Published online Oct 18, 2025. doi: 10.5312/wjo.v16.i10.112086
Revised: August 7, 2025
Accepted: September 11, 2025
Published online: October 18, 2025
Processing time: 91 Days and 19.7 Hours
Developmental dysplasia of the hip (DDH) remains a significant public health challenge, particularly in developing countries where cultural factors and limited access to appropriate medical equipment complicate optimal management.
To evaluate the difficulties encountered in the management of DDH in our healthcare setting.
A retrospective, single-center study was conducted over nine years (2015-2023), including 20 patients (26 hips) with idiopathic DDH. Patients with post-traumatic or post-infectious hip dislocations were excluded. Data collected included age at diagnosis, laterality, Tönnis classification, duration of traction, and surgical interventions.
The mean age at diagnosis was 35.6 months (4 months to 10.8 years). Dislocation was unilateral in 70% of cases; 69.2% were classified as Tönnis stage 3 or 4. The average traction duration was 57.5 days. Surgery was performed in 8 hips. Among 16 patients with regular follow-up, 10 showed good outcomes, 3 excellent, and 3 fair according to the McKay score. Older age at treatment (> 1 year), bilateral dislocation, and higher severity (Tönnis stages 3 and 4) were associated with worse functional outcomes. No significant correlation was found between functional and radiological results. Residual dysplasia occurred in 3 patients, and 1 re-dislocation was noted.
Delayed diagnosis and advanced severity at presentation are key challenges in managing DDH in our context. Nevertheless, appropriate management can achieve generally favorable outcomes, despite complications linked to prolonged treatment and resource limitations.
Core Tip: Developmental dysplasia of the hip management in resource-limited settings faces delays and severe cases at diagnosis. Appropriate treatment yields good outcomes despite prolonged traction and equipment challenges.
- Citation: Manasse H, Poiri A, Moullac D, Rasamoelina MB, Daoulas T, Solofomalala GD. Challenges in the management of hip dislocation disease in Madagascar. World J Orthop 2025; 16(10): 112086
- URL: https://www.wjgnet.com/2218-5836/full/v16/i10/112086.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i10.112086
Developmental dysplasia of the hip (DDH) is defined as an abnormality of the hip joint, characterized by a loss of anatomical congruence between the femoral head and the acetabulum, and can be detected at birth[1]. It represents a significant public health concern due to its prevalence and its physical, psychological, and social consequences, and remains a current topic of interest[2]. In France, the incidence of late-diagnosed DDH (after the age of 12 months) is estimated at 10 cases per 100000 per year[3].
In Madagascar, estimating the true incidence of this condition is challenging due to several barriers to diagnosis and management. These include limited access to healthcare for much of the population, lack of public awareness and education, frequent recourse to traditional medicine, and certain cultural beliefs—such as attributing congenital malformations to divine will. These factors contribute to a very low rate of consultations for congenital abnormalities detected at birth.
Screening still relies primarily on repeated clinical examinations and hip ultrasound in the presence of risk factors[2,3]. Clinical signs include limited hip abduction, congenital pelvic asymmetry, and joint instability. The treatment principle is to achieve reduction of the femoral head, either through orthopedic or surgical means[4].
Like many congenital pathologies, DDH represents a major challenge in developing countries. The condition is often more severe than in Europe, as it frequently goes untreated[5]. In our setting, DDH management remains controversial, particularly due to the advanced age at presentation, limited availability of appropriate medical equipment, and the inherent complexity of the disease itself. The aim of this study was to assess the factors contributing to the difficulties in managing DDH in a developing country context.
Oral consent was obtained from the parents or legal guardians of all participants. Prior approval was granted by the institutional ethics committee.
This retrospective, analytical study included 20 patients (26 hips) diagnosed with idiopathic DDH. All were examined in outpatient consultation by a senior orthopedic surgeon and subsequently hospitalized in the Surgery Department of CHU Anosiala, Madagascar, between June 2015 and June 2023 (8 years). Inclusion criteria were: (1) Idiopathic DDH confirmed clinically and radiologically; (2) No prior treatment; and (3) complete medical records and follow-up allowing for radioclinical evaluation. Exclusion criteria were: Post-traumatic or post-infectious hip dislocations, teratologic dis
| Group | Radiographic appearance | Vertical center edge angle |
| I | Normal | |
| I-a | Normal | > 19° (ages 6-13) |
| I-b | Normal | > 25° (older than 13 years) |
| II | Mild deformity of the femoral head, neck, or acetabulum | Same as group I |
| III | Dysplasia without subluxation | < 15° (ages 6-13) < 20° (> 13 years) |
| IV | ||
| IV-a | Moderate subluxation | ≥ 0° |
| IV-b | Severe subluxation with neo-hip joint formation at the upper margin of the acetabulum | < 0° |
| V | Recurrent dislocation |
Orthopedic treatment: Initial treatment consisted of continuous skin traction using adhesive bands fixed to both lower limbs. The patient was positioned supine, with the hips in extension and slight abduction, and the traction angle ranged from 10° to 30° from the bed plane (Figure 1). Traction force ranged from 10% to 50% of body weight depending on the degree of femoral head displacement. Radiographs were obtained every 15 days to assess reduction and adjust traction in abduction and/or internal rotation. Once the femoral head was centered, traction was followed by the application of a pelvipedal cast (PPC) under general anesthesia and fluoroscopic guidance. The cast was maintained for 2 to 4 months. This sequence was applied to all patients as definitive treatment or to prepare soft tissue for surgery.
Surgical treatment: Surgery was indicated either in the event of failure of conservative treatment or for residual dysplasia requiring correction. Surgical procedures included: Adductor and/or iliopsoas tenotomy, performed percutaneously with the limb in flexion-abduction. This was indicated in case of persistent subluxation despite 3-4 weeks of traction (i.e., femoral head still not concentric under the Hilgenreiner line[9]). This was followed by axial traction and PPC. Salter innominate osteotomy[10], performed in an 8-year-old girl with acetabular dysplasia, followed by PPC for 2 months. Supra-acetabular shelf procedure, performed in an 11-year-old girl with acetabular dysplasia, followed by PPC for 45 days.
Data were collected retrospectively from hospital records and supplemented by follow-up phone interviews with families. All cases were reviewed by a single observer. Data were analyzed using SPSS version 26.0. Quantitative variables were expressed as means with standard deviations and ranges (minimum and maximum). Qualitative variables were presented as proportions and percentages. Comparisons of quantitative data were performed using the Student's t-test. A P-value < 0.05 was considered statistically significant.
At the time of initial management, the mean age was 35.6 ± 23.1 months (range: 4 months to 10.8 years), corresponding to an average of 2.9 years. Sixteen children (20 hips) were retained for final analysis after applying the exclusion criteria. Of these, 13 children (80.76%) were seen after the age of 12 months, with a marked female predominance (sex ratio: 0.23, i.e., 3 boys for 13 girls). Limping was the main presenting symptom (69.23%, i.e., 9 of 13 patients). Unilateral involvement was found in 14 hips (70%). According to the Tönnis classification, 18 hips (69.2%) were graded as stage III or IV. Surgical treatment was performed on 8 hips (30.76%), either as a primary or complementary approach. Table 2 summarizes the population characteristics.
| Variable | Frequency (n = 26) | Proportion (%) |
| Age at diagnosis (years) | ||
| < 1 | 5 | 19.23 |
| 1-3 | 12 | 46.15 |
| > 3 | 9 | 34.61 |
| Sex | ||
| Female | 21 | 80.75 |
| Male | 5 | 19.25 |
| Reason for consultation | ||
| Limping | 18 | 69.23 |
| Delayed walking acquisition | 6 | 23.07 |
| Follow-up radiograph | 2 | 7.69 |
| Tönnis classification | ||
| Grade 1 | 3 | 19.23 |
| Grade 2 | 5 | 34.61 |
| Grade 3 | 8 | 30.76 |
| Grade 4 | 10 | 38.46 |
| Treatment performed | ||
| Closed reduction then hip spica | 18 | 69.23 |
| Closed reduction →adductor tenotomy →hip spica | 6 | 23.07 |
| Closed reduction →salter osteotomy →hip spica | 1 | 3.84 |
| Closed reduction →acetabular shelf procedure →hip spica | 1 | 3.84 |
Patients were followed for a mean duration of 2.9 years (range: 1-7.5 years). A total of five patients were excluded due to traumatic or infectious etiologies of hip dislocation; although this number is relatively high (20% of initial cases), they were not comparable to congenital forms in terms of pathophysiology and treatment protocol. We acknowledge this exclusion may limit generalizability. The average duration of traction was 57.5 ± 37.4 days (range: 30-180 days), which was significantly correlated with the severity of the lesion [P < 0.05, 95%CI; relative risk (RR) = 2.4]. Patients with Tönnis stage III and IV dislocations required an average of 8 weeks of traction, compared to 4 weeks for stages I and II. A linear regression model confirmed that Tönnis stage was a significant predictor of traction duration (R² = 0.44, P = 0.003). Although socioeconomic and geographic factors were not directly assessed due to limited retrospective data, we acknowledge their potential influence on access to care, treatment adherence, and follow-up continuity, and discuss this in the discussion section.
Functional outcomes were assessed in 16 children (20 hips) at regular intervals after discharge: Day 30, day 60, month 6, one year, and every two years thereafter. At the latest follow-up, 10 hips (50%) had a good outcome, and 3 hips (15%) had an excellent outcome according to the McKay score. Seven children presented with a mild limp while walking, but had no pain, stable hips, and a negative Trendelenburg sign. Three children were asymptomatic with excellent outcomes (no limp, no pain, no instability, negative Trendelenburg sign).
There was no statistically significant difference between gender and functional outcome (P = 0.34). However, older age at the time of treatment (> 12 months), higher Tönnis stage, and bilateral involvement were associated with poorer outcomes (P < 0.05). Older age was associated with a RR of 1.4 for poor functional outcome, bilaterality with RR = 0.4, and Tönnis stage III-IV with RR = 3.0. These associations remained significant in a multivariate logistic regression model (P = 0.01), suggesting that these variables were independent predictors of outcome. Confidence intervals for the main risk ratios were: Tönnis III-IV RR: 3 (95%CI: 1.2-7.5); age > 12 months RR: 1.4 (95%CI: 1.05-2.8); bilateral involvement RR: 0.4 (95%CI: 0.1-0.95).
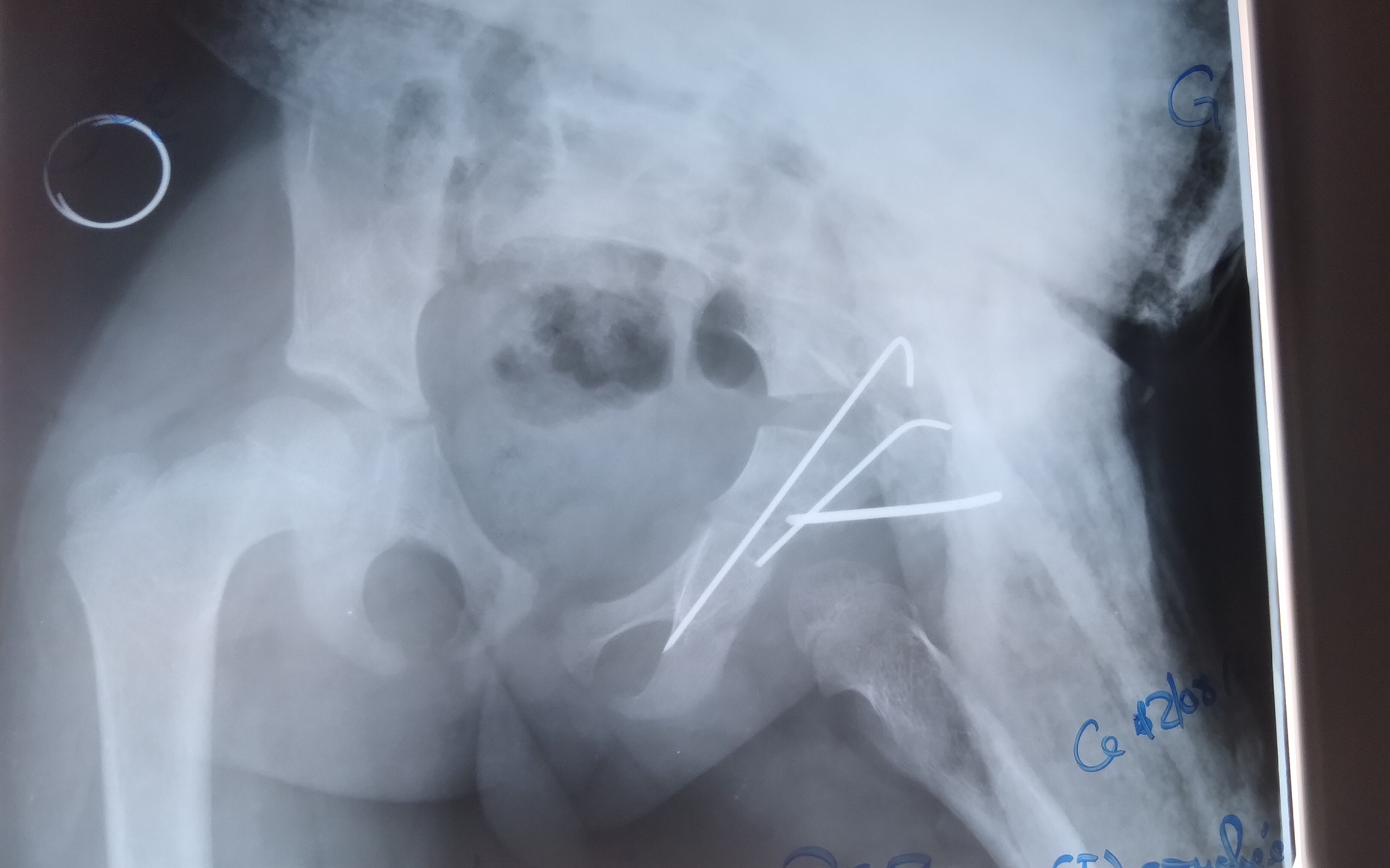
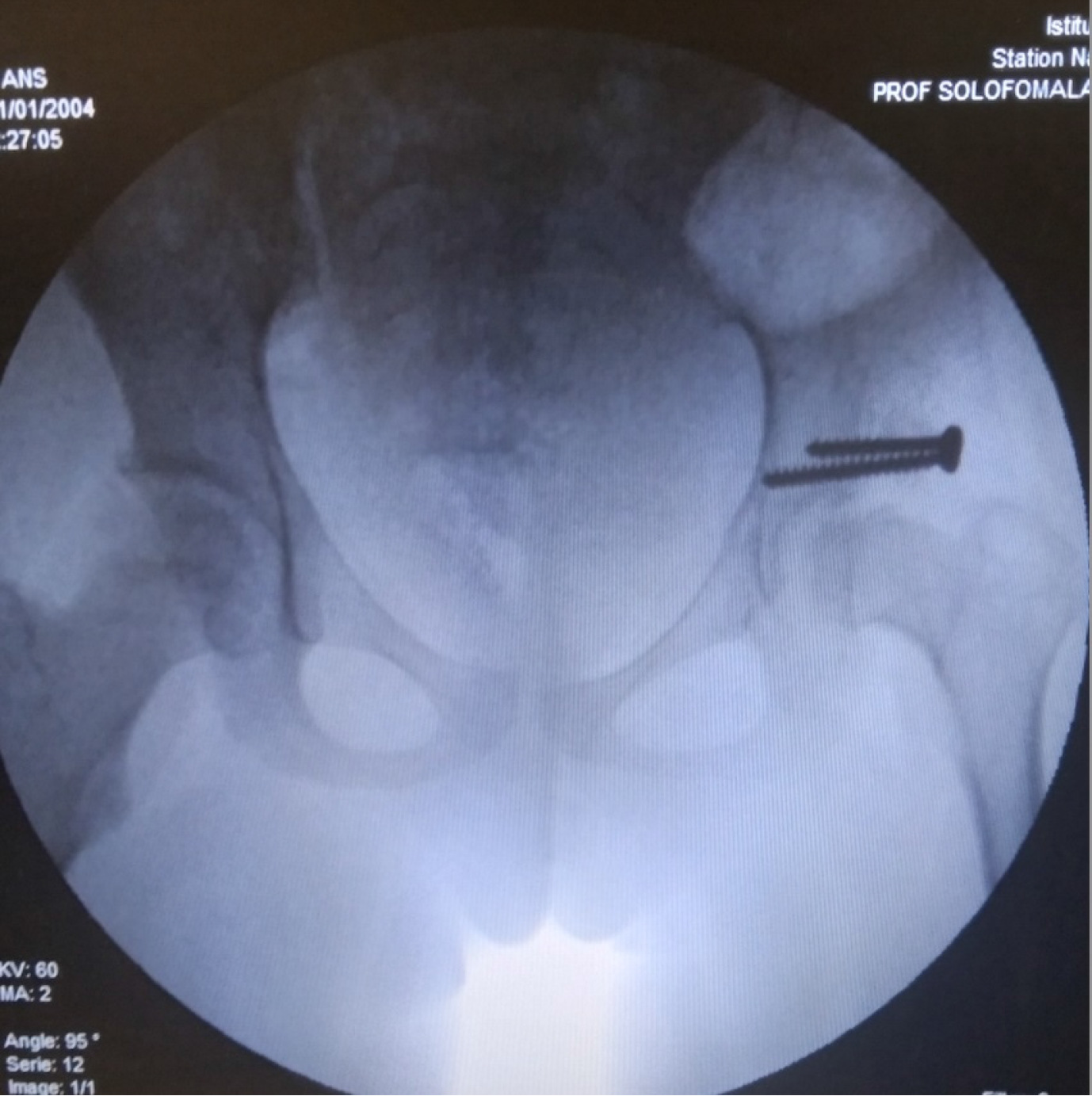
Radiological outcomes were assessed using the Severin classification. Each child underwent a mean of four radiographs during the treatment course. At final follow-up, 22 hips (84.6%) were classified as Severin stage I or II, indicating concentric and congruent reduction. Three hips (11.5%) showed residual dysplasia without subluxation. One case of redislocation (Severin stage V) was observed in a 21-month-old child with a unilateral Tönnis stage IV DDH, initially managed with 2 months of adhesive traction followed by spica casting. This required re-hospitalization and repeat treatment with traction and PPC. No further complications occurred (Figures 2 and 3).
There was no statistically significant correlation between functional and radiological outcomes (P = 0.24). A Cox proportional hazards model was tested to assess time to hip stabilization (defined as Severin I-II), but the sample size limited statistical power and results were not significant. All radiological and clinical evaluations were performed by the same orthopedic surgeon to limit interobserver variability.
DDH remains a public health challenge. Its incidence is estimated at 6 to 20 per 1000 Live births in France[4,11], corresponding to between 4900 and 16400 DDH cases diagnosed at birth[12]. In our study, twenty-three children with DDH were seen in outpatient consultations over a 7-year period, all either self-referred or referred by independent physicians for further management. However, this figure may be underestimated due to the lack of accurate epidemiological data in our context, the prevalence of home births, and general unawareness of the condition among the population. The absence of systematic screening, as well as poor understanding of screening procedures among healthcare and paramedical staff, may also contribute. In the study by Wicart et al[13], the incidence of DDH diagnosed after one year of age in children born in 2009 was 2.9 per 100000 Live births. The literature consistently reports a predominance of female patients[13,14], as reflected in our study with a sex ratio of 0.2, making female sex a major risk factor.
Diagnosis after three months of age is considered delayed[12]. In our series, the mean age at diagnosis was 35.6 months, with over 80% of children seen after the age of one year. This highlights the central role of diagnostic delay in shaping treatment strategies: Late diagnosis is often associated with more advanced radiographic stages (Tönnis III or IV), which require longer and more invasive treatment protocols. This delay could be explained by failures in systematic neonatal screening[14] and insufficient training of healthcare personnel, both in primary care centers and referral hospitals. However, systematic screening for DDH should begin at birth via clinical examination and be repeated at all well-baby visits during the first year of life, specifically looking for signs of hip instability or dislocation. The lack of national screening programs in Madagascar represents a missed opportunity for early, non-invasive intervention. To address this, a structured screening strategy could include mandatory hip examinations during vaccination visits, systematic use of the Ortolani and Barlow maneuvers by all front-line health workers, and gradual introduction of targeted ultrasound screening in high-risk newborns. This raises the question of implementing an organized national screening program in Madagascar, as currently recommended in France, and of identifying risk factors specific to the Malagasy population. Detecting DDH at the onset of ambulation requires a lengthy, restrictive, and costly orthopedic management, sometimes necessitating surgery. Conversely, neonatal diagnosis usually allows for short-term conservative treatment with complete anatomical and functional recovery in most cases[3,15]. Ultrasound screening is recommended between one and three months of age[11,13].
Socioeconomic and geographic factors likely contribute to the observed diagnostic delays and treatment outcomes. Lack of parental awareness about the disease and its potential consequences may explain why, in our series, 92.3% of consultations occurred after the onset of limping or delayed walking. Families living in remote or rural areas may also face geographic and financial barriers to early referral and follow-up care, impacting adherence and limiting access to specialized treatment. Therefore, a public awareness campaign could be beneficial, targeting both the general population and healthcare professionals, particularly midwives, even in remote areas. Such campaigns should emphasize the importance of routine newborn screening, educate families on early signs of DDH, and promote timely medical con
According to several studies, bilateral involvement occurs in one-third of cases[1,14]. Orthopedic treatment in such cases is more complex, requiring bilateral traction or staged treatment[16]. Tönnis radiographic classification grades III and IV are considered severe forms of DDH[6]. In our series, a correlation was found between severity and treatment burden, as more severe forms required significantly longer durations of traction (P < 0.05; 95%CI; RR = 3.1), consistent with the findings of Kaneko et al[17].
According to the French Society of Pediatric Orthopedics (SOFOP), the standard conservative treatment for DDH diagnosed after one year of age involves continuous traction in bed for 2 weeks to 2 months depending on age and dislocation severity, followed by a closed reduction under general anesthesia and immobilization in a hip spica cast for 2-6 months[14]. This method is similar to that described by Petit Morel for children diagnosed after walking age, during which tenotomy and/or osteotomy are not performed systematically[18]. Our management protocol followed similar principles but with longer traction durations (average of 57 days), due to the traction devices being adapted from available materials in Madagascar. This occasionally caused skin irritation (e.g., leg blisters), foot pain, and required temporary traction interruption (2-3 days). Nevertheless, Yamada et al[19] reported excellent results with minimal complications after two months of prolonged traction followed by closed reduction performed at the bedside without general anesthesia. However, this approach required prolonged hospitalization, higher costs, and considerable patience from both the child and their family. Given the low average income in Madagascar and the absence of hospital social support services, the socioeconomic burden of such treatment protocols cannot be overstated. In our experience, treatment abandonment was primarily linked to this socioeconomic pressure. To reduce dropout, we recommend structured counseling sessions with families upon admission, setting realistic expectations about treatment length and potential outcomes. When feasible, providing accommodation or meal vouchers for accompanying parents, even at a minimal level, could also improve adherence. This can lead to treatment dropout, increased hospitalization costs, and indirect costs such as travel expenses and loss of income for parents who must stay at the hospital full-time, sometimes resulting in job loss. In this context, family involvement is crucial as they must also provide food and basic care for the child.
According to SOFOP, surgical treatment is recommended for children over the age of 3 or in case of failure of conservative treatment. Surgery is usually performed after a period of traction (48 hours to 1 month), and includes capsulorrhaphy, adductor and/or psoas tenotomy, and occasionally pelvic osteotomy[13]. However, surgical treatment can be indicated up to the age of 8 in late-diagnosed dislocations[20]. In our series, 8 hips (30.75%) required surgical treatment. This included one child diagnosed with DDH at age 8 who underwent Salter osteotomy, a technically feasible and relatively simple procedure in our context, although recent consensus favors its use in children aged 3-5 years, especially in cases of residual dysplasia[21]. In Ait Ziane et al[1], surgical treatment was performed in 95.6% of cases, and in Wicart et al[13], in 56%. Our lower rate may be explained by favorable outcomes following conservative treatment, albeit at the cost of prolonged traction and hospitalization (18 hips with at least 8 weeks of traction). It is therefore essential to clearly explain to parents the management plan, expected treatment duration, and potential need for surgical intervention, to prevent treatment abandonment, which occurred in 3 patients in our study. Furthermore, surgery is not without complications, including recurrent dislocation and avascular necrosis of the femoral head. As such, conservative management remains the first-line treatment for late-diagnosed DDH[20].
Despite prolonged traction, 3 hips (11%) showed residual dysplasia, due to severe initial lesions and delayed treatment. This rate is consistent with literature reports, which range from 2% to 20% of treated hips, with recent series reporting 2.7%[21]. Mazaleyrat et al[18] reported a failure rate of 5.7%.
The relatively small sample size (20 patients, 26 hips) constitutes a limitation of this study, reducing the generalizability of our findings. Nevertheless, statistically significant associations were found between severity and treatment duration, suggesting a strong underlying effect. Future studies with larger cohorts should aim to confirm these trends and evaluate the role of additional variables. Particularly, integrating socioeconomic, geographic, and health literacy indicators would offer deeper insight into risk factors for delayed diagnosis and treatment non-compliance. Including socioeconomic or geographic indicators in future analyses would provide a more comprehensive understanding of DDH management in low-resource settings. Most patients in our series experienced long hospital stays due to the traction-based conservative treatment. Radiographic monitoring under traction was necessary to assess reduction, with the number of X-rays correlating with traction duration. Despite these challenges, outcomes were favorable in most cases (≥ 75% good or excellent results) according to McKay’s clinical score.
This study highlights how late diagnosis, often resulting from systemic failures in screening and public education, directly influences both the severity of DDH and the complexity of its treatment. The management of DDH upon admission is often hindered by several factors: Delayed diagnosis and initiation of appropriate treatment, a high frequency of severe (Tönnis grade III or IV) and bilateral dislocations, and the prolonged duration of treatment. However, with a well-adapted therapeutic strategy, outcomes are generally favorable. To improve care in resource-limited contexts, national early screening programs and public health interventions are urgently needed. Early screening and standardized technical equipment could significantly improve management, which remains multidisciplinary. Furthermore, socioe
| 1. | Ait Ziane S, Belabbassi H, Benbellal A, Arkam M, Hamza H, Louz K, Kaced H. Evaluation of the orthopedic and functional status of congenital dislocations of neglected hips, surgically treated. Batna J Med Sci. 2019;6:104-108. [DOI] [Full Text] |
| 2. | Salut C, Moriau D, Pascaud E, Layré B, Peyrou P, Maubon A. [Initial results from an ultrasound screening program for the detection of developmental dysplasia of the hip in girls]. J Radiol. 2011;92:920-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Blondiaux E, Morel B, Maheux A, Ducou le Pointe H. Dépistage et diagnostic de la luxation congénitale de hanche: coupes échographiques types et interprétation. J Diagn Int Imaging. 2018;1:161-167. [DOI] [Full Text] |
| 4. | Fassier A. Luxation congénitale de hanche, les enjeux du premier mois. Sages-Femmes. 2024;23:39-43. [DOI] [Full Text] |
| 5. | Sales de Gauzy J. Orthopédie pédiatrique en mission humanitaire. Conférences D'enseignement 2016. Paris: Elsevier, 2016: 165-177. [DOI] [Full Text] |
| 6. | Ramo BA, De La Rocha A, Sucato DJ, Jo CH. A New Radiographic Classification System for Developmental Hip Dysplasia is Reliable and Predictive of Successful Closed Reduction and Late Pelvic Osteotomy. J Pediatr Orthop. 2018;38:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | McKay DW. A comparison of the innominate and the pericapsular osteotomy in the treatment of congenital dislocation of the hip. Clin Orthop Relat Res. 1974;124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Ward WT, Vogt M, Grudziak JS, Tümer Y, Cook PC, Fitch RD. Severin classification system for evaluation of the results of operative treatment of congenital dislocation of the hip. A study of intraobserver and interobserver reliability. J Bone Joint Surg Am. 1997;79:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Classic. Translation: Hilgenreiner on congenital hip dislocation. J Pediatr Orthop. 1986;6:202-214. [PubMed] |
| 10. | Salter RB. The classic. Innominate osteotomy in the treatment of congenital dislocation and subluxation of the hip by Robert B. Salter, J. Bone Joint Surg. (Brit) 43B:3:518, 1961. Clin Orthop Relat Res. 1978;2-14. [PubMed] |
| 11. | Tréguier C, Chapuis M, Branger B, Grellier A, Chouklati K, Bruneau B, Fraisse B, Violas P, Pladys P, Darnault P, Gandon Y. [Developmental dysplasia of the hip]. J Radiol. 2011;92:481-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Schirrer J, de Billy B, de Billy M. [Tracking dysplasia and congenital luxation of the hip]. Arch Pediatr. 2005;12:789-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Wicart P, Bocquet A, Gelbert N. Stratégie actuelle du dépistage de la luxation congénitale de hanche. e-memoires de l’Académie nationale de chirurgie 2015: 115-119. [DOI] [Full Text] |
| 14. | Wicart P, Bocquet A, Gelbert N, Beley G, Proslier R, Pracos-deffrenne P, Vie le Sage F, Assathiany R, Chapuis M, Fron D, Guillard S, Mainard-Simard L, Ducou le Pointe H, Kohler R, Seringe R, Morin C. Luxation congénitale de la hanche: quel dépistage pour 2014? J Orthop Surg Trauma. 2014;100:S184-S191. [DOI] [Full Text] |
| 15. | Wang JH, Yao SH, Wang TM, Lin CJ, Lin CH, Chen CH. Effectiveness of incorporating the graf method into a universal neonatal ultrasound screening program for early diagnosis of developmental dysplasia of the hip in the Taiwanese population. BMC Musculoskelet Disord. 2025;26:551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Farsetti P, Caterini R, Potenza V, Ippolito E. Developmental Dislocation of the Hip Successfully Treated by Preoperative Traction and Medial Open Reduction: A 22-year Mean Followup. Clin Orthop Relat Res. 2015;473:2658-2669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Kaneko H, Kitoh H, Mishima K, Matsushita M, Ishiguro N. Long-term outcome of gradual reduction using overhead traction for developmental dysplasia of the hip over 6 months of age. J Pediatr Orthop. 2013;33:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Mazaleyrat M, Lacroix R, Lakhal W, Morel B, Bonnard C, Odent T. Traitement de la maladie luxante de hanche après l’âge de la marche par la méthode de Petit-Morel. Étude rétrospective de 34 patients. J Orthop Surg Trauma. 2022;108:122-126. [DOI] [Full Text] |
| 19. | Yamada N, Maeda S, Fujii G, Kita A, Funayama K, Kokubun S. Closed reduction of developmental dislocation of the hip by prolonged traction. J Bone Joint Surg Br. 2003;85:1173-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Glorion C. Surgical reduction of congenital hip dislocation. Orthop Traumatol Surg Res. 2018;104:S147-S157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | de Courtivron B, Brulefert K, Portet A, Odent T. La dysplasie résiduelle de la hanche. J Orthop Surg Trauma. 2021;107:S138-S148. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/