INTRODUCTION
Neutrophils account for approximately 70% of all leukocytes in the peripheral blood and about 1 billion neutrophils are produced per kilogram of body weight in the bone marrow every day[1]. Under normal physiological conditions, most neutrophils are replaced by new cells within 8 hours. Therefore, neutrophils are the shortest-lived leukocytes and rely on replenishment from the bone marrow. In damaged and infectious sites, neutrophils were the first type of immune cells to fight against bacterial infections[2,3]. Activated neutrophils have plentiful biological functions, including degranulation, phagocytosis, production of neutrophil extracellular traps (NETs)[4]. However, different types of neutrophils have different effector functions during tissue repair, and the dysregulation of neutrophil heterogeneity and production of cytokine or phagocytosis may result in negative effects for tissue repair. Of course, NETs can also pose harmful effects, such as induction of heterotopic ossification (HO) and promotion of autoimmunity, resulting from their cytotoxicity and autoantigenicity[5].
HO is defined as the pathological bone formation in soft tissues, such as joints, muscles and vessels, which were classified into traumatic HO (THO), genetic HO, and neurogenic HO[6]. In addition to the above types, ankylosing spondylitis (AS) can also present as progressive HO. THO was commonly induced by trauma or surgery, followed by chronic inflammation and subsequent events, which activate osteochondrogenic or osteogenic mechanisms inappropriately. Patients with HO may suffer from muscle pain and joint ankylosis, which was difficult to treat. Inflammatory cytokines recruit immune cells to fabricate a microenvironment appearing to be indispensable triggers for THO. Recent researches have shown that neutrophils and NETs played an important role in the THO process. The commented paper has effectively elucidated the progress of NETs in common osteoarticular diseases[7]. Sun et al’s findings[7] have elucidated the formation mechanism of NETs and the roles of NETs in various osteoarticular diseases including rheumatoid arthritis, gouty arthritis, osteoarthritis, and fibrosis. This article focuses on the role of neutrophils/NETs in the pathogenesis of HO, and clarifies potential treatment measures, which is an effective supplement to Sun et al’s research[7]. However, identifying the molecular drivers of THO were crucial for developing effective therapeutic strategies.
MECHANISMS OF NEUTROPHILS/NETS IN HO
In addition to fighting against fungi and bacteria, the role of neutrophils in tissue regeneration is becoming better understood. For example, neutrophils could significantly promote angiogenesis to accelerate the bone fracture healing[8]. Skeletal muscle acute injuries commonly caused by contusions, lacerations and burns. At injured sites, damage-associated molecular pattern and pathogen-associated molecular pattern signals are released to recruit immune cells. Moreover, neutrophil-derived proteolytic enzymes and NETs are released to defend against microorganisms. However, if the NETs cannot be removed in time, it will cause a persistent inflammatory microenvironment in local tissue. NETs possess dual characteristics, having both antibacterial properties and the ability to regulate inflammatory responses. It has been reported that NETs and mesenchymal stem cells (MSCs) are interrelated in tissue repair. Imbalanced NETs-related pathways, including nuclear factor kappa B and Wnt/β-catenin, may result in oxidative inflammation and hinder tissue repair[9]. Meanwhile, the high levels of interleukin (IL)-1β and the abnormally activated Wnt/β-catenin pathway may directly lead to the osteogenic differentiation of MSCs.
Neutrophils use complex machineries to recognize and respond to pathogen recognition. In injured soft tissue, the infiltrated neutrophils release matrix metalloproteinase-9 and vascular endothelial growth factor to promote angiogenesis, which accelerate wound healing[10]. In addition, the infiltration of neutrophils also facilitates the activation of satellite cells through the signal transducer and activator of transcription pathway for muscle regeneration[11]. Whether antibacterial or promoting angiogenesis function, the early infiltration of neutrophils is beneficial for tissue repair. However, the excessive activation of neutrophils leads to persistent inflammatory activation, which in turn causes tissue damage. The nuclear factor kappa B signaling pathway within the over-activated neutrophils is activated, resulting in the massive production of reactive oxygen species which directly damages the vascular endothelial cells and ultimately hinders vascular formation[12].
Three factors have been considered as necessary to induce HO: Osteogenic precursor cells, activation of the osteogenic signaling pathway and an appropriate osteoconductive environment[13]. The bone morphogenetic protein (BMP) family is widely recognized for its ability to promote bone formation. In the muscle tissues of human blast injuries, the high expression of BMP-1, BMP-2 and BMP-4 has been confirmed[14,15]. It is worth noting that the neutrophils can increase the levels of BMP-2 and BMP-4[16]. In the model of achilles tendon injury, neurogenesis occurs near nerve growth factor (NGF)-positive cells, and the imbalance of the NGF and transforming growth factor β signaling pathways may limit tendon repair, ultimately resulting in the occurrence of HO in tendons[17]. In the optic nerve crush model, neutrophils could adjust the NGF expression level, indicating that neutrophils may induce HO via NGF signaling pathway[18].
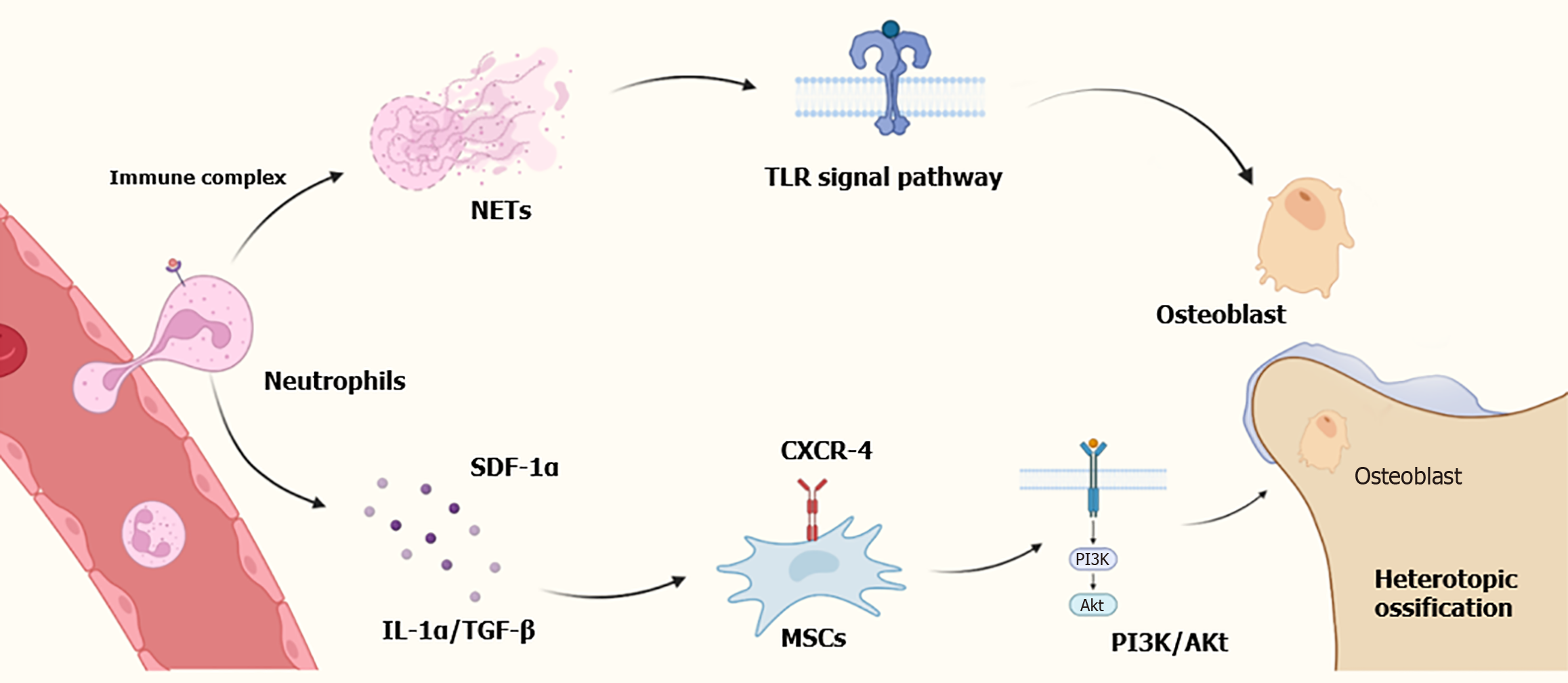
Through molecular and transcriptional analyses, Nunez et al[19] found that NETs were restricted to the THO site, which not manifested in the blood or bone marrow[17]. Furthermore, the NETs formation at the skeletal injury sites was notably high in the THO group on day 3 than in the control group on day 7. Moreover, they also ascertained that the formation of NETs corresponded with high level of toll-like receptor signaling pathways in neutrophils at injury sites[19]. Post-traumatic inflammation is crucial for normal wound healing, but if it persists for too long or is uncontrolled, it may cause damage to physiological healing[20]. NETs are a component of the neutrophil response to trauma and are associated with a range of pro-inflammatory diseases including lupus, rheumatoid arthritis and vascular thromboses. Study have shown that the formation of NETs significantly increased in HO model at early stage through enzyme-linked immunosorbent assay and single-cell sequencing, and early inhibition of NETs formation can alleviate HO. Besides, neutrophils could activate MSCs and promote osteogenic differentiation by upregulating the IL-1α and transforming growth factor β levels[21]. Following IL-8 implantation, the neutrophils are recruited and transformed into N2 type, which subsequently secrete stromal cell-derived factor-1α influencing C-X-C motif chemokine receptor (CXCR) 4, leading to phosphatidylinositol 3-kinase/protein kinase B pathway and β-catenin-mediated migration of MSCs, and ultimately contributing to ossification[22]. The mechanism diagram is shown in Figure 1.
Figure 1 Role of neutrophils and neutrophil extracellular traps in heterotopic ossification.
NETs: Neutrophil extracellular traps; SDF-1α: Secrete stromal cell-derived factor-1α; IL-1α: Interleukin-1α; TGF-β: Transforming growth factor β; TLR: Toll-like receptor; CXCR-4: C-X-C motif chemokine receptor 4; MSCs: Mesenchymal stem cells; PI3K: Phosphatidylinositol 3-kinase; Akt: Protein kinase B.
AS was an autoimmune disease, characterized by chronic inflammation and progressive ossification[23]. Recent studies have shown that NETs may be related to the occurrence and progression of AS[24,25]. Firstly, neutrophils release NETs in response to p30 in AS, and there may be a correlation between NETs and chronic inflammation[24]. Moreover, Papagoras et al[25] hold the view that NETs are the important sources of IL-17 and IL-1β, leading to inflammation and new bone formation in AS.
TARGETING NEUTROPHILS/NETS IN HO
A deeper understanding of neutrophils or NETs in HO could help us to view them as promising therapeutic targets. Recently, various clinical studies have suggested the significant efficacy of MSC-based therapies in development of treatment strategies[26]. Study have shown that transplanting umbilical cord MSCs can ameliorate acute inflammation by attenuating neutrophil infiltration[27]. Chemokines are crucial for coordinating the migration of neutrophils. The most crucial neutrophil chemokines factor receptors involved in neutrophil recruitment is CXCR-2. The use of CXCR-2 inhibitor QBM076 in patients with chronic obstructive pulmonary disease did improve the condition, but its side effect was an increase in liver transaminase levels[2].
Masuda et al[28] demonstrated the capacity of batroxobin to eliminate NETs, which promoted myoblast regeneration and suppressed inflammation. Besides, the NETs-mediated pathology is also targeted by the DNA-degrading enzyme DNase I, which can effectively alleviate inflammation in animal models. Therefore, the degradation of NETs mediated by DNase I may be a potential therapeutic strategy for HO[29].
In several cases, the formation of NETs was dependent on the activity of NADPH oxidase. In the nucleus, neutrophil elastase facilitates nuclear decondensation, but the activity of elastase was enhanced by myeloperoxidase, meaning that the two enzymes can regard as therapeutic targets in NETs-mediated disorders[30]. Peptidylarginine deaminase 4 was also thought to be necessary for NETs formation and a promising therapeutic target in NETs-associated diseases[31]. Recent study has shown that Gasdermin D is involved in NETs formation. During this process, Gasdermin D plays a positive feedback role, thereby promoting the formation of NETs. Therefore, the Gasdermin D inhibitor, necrosulfonamide, may have therapeutic value in HO[32]. Given the role of neutrophils/NETs in the antibacterial host defense, therapeutic inhibition targeting neutrophils/NETs may lead to serious infection events. Moreover, the use of some monoclonal antibodies may also increase the risk of developing tumors and tuberculosis. Therefore, when choosing a treatment plan, it is necessary to assess the therapeutic window and side effects.
CONCLUSION
The overactivation of neutrophils and persistent existence of NETs play an indispensable role in the pathogenesis of HO. At the early stage of soft injury, neutrophils may play dual roles, either by contributing to tissue repair or inducing the progression of HO. Therefore, there are also numerous controversies related to a number of aspects of neutrophils, such as transcriptional activity and roles in HO. However, recent development has shown that precise therapeutic interventions for neutrophils or NETs provide therapeutic benefit without harmful effects. Currently, research on the relationship between neutrophils and HO is still scarce, and the related mechanisms remain to be elucidated. We must recognize that most of the research on the mechanisms of neutrophils/NETs in the pathogenesis of HO relies on animal models, while studies based on human samples are very few. Future research can focus on the targeted therapeutic effect of NETs in HO and apply it in clinical practice.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade B
Novelty: Grade B, Grade B
Creativity or Innovation: Grade B, Grade B
Scientific Significance: Grade B, Grade B
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Ren SQ, MD, China S-Editor: Bai Y L-Editor: A P-Editor: Zhao YQ