Published online Oct 18, 2025. doi: 10.5312/wjo.v16.i10.111955
Revised: July 29, 2025
Accepted: September 9, 2025
Published online: October 18, 2025
Processing time: 94 Days and 13.9 Hours
Two-stage revision is the most common treatment for chronic periprosthetic joint infection of the hip, involving a resection arthroplasty with or without placement of an antibiotic-loaded spacer, followed by antibiotic therapy before reimplan
To compare the outcomes and complications of two consecutive treatment pro
In this retrospective study, two consecutive cohorts were compared. Group A (2017-2020) underwent two-stage revision with a Girdlestone and an antibiotic holiday before reimplantation, while Group B (2020-2023) received CUMARS whenever possible, and no antibiotic holiday, or a Girdlestone if indicated. The primary outcome was successful infection eradication after one year. Secondary outcomes included surgical duration, length of hospital stay, weight-bearing allowance, discharge destination, and complications.
A total of 98 patients were included: 39 patients in Group A and 59 patients in Group B. Successful infection eradication after one year was achieved in 69% of Group A and 83% of Group B (P = 0.164). Patients in Group B were more frequently allowed to bear weight (64% vs 18%, P < 0.001), had a shorter in-hospital stay (9 vs 16 days, P < 0.001), and were more often discharged home after the first surgery (48% vs 24%, P = 0.048). No significant differences were found in (mechanical) complications.
A protocol including CUMARS is a safe and effective treatment, offering faster recovery, shorter length of hospital stay, and enabling more patients to return home during the interval. This reduces strain on patients and the healthcare system, potentially saving costs, without compromising infection control or increasing (mechanical) complications.
Core Tip: This retrospective cohort study compares two consecutive treatment protocols for two-stage hip revision in periprosthetic joint infection: One using only Girdlestone and another incorporating custom-made articulating spacers (CUMARS) whenever possible, or a Girdlestone when necessary. While infection eradication rates were similar, the introduction of CUMARS was associated with shorter hospital stays, earlier mobilization, and more frequent discharge to home. These results suggest that CUMARS facilitates a faster recovery for suitable patients and reduce healthcare burden without compromising infection control, making them a valuable addition to the treatment algorithm. For patients with contraindications, a Girdlestone remains a viable option.
- Citation: Stelwagen MH, Van Oldenrijk J, Croughs PD, Yusuf E, Bos PK, Veltman ES. Impact of introducing custom-made articulating spacers: A retrospective cohort study on two protocols for two-stage hip revision. World J Orthop 2025; 16(10): 111955
- URL: https://www.wjgnet.com/2218-5836/full/v16/i10/111955.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i10.111955
Two-stage revision arthroplasty is the most common treatment for chronic periprosthetic joint infection (PJI) of the hip, consisting of two surgeries and antibiotic therapy of 6 to 12 weeks. Traditionally, a Girdlestone resection arthroplasty was performed, frequently with an antibiotic holiday before reimplantation. The absence of a functional hip joint severely immobilized patients, prolonging hospital stays and often requiring admission to an extended care facility[1-3].
Over the years, treatment protocols have changed: Antibiotic holidays are no longer mandatory, intervals can be long or short, and antibiotic-loaded interval spacers are advocated[4-6]. More recently, the use of functional/custom-made articulating spacers (CUMARS) has increased. First described by Tsung et al[7], CUMARS utilize standard commercially available hip implants coated in antibiotic-loaded cement tailored to the causative pathogen. CUMARS maintain soft tissue tension, preventing fibrosis and retraction, which facilitates easier reimplantation and reduces leg length discrepancy and dislocation risk after reimplantation[1-3,8]. CUMARS improve functional outcomes while maintaining locally sufficient antibiotic levels until the second-stage surgery[3,9-11]. However, spacers introduce the risk of mechanical complications, including spacer dislocation, (peri)-spacer fracture, and acetabular bone loss[11,12]. These complications are particularly associated with prefabricated/molded spacers and spacers without an acetabular component. They are less frequently or not reported in CUMARS, which also have the advantage of allowing (partial) weight-bearing, potentially shortening hospital stays and enabling patients to return home between stages[13-18].
Traditionally, following the first-stage surgery and subsequent antibiotic treatment, a period without antibiotics, known as the antibiotic holiday, was recommended. The rationale behind this approach was to determine whether it was safe to proceed with second-stage surgery, as any residual infection would have the opportunity to reemerge during this drug-free interval[6]. Secondarily, if tissue cultures remained negative after the second stage procedure, antibiotics could be ceased. However, this approach has proven to be challenging, as there are currently no reliable preoperative tests to definitively exclude any persistent infection prior to reimplantation[6,19,20]. Consequently, the antibiotic holiday is now frequently discouraged, with continuous antibiotic treatment being advocated instead[20,21]. Nevertheless, robust evidence remains limited, as available studies are scarce and present conflicting results[19,22,23]. The International Consensus Meeting on infection also acknowledged the lack of conclusive evidence and, consequently, was unable to issue a formal recommendation on the need for and/or the ideal length of an antibiotic holiday[24].
Motivated by the significant impact of a Girdlestone on a patient's mobility and health, and a strive to limit in-hospital length of stay, we introduced a new protocol including CUMARS and no antibiotic holiday in our center in 2020[25]. We hypothesize that the introduction of the new protocol leads to faster recovery, fewer postoperative complications, and easier reimplantation compared to a Girdlestone interval with an antibiotic holiday for two-stage revision of the infected hip, while maintaining the same infection eradication rate.
A retrospective single-center cohort study was conducted at a tertiary referral center for PJI in the Netherlands. All patient records of individuals who underwent a two-stage revision arthroplasty of the hip for infection, as determined by a multidisciplinary team based on the EBJIS criteria for PJI, between 2017 and 2023 were reviewed for inclusion in the study. Patients with a two-stage hip arthroplasty for infection were included in the study. Exclusion criteria included one-stage revisions, intentional definitive Girdlestone procedures, and cases in which the first or second-stage surgery was performed at other institutions.
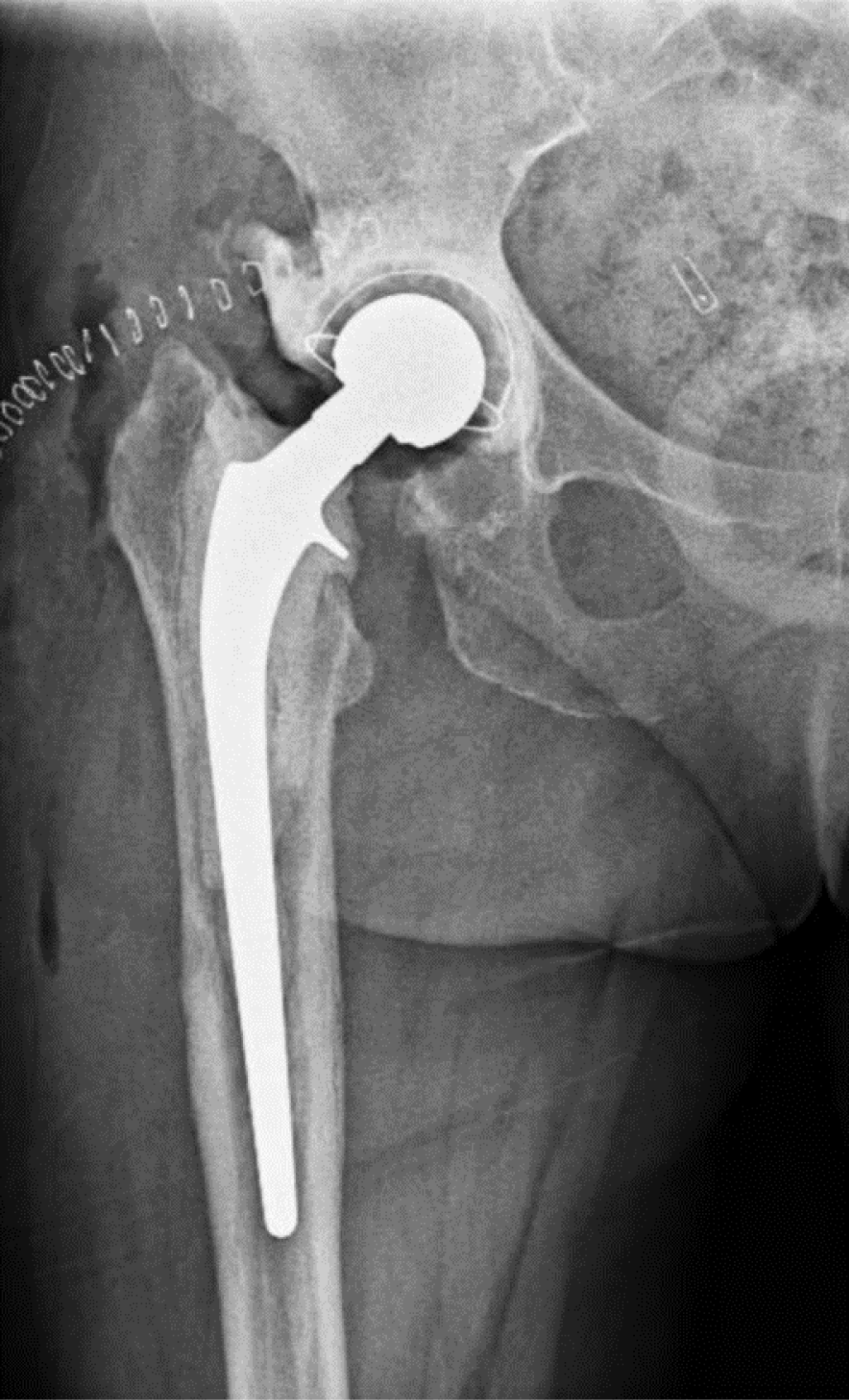
This study involves two consecutive cohorts. In the first cohort, from 2017 to 2020, only Girdlestone (Figure 1) intervals were used (Group A). At the beginning of this period, gentamicin beads were left in place during the first-stage surgery and were removed 2 weeks later. Patients were instructed to avoid weight-bearing during the interim period to limit leg length discrepancy. Antibiotic treatment was discontinued for at least two weeks before the second-stage procedure. After reimplantation, patients received intravenous antibiotics for five days, which were discontinued if cultures remained negative on day five. If cultures were positive, patients received an additional course of targeted antibiotic therapy for at least six weeks.
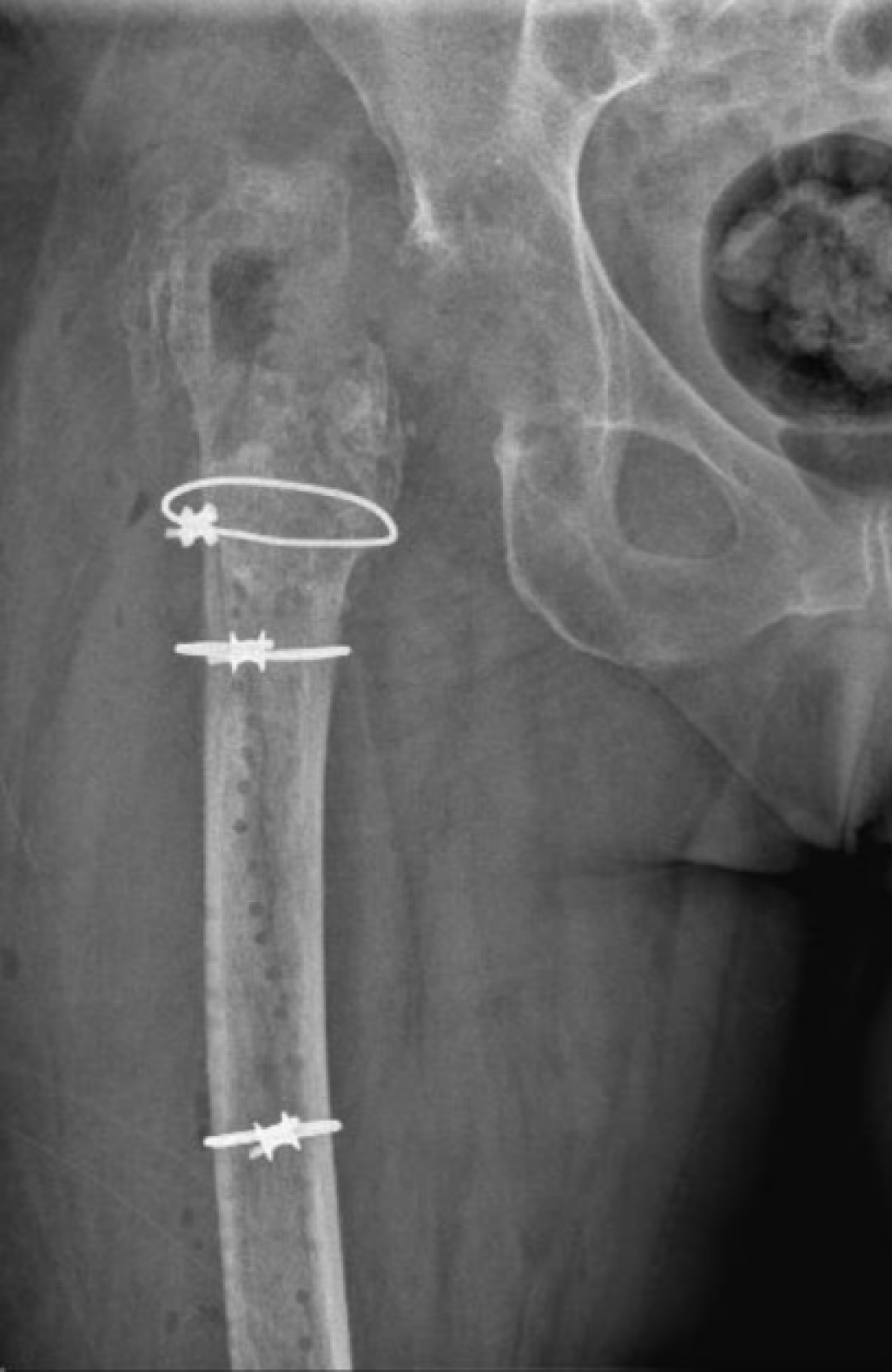
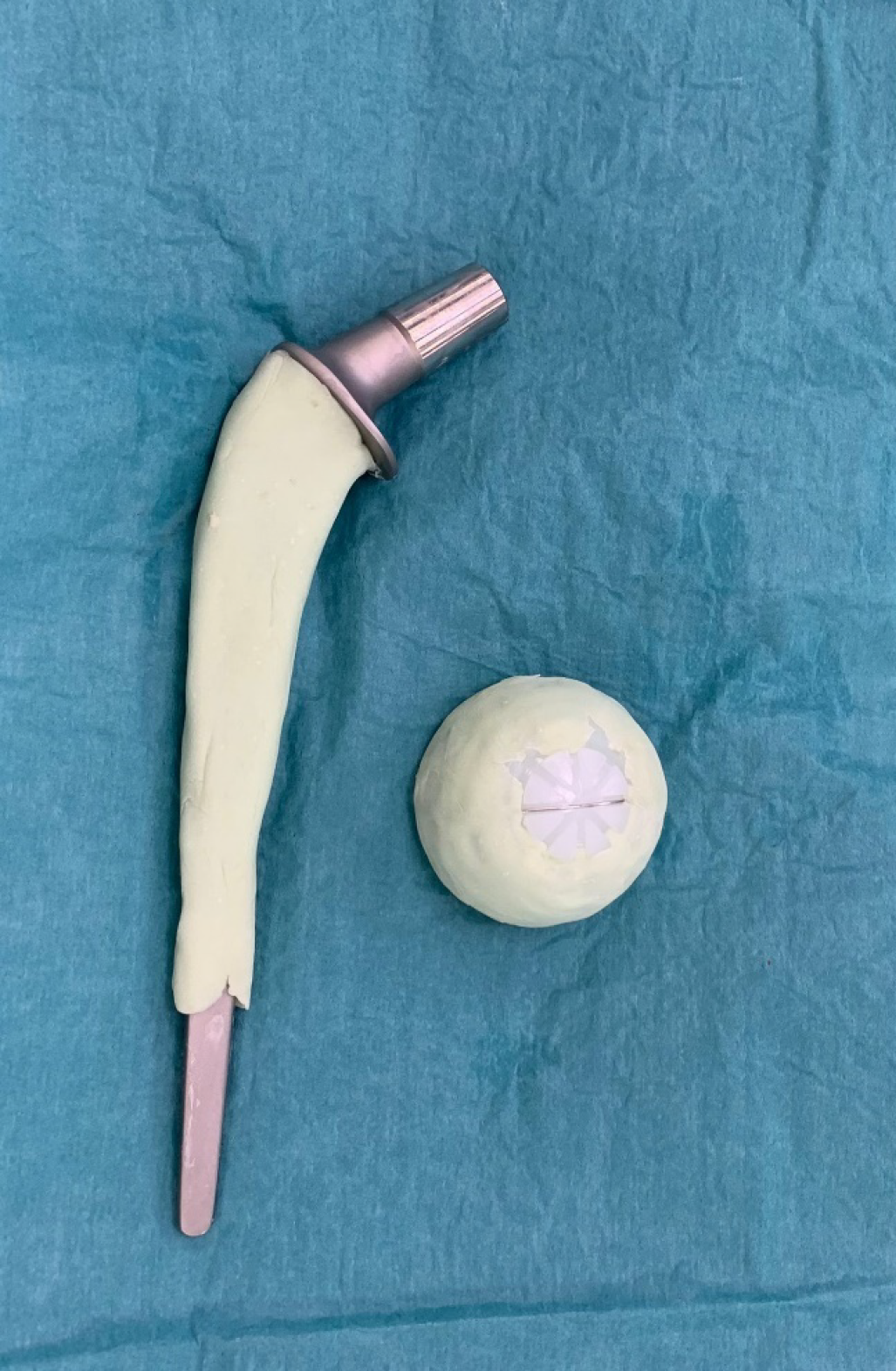
In the second cohort, from 2020 to 2023, a new protocol was introduced in our center (Group B). After the resection arthroplasty, a CUMARS (Figure 2) was created by coating commonly used femoral and acetabular components (Link® Lubinus Classic Plus Stem and Link® IP Acetabular Cup (Waldemar Link GmbH & Co. KG, Hamburg, Germany)) with antibiotic-loaded cement (Palacos® R+G bone cement, Heraeus Medical GmbH, Wehrheim, Germany), with additional antibiotics (1 g per batch of cement) tailored to the patient's specific pathogen (Figure 3). The spacer was then loosely implanted when the cement had a doughy consistency, ensuring easy removal during the second-stage surgery. Rotational stability of the CUMARS is achieved by folding the cement around the edges of the proximal femur and acetabulum. Patients were permitted partial weight-bearing (up to 50% of bodyweight) during the interval based on a recommendation by the operating surgeon. Patients were continuously treated with a minimum of 6 weeks of antibiotics following the first-stage surgery, including 2 weeks intravenously, up until reimplantation. After reimplantation, they received an additional 6-week course of antibiotics, with 1 week administered intravenously, totaling a minimum antibiotic duration of 12 weeks in total. In Group B, a Girdlestone interval was used in case a CUMARS was deemed contraindicated by the treating orthopedic surgeon intraoperatively based on bone stock and fracture risk. These patients received antibiotic treatment according to the protocol used in Group A.
All patients in both groups received preoperative antibiotic prophylaxis. The surgeries were performed through a posterolateral approach by three senior orthopedic surgeons. During the first-stage surgery, the infected prosthesis, any osteosynthesis material if present, and/or the native femoral head were removed. An extended trochanteric osteotomy (ETO) was performed to remove all cement if necessary. All prostheses were sent for sonication, and at least five cultures were obtained. Postoperatively, after both the first and second-stage surgery, patients were discharged home or to an extended care facility, depending on their mobility and care needs. If necessary, patients could receive intravenous antibiotic treatment both at home and in the extended care facility. The second-stage surgery was generally performed after 6-8 weeks.
General patient characteristics, surgical and treatment details, complications, and infection status were collected from the patient records (Table 1). The primary outcome was successful infection eradication, defined as the absence of clinical suspicion of infection, no requirement for an additional (two-stage) revision due to infection, and no PJI-related death at one year postoperative. Secondary outcomes included surgical time, blood loss, length of hospital stay, postoperative weightbearing allowance, complications, and discharge destination.
| Variable | Group A (n = 39) | Group B (n = 59) | P value |
| Age, mean (SD) | 66 (11.5) | 64 (14.6) | 0.41 |
| Gender male | 25 (64) | 28 (47) | 0.15 |
| BMI, mean (SD) | 28 (5.4) | 29 (6.3) | 0.32 |
| Diabetes | 4 (10) | 9 (15) | 0.56 |
| ASA score | 0.53 | ||
| 1 | 0 (0) | 2 (3) | |
| 2 | 22 (56) | 34 (58) | |
| 3 | 17 (44) | 22 (37) | |
| 4 | 0 (0) | 1 (2) | |
| Infected material | 0.45 | ||
| Primary prosthesis | 22 (58) | 27 (49) | |
| Revision prosthesis | 12 (32) | 17 (30) | |
| Internal fixation | 4 (11) | 7 (13) | |
| Septic arthritis | 0 (0) | 4 (7) | |
| Post-traumatic indication | 10 (56) | 15 (42) | 0.39 |
| Infection duration, days (SD) | 468 (498.3) | 411 (407.9) | 0.57 |
| Earlier infection related surgery | 19 (49) | 26 (48) | 1.00 |
| Earlier two-stage revision | 4 (11) | 7 (14) | 0.75 |
| Mean months of follow-up (SD) | 32 (22.0) | 19 (10.3) | < 0.001 |
Descriptive statistics are reported as number (percentage) or mean (SD), as appropriate. The student’s t-test was used to compare numerical variables, while Fisher's exact or χ2 test was applied for categorical variables. Statistical significance was set at P < 0.05. Statistical analysis was performed using IBM SPSS Statistics (version 28.0.1.0).
From 2017 to 2023, a total of 98 patients underwent a two-stage revision of the hip: 39 patients in Group A received a Girdlestone, while 35 and 24 patients in Group B received a CUMARS or a Girdlestone, respectively. The groups were comparable at baseline in terms of general patient and infection characteristics (Table 1). The mean age was 65 years (SD 13.4), 54% were male, and the mean follow-up duration was 24 months (SD 16.9). The distribution of the causative pathogens is detailed in Table 2.
| Pathogen | Group A (n = 39) | Group B (n = 59) |
| Polymicrobial | 8 (21) | 7 (12) |
| S. Aureus | 9 (23) | 12 (20) |
| S. epidermidis | 4 (10) | 11 (19) |
| Other CoNS | 5 (13) | 9 (15) |
| Cutibacterium acnes | 0 (0) | 5 (6) |
| Streptococci | 5 (13) | 3 (5) |
| E. Coli | 1 (3) | 3 (5) |
| Enterococci | 3 (8) | 2 (3) |
| P. Aeruginosa | 1 (3) | 1 (2) |
| Other | 2 (5) | 1 (2) |
| Culture negative | 1 (3) | 5 (9) |
Successful infection eradication at one year of follow-up was achieved in 69% and 84% of patients in Group A and Group B, respectively (P = 0.16). In Group A, there were six (21%) cases of persistent infection and three (10%) cases of reinfection with a different pathogen. In Group B, four (8%) cases of persistent infection and four (8%) cases of reinfection caused by a different pathogen.
Treatment details per group are provided in Table 3. The mean operative time for the first stage was significantly shorter in Group A. The mean operative time for the second-stage procedure was comparable between the groups. Patients in Group B were permitted significantly greater weight-bearing during the interim period, had a shorter duration of hospital stay, and were more frequently discharged home following the first-stage surgery. These differences were no longer observed following the second-stage surgery.
| Variables | Group A | Group B | P value |
| First-stage surgery | n = 39 | n = 59 | |
| ETO | 12 (35) | 19 (41) | 0.38 |
| Gentamicin beads | 6 (15) | 0 (0) | 0.07 |
| Mean surgical time, min (SD) | 149 (49.4) | 179 (60.0) | 0.02 |
| Mean blood loss, mL (SD) | 1128 (581.6) | 984 (531.5) | 0.32 |
| Interval weightbearing allowance1 | < 0.001 | ||
| 0% | 32 (82) | 21 (36) | |
| 10% | 7 (18) | 29 (49) | |
| 50% | 0 (0) | 9 (15) | |
| Mean length of hospital stay, days (SD) | 16 (8.3) | 9 (6.5) | < 0.001 |
| Discharge destination | 0.048 | ||
| Home | 9 (26) | 28 (48) | |
| Extended care facility | 26 (74) | 30 (52) | |
| Second-stage surgery | n = 35 | n = 58 | |
| Mean interval duration, days (SD) | 213 (266.3) | 110 (113.9) | 0.01 |
| Mean surgical time, min (SD) | 157 (40.1) | 165 (54.7) | 0.51 |
| Mean blood loss, mL (SD) | 1042 (710.1) | 886 (586.0) | 0.36 |
| Postoperative weightbearing allowance1 | 0.71 | ||
| 0% | 0 (0) | 2 (4) | |
| 10% | 3 (9) | 5 (9) | |
| 50% | 14 (40) | 23 (41) | |
| 100 | 18 (51) | 26 (46) | |
| Mean length of hospital stay, days (SD) | 10 (10.9) | 8 (5.6) | 0.16 |
| Discharge destination | 0.83 | ||
| Home | 20 (61) | 31 (57) | |
| Extended care facility | 13 (39) | 23 (43) |
In Group A, reimplantation was not performed in four patients, compared to one patient in Group B (P = 0.08). In Group A, two patients declined further surgery and two had died of PJI-related causes during the interval. The patient in Group B, who received a Girdlestone, was deemed medically unfit for reimplantation and was subsequently treated with suppressive antibiotic therapy.
Complications are listed in Table 4. No significant differences were observed between the groups for any complication, except for total PJI-related mortality. In Group A, five patients died, whereas no deaths occurred in Group B (P = 0.01). Two patients died during the interval, and three patients died after reimplantation. Three patients died from sepsis, one from complications of intraoperative bleeding, and one from hospital-acquired pneumonia. A total of 55 reoperations were performed in 28 patients, with no significant differences observed between the groups (Table 5).
| Variables | Group A | Group B | P value |
| During interval | n = 39 | n = 59 | |
| Any complication | 20 (51) | 31 (53) | 1.00 |
| Anemia | 13 (33) | 21 (36) | 1.00 |
| AKI | 4 (10) | 4 (7) | 0.71 |
| Delirium | 3 (8) | 1 (2) | 0.30 |
| Decubitus | 1 (3) | 3 (5) | 1.00 |
| Pneumonia | 1 (3) | 0 (0) | 0.40 |
| PJI related death | 2 (5) | 0 (0) | 0.16 |
| Cup loosening | 0 (0) | 1 (2) | 1.00 |
| Spacer dislocation | 0 (0) | 1 (2) | 1.00 |
| After second-stage surgery1 | n = 35 | n = 58 | |
| Any complication | 17 (49) | 34 (59) | 0.39 |
| Anemia | 9 (26) | 17 (29) | 0.81 |
| AKI | 1 (3) | 5 (9) | 0.40 |
| Delirium | 1 (3) | 1 (2) | 1.00 |
| Decubitus | 0 (0) | 0 (0) | |
| Pneumonia | 1 (3) | 1 (2) | 1.00 |
| PJI related death | 3 (9) | 0 (0) | 0.05 |
| Dislocation | 3 (8) | 6 (10) | 1.00 |
| Mean dislocations per patient (SD) | 3.0 (1.0) | 2.6 (1.6) | 0.67 |
| Aseptic loosening | 0 (0) | 2 (3) | 0.53 |
| Variable | Group A (n = 39) | Group B (n = 59) | P value |
| Any reoperation | 8 (21) | 20 (34) | 0.27 |
| Mean surgeries per patient (SD) | 2.25 (1.5) | 1.85 (1.2) | 0.47 |
| During interval | |||
| Removal of spacer due to infection | 0 (0) | 1 (2) | 1.00 |
| Debridement | 1 (3) | 0 (0) | 0.40 |
| After second-stage surgery | |||
| DAIR | 5 (14) | 16 (27) | 0.20 |
| Two-stage revision | 4 (11) | 5 (9) | 0.72 |
| Revision (non-infectious reasons) | 2 (6) | 3 (5) | 1.00 |
| Plate fixation | 1 (3) | 1 (2) | 1.00 |
When dividing Group B based on the type of treatment received, patients with a Girdlestone were significantly younger [mean age 59 (SD 14.2) vs 67 (SD 14.2) years, P = 0.049]. No other significant differences were observed at baseline. Infection eradication rates at one year were 86% for CUMARS and 82% for Girdlestones (P = 1.00). CUMARS patients had three persistent infections and one reinfection with a new pathogen, while Girdlestone patients had one and three, respectively. Patients with a CUMARS had a significantly shorter second-stage operative time, with a mean duration of 143 (SD 64.5) minutes compared to 196 (SD 33.6) for those treated with a Girdlestone (P < 0.001). Patients with CUMARS were more frequently discharged home: 59% vs 33% after the first-stage surgery (P = 0.07) and 65% vs 45% after the second-stage surgery (P = 0.25) for CUMARS and Girdlestones, respectively.
No statistically significant differences in complications were observed. Following the first-stage surgery, 18 (51%) patients with CUMARS and 13 (54%) patients with a Girdlestone experienced any complication (P = 1.00). After the second-stage surgery, this was the case for 17 (49%) and 17 (74%) patients with CUMARS and Girdlestone, respectively (P = 0.06). Anemia occurred in 20% of CUMARS and 43% of Girdlestone patients following the second-stage surgery (P = 0.08). Dislocations after reimplantation occurred in two (6%) patients with CUMARS and five (22%) patients with a Girdlestone (P = 0.10). Nine (26%) patients with CUMARS needed additional surgery after reimplantation compared to 11 (46%) Girdlestone patients (P = 0.16).
This study aimed to evaluate two treatment protocols for two-stage revision arthroplasty of the hip by comparing two consecutive patient cohorts: The first received a Girdlestone interval with an antibiotic holiday, while the second cohort was treated with a CUMARS and no antibiotic holiday. Successful eradication of infection after one year was not statistically significantly different between the groups [69% and 84% of patients in groups A and B, respectively (P = 0.16)]. Interpretation of these results is complicated, as it is difficult to determine which portion of the outcomes can be attributed to the use of CUMARS, the application of antibiotic-loaded cement, or the presence or absence of an antibiotic holiday. Direct comparison of these results with other studies is challenging due to heterogeneity in study designs, treatment protocols, spacer types, and the limited availability of comparable studies. Nevertheless, similar to the findings of the present study, most studies report a trend toward a higher infection eradication rate associated with the use of spacers, without reaching statistical significance[3,11]. With reported infection eradication rates ranging from 67% to 97%, our results are in concordance with current literature[2,3,11,26]. A study by Cabrita et al[1] is the only study to date to report a statistically significant difference, demonstrating fewer reinfections with the use of spacers. This could be caused by the absence of local antibiotics or the hematoma-filled dead space resulting from prosthesis removal, which is more susceptible to infection.
By introduction of the new protocol in Group B, the mean length of hospital stay was nearly halved, patients were allowed to bear more weight, and more patients were discharged home following the first-stage surgery. These findings are consistent with other literature and can probably mostly be attributed to the CUMARS[1-3]. Contrary to the study by Cabrita et al[1], in our findings these differences were no longer present after the second-stage surgery. This protocol reduces the overall burden on patients due to the improved mobility and a quicker return home during the interval period. Although treatment costs were not analyzed in this study, the shorter hospital stay and reduced need for extended care facility admission suggest that overall costs are likely to be lower in Group B.
Unfortunately, aside from the orthopedic surgeon’s weight-bearing recommendation, functional outcomes could not be assessed in this study due to a lack of available data. However, comparable studies report superior Harris Hip Score following two-stage revisions with an interval spacer compared to a Girdlestone[3,11]. Additionally, improved interim function has been described with the use of CUMARS compared to molded spacers[13,14]. We believe the shorter hospital stay and increased ability for our patients with a CUMARS to return home during the interval, are an expression of improved mobility compared to the Girdlestone patients.
Logically, the duration of surgery for the first-stage procedure was longer in group B than in group A, which is caused by the time it takes to fabricate and implant the CUMARS. No difference in surgical time was observed during the second-stage surgery when comparing the two consecutive cohorts. However, a significant difference was present when comparing second-stage surgery times of patients with Girdlestones to those with CUMARS, in favor of the latter. This finding aligns with existing literature and is likely due to the preservation of tissue tension and joint movement by the spacer during the interim period, making surgical planes and bony landmarks easier to identify, and allowing for simpler reimplantation procedure[1-3,11].
We expected to observe more complications such as delirium, decubitus, and pneumonia in Group A due to reduced mobility; however, this was not the case. No significant differences were found between the groups regarding any individual complication or the total number of complications, except for PJI-related mortality. Most existing literature similarly reports no significant differences in complications, except for dislocations after reimplantation, which contrasts with our findings[2,3,11]. This discrepancy may be explained by our comparison of two consecutive cohorts. Within Group B, when comparing Girdlestone with CUMARS, there was no significant difference in the number of dislocations (P = 0.10), however this may be caused by the limited number of patients to compare within this group. There were significantly more deaths in Group A compared to Group B (5 vs 0, P = 0.01). This suggests that a protocol involving CUMARS and continuous antibiotic therapy may be associated with improved survival. Cabrita et al[1] is the only comparable study reporting on PJI-related mortality and also observed fewer deaths with the use of spacers, although this did not reach statistical significance. Larger studies are therefore necessary to confirm these findings.
There were two mechanical complications among 35 patients with a CUMARS related to spacer use (6%): One dislocation and one loosening of the cup. Peri-spacer fracture was not observed. This complication rate is considerably lower than the reported average of 16.3% for mechanical complications of hip spacers[12]. Jones et al[16] found that dislocation is associated with a reduced offset, whereas peri-spacer fractures are linked to an increased offset. Furthermore, they demonstrated that CUMARS are the most effective type of spacer for restoring offset compared to other spacer designs[16]. CUMARS offer the same range of options as standard total hip prostheses, whereas molded or prefabricated spacers are only available in a limited range of sizes. Additionally, acetabular complications such as pelvic protrusion were also absent in this study. Burastero et al[17] demonstrated that spacers without an acetabular component cause significantly more acetabular bone loss. Increased acetabular bone loss leads to a greater need for complex revision hardware[27].
This study has several limitations, including a relatively small sample size and its retrospective design, which is prone to missing data and may introduce selection, inclusion, and information biases. A small sample size can lead to a type II error, and results should therefore be interpreted with caution. We have made efforts to minimize the risk of selection and inclusion bias-a common issue in retrospective cohort studies, as not all patients are suitable candidates for spacers - by not selecting groups based on the type of treatment, but instead using two consecutive cohorts including all treated patients during the specific timeframe. However, some heterogeneity between the two groups was still present as the follow-up period for Group B was shorter. Immortal time bias was limited by choosing a determined endpoint of follow-up at one year for the primary outcome. However, this period is relatively short, a more detailed analysis could be conducted after five years of follow-up. Furthermore, as previously mentioned, the lack of available objective data on the functional outcomes of both groups means these results should be interpreted with caution.
To our knowledge, this is the first study to directly compare the outcomes of patients treated with consecutive treatment protocols for two-stage revision of the hip including the introduction of CUMARS. The results of our study confirm swifter recovery during the treatment interval when using CUMARS for two-stage revision arthroplasty of the infected hip.
Although it was not the objective of this study, CUMARS offers the advantage of potentially delaying or even avoiding reimplantation[13,28]. In this study, the longest spacer interval was 114 days, and no patient retained their spacer indefinitely. However, this can potentially be a significant benefit for elderly patients with multiple comorbidities and low functional demands. In a study by Wang et al[13], as many as 58% of patients opted to retain their spacer while maintaining a comparable infection eradication rate to the classic two-stage revision group.
A protocol including CUMARS and no antibiotic holiday is a safe and effective treatment option for suitable patients, as it enables faster recovery during the interval period, while maintaining an adequate infection eradication rate and without increasing mechanical complications. Additionally, CUMARS seem to reduce the strain on the healthcare system by shortening the average hospital stay for the entire cohort and enabling more patients to be discharged home, thereby potentially leading to significant cost savings. We believe a CUMARS should be the preferred type of interval spacer over a Girdlestone or other types of spacers in any patient treated with two-stage revision surgery for PJI of the hip. For patients with severe bone loss where a CUMARS is deemed contraindicated, a Girdlestone remains a viable option. The optimal revision strategy for infection eradication remains a subject for further investigation. Whether the indications for a CUMARS can be stretched in unfavorable situations (for instance in case of massive bone loss at the femoral and/or acetabular side, or in case of necessity of a long ETO for cement removal) and if frail patients could benefit from retaining a CUMARS should be further evaluated.
| 1. | Cabrita HB, Croci AT, Camargo OP, Lima AL. Prospective study of the treatment of infected hip arthroplasties with or without the use of an antibiotic-loaded cement spacer. Clinics (Sao Paulo). 2007;62:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Hsieh PH, Chang YH, Chen SH, Shih CH. Staged arthroplasty as salvage procedure for deep hip infection following intertrochanteric fracture. Int Orthop. 2006;30:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Fiore M, Rondinella C, Paolucci A, Morante L, De Paolis M, Sambri A. Functional Outcome after Reimplantation in Patients Treated with and without an Antibiotic-Loaded Cement Spacers for Hip Prosthetic Joint Infections. Hip Pelvis. 2023;35:32-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Abdel MP, Barreira P, Battenberg A, Berry DJ, Blevins K, Font-Vizcarra L, Frommelt L, Goswami K, Greiner J, Janz V, Kendoff DO, Limberg AK, Manrique J, Moretti B, Murylev V, O'Byrne J, Petrie MJ, Porteous A, Saleri S, Sandiford NA, Sharma V, Shubnyakov I, Sporer S, Squire MW, Stockley I, Tibbo ME, Turgeon T, Varshneya A, Wellman S, Zahar A. Hip and Knee Section, Treatment, Two-Stage Exchange Spacer-Related: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34:S427-S438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Craig A, King SW, van Duren BH, Veysi VT, Jain S, Palan J. Articular spacers in two-stage revision arthroplasty for prosthetic joint infection of the hip and the knee. EFORT Open Rev. 2022;7:137-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Sousa R, Carvalho A, Soares D, Abreu MA. Interval between two-stage exchanges: what is optimal and how do you know? Arthroplasty. 2023;5:33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Tsung JD, Rohrsheim JA, Whitehouse SL, Wilson MJ, Howell JR. Management of periprosthetic joint infection after total hip arthroplasty using a custom made articulating spacer (CUMARS); the Exeter experience. J Arthroplasty. 2014;29:1813-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Charlton WP, Hozack WJ, Teloken MA, Rao R, Bissett GA. Complications associated with reimplantation after girdlestone arthroplasty. Clin Orthop Relat Res. 2003;119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Fink B, Vogt S, Reinsch M, Büchner H. Sufficient release of antibiotic by a spacer 6 weeks after implantation in two-stage revision of infected hip prostheses. Clin Orthop Relat Res. 2011;469:3141-3147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Anagnostakos K, Meyer C. Antibiotic Elution from Hip and Knee Acrylic Bone Cement Spacers: A Systematic Review. Biomed Res Int. 2017;2017:4657874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Khanna A, Carter B, Gill I. Two-Stage Revision Hip Arthroplasty with or without the Use of an Interim Spacer for Managing Late Prosthetic Infection: A Systematic Review of the Literature. Orthop Surg. 2021;13:384-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Sambri A, Fiore M, Rondinella C, Morante L, Paolucci A, Giannini C, Alfonso C, De Paolis M. Mechanical complications of hip spacers: a systematic review of the literature. Arch Orthop Trauma Surg. 2023;143:2341-2353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Wang B, Li M, Wang J, Han P, Wang Q, Shen H. Use of 1.5-Stage Functional Articulating Hip Spacers for Two-Stage Treatment of Hip Infection. J Arthroplasty. 2024;39:2591-2599.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 14. | Zhang W, Fang X, Shi T, Cai Y, Huang Z, Zhang C, Lin J, Li W. Cemented prosthesis as spacer for two-stage revision of infected hip prostheses: a similar infection remission rate and a lower complication rate. Bone Joint Res. 2020;9:484-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Kugelman D, Roof M, Egol A, Guanche I, Chen AF, Schwarzkopf R, Aggarwal VK. Comparing Articulating Spacers for Periprosthetic Joint Infection After Primary Total Hip Arthroplasty: All-Cement Versus Real-Component Articulating Spacers. J Arthroplasty. 2022;37:S657-S663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Jones CW, Selemon N, Nocon A, Bostrom M, Westrich G, Sculco PK. The Influence of Spacer Design on the Rate of Complications in Two-Stage Revision Hip Arthroplasty. J Arthroplasty. 2019;34:1201-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Burastero G, Basso M, Carrega G, Cavagnaro L, Chiarlone F, Salomone C, Papa G, Felli L. Acetabular spacers in 2-stage hip revision: is it worth it? A single-centre retrospective study. Hip Int. 2017;27:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Veltman ES, Moojen DJ, Glehr M, Poolman RW. Similar rate of infection eradication for functional articulating, prefabricated and custom-made spacers in 2-stage revision of the infected total hip: a literature review. Hip Int. 2016;26:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Ascione T, Balato G, Mariconda M, Rotondo R, Baldini A, Pagliano P. Continuous Antibiotic Therapy Can Reduce Recurrence of Prosthetic Joint Infection in Patients Undergoing 2-Stage Exchange. J Arthroplasty. 2019;34:704-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Li C, Renz N, Trampuz A, Ojeda-Thies C. Twenty common errors in the diagnosis and treatment of periprosthetic joint infection. Int Orthop. 2020;44:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 21. | Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection: current concepts and outlook. EFORT Open Rev. 2019;4:482-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 436] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 22. | Bejon P, Berendt A, Atkins BL, Green N, Parry H, Masters S, McLardy-Smith P, Gundle R, Byren I. Two-stage revision for prosthetic joint infection: predictors of outcome and the role of reimplantation microbiology. J Antimicrob Chemother. 2010;65:569-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Chang CH, Tsai SW, Hsu KH, Chang MC, Chen WM, Su YP. The efficacy of a drug holiday test on two-stage revision for infected total knee arthroplasty. J Chin Med Assoc. 2019;82:500-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Fayaz H, Higuera CA, Shubnyakov I. Question 4: What is the importance of two-week antibiotic holiday prior to reimplantation? In: Proceedings of the International Consensus Meeting on Musculoskeletal Infection. Hip Knee Section. 2018 [cited 5 May 2025]. Available from: https://www.icmortho.org/_files/ugd/0540e9_f047ffe9ecc543279bba6b84688bc27e.pdf. |
| 25. | Vincenten CM, Den Oudsten BL, Bos PK, Bolder SBT, Gosens T. Quality of life and health status after Girdlestone resection arthroplasty in patients with an infected total hip prosthesis. J Bone Jt Infect. 2019;4:10-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Marczak D, Synder M, Sibiński M, Polguj M, Dudka J, Kowalczewski J. Two stage revision hip arthroplasty in periprosthetic joint infection. Comparison study: with or without the use of a spacer. Int Orthop. 2017;41:2253-2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Grosso MJ, Kozaily E, Cacciola G, Parvizi J. Characterizing Femoral and Acetabular Bone Loss in Two-Stage Revision Total Hip Arthroplasty for Infection. J Arthroplasty. 2021;36:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Tseng J, Oladipo VA, Acuña AJ, Jones CM, Tsintolas J, Levine BR. Evaluating Modern Spacer Options and Outcomes in Revision Hip Arthroplasty. J Arthroplasty. 2024;39:S236-S242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/