dysplasia[39,40]. Soft tissue advanced imaging should also be performed to evaluate deficiencies or abnormalities of the capsule or labrum. Dynamic methods to diagnose hip instability also exist, such as utilizing fluoroscopy to visualize the extent of hip joint distraction once a force has been applied[41]. However, it should be noted that to date, there is no gold standard to establish the diagnosis of hip instability[35].
Management: The mainstay of treatment is centered around physical therapy, strengthening muscles to best support the hip, with a focus on core stability. In the event of persistent symptoms that are refractory to physical rehabilitation, surgical interventions can be considered to improve hip stability in the long term. Surgical methods that have been described as treatment options include arthroscopic thermal capsulorrhapy, as well as arthroscopic capsular plication, even with good outcomes with just suture capsular plication and no bony procedures performed concurrently[42]. In cases of traumatic hip instability with associated soft tissue injuries such as labral tears, the concurrent pathology is usually addressed in the same setting.
Internal snapping hip syndrome
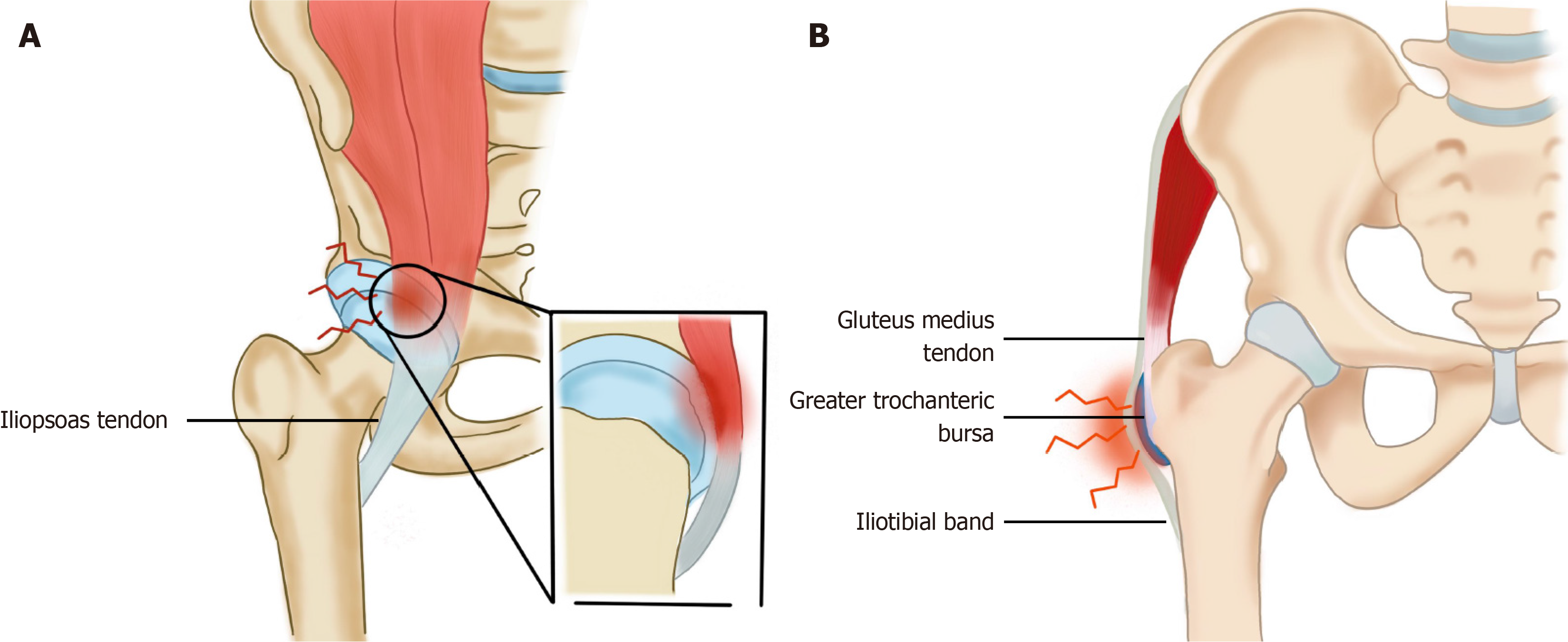
Relevant anatomy: The iliopsoas muscle is formed by the fusion of two separate muscles - the iliacus and the psoas. These two muscles arise from the iliac crest and the lumbar vertebrae respectively and combine at the level of the inguinal ligament to form the iliopsoas muscle which inserts onto the lesser trochanter. The iliopsoas tendon insertion has been described as an inverted teardrop-shaped region that covers the posterior surface of the lesser trochanter completely[43]. The proximal extent of the iliopsoas tendon insertion is closely related to the inferior insertion of the hip joint capsule, where it may either be susceptible to insult from, or contribute directly to adjacent intra-articular hip pathology. Iliopsoas impingement is also known as internal snapping hip syndrome and it involves an audible or palpable click or clunk of the hip joint when it moves from a flexed and externally rotated position to an extended and internally rotated position[44]. What actually happens on an anatomical level is that the iliopsoas tendon abuts against the femoral head or the iliopectineal eminence in this specific position which may cause a click (Figure 2A). Repetitive mechanical irritation of the musculoskeletal junction on the adjacent labrum at the 3 o’ clock position of the right hip or the 9 o’ clock position of the left hip can also lead to labral pathology[45].
Figure 2 The illustration of hip syndromes.
A: Internal snapping hip syndrome; B: Greater trochanteric pain syndrome.
Clinical features and evaluation: It is important to differentiate between snapping from painful snapping because simple snapping alone is not pathological and a commonly encountered symptom in up to 40% of patients without any groin pain[46]. Clinical examination of this condition will yield focal tenderness over the iliopsoas tendon at the anterior joint line, pain on active resisted straight leg raise as well as positive hip impingement tests. However, these tests are not specific for the condition, with further investigations being required. X-rays and MRI scans can be used to pick up acetabular dysplasia which is a possible cause of iliopsoas impingement. Labrum tears on these scans also signify underlying iliopsoas impingement. Ultrasound guided iliopsoas peritendinous injections are a further diagnostic and therapeutic tool, with the site of injection being useful to distinguish between intra-articular and extra-articular causes of groin pain.
Management: Treatment can be divided into conservative or surgical treatment options. Conservative measures include physical therapy to stretch and relax the iliopsoas tendon in cases of shortened or tightened muscles, reducing the inflammation or correcting underlying posture through pelvic mobilization to alleviate the impingement. The therapy should be combined with activity modification, deep massage and pharmacotherapy to achieve symptom improvement and relief. There is currently no standardized physiotherapy regimen with proven efficacy. Hip arthroscopy is currently the most useful technique in the surgical treatment of iliopsoas impingement. It is minimally invasive and has better outcomes compared to the traditional open surgery method[47]. An arthroscopic or endoscopic release of the iliopsoas tendon can be performed at various locations - the central compartment, the peripheral compartment and at the lesser trochanter with similar outcomes observed between different techniques[48].
LATERAL HIP SYMPTOMS
Greater trochanteric pain syndrome
Relevant anatomy: The pain generators on the lateral aspect of the hip are the greater trochanteric bursa, gluteus medius tendon and the iliotibial band (ITB, Figure 2B). Gluteus medius tendinopathy or tears are now thought to be more common than greater trochanter bursitis[49].
Clinical features and evaluation: Injury, inflammation or repetitive stress to any of these structures can cause lateral sided hip pain, aggravated by ambulation or other physical activities such as lying on the affected hip or being seated for long durations. Greater trochanteric pain syndrome most commonly affects women of 40 to 60 years of age, most of the times without any prior trauma[49]. The patient will have focal tenderness over the greater trochanter upon palpation, with resisted external rotation of the affected hip reproducing similar pain and discomfort over the lateral aspect of the hip. In more severe cases of gluteus medius pathology, patients may walk with a Trendelenburg gait or have a positive Trendelenburg test. In the setting of persistent pain and weakness, an MRI or ultrasound scan is strongly recommended to exclude a gluteus medius tendon tear.
Management: First line treatment consists of anti-inflammatory medications and physical therapy. Surgery is indicated in patients for large partial or complete gluteus medius tendon tear as outcomes are better than conservative treatment in patients with this condition. For patients without tendon tears, targeted combined steroid and local anaesthetic injections to the point of tenderness to the bursa or ITB will also help to alleviate symptoms. Platelet rich plasma injections have also become an effective alternative treatment for patients with recalcitrant greater trochanteric pain syndrome[50].
External snapping hip syndrome
Relevant anatomy: The culpable structures in external snapping hip syndrome are commonly the proximal ITB or occasionally the gluteus maximus tendon as it slides anteriorly over the posterior aspect of the greater trochanter. The ITB in this condition is always taut due to its proximal muscular attachments and distal bony attachments, with increased tension providing little opportunity for smooth gliding of the ITB through the range of motion over the prominent greater trochanter[51].
Clinical features and evaluation: Patients typically complain of a snapping sensation that sometimes manifests as a distinct audible pop or snap heard. Occasionally, some may experience pain or discomfort after repetitive snapping after prolonged activity. There is often a visible clunk over the posterolateral aspect of the greater trochanter as the hip performs the movement from an extended to a flexed position. Snapping is often palpable and can be mitigated by applying direct focal pressure over the gliding ITB[52]. Medical history and physical examination are the primary diagnostic methods, but a dynamic ultrasound scan can be utilized to document the tendon moving over the greater trochanter. An MRI scan for external snapping hip syndrome can show hypertrophied gluteus maximus, or edema over the tense proximal ITB[53].
Management: Conservative management is the primary treatment methodology and comprises of rest, stretching, physical therapy, and anti-inflammatory medication targeted towards the affected muscle tendons. These non-invasive measures have a high success rate[54]. More invasive procedures such as anaesthetic and corticosteroid injections into the trochanter bursa or tendon sheath can provide pain relief. Minimally invasive surgery is considered if there are persistent symptoms despite 3 months of conservative treatment, and usually involves release of the offending ITB or gluteus maximus tendon endoscopically[55].
POSTERIOR HIP SYMPTOMS
Deep gluteal syndrome
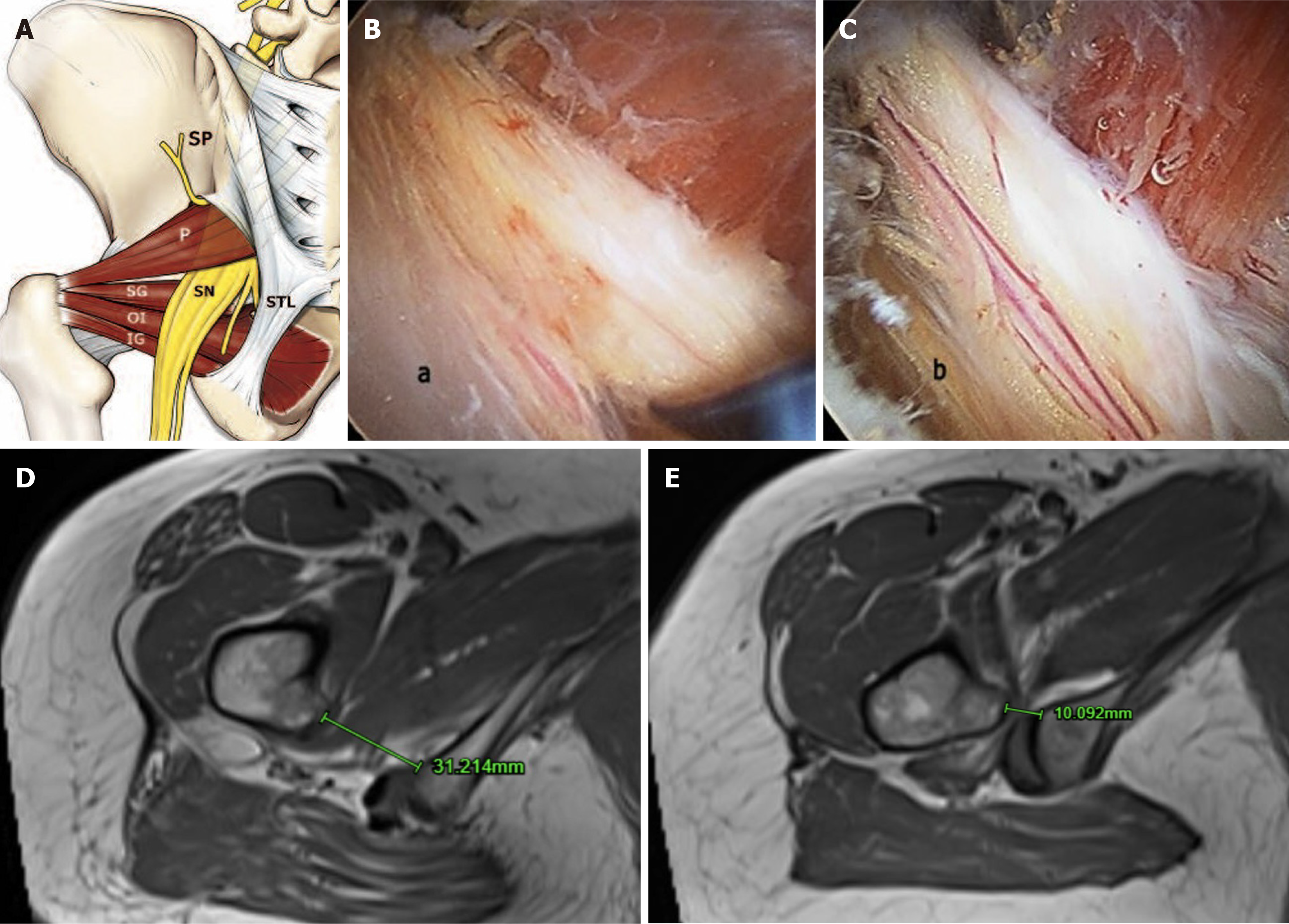
Relevant anatomy: The deep gluteal space is anterior and beneath the gluteus maximus muscle, and posterior to the posterior border of the femoral neck. It is bound superiorly by the inferior margin of the sciatic notch, inferiorly by the hamstring origin at the ischial tuberosity, laterally by the linea aspera on the posterior aspect of the femur, and medially by sacrotuberous ligament[56]. The contents of this space include the piriformis muscle which occupies a central position in the buttock, and the cluster of short external rotators (superior gemellus, obturator internus, inferior gemellus, quadratus femoris) distal to the piriformis (Figure 3A). The course of the sciatic nerve relative to the piriformis has been described in different anatomical relationships[57], with DGS referring to focal pain, dysesthesia or radicular pain in the buttock area or posterior thigh due to non-discogenic sciatic nerve entrapment in this space (Figure 3B). Specific entrapments that may be culpable include fibrous bands, piriformis syndrome, obturator internus/gemellus syndrome, quadratus femoris and ischiofemoral pathology.
Figure 3 Deep gluteal syndrome and ischiofemoral impingement.
A: Deep gluteal syndrome anatomy; B: Endoscopic view showing edematous and flattened sciatic nerve due to fibrovascular entrapment in a patient with ischemic neuritis; C: Normal vascularization recovery after sciatic nerve neurolysis; D: Normal ischiofemoral space between the medial cortex of the lesser trochanter and lateral cortex of ischial tuberosity (> 20mm); E: Reduced ischiofemoral space signifying underlying ischiofemoral impingement. Figure 3A-C is reproduced from Hernando et al[68]. Copyright © 2015 by Springer Nature. Published by Springer Nature. The authors have obtained the permission for figure using (Supplementary material). SP: Sacral plexus; SN: Sciatic nerve; STL: Sacrotuberous ligament; P: Piriformis muscle; SG: Superior gemellus; OI: Obturator internus muscle; IG: Inferior gemellus muscle.
Clinical features and evaluation: Evaluation of patients with DGS is challenging as symptoms are vague and unspecific and are often mistaken as lumbar or intra-articular hip pathology. Common symptoms include posterior hip or buttock pain with tenderness in the gluteal and retro-trochanteric region. The pain can resemble sciatica and is often unilateral. Rotation of the hip in flexion with the knee in extension can typically exacerbate symptoms. The active piriformis and seated piriformis stretch tests demonstrate high sensitivity and specificity for the diagnosis of sciatic nerve entrapment especially when used in combination[57]. The seated piriformis stretch test is a hip flexion/adduction with internal rotation test performed with the patient in a seated position, while the active piriformis test is performed by the patient pushing the heel down into the table, while abducting and external rotating the leg against resistance while the examiner monitors the piriformis for pain. High resolution magnetic resonance neurography are the investigation of choice for this condition and has shown to discriminate anatomy and small elements of the lumbo-sacral plexus and sciatic nerve if implicated in DGS[58].
Management: First line treatment consists mainly of rest, muscle relaxants and anti-inflammatory medications and physical therapy for a recommended duration of six weeks. The injection test is a useful part of the treatment algorithm, enabling diagnosis and excluding articular pathology. Guided injections through the piriformis muscle into the perineural sciatic fat or within the nerve sheath is recommended. Endoscopic decompression of the deep gluteal space with neurolysis of the sciatic nerve (Figure 3C) is a possible treatment option that has been described to improve function and diminish posterior hip pain[56].
Ischio-femoral impingement
Relevant anatomy: The ischiofemoral space (IFS) is a region in the proximal posterior thigh, between the lesser trochanter and the ischial tuberosity. The basic pathology in IFI is that this space is reduced and hence causing compression of the quadratus femoris muscle leading to pain. With the insertion of the psoas tendons on the lesser trochanter, and the hamstrings on the ischial tuberosity, bursitis of these tendons may also exist, either as a cause or a result of IFI[59].
Clinical features and evaluation: Patients who suffer from IFI tend to be older in their early fifties on average in comparison with other types of impingement. IFI is more common amongst females due to the pelvis being shallower and a wider ischial tuberosity[60]. Patients feel pain deep in the groin and/or buttock with radiation occasionally down the medial side of the thigh. Pain is usually brought upon by hip extension, adduction and external rotation. These symptoms are classically worsened with longer strides with pain felt just lateral to the ischium in the gluteal region. The IFI test involves passively extending the leg with the hip in an adducted or neutral position as the patient is in a contralateral decubitus position[61]. Reproduction of the symptoms with this manoeuvre represents a positive IFI test. Repeating the test with the hip abducted and should not elicit symptoms as the IFS is now increased. Plain X-ray radiographs can be used to screen for narrowing between the lesser trochanter and ischium but the MRI scan is the useful imaging modality of choice to demonstrate hyperintense signal surrounding the quadratus femoris muscle, and a reduced ischiofemoral distance which is measured as the smallest distance between the medial cortex of the lesser trochanter and the lateral cortex of the ischial tuberosity (Figure 3D and E)[60].
Management: Rest, activity modification and anti-inflammatory medications are the cornerstones of management of IFI. Guided anaesthetic and corticosteroid injections around the quadratus femoris is a subsequent intervention that can be considered in patients who fail non-invasive measures and may help to reduce pain. Only a minority of patients diagnosed with IFI eventually undergo surgical intervention in the form of open or endoscopic lesser trochanteric resection to aid in increasing the IFS[62].
Proximal hamstring injuries
Relevant anatomy: There are three hamstring muscles - the semimembranosus, semitendinosus, and the long head of the biceps femoris with all sharing a common origin at the ischial tuberosity. The semitendinosus and biceps femoris long head form a conjoint tendon, originating at the superomedial ischial tuberosity, while the semitendinosus originates from the superolateral ischial tuberosity. Together the hamstring muscles aid in hip extension, knee flexion and tibia internal rotation. Hamstring injuries are one of the most prevalent injuries occurring in sports, accounting for 12% to 29% of all injuries amongst athletes, with reinjury rates occurring as high as 22% to 34%[63]. These injuries can occur at different levels of the musculotendinous units, with injury severity ranging from strain to complete rupture.
Clinical features and evaluation: The distribution of proximal hamstring injuries is bimodal - occurring either in young athletes or middle-aged individuals. The typical mechanism is eccentric loading during hip flexion and knee extension that causes too much hamstring tension and can be a result of an acute injury or from chronic repetitive overuse. Patients who suffer an acute proximal hamstring injury usually report a “popping” sensation with sharp pain in the ischium and buttock region, with the injury associated with bruising in the posterior thigh that may track towards the knee. Clinical examination should include an inspection of the entire posterior thigh for swelling and bruising. There is also occasionally a palpable local mass or retracted muscle just distal to the ischial tuberosity with associated tenderness when palpating at the ischial tuberosity. Patients will also present with painful resisted hip flexion and extension and avoidance of simultaneous hip flexion and knee extension to avoid pain resulting in a stiff-leg gait in the early post-injury phase.
An X-ray radiograph of the pelvis should be performed to rule out a bony avulsion of the ischial tuberosity. Occasionally, it is possible to pick up subtle clues of chronic overuse in the form of hamstring enthesopathy. However, the gold standard of diagnostic imaging is either an ultrasound or MRI scan. If resources are available, ultrasound can be performed in the consultation room to identify the severity and level of proximal hamstring injury[64,65]. An MRI scan should be considered in patients with persistent symptoms refractory to conservative treatment, with its utility being in the ability to pick up tendinopathy, number of tendons involved, partial vs complete rupture as well as degree of retraction of the tendons[66].
Management: Conservative treatment in the form of rest, non-steroidal anti-inflammatory drugs and stretching is usually recommended for low grade, partial tears or insertional tendinosis of proximal hamstring tendons in patients who do not require a high functional demand. An ultrasound-guided corticosteroid injection can be considered in patients with non-resolution of symptoms after a month, which has shown to provide some relief[67]. Additional non-surgical options include platelet-rich plasma injections as well as shockwave therapy. Surgical management of proximal hamstring injuries are usually reserved for complete and proximal soft tissue avulsions, or partial avulsions that involve more than two tendons with 2cm retraction in young and active patients[63]. Numerous surgical technique options have been described over recent decades, and can be done effectively via both endoscopic or direct open repair methods.
STRENGTHS, LIMITATIONS AND FUTURE DIRECTIONS
Non-arthritic hip pain is a common complaint with a wide range of differential diagnosis. Symptoms are often non-specific, and pain may also be referred from neighbouring anatomical structures from the lumbosacral spine, pelvis, or perineum. By categorising the causes of non-arthritic hip pain based on predominant symptom location, this review article aids physicians’ understanding of relevant anatomy and complements their clinical evaluation to allow for accurate and precise diagnosis. Latest advances in imaging modalities and hip arthroscopy techniques have also been highlighted in the article to enhance physicians’ assessment and management of non-arthritic hip pain. There are still many questions to be answered with regards to a large proportion of conditions described in this article. We have yet to fully comprehend the natural history and outcomes of both conservative and surgical management of these conditions, and more focus must be given to research of these areas in the future. Future studies, including scoping and systematic reviews, will allow physicians to stay current with the latest evaluation methods and surgical techniques while identifying potential knowledge gaps that need further exploration.