Published online Oct 18, 2025. doi: 10.5312/wjo.v16.i10.110009
Revised: June 10, 2025
Accepted: September 8, 2025
Published online: October 18, 2025
Processing time: 142 Days and 0.4 Hours
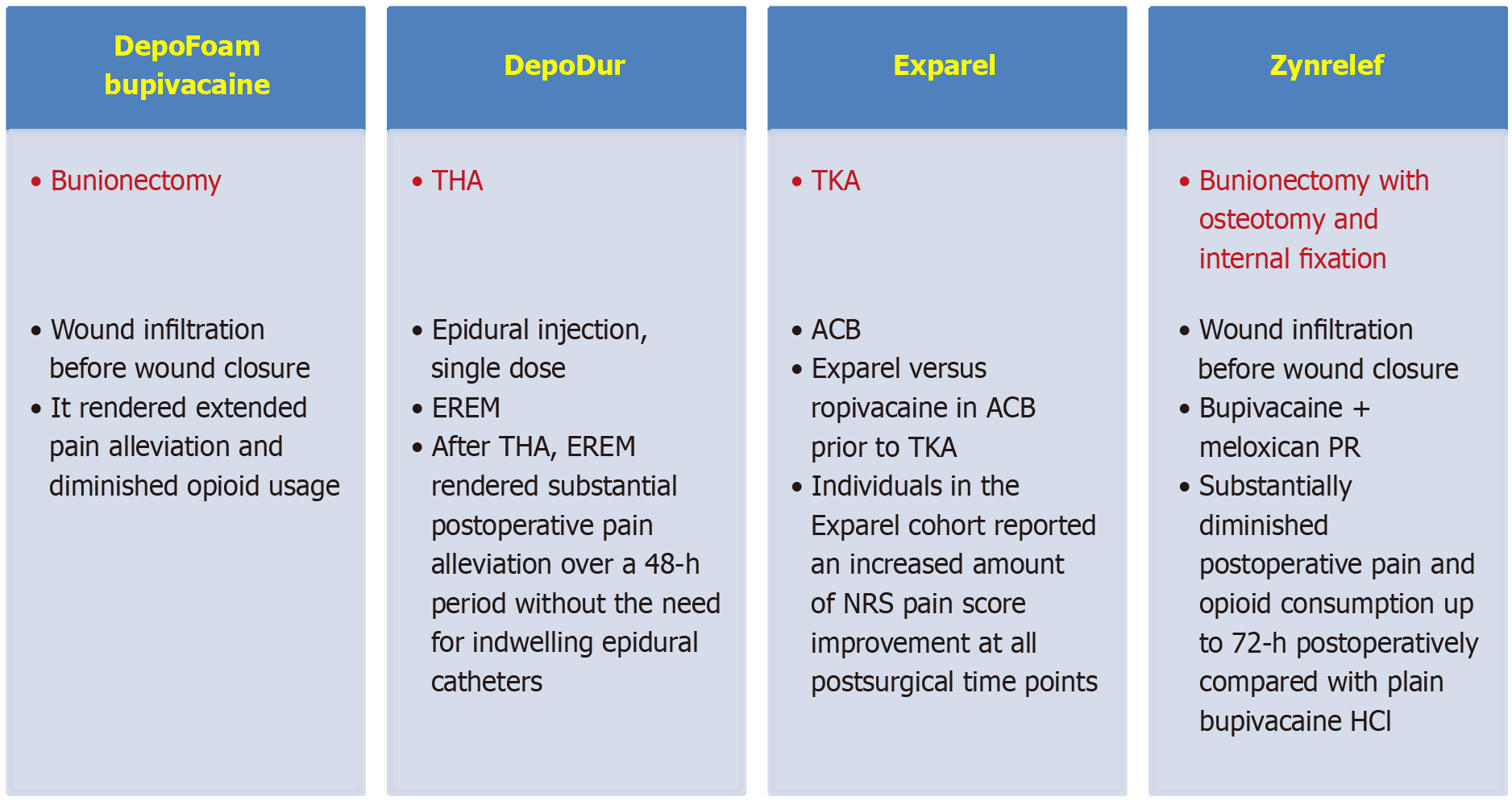
This narrative review evaluated the potential of nanotechnology platforms (DepoFoam bupivacaine®, DepoDur®, Exparel®, Zynrelef®, NeuroCuple™) in the control of postoperative pain following orthopedic procedures. In individuals experiencing bunionectomy DepoFoam bupivacaine® 120 mg and placebo via wound infiltration before wound closure was compared. The area under the curve for numeric rating scale scores was substantially less in individuals treated with DepoFoam bupivacaine® vs individuals receiving placebo at 24 h and 36 h. In individuals undergoing total hip arthroplasty, a single dose of 15 mg, 20 mg, or 25 mg DepoDur® [extended-release epidural morphine (EREM)] and placebo were compared. All EREM dosages diminished the mean fentanyl usage vs placebo and delayed the median time to first dose of fentanyl. All EREM cohorts had substantially improved pain control at rest for up to 48 h post-surgery compared with placebo. No supplementary analgesia was required in 25% of individuals treated with EREM and 2% of individuals treated with placebo at 48 h. In individuals undergoing total knee arthroplasty, iPACK (local anesthetic infiltration of the interspace between the popliteal artery and the posterior knee capsule) adductor canal block was compared to adductor canal block with Exparel®. Individuals in the Exparel® cohort experienced an improvement in numeric rating scale pain scores at all postsurgical time points. These individuals also utilized a lower dose of inpatient opioids. In individuals undergoing bunionectomy a single intraoperative dose of Zynrelef® prolonged release (PR) was compared to bupivacaine HCl 0.5% via wound infiltration before wound closure. Zynrelef® PR diminished pain intensity by 18% compared with bupivacaine HCl. Opioid consumption was reduced by 37% in the Zynrelef® PR cohort vs 25% in the bupivacaine HCl cohort. In individuals experiencing total knee arthroplasty and total hip arthroplasty, the use of a nanotechnology-based NeuroCupleTM device diminished postoperative pain at rest by 34% and reduced pain with movement by 18%.
Core Tip: Nanotechnology offers encouraging alternatives for the management of pain in orthopedic surgery either as drug transporters or as capacitor devices/patches. As drug transporter-based formulations for morphine, bupivacaine, and lidocaine, nanotechnology permits lengthening the duration of these drugs and increasing the effective analgesic dose, consequently diminishing potential drug toxicity. The nanocapacitor-based technology, the NeuroCupleTM device, possibly represents an efficacious non-pharmacology method.
- Citation: Rodriguez-Merchan EC, De Andres-Ares J, Delgado-Martinez AD, Ribbans WJ. Nanotechnology for pain management in orthopedic surgery. World J Orthop 2025; 16(10): 110009
- URL: https://www.wjgnet.com/2218-5836/full/v16/i10/110009.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i10.110009
The main current strategies for treating postoperative pain in orthopedic surgery are the enhanced recovery after surgery (ERAS) protocols[1]. ERAS protocols include four fundamental components: (1) Optimization of preoperative care; (2) Reduction of physical stress of surgery; (3) Improvement of postoperative comfort; and (4) Optimization of postoperative care[2]. ERAS protocols use opioids in a manner considered appropriate in terms of timing and dosage together with regional nerve blocks and non-opioid medications (nonsteroidal anti-inflammatory drugs and acetaminophen). However, there are several gaps regarding the efficacy and safety of ERAS protocols that are not yet sufficiently defined, such as optimal drug combinations, dosing regimens, and patient-specific factors. The purpose of ERAS protocols is to find a balance between patient safety and postoperative pain control.
Some publications indicate that nanotechnology could address the aforementioned gaps. The rationale for undertaking this review was to analyze publications on nanotechnology in the treatment of postoperative pain in orthopedic surgery in order to determine whether nanotechnology could contribute positively to ERAS protocols[1]. Advances in nanotechnology and quantum technology have resulted in an increasingly diverse number of medical applications[3]. Nanome
In vivo applications have established a basis for the employment of nanotransporters, such as liposomes, nanofibers, nanoparticles, nanoscaffolds, micelles, and quantum dots[3]. These forms of nanotransporters have permitted the creation of medicinal formulas with little drug toxicity, extended drug efficacy, and, in some instances a more targeted form of release[3,5]. Nanocapacitor devices/patches have been created due to advances in nanotechnology. The use of nanocapacitor devices/patches has been shown to be somewhat effective in the treatment of acute and chronic musculoskeletal pain[3,6].
Nanomaterials already approved by the Food and Drug Administration (FDA) cover liposomes, poly lactic-co-glycolic acid, and other carbon-based polymer nanomaterials[7,8]. This review evaluated recent clinical trials and FDA-approved nanotechnology platforms for postoperative pain control in orthopedic procedures. For this purpose a search was performed in PubMed, EMBASE, and the Cochrane Library on May 30, 2025 using “nanotechnology musculoskeletal pain” and “nanocarriers postoperative analgesia” as keywords. The bibliographic search was conducted using search engines and including only articles published in English. One hundred and twenty-eight articles were found. In our opinion only 27 of them were related to the title of this article. Additionally, only five studies were considered to be strictly focused on nanotechnology for pain management in orthopedic surgery[9-13].
The employment of transporters based on nanotechnology for pain management has concentrated on liposomal transporters. The objectives of these medications were to prolong the time span of pain control and diminish their toxic effects[6].
DepoFoam bupivacaine® is a liposome-based formulation with multiple benefits (Table 1)[6].
| Benefits |
| High drug-loading capacity combined with controlled release, resulting in enhanced analgesic efficacy |
| Uniform and lengthy medication liberation |
| Increased encapsulation yield |
| Structural stability |
| Inferior exposure and toxicity in another location |
| Negligible negative complications |
| Greater medication half-life |
| Biocompatibility for medication liberation into frail zones (e.g. epidural, intrathecal) |
In patients experiencing bunionectomy Golf et al[9] compared DepoFoam bupivacaine® (Pacira Pharmaceuticals, Inc., San Diego, CA, United States), a slow liberation liposomal bupivacaine-based pain killer, with placebo for postoperative pain decline after the surgical procedure. Individuals were treated with placebo (n = 96) or DepoFoam bupivacaine® 120 mg (n = 97) by infiltrating the wound before closing it[9]. The degree of pain intensity was evaluated utilizing a numeric rating scale (NRS) from time 0 to 3 days after surgery. The principal effectiveness parameter was estimated by calculating the area under the curve (AUC) of NRS scores up to 1 day. Other effectiveness parameters were the AUC of NRS at other times, the percentage of individuals who were pain-free, the time of first opioid utilization, and the use of supplemental opioids during the postoperative period.
Complications were evaluated[9]. At 1 day and 36 h the AUC for NRS scores was substantially inferior in individuals managed with DepoFoam bupivacaine® than in individuals managed with placebo. More individuals treated with DepoFoam bupivacaine® averted the utilization of rescue opioids during the first day. They were pain-free at 2 h, 4 h, 8 h, and 2 days. The average time to first utilization of opioids was 7.2 h in the DepoFoam bupivacaine® group vs 4.3 h in the placebo group (P < 0.0001).
Patients treated with DepoFoam bupivacaine® (59.8%) had fewer complications than those treated with placebo (67.7%). In comparison with placebo the duration of pain alleviation in the DepoFoam bupivacaine® group was superior. DepoFoam bupivacaine® diminished opioid usage after bunionectomy more than placebo[9].
DepoFoam mixed with morphine [DepoDur® (Endo Pharmaceuticals Inc., Chadds Ford, PA, United States; Skye Pharma, Inc., San Diego, CA, United States)] has been used for epidural administration[6].
Viscusi et al[10] assessed the effectiveness of extended-release epidural morphine (EREM) (DepoDur®) in pain al
All doses of EREM reduced the mean use of fentanyl more than placebo use (510 µg vs 2091 µg) and postponed the mean time to first dose of fentanyl (21.3 h vs 3.6 h). All EREM cohorts had substantially improved pain control at rest for up to 48 h post-surgery compared with placebo. More individuals in the EREM group considered their pain control to be good or very good compared with the placebo group (at 1 day: 90% vs 65%; at 2 days: 83% vs 67%). At 2 days after surgery in the EREM group, 25% of patients did not require supplemental analgesics while in the placebo group that percentage was only 2%. The safety of EREM was similar to that of other epidurally administered opioids. It appeared that EREM rendered substantial postoperative pain alleviation over a 2-day period following THA without the need to use epidural catheters[10].
Additionally, DepoFoam has been utilized to create a liposome-bupivacaine formulation for local injection (Exparel®, Pacira Pharmaceutical Inc., Parsippany-Troy Hill, NJ, United States)[11]. Even though this advancement permitted consistent liberation of the drug over a lengthy period, some publications have not shown that it relieves postoperative pain better than ordinary bupivacaine[9]. Exparel® has been validated by the FDA as a local analgesic for postoperative pain control and for interscalene nerve block and adductor canal and sciatic nerve blocks[11].
In 2022 Malige et al[11] compared the utilization of Exparel® vs ropivacaine in adductor canal block (ACB) prior to unilateral total knee arthroplasty (TKA) in 100 individuals. In a prospective, double-blinded randomized controlled trial each individual received an iPACK (local anesthetic infiltration in the interspace between the popliteal artery and the posterior capsule of the knee) block using ropivacaine. Additionally, each patient was randomized to receive an ACB with Exparel® (n = 50) or ropivacaine (n = 50). The Exparel® cohort had an inferior hospital length of stay (LOS) compared with the control cohort (36.3 h vs 49.7 h). Individuals in the Exparel® cohort experienced an improvement in NRS pain scores at all postsurgical time points. These individuals also used fewer opioids during their hospital stay [40.9 morphine milligram equivalents (MME)/day) vs 47.3 MME/day)] but a similar amount after discharge (33.4 MME/day vs 32.1 MME/day). Additionally, the Exparel® cohort showed better Western Ontario and McMaster Universities Osteoarthritis Index subscores and total scores than the control group at almost all postoperative moments. In conclusion compared with the control group Exparel® peripheral regional nerve blocks were more effective in relieving pain, improving Western Ontario and McMaster Universities Osteoarthritis Index scores, and reducing opioid use during hospitalization and LOS. Exparel® in the form of ACB blocks before TKA appeared to be safe and effective in controlling postoperative pain and decreasing LOS[11].
Zynrelef® (Heron Therapeutics, San Diego, CA, United States) is a liposome preparation of bupivacaine/meloxicam that is applied to the soft tissue incision at the time of wound closure for the treatment of acute pain. Zynrelef® was validated by the FDA in 2021 to be utilized at the time of closure in the area of the surgical incision for TKA and bunionectomy[6]. In individuals who experienced bunionectomy with osteotomy and internal fixation under regional anesthesia, Viscusi et al[12] compared the effectiveness of three groups: (1) Bupivacaine/meloxicam PR; (2) Bupivacaine HCl; and (3) Placebo. Zynrelef® was more effective than bupivacaine HCl in reducing pain until the third postoperative day. In addition opioid consumption was lower with Zynrelef® than with bupivacaine HCl. Safety was similar across the three study groups, but the Zynrelef® group had fewer complications related to opioid use[12].
In 2024 the indications of Zynrelef® were expanded to entail open surgical procedures of the shoulder and spine. Before the instillation of Zynrelef™, it must be reconstituted utilizing a needle-free syringe and a Luer-lock applicator. Contraindications to the use of Zynrelef® are shown in Table 2[6].
| Contraindications |
| Individuals with proven hypersensitivity to local anesthetics and NSAIDs |
| Individuals with a history of asthma, urticaria, or allergic reaction related to aspirin or other NSAIDs |
| Individuals receiving obstetric paracervical block anesthesia |
| Individuals experiencing coronary bypass surgery |
In 2023 Goel et al[14] found that the utilization of Zynrelef® combined with unilateral ACB (20 mL of either bupivacaine 0.325% or ropivacaine 0.5%) was safe when performed before three TKAs under spinal anesthesia. No symptoms suggestive of local anesthetic toxicity were reported. This quality improvement study investigated efficacy and safety of the combination of Zynrelef® and ACB for TKA[14]. Table 3 summarizes the main studies performed on liposome-based formulations (DepoFoam®, DepoDur®, Exparel®, Zynrelef®) in postoperative pain management in orthopedic surgery[9-12].
| Study | Ref. | Number of patients (n), type of study | Treatment vs comparison | Results |
| DepoFoam bupivacaine® (a slow-release liposomal bupivacaine-based analgesic) | ||||
| Bunionectomy | Golf et al[9] | n = 216. Randomized, multicenter, double-blind phase 3 clinical study | Individuals received placebo (n = 96) or DepoFoam bupivacaine® 120 mg (n = 97) via wound infiltration before wound closure | The area under the curve for NRS scores was substantially less in individuals treated with DepoFoam bupivacaine® vs individuals receiving placebo at 24 h and 36 h. More individuals treated with DepoFoam bupivacaine® avoided the use of opioid ‘rescue’ medication during the first 24 h and were pain-free (NRS ≤ 1) at 2 h, 4 h, 8 h, and 48 h |
| DepoDur® (DepoFoam mixed with morphine) | ||||
| THA | Viscusi et al[10] | n = 200. Patients scheduled to undergo THA were randomized to receive a single dose of 15 mg, 20 mg, or 25 mg EREM or placebo | 15 mg, 20 mg, or 25 mg DepoDur® (single dose, epidural injection) vs saline | All EREM dosages diminished the mean fentanyl usage vs placebo (510 µg vs 2091 µg) and delayed the median time to first dose of fentanyl (21.3 h vs 3.6 h). All EREM cohorts had substantially improved pain control at rest for up to 48 h post-surgery compared with placebo. More EREM-treated individuals rated their pain control as good or very good compared with placebo (at 24 h: 90% vs 65%; at 48 h: 83% vs 67%). No supplementary analgesia was required in 25% of EREM-treated individuals and 2% of placebo-treated individuals at 48 h |
| Exparel® (bupivacaine liposome injectable suspension) | ||||
| TKA | Malige et al[11] | n = 100. Prospective, double-blind randomized controlled trial. Each patient received an iPACK block utilizing ropivacaine and was additionally randomized to receive an ACB with Exparel® or ropivacaine | Liposomal bupivacaine 20 mL with 5 mL of 0.5% bupivacaine in ACB and 20 mL of 0.2% ropivacaine in iPACK (local anesthetic infiltration of the interspace between the popliteal artery and the posterior knee capsule) block vs 25 mL of 0.2% ropivacaine in ACB and 20 mL of 0.2% ropivacaine in iPACK block prior to TKA | The Exparel® cohort had a lower hospital length of stay compared to the control cohort (36.3 h vs 49.7 h). Individuals in the Exparel® cohort experienced an improvement in NRS pain score at all postsurgical time points. These individuals also utilized a lower level of inpatient opioids (40.9 MME/day vs 47.3 MME/day) but a similar amount of outpatient opioids (33.4 MME/day vs 32.1 MME/day). Additionally, the Exparel® group showed increased improvements in all Western Ontario and McMaster Universities Osteoarthritis Index sub scores and total scores at most timepoints compared with the control cohort |
| Zynrelef® (liposome preparation of bupivacaine/meloxicam) | ||||
| Bunionectomy | Viscusi et al[12] | n = 412. EPOCH 1 was a randomized, double-blind, placebo-controlled and active-controlled phase III study in subjects undergoing a primary unilateral, distal, first metatarsal bunionectomy in which subjects received either a single intraoperative dose of HTX-011, immediate-release bupivacaine HCl or saline placebo | Zynrelef® PR 60/1.8 mg vs bupivacaine HCl by 0.5%. 50 mg via wound infiltration before wound closure | Zynrelef® PR diminished pain intensity by 18% compared to bupivacaine HCl. Opioid consumption was reduced by 37% in the bupivacaine/meloxicam cohort vs 25% in the bupivacaine HCl cohort |
Figure 1 summarizes the most important information on the use of nanotechnology-based transporters in the treatment of postoperative pain in orthopedic surgery.
A retrospective cohort study analyzed 489 individuals who underwent inpatient (> 23-h) hospitalization for primary anatomic or reverse total shoulder arthroplasty; patients received either liposomal interscalene bupivacaine (LIB) (n = 316) or a non-LIB (n = 173) for perioperative analgesia. Pain intensity at 3 h, 6 h, 12 h, and 48 h after surgery was similar. However, the LIB cohort had better pain scores at 24 h and 36 h after surgery[15].
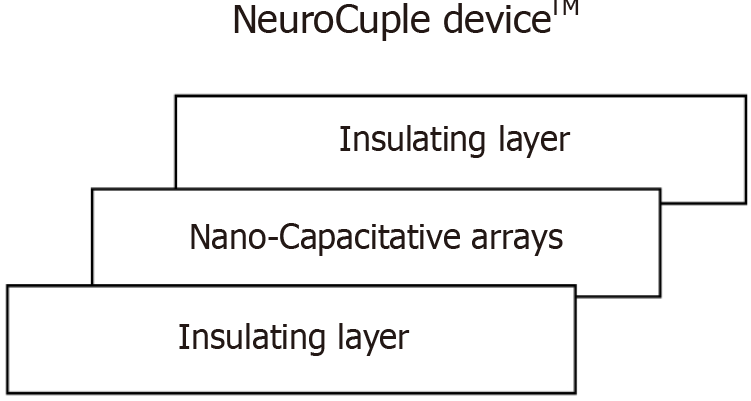
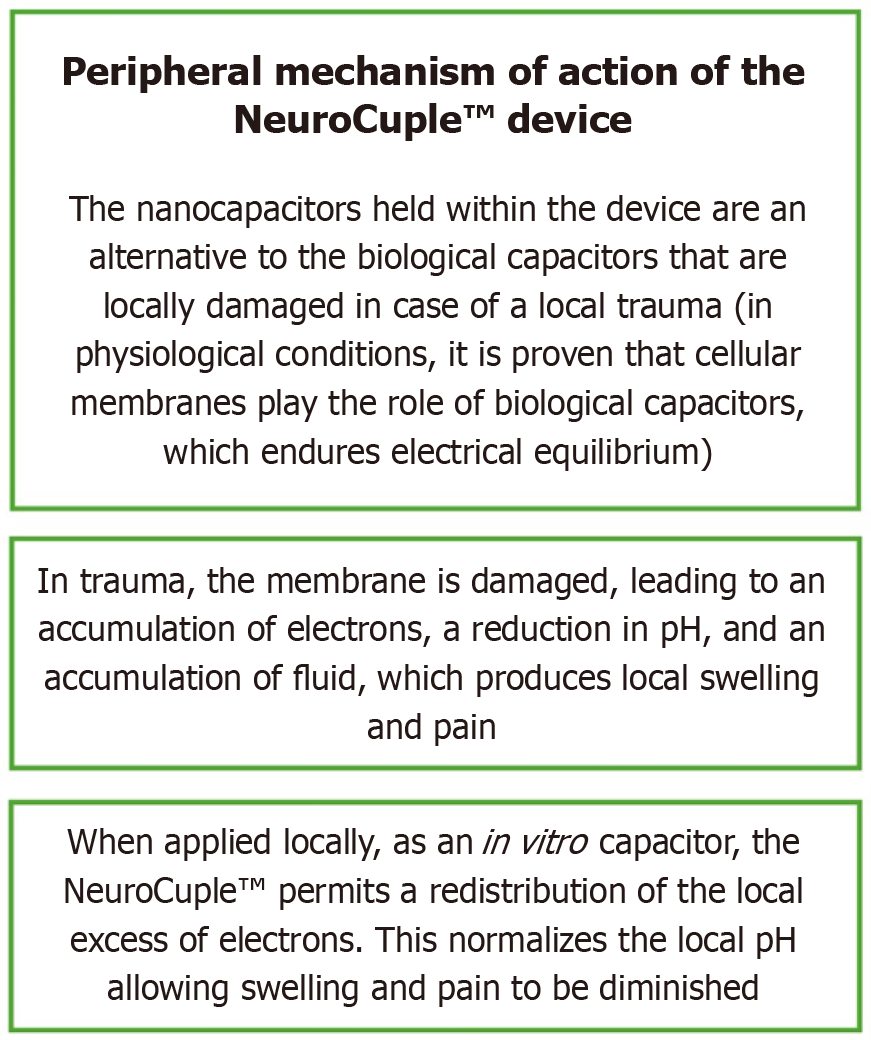
The NeuroCuple™ device/patch (nCap Medical LLC, Heber City, UT, United States) has three areas. They are shown in Figure 2. The local application of NeuroCupleTM normalizes the local pH and diminishes pain[6,16]. The peripheral mechanism of action of the NeuroCuple™ device is shown in Figure 3. The nanotechnology-based device/patch contains no drug or energy source. It can be utilized repeatedly. So far no complications have been published during the utilization of nanotechnology-based devices/patches[6].
In a prospective, randomized study, Chelly et al[13] compared the analgesic efficacy of two NeuroCupleTM devices/patches plus standard of care (study group) vs standard of care (control group) in individuals experiencing TKA and THA. It was found that the application of NeuroCupleTM devices/patches diminished postoperative pain at rest by 34% and reduced pain with movement by 18%. Besides the original NeuroCupleTM devices/patches, two other devices are accessible: (1) One created and distributed by Signal Relief (Salt Lake City, UT, United States); and (2) One by Kailo (Salt Lake City, UT, United States) (Figure 4).
The most commonly employed form of polymeric nanoparticle is poly lactic-co-glycolic acid (PLGA)[16]. It has been used to encapsulate bupivacaine to prolong its effect. It has also been used to attenuate side effects in neuropathic pain combined with lamotrigine[17] and to treat bone pain combined with alendronate and cabazitaxel[18].
Most long-acting injectable products use PLGA polymers[19]. Chitosan nanoparticles have shown better bioavailability, sustained drug liberation, and targeted delivery than standard chitosan formulations, resulting in better therapeutic results in preclinical models of osteoarthritis[20]. In both in vitro and in vivo models of osteoarthritis, Park et al[21] have encountered that polydopamine-coated selenium nanoparticles promote cartilage repair, enhance extracellular matrix synthesis, and diminish knee pain.
In 2014 Pek et al[22] demonstrated in an experimental model of postoperative knee pain in goats that PLGA-loaded bupivacaine in the form of core-shell polymer microspheres released bupivacaine and relieved postoperative pain for 2 weeks. The microspheres consisted of a PLGA core and a poly (l-lactide) shell.
In 2017 Fu et al[23] studied the efficacy of a thermosensitive PLGA-poly(ethylene glycol)-PLGA gel used at the surgical incision site in experimental rat models. Pain relief lasted for more than 2 days, lasting significantly longer than when ropivacaine was used alone.
According to Liang et al[24] local anesthetics such as lidocaine and propivacaine were used to relieve postoperative pain because they block the sodium channels of the cell membranes of neurons. However, local anesthetics have some systemic toxicity in the form of allergic reactions, hypotension, headaches, muscle tremors, convulsions, or paralysis.
There are several scavengers that could relieve both acute and chronic pain. The most important are nucleic acid-binding scavengers and reactive oxygen scavengers (ROS). These ROS include alpha-phenyl N-tertiary-butyl nitrone, 5,5-dimethyl-pyrroline N-oxide, 2,2,6,6-tetramethylpiperidine-N-oxyl, 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl, and vitamin E. Nanoparticles can also be used to scavenge ROS[25]. Holl et al[25] performed in vitro studies (mouse) to evaluate the effect of nucleic acids on pathologic scar-associated fibroblast activity.
Yao et al[26] have published a nanomaterial that promotes wound healing, i.e., it accelerates wound repair thanks to its anti-inflammatory and reactive oxygen species scavenging capabilities. These are chitosan-derived carbon quantum dots.
Lai et al[27] have published that the use of a liposome responsive to ROS appears to be a promising option for the treatment of osteoarthritis. Laboratory tests and animal studies (osteoarthritis in a mouse model induced by anterior cruciate ligament transection) demonstrated that the liposome developed by Lai et al[27] has remarkable anti-inflammatory properties, resulting in substantial improvement in synovial inflammation. In addition it appeared to protect damaged cartilage and reverse changes in the subchondral bone. In other words it slowed the progression of osteoarthritis[27].
Research has been published upon the effectiveness of liposome-based transporters such as DepoFoam bupivacaine®, DepoDur®, Exparel®, and Zynrelef®. All of them have shown postoperative pain alleviation in patients after bunionectomy, THA, and TKA. The NeuroCupleTM device has also been shown to reduce postoperative pain following TKA. However, further research is needed to determine the real efficacy of nanotechnology for the management of pain in orthopedic surgery. Many studies (e.g., those on DepoFoam and NeuroCuple) were industry-funded; therefore, there could be potential conflicts of interest.
| 1. | Grossi P. Enhanced Recovery After Surgery (ERAS) Protocols in Orthopaedic Surgery: Opioids or Not Opioids? J Pain Res. 2025;18:1683-1695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 2. | White JJ, Houghton-Clemmey R, Marval P. Enhanced recovery after surgery (ERAS): an orthopaedic perspective. J Perioper Pract. 2013;23:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Bhansali D, Teng SL, Lee CS, Schmidt BL, Bunnett NW, Leong KW. Nanotechnology for Pain Management: Current and Future Therapeutic Interventions. Nano Today. 2021;39:101223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 4. | Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1332] [Cited by in RCA: 1237] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 5. | Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L, Bernardino L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J Control Release. 2016;235:34-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 1014] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 6. | Chelly JE, Goel SK, Kearns J, Kopac O, Sadhasivam S. Nanotechnology for Pain Management. J Clin Med. 2024;13:2611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 7. | Koudelka S, Turánek J. Liposomal paclitaxel formulations. J Control Release. 2012;163:322-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 278] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 8. | Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel). 2011;3:1377-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2486] [Cited by in RCA: 2973] [Article Influence: 198.2] [Reference Citation Analysis (0)] |
| 9. | Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam® bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther. 2011;28:776-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Viscusi ER, Martin G, Hartrick CT, Singla N, Manvelian G; EREM Study Group. Forty-eight hours of postoperative pain relief after total hip arthroplasty with a novel, extended-release epidural morphine formulation. Anesthesiology. 2005;102:1014-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Malige A, Pellegrino AN, Kunkle K, Konopitski AK, Brogle PJ, Nwachuku CO. Liposomal Bupivacaine in Adductor Canal Blocks Before Total Knee Arthroplasty Leads to Improved Postoperative Outcomes: A Randomized Controlled Trial. J Arthroplasty. 2022;37:1549-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Viscusi E, Gimbel JS, Pollack RA, Hu J, Lee GC. HTX-011 reduced pain intensity and opioid consumption versus bupivacaine HCl in bunionectomy: phase III results from the randomized EPOCH 1 study. Reg Anesth Pain Med. 2019;rapm-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Chelly JE, Klatt BA, Groff Y, O'Malley M, Lin HS, Sadhasivam S. Role of the NeuroCuple™ Device for the Postoperative Pain Management of Patients Undergoing Unilateral Primary Total Knee and Hip Arthroplasty: A Pilot Prospective, Randomized, Open-Label Study. J Clin Med. 2023;12:7394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Goel S, Luke C, Holtzman M, Davies B, O'Malley M, Lavage D, Siedlecki C, Chelly JE. Safety and Efficacy of Zynrelef(®) in Combination with a Single Unilateral or Bilateral Nerve Block Performed Prior to Surgery. J Pain Relief. 2023;12:1000002. [PubMed] |
| 15. | Lorentz S, Levin JM, Warren E Jr, Hurley ET, Mills FB, Crook BS, Poehlein E, Green CL, Bullock WM, Gadsden JC, Klifto CS, Anakwenze O. Single-shot interscalene block with liposomal bupivacaine vs. non-liposomal bupivacaine in shoulder arthroplasty. J Shoulder Elbow Surg. 2025;34:750-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Torpey A, Bellow E, Samojedny V, Ahluwalia S, Desai A, Caldwell W, Bergese S. Nanotechnology in Pain Management. Pharmaceutics. 2024;16:1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Lalani J, Patil S, Kolate A, Lalani R, Misra A. Protein-functionalized PLGA nanoparticles of lamotrigine for neuropathic pain management. AAPS PharmSciTech. 2015;16:413-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Gdowski AS, Ranjan A, Sarker MR, Vishwanatha JK. Bone-targeted cabazitaxel nanoparticles for metastatic prostate cancer skeletal lesions and pain. Nanomedicine (Lond). 2017;12:2083-2095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Park K. PLGA-based long-acting injectable (LAI) formulations. J Control Release. 2025;382:113758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 20. | Novy TCT, Joni IM, Lesmana R, Biben V, Setiawan. Chitosan Nanoparticles as an Alternative Therapeutic Approach for Knee Osteoarthritis Treatment: A Systematic Review. Int J Nanomedicine. 2025;20:6187-6203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Park KC, Choi J, Choi S, Lee G, An HJ, Yun H, Lee S. Therapeutic potential of Polydopamine-Coated selenium nanoparticles in Osteoarthritis treatment. Int J Pharm. 2025;675:125568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Pek YS, Pitukmanorom P, Ying JY. Sustained release of bupivacaine for post-surgical pain relief using core-shell microspheres. J Mater Chem B. 2014;2:8194-8200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Fu X, Zeng H, Guo J, Liu H, Shi Z, Chen H, Li D, Xie X, Kuang C. A PLGA-PEG-PLGA Thermosensitive Gel Enabling Sustained Delivery of Ropivacaine Hydrochloride for Postoperative Pain Relief. Chem Pharm Bull (Tokyo). 2017;65:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Liang T, Gao J, Feng R, Zheng Y, Tian K, Chen J, Xu X. Recent progress in poly (lactic-co-glycolic acid)-based biodegradable drug delivery carriers for pain management. Processes. 2024;12:1372. [DOI] [Full Text] |
| 25. | Holl EK, Bond JE, Selim MA, Ehanire T, Sullenger B, Levinson H. The nucleic acid scavenger polyamidoamine third-generation dendrimer inhibits fibroblast activation and granulation tissue contraction. Plast Reconstr Surg. 2014;134:420e-433e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Yao M, He Q, Tao Y, Kang X, Wu X, Shi F, Wei Y, Liu J, Meng Z, Gu R, Gan H, Dou G, Liu S, Sun Y. Chitosan-Derived Carbon Quantum Dots with Dual ROS Scavenging and Anti-inflammatory Functionalities for Accelerated Wound Repair. ACS Appl Mater Interfaces. 2025;17:40157-40172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | Lai P, Ma Y, Sang W, Zhou Q, Chen H, Wang C, Yin J, Wang T, Zhu L, Zhou X, He C, Ma J. Reprogramming Macrophage Phenotype Using a Reactive Oxygen Species-Responsive Liposome Delivery System for Inflammation Microenvironment Remodeling and Osteoarthritis Treatment. ACS Appl Mater Interfaces. 2025;17:17932-17947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/