INTRODUCTION
Slipped capital femoral epiphysis (SCFE) is a prevalent disorder of the hip joint among adolescents, characterized by displacement of the femoral head epiphysis at the growth plate (GP). This condition may result in serious complications, including pain, gait abnormalities, and femoral head necrosis[1-4]. While its clinical manifestations are well established, the underlying pathogenesis remains incompletely understood. Accumulating evidence indicates that endocrine dysfunction, metabolic disturbances, and genetic predispositions represent key contributing factors[5-10].
The core value of molecular mechanism research lies in elucidating molecular pathological processes - such as abnormal GP chondrocyte (GPC) function, dysregulation of hormone signaling pathways, and the interplay between metabolic and inflammatory responses - that not only underpin the pathogenesis of SCFE but also offer novel perspectives for early disease detection and targeted intervention. For instance, in-depth investigation into the regulatory roles of signaling pathways such as Wnt/β-catenin and Hedgehog in chondrocyte differentiation, together with genetic susceptibility factors like COL2A1 gene mutations and obesity-associated metabolic-inflammatory networks, may enable the development of a molecular subtype-based risk prediction model and innovative therapies targeting inflammatory pathways [e.g., nuclear factor kappa B (NF-κB)] or chondroprotective mechanisms [e.g., SRY-box transcription factor 9 (SOX9) regulation][11-16]. This molecular-level research paradigm has the potential to drive the transition of SCFE management from conventional symptom-oriented treatment toward mechanism-guided precision medicine.
Based on existing literature, this article systematically summarizes various disease conditions associated with SCFE and explores its potential molecular mechanisms from perspectives such as chondrocyte dysfunction in the GP, hormonal imbalances, and inflammatory/metabolic factors, aiming to provide a theoretical foundation for the prevention and treatment of SCFE.
PRIMARY INTERNAL DISEASES ASSOCIATED WITH SCFE
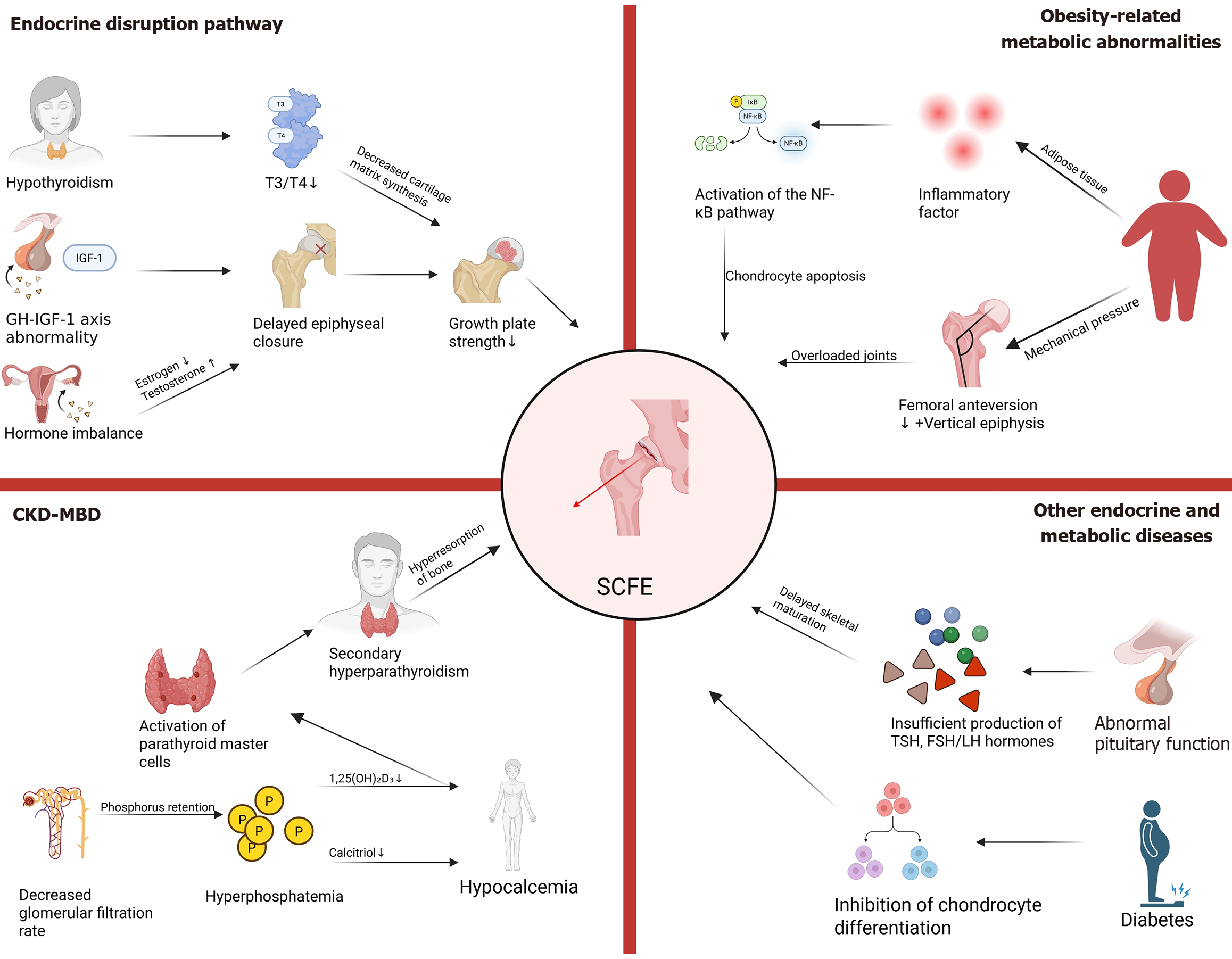
The primary internal diseases associated with SCFE include endocrine disorders, obesity-related metabolic abnormalities, renal osteodystrophy, and other conditions such as pituitary dysfunction, diabetes, and insulin resistance (Figure 1). These diseases elevate the risk of SCFE by influencing bone development, GP structure and function, mechanical stress distribution, and metabolic equilibrium.
Figure 1 Primary internal diseases associated with slipped capital femoral epiphysis.
NF-κB: Nuclear factor kappa B; IGF1: Insulin-like growth factor 1; GH-IGF-1: Growth Hormone-insulin-like growth factor-1 axis; CKD: Chronic kidney disease; MBD: Mineral and bone disorder; SCFE: Slipped capital femoral epiphysis; TSH: Thyroid-stimulating hormone; FSH: Follicle-stimulating hormone; LH: Luteinizing hormone.
Obesity-related metabolic abnormalities
Obesity is one of the primary risk factors for SCFE[4,17,18]. Studies have shown that over 80% of children diagnosed with SCFE are obese, defined as having a body mass index greater than the 95th percentile for their age and sex[19]. Obesity-induced hyperinsulinemia and adipokine dysregulation contribute to altered chondrocyte proliferation and matrix remodeling.
Mechanical pressure increases: Obese children exhibit reduced femoral anteversion[20] and a more vertically oriented proximal femoral epiphysis[21]. These anatomical changes result in abnormal shear forces acting on the hip joint in obese children[14,17]. Biomechanical models demonstrate that during stair climbing, the hip abduction moment in obese children increases by 23%, while the peak moment of the hip flexors rises by 26%[22]. Such alterations may lead to joint overload and early fatigue, potentially increasing the risk of hip joint instability. Additionally, overweight children have relatively smaller bone areas and lower bone mass compared to normal-weight children[23]. Consequently, a greater load must be supported and transmitted by a structurally weaker bone framework. This is particularly relevant in the posteromedial region of the femoral neck, which is the common site of SCFE.
Inflammatory responses mediated by adipokines: Inflammatory factors secreted by adipose tissue, such as interleukin (IL)-6, tumor necrosis factor-alpha, and leptin, activate the NF-κB pathway, thereby inducing local inflammation in the GP and promoting chondrocyte apoptosis and matrix degradation[24,25]. Lee et al[25] conducted experiments using the 5th to 6th generation of human umbilical vein endothelial cells and human osteoblasts, treating them with human recombinant adiponectin or IL-1β in serum-free medium. The results demonstrated that both adiponectin and IL-1β could induce the secretion of IL-6 and IL-8 by endothelial cells and osteoblasts. Studies have indicated that, based on physiological concentrations of adiponectin, its role in stimulating IL-6/IL-8 production by endothelial cells may be more pronounced than that of IL-1β. Furthermore, adiponectin-mediated IL-8 release by endothelial cells may play a critical role in neutrophil recruitment in the joints of arthritis patients.
Animal experiments have revealed that high-fat diet (HFD)-induced obesity upregulates matrix metalloproteinase-13 expression in the GP, accelerating collagen degradation and potentially contributing to GP instability[26,27].
Endocrine disorders
The integrity of the endocrine system plays a critical role in the normal development of GPs. Disruption of this balance can lead to a range of endogenous disorders that compromise GP stability through specific pathophysiological mechanisms, thereby increasing the risk of SCFE.
Hypothyroidism: Thyroid hormones [triiodothyronine (T3) and thyroxine (T4)] promote GP ossification by regulating chondrocyte proliferation and differentiation[28-31]. The presence of thyroid hormone receptors (TRα1, TRα2, TRβ1) in the GP indicates that thyroid hormones (T3/T4) can directly act on chondrocytes to accelerate endochondral ossification. WALKER[32] demonstrated that immature rats treated with thyroid hormones exhibited epiphyseal maturation at 8 days of age equivalent to that of normal rats at 18 days of age, significantly shortening the bone maturation period. This highlights the rapid promotion of chondrocyte differentiation and ossification by thyroid hormones.
Congenital or acquired hypothyroidism (e.g., Hashimoto’s thyroiditis) reduces the synthesis of cartilage matrix in the GP and disrupts collagen alignment, thereby weakening the mechanical strength of the GP[33,34]. Studies have shown that the incidence of SCFE in hypothyroid patients is 3-5 times higher than in the general population[35]. Hervas suggested that thyroid hormones may stimulate longitudinal bone growth indirectly by promoting growth hormone (GH) secretion; however, administration of GH alone does not adequately stimulate cartilage maturation.
GH and insulin-like growth factor-1 deficiency: The GH-insulin-like growth factor-1 (IGF-1) axis serves as a critical regulatory pathway for longitudinal bone growth[28,36,37]. GH deficiency or insufficient IGF-1 secretion can delay epiphyseal plate closure and prolong the “vulnerable period” of the GP[36], thereby increasing its susceptibility to SCFE under mechanical stress[38]. GH regulates the proliferation and differentiation of epiphyseal plate chondrocytes via two distinct pathways. First, GH directly acts on the resting zone of the epiphyseal plate, promoting local IGF-1 production and stimulating clonal expansion of chondrocytes in the proliferative zone through autocrine/paracrine mechanisms. Second, GH promotes hepatic IGF-1 synthesis and exerts its effects on the epiphyseal plate via an endocrine mechanism, further enhancing chondrocyte proliferation. These dual mechanisms synergistically regulate bone growth and development. Clinical studies indicate that the incidence of SCFE in patients with GH deficiency is significantly higher compared to normal children[39].
Sex hormone imbalance: Fluctuations in sex hormones during puberty, such as estrogen and testosterone, influence the risk of SCFE by modulating the timing and rate of GP closure[40]. Estrogen deficiency can delay epiphyseal closure, leading to prolonged GP activity and increased stature[15], while premature elevation of estrogen may result in abnormal GP structure, including premature closure (epiphyseal fusion)[41,42]. The GH-IGF-1 axis serves as a major regulator of longitudinal bone growth and exerts both direct and indirect effects on chondrocyte proliferation and differentiation. Estrogen can directly stimulate local IGF-1 production and other growth factors within the GP. Conversely, low levels of estrogen stimulate GH secretion, indirectly elevating circulating IGF-1 levels, which subsequently promote chondrocyte proliferation in the proliferative zone and enhance clonal expansion. Additionally, testosterone indirectly alters hip joint load distribution by increasing muscle mass, thereby potentially increasing the mechanical stress on the GP and the risk of SCFE[43].
Hypothyroidism, abnormalities in the GH-IGF-1 axis, and sex hormone imbalances are key endocrine factors influencing bone development and the pathogenesis of SCFE. Thyroid hormones directly regulate chondrocyte differentiation and ossification, accelerating bone maturation. Hypothyroidism reduces GP strength and increases its susceptibility to mechanical stress, thereby elevating the risk of SCFE. The GH-IGF-1 axis promotes chondrocyte proliferation through both local autocrine/paracrine mechanisms and systemic endocrine pathways. Its deficiency prolongs the vulnerable period of the GP, increasing the likelihood of SCFE under mechanical stress. Among sex hormones, estrogen imbalance affects chondral stability by regulating the timing of epiphyseal closure and modulating the GH-IGF-1 pathway, while testosterone influences hip joint biomechanics by altering load distribution. The interplay among these three factors collectively determines bone homeostasis and GP integrity.
Renal osteodystrophy
Chronic kidney disease-mineral and bone disorder disrupts GP homeostasis through multiple interacting pathways[44,45]. The pathological cascade originates from phosphorus retention due to reduced glomerular filtration rate. Hyperphosphatemia disrupts calcium-phosphorus balance via a dual mechanism: First, it inhibits 1α-hydroxylase activity, thereby reducing the synthesis of calcitriol; second, it accelerates the degradation of vitamin D metabolites, leading to hypocalcemia. The resultant decrease in serum calcium activates the calcium-sensing receptor in parathyroid chief cells, triggering upregulation of the parathyroid hormone (PTH) gene and parathyroid gland hyperplasia, which culminates in secondary hyperparathyroidism (SHPT). The chronically elevated PTH levels drive osteoclast activation and excessive bone resorption, resulting in high-turnover bone disease. Characteristic pathological changes include trabecular fibrosis, disordered bone mineralization fronts, and microstructural damage, ultimately contributing to renal osteodystrophy[44-53].
Dysregulation of calcium and phosphorus metabolism: Hyperphosphatemia (serum phosphorus > 1.45 mmol/L)[46]: It is a critical factor in the pathogenesis and progression of SHPT, as it suppresses calcitriol synthesis, accelerates its degradation[49], induces hypocalcemia, and upregulates PTH gene expression[47,48].
Disorders of vitamin D metabolism: Inadequate production of 1,25(OH)2D3 markedly impairs calcium absorption, exacerbates hypocalcemia, and triggers feedback-mediated parathyroid hyperplasia[48]. Additionally, when 1,25(OH)2D3 levels are deficient (< 15 nmol), bone formation and mineralization in trabecular bone may also be compromised[49,50].
SHPT: In SHPT, the pathological manifestations of PTH are characterized as follows: Chronic hypocalcemia and/or hyperphosphatemia stimulate excessive PTH secretion. This, in turn, enhances calcium reabsorption in the proximal renal tubules while inhibiting phosphorus reabsorption (thereby increasing urinary phosphorus excretion). Simultaneously, PTH drives high bone turnover by promoting osteoclastic activity, leading to the release of calcium and phosphorus from cortical bone into the bloodstream. Furthermore, PTH stimulation of osteoblasts elevates fibroblast growth factor 23 levels, exacerbating systemic phosphorus metabolism disorders. The resultant increase in serum calcium and phosphorus concentrations establishes a positive feedback loop, accelerating parathyroid hyperplasia and dysregulating PTH secretion. This vicious cycle not only induces bone microstructural damage (e.g., fibrous osteitis, increased bone fragility) but also facilitates ectopic calcium-phosphorus deposition in the vascular wall via the “bone-vascular axis”, serving as a central mechanism underlying the progression of chronic kidney disease-mineral and bone disorder and associated cardiovascular complications[51-53].
Other disorders of the endocrine and metabolic systems
Additionally, dysfunctions of the pituitary gland and other endocrine or metabolic disorders, such as diabetes or insulin resistance, may contribute to aberrant proliferation and differentiation of chondrocytes.
Malfunction of the pituitary gland: Hypopituitarism, particularly panhypopituitarism, which results from inadequate secretion of multiple tropic hormones including TSH and gonadotropins (follicle-stimulating hormone/Luteinizing hormone), indirectly contributes to delayed skeletal maturation[54,55].
Diabetes or insulin resistance: Animal experiments have demonstrated that in mice fed a high-fat, high-calorie diet, hyperinsulinemia may promote GP cartilage formation and longitudinal bone growth[56,57]. To explore whether insulin directly influences GP cartilage formation and longitudinal bone growth, Wu et al[57] generated knockout mice lacking insulin receptors specifically in cartilage tissue. At three weeks of age, the mice were divided into four groups: Two standard diet (SD) groups and two HFD groups. By the end of the study, mice fed the HFD exhibited significantly greater weight gain compared to their counterparts on the SD. The body weight, tibia length, and GP height of the HFD-fed control mice were higher than those of the SD-fed mice; however, no significant differences were observed between the two groups regarding body weight, tibia length, or GP height. This study not only elucidated the role of insulin in stimulating GP cartilage formation and longitudinal bone growth but also examined the underlying mechanisms by which insulin affects these processes. Insulin has been shown to stimulate chondrocyte proliferation[58-61] and exert differential effects on chondrocyte differentiation[57-61]. Additionally, insulin resistance can decrease the bioavailability of IGF-1, thereby exacerbating GP dysfunction[18].
In summary, endocrine disorders, including hypothyroidism, abnormalities in the GH-IGF-1 axis, sex hormone imbalances, obesity, and renal osteodystrophy, disrupt bone homeostasis by influencing chondrocyte function, epiphyseal closure, mechanical stress distribution, inflammatory responses, and metabolic balance. These disruptions consequently increase the risk of SCFE. Moreover, these factors interact synergistically, further exacerbating GP vulnerability and lesion risk. To elucidate the etiology of SCFE, it is essential to investigate the underlying molecular mechanisms contributing to its pathogenesis.
POTENTIAL MOLECULAR MECHANISMS OF SCFE
The occurrence of SCFE can be attributed to the synergistic action of multiple molecular mechanisms, encompassing various levels including cellular function of GP cartilage, inflammatory response, mechanical stress, and genetic epigenetic regulation. The interplay among these factors exacerbates the disruption of GP homeostasis.
Aberrant functionality of GPCs
The functional homeostasis of GPCs serves as a fundamental basis for maintaining normal bone development. Disruptions in this function can directly compromise the structural integrity and mechanical properties of the GP by interfering with hormone signal transduction and cellular differentiation processes.
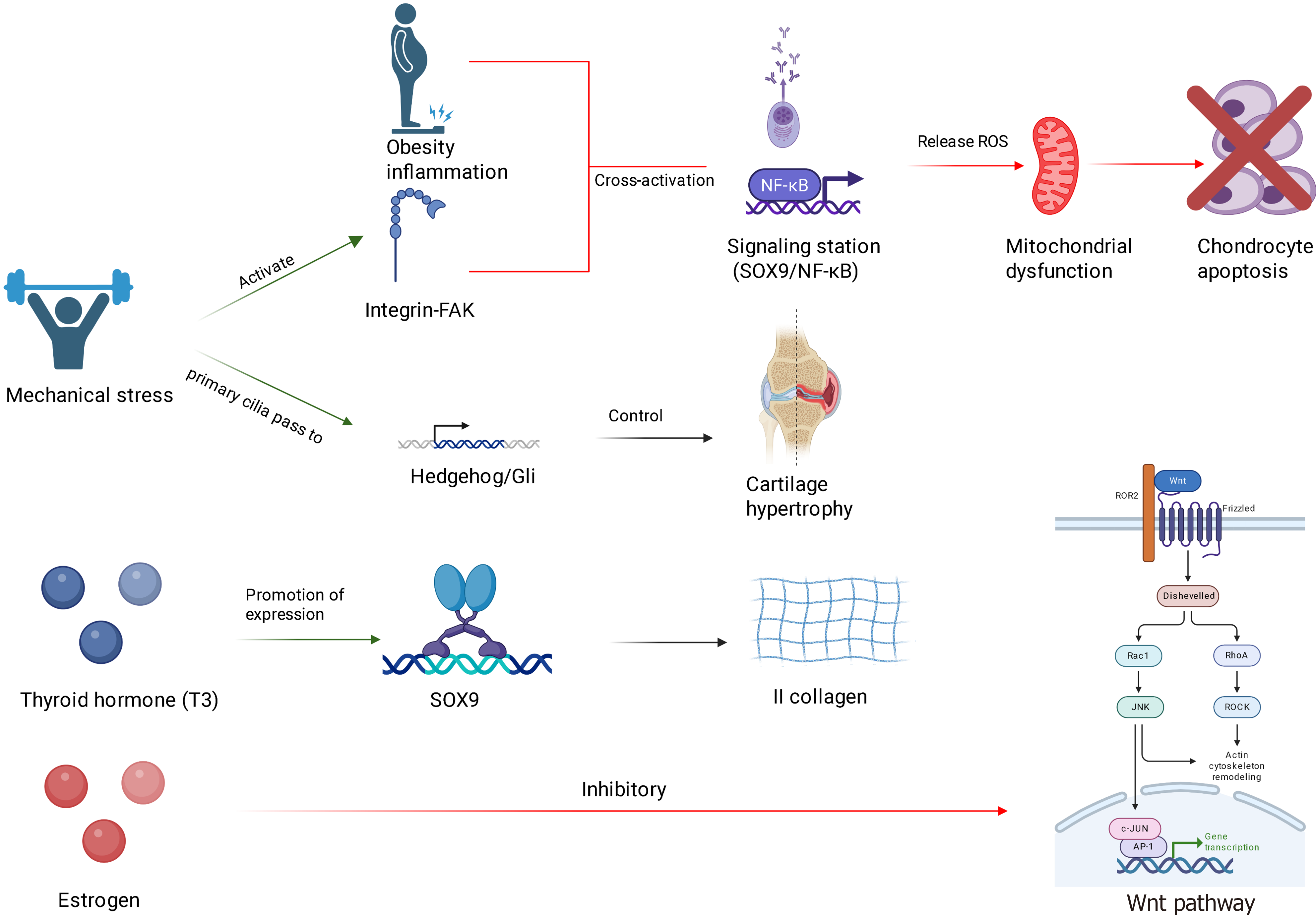
Dysregulation of hormonal signaling pathways: Disruption of the thyroid hormone signaling pathway[62-65] includes T3 upregulates the expression of SOX9 and COL2A1 by binding to the TRβ[66], thereby promoting type II collagen synthesis[67]. In cases of hypothyroidism, the suppression of SOX9 expression results in a loosened cartilage matrix[68]. Freitas conducted experiments to investigate the effects of thyroid hormone T3 and its receptor TRβ on bone development. Six groups of female Wistar rats were utilized: (1) Normal rats; and (2) Hypothyroid rats (Hypo); and four additional Hypo groups treated intraperitoneally with 0.3 μg/100 g body weight/day of T3 (1 × T3), 5 × T3, or equimolar doses of the TRβ-specific agonist sobetirome (GC-1) (1 × GC-1 and 5 × GC-1) for five weeks. The findings revealed that hypothyroid individuals exhibited reduced longitudinal body growth, bone mineral density, delayed ossification, and disorganized chondrocyte columns, along with a decreased number of hypertrophic chondrocytes (HCs). Compared to normal rats, serum IGF-I levels were significantly diminished, and IGF-I protein as well as collagen II and X mRNA expression were undetectable in the GPs of Hypo rats. However, these indicators were nearly normalized in rats treated with 5 × T3. Conversely, GC-1 did not influence serum IGF-I levels or IGF-I expression in the epiphyseal GP, nor could it restore the integrity of proliferating chondrocyte columns or significantly affect longitudinal growth. Nonetheless, GC-1 demonstrated efficacy in inducing ossification, HC differentiation, and the expression of collagen II and X mRNA, while also increasing the thickness of the epiphyseal GP to normal values. Additionally, bone mineral density was higher in GC-1-treated rats compared to Hypo rats. This study confirmed the roles of T3 and TRβ in GP cartilage and explored their underlying mechanisms, though it provided limited insight into the entire process of GP development. Future research could expand upon this experimental framework by investigating the contributions of other hormones and receptors.
The effects mediated by sex hormone receptors encompass the regulation of osteoblast differentiation via estrogen-related receptor α through Wnt/β-catenin signaling[69]. Estrogen regulates chondrocyte growth via estrogen receptor (ER) α/β receptors and maintains the homeostasis of GPs. ERβ knockout mouse models exhibit an increased number of proliferating chondrocytes and a reduced number of HCs[70]. To elucidate the role of ERα/β receptors in regulating chondrocyte growth, Chagin et al[70] conducted a long-term observational study comparing appendicular and axial bone growth in female Erα-/-, ERβ-/-, and ERα-/-β-/- mice. The findings indicate that in ERβ-/- mice, there is an increase in the number of proliferating chondrocytes and a decrease in HCs, whereas in ERα-/- and ERα-/-β-/- mice, the numbers of proliferating and HCs are comparable. However, compared to ERα-/-β-/- mice, the column density in ERα-/- mice is lower. Through comprehensive analysis of GPs and related indicators such as body length and age in mice, it was concluded that ERβ acts as a physiological inhibitor of appendicular and axial bone growth in young adult female mice, while ERα primarily mediates estrogen-induced GP closure.
In ERα knockout mouse models, longitudinal bone growth is impaired and correlates with alterations in the GH/IGF-1 axis. However, in mice with ERα specifically inactivated in cartilage, bone growth during sexual maturation remains normal[71].
Inflammatory responses and oxidative stress
Chronic low-grade inflammation in obese or metabolic syndrome patients plays a critical role in the pathogenesis of SCFE via activation of the NF-κB pathway. In obesity, the number of macrophages in white adipose tissue increases and becomes activated, interacting with adipocytes to promote the release of pro-inflammatory factors through pathways such as toll-like receptor 4/myeloid differentiation primary response 88/NF-κB, thereby establishing a chronic low-grade inflammatory state[72,73]. Zhang et al[72] explored the effects of irisin on bone metabolism mediated by adipocytes within the bone marrow microenvironment. In their study, irisin precursor protein (FNDC5) knockout mice (FNDC5-/-) were fed either a HFD or a SD for 10 weeks. After sacrifice, samples were collected for analysis. Staining results revealed that trabecular bone mass beneath the epiphyseal plate was reduced and disordered in the HFD group. Notably, compared with SD-fed mice, collagen fibers beneath the GP of the femur were diminished in HFD-fed mice, suggesting that irisin deficiency exacerbates bone loss. RNA sequencing and blocking experiments demonstrated that irisin reduces IL-6 production in adipocytes by downregulating the toll-like receptor 4/myeloid differentiation primary response 88/NF-κB pathway. Several studies have confirmed that IL-6 acts as an osteogenic inhibitor. Specifically, IL-6 regulates the activity of mitogen-activated protein kinase 2 and AKT serine/threonine kinase 2 via SH2 domain-containing protein tyrosine phosphatase 2 to inhibit osteogenic differentiation and reduce bone mineralization, while also suppressing osteoblast differentiation by attenuating mitogen-activated protein kinase signaling through Janus kinase/signal transducer and activator of transcription 3. Moreover, elevated IL-6 receptor and IL-6 expression in obese individuals contribute positively to this process. Their findings indicated that IL-6 secretion in the bone marrow cavity of FNDC5-/- mice was significantly higher than in wild-type mice, leading to osteoporosis. Although IL-6 produced by adipocytes in the bone marrow microenvironment inhibits bone formation derived from mesenchymal stem/stromal cells, irisin alleviates this inhibition by suppressing IL-6 production and promoting bone mass. tumor necrosis factor-alpha phosphorylates inhibitor of kappa B via the inhibitor of kappa B kinase complex, enabling NF-κB to translocate into the nucleus and induce the expression of pro-inflammatory factors such as IL-1β and cyclooxygenase-2, thereby abnormally activating chondrocytes[74,75].
Accumulation of reactive oxygen species (ROS) induces mitochondrial dysfunction and subsequently triggers chondrocyte apoptosis[76,77]. Bolduc et al[77] have extensively investigated the interplay between mitochondria and ROS in their studies. The findings indicate that mitochondrial dysfunction leads to elevated ROS levels in the body. Concurrently, the activity of superoxide dismutase 2 decreases, and excessive oxidative stress inactivates peroxiredoxin, thereby impairing the antioxidant capacity of chondrocytes. This ROS-induced mitochondrial dysfunction results in a cascade of adverse effects. First, ROS can cause oxidative damage to proteins, lipids, and DNA within cells, potentially leading to cell death under severe conditions. Second, ROS disrupts redox-regulated cell signaling pathways by oxidizing protein sulfhydryl, thereby affecting cellular homeostasis.
Mechanical stress and biomechanical changes
During the growth period of children, mechanical loads and weight changes on bones may induce biomechanical alterations in the femoral head region. Insufficient supporting force between the femoral head and the epiphysis or excessive shear stress can lead to SCFE. The occurrence of SCFE may be associated with multiple molecular mechanisms. Specifically, the disruption of the balance between osteoblasts and osteoclasts affects bone tissue remodeling and normal growth. Additionally, growth factors such as insulin-like growth factor IGF-1 and transforming growth factor transforming growth factor-β play critical roles in SCFE by regulating bone development and growth. Notably, the reactive force on the hip joint in obese children increases significantly with body weight[78,79], leading to a marked rise in shear stress on the proximal femoral GP[1,14], thereby activating following mechanically sensitive pathways.
Hedgehog signaling pathway: The Hedgehog signaling pathway plays a crucial role in bone development and repair, mainly regulating bone development. Hedgehog signaling is essential for the proliferation and differentiation of osteoblasts. It promotes bone formation and regulates normal bone growth. When mechanical stress is excessive, the regulation of Hedgehog signaling may be affected, interfering with the normal function of bone cells and increasing the risk of femoral head dislocation. The Hedgehog signaling pathway not only affects osteoblasts but also maintains the dynamic balance of bones by regulating other cells such as osteoclasts and chondrocytes. Changes in mechanical stress may lead to alterations in Hedgehog signaling expression, thereby influencing cell-to-cell interactions. Under biomechanical stress conditions, the activation of Hedgehog signaling may help cells adapt to mechanical stress. If this signaling pathway is dysfunctional, it may lead to poor bone tissue remodeling and increase the risk of epiphyseal dislocation. Mechanical stress activates Gli transcription factors through primary cilia, promoting the differentiation and maturation of fibrocartilage[80-85].
Integrin-focal adhesion kinase pathway: Integrins are a class of cell membrane receptors that regulate cell adhesion, migration, proliferation, and survival by binding to the extracellular matrix. The integrin-focal adhesion kinase (FAK) pathway serves as a critical signal transduction pathway for cells to respond to mechanical stress. FAK, acting as a downstream signaling molecule of integrins, mediates signal transmission between cells and the extracellular matrix. Integrins can sense mechanical stress in the cellular microenvironment and transmit these signals intracellularly, activating downstream signaling pathways via FAK. Changes in mechanical stress can modulate the expression and activity of integrins, thereby altering the phosphorylation state of FAK and influencing cellular functions. In the pathogenesis of SCFE, the adhesion and migration capabilities of bone cells are regulated, with FAK playing a pivotal role in this process. Abnormal mechanical stress may disrupt the regulation of the integrin-FAK signaling pathway, thereby affecting the functions of osteoblasts and osteoclasts and increasing the risk of SCFE. FAK contributes to bone tissue remodeling by regulating cell growth, differentiation, and promoting intercellular interactions. If the function of the integrin-FAK pathway is impaired due to altered mechanical stress, it will compromise the normal development and remodeling of bones. Mechanical stress and biomechanical changes significantly impact the occurrence of SCFE in children, with the integrin-FAK signaling pathway playing a key regulatory role in this process. Through in-depth investigation of the relationship between mechanical stress and the integrin-FAK pathway, a deeper understanding of the pathological mechanisms underlying SCFE can be achieved, providing novel strategies for its prevention and treatment[86].
Genetic and epigenetic factors
The occurrence of SCFE is likely the result of the combined effects of multiple factors. Genetic susceptibility may indirectly contribute to the disease by influencing bone development, hormone metabolism, and obesity risk, while epigenetic regulation may serve as a bridge in the interaction between environmental factors and genetic predisposition. Studies have shown that SCFE exhibits a certain degree of familial aggregation, particularly in some families where genetic predisposition appears more pronounced. Although no specific single gene mutation has been identified as directly causing SCFE thus far, several studies suggest that genes involved in bone development and growth (such as those regulating GH and insulin-like growth factor pathways) may play a critical role in its pathogenesis. Additionally, genetic variations affecting bone density and morphology (such as COL1A1, BMP, etc.) are also suspected to be associated with an increased risk of SCFE. Epigenetics investigates heritable changes in gene expression that occur without alterations in the DNA sequence.
The occurrence of SCFE is not only associated with genetic susceptibility but also intricately linked to the interplay of environmental factors. Environmental factors, including childhood obesity, exercise patterns, and hormone levels, may modulate genetic risk by altering epigenetic states. Certain genetic variations, while not directly causing disease, may increase the risk of SCFE by influencing their expression and function through epigenetic mechanisms. For example, mutations in the COL2A1 gene (such as c.3505G>A) result in abnormal type II collagen structure, thereby reducing the shear resistance of the cartilage matrix[87-89]. Mutations in the ACAN gene lead to defects in proteoglycan synthesis, affecting matrix hydration[90-92]. Abnormal DNA methylation in the GP, such as hypermethylation of the SOX9 promoter, can inhibit chondrocyte differentiation[93,94].
Haseeb et al[93] were the first to investigate whether SOX9 expression in chondrocytes changes as mice age and transition into adulthood, focusing on the proximal tibial GP and knee joint. At 4 weeks of age, the GP volume was larger; however, two weeks later (6 weeks of age), when the mice entered adulthood, its height decreased by two-thirds, and it further atrophied by 1 year of age. The primary trabeculae in the GP gradually degenerated during adulthood, confirming the cessation of endochondral ossification. The expression levels of SOX9 protein and SOX9 RNA in GPCs were higher in young mice but significantly diminished in aging mice. The thickness and tissue structure of articular cartilage (AC) remained similar between young and aging mice, yet SOX9 expression markedly decreased with aging. This decline in SOX9 expression, coupled with the reduced function of the GP and the aging of AC, underscores the critical role of SOX9 in maintaining the vitality of the GP and the homeostasis of AC during youth. To elucidate the pivotal role of SOX9 in postnatal cartilage, they developed a SOX9fl/fl (conditional knockout allele) mouse model. Upon inducing SOX9 inactivation at 4 weeks of age, GP closure occurred within 2 weeks, initially characterized by proteoglycan loss and disappearance of the hypertrophic zone. Mutant mice exhibited significantly reduced body size compared to their littermate controls but otherwise maintained normal physiological states. Non-mineralized AC also demonstrated proteoglycan depletion, yet its overall structure remained intact. Inducing SOX9 inactivation in 3-month-old mice similarly resulted in analogous phenotypes.
Moreover, the expression of obesity-related miRNAs (such as miR-140) was downregulated, leading to uncontrolled upregulation of ADAMTS-5 (proteoglycanase), which accelerated matrix degradation[95,96].
The onset of SCFE results from the synergistic effects of endocrine disorders (thyroid hormones, sex hormones, GH-IGF-1 axis), metabolic abnormalities (obesity-related inflammation), mechanical stress imbalance, and genetic/epigenetic regulatory dysfunctions. For example, obesity activates the Hedgehog and integrin signaling pathways via mechanical load and induces NF-κB inflammatory responses through adipokines; sex hormone imbalance affects chondrocyte differentiation via ERβ and interacts with the GH/IGF-1 axis. These mechanisms are interdependent and mutually reinforcing, collectively compromising the structural integrity and functional stability of the GP, ultimately resulting in SCFE. Future research should further delineate the precise interactions among these mechanisms to provide a stronger theoretical foundation for the precise prevention and treatment of SCFE.
CLINICAL SIGNIFICANCE AND TREATMENT APPROACHES
Early diagnosis and multidisciplinary intervention
Screening of high-risk populations: Regular hip joint magnetic resonance imaging examinations and hormone level assessments should be conducted for obese children, those with thyroid dysfunction, or those experiencing precocious puberty. Community and primary healthcare providers should enhance their recognition of SCFE -related symptoms (e.g., thigh and groin pain, limping, and referred knee pain) to promote early detection and timely intervention. Delayed diagnosis is strongly associated with increased severity of the slip, which can lead to a poorer long-term prognosis[4].
Metabolic management: Correct calcium and phosphorus metabolic imbalances through dietary adjustments, vitamin D supplementation, and phosphate-lowering medications (e.g., sevelamer).
Combined treatment of surgery and pharmacotherapy
Surgical fixation: In situ screw fixation remains the most commonly adopted first-line treatment for stable SCFE[97]. However, severe cases of SCFE typically require osteotomy correction to restore anatomical alignment and biomechanical stability. Eduardo highlighted in his review that even in patients with mild SCFE, the incidence of early AC damage is significantly elevated. Traditional treatment strategies primarily focus on stabilizing the epiphysis to prevent secondary displacement and complications such as avascular necrosis of the femoral head, thereby aiming to restore hip joint function and delay the progression of osteoarthritis (OA). Nevertheless, the widely used traditional in situ screw fixation may not fully achieve these objectives. For mild SCFE, a combined treatment approach - combining epiphyseal screw fixation with femoral head-neck osteochondroplasty - can stabilize the SCFE while simultaneously achieving proximal femoral morphological remodeling through dual mechanisms, thereby reducing the risk of femoroacetabular impingement. In moderate to severe SCFE cases, intertrochanteric osteotomy is often indicated to restore the biomechanical axis, reduce abnormal stress distribution on the articular surface, and effectively delay the progression of OA. Clinical follow-up data indicate that after intertrochanteric osteotomy, patients demonstrated an excellent/good rate of 90% based on the Southwick criteria after an average follow-up of 9 years (range: 2-24 years). Additionally, a long-term study with an average follow-up of 24 years (range: 20-29 years) confirmed that 55% of patients with severe SCFE did not exhibit OA-related indicators or clinical pain postoperatively.
Targeted therapy exploration: Clinical trials have evaluated the potential of Wnt inhibitors (such as SM04690) or anti-inflammatory drugs (such as IL-1β antagonists)[98-100]. While these agents may exhibit certain effects, further research is required to confirm their specific efficacy and safety profiles. Schieker et al[100] investigated whether canakinumab, a human therapeutic monoclonal antibody targeting IL-1β, could reduce the incidence of total hip or knee arthroplasty (total hip replacement/total knee replacement). In their study, 10061 men and women with elevated high-sensitivity C-reactive protein levels and a history of myocardial infarction were randomly assigned to either a placebo group or a canakinumab treatment group. Participants received subcutaneous injections every three months over a five-year period. The final follow-up results indicated that inhibiting IL-1β with canakinumab significantly reduced the incidence of total hip and knee arthroplasty as well as OA-related symptoms.
This study provides insights into the role of IL-1β in the incidence of total hip and knee arthroplasty and OA-related symptoms, offering valuable guidance for future research. However, the study primarily relied on statistical surveys and lacked experimental validation at the cellular or molecular level. To address this limitation, relevant animal and cell experiments could be designed specifically for SCFE to elucidate the underlying mechanisms and accelerate the development of targeted therapies.
The prevention and treatment of SCFE necessitates the integration of multi-disciplinary resources, including orthopedics, endocrinology, and nutrition, along with the support of community-based primary healthcare institutions, adhering to the principle of “early screening - precise classification - stepwise treatment”. Currently, surgical interventions primarily aim at restoring biomechanical stability, whereas targeted drug research focuses on modulating inflammation (via the IL-1β/NF-κB pathway) and protecting cartilage (through the Wnt/SOX9 pathway). Looking ahead, researchers must further elucidate the interaction mechanisms among mechanical stress, inflammation, and metabolic pathways through fundamental research. This will facilitate the development of personalized targeted therapies based on molecular subtyping, such as gene therapy for COL2A1 mutations or miR-140 mimics for tissue repair, ultimately achieving precise prevention and treatment of SCFE.
CONCLUSION
The onset of SCFE results from the combined effects of genetic susceptibility, endocrine imbalance, metabolic abnormalities, and mechanical stress (Figure 2). The molecular regulatory mechanisms underlying these processes warrant further systematic investigation. Current research has elucidated key mechanisms, including dysfunctions in GPCs (e.g., imbalances in Wnt/β-catenin and Hedgehog signaling pathways), activation of inflammatory pathways (e.g., NF-κB-mediated cartilage degradation), and disruptions in mechanical stress regulation. However, the interplay between hormonal factors and metabolic regulators (such as adipokines) and the influence of epigenetic modifications (e.g., non-coding RNAs and DNA methylation) on GP function remain poorly understood. Clinically, it is essential to monitor internal diseases such as obesity, thyroid dysfunction, and precocious puberty, with early intervention facilitated through advanced imaging techniques (e.g., magnetic resonance imaging) and hormone level assessments. In treatment, surgical stabilization of SCFE area should be integrated with correction of underlying endocrine or metabolic disorders. To advance precision medicine for SCFE, a pediatric database integrating genomic, metabolomic, and imaging data should be established, enabling the development of a risk prediction model based on multi-omics data. This approach will facilitate the establishment of a precise prevention and treatment system, ultimately reducing the disability rate associated with SCFE.
Figure 2 Molecular pathways potentially involved in the pathogenesis of slipped capital femoral epiphysis.
NF-κB: Nuclear factor kappa B; FAK: Focal adhesion kinase; SOX9: SRY-box transcription factor 9; ROS: Reactive oxygen species; ROR2: Receptor tyrosine kinase-like orphan receptor 2; Rac1: Ras-related C3 botulinum toxin substrate 1; RhoA: Ras homolog family member A; ROCK: Rho-associated protein kinase; JNK: C-Jun N-terminal kinase; c-JUN: Cellular Jun N-terminal kinase substrate; AP-1: Activator protein 1.