Published online Apr 18, 2024. doi: 10.5312/wjo.v15.i4.355
Peer-review started: December 31, 2023
First decision: January 16, 2024
Revised: January 30, 2024
Accepted: March 18, 2024
Article in press: March 18, 2024
Published online: April 18, 2024
Processing time: 107 Days and 8 Hours
Enzymatic fasciotomy with collagenase clostridium histolyticum (CCH) has revolutionized the treatment for Dupuytren’s contracture (DC). Despite its benefits, the long-term outcomes remain unclear. This study presented a comprehensive 10-year follow-up assessment of the enduring effects of CCH on patients with DC.
To compare the short-term (12 wk) and long-term (10 years) outcomes on CCH treatment in patients with DC.
A cohort of 45 patients was treated with CCH at the metacarpophalangeal (MCP) joint and the proximal interphalangeal (PIP) joint and underwent systematic re-evaluation. The study adhered to multicenter trial protocols, and assessments were conducted at 12 wk, 7 years, and 10 years post-surgery.
Thirty-seven patients completed the 10-year follow-up. At 10 years, patients treated at the PIP joint exhibited a 100% recurrence. However, patients treated at the MCP joint only showed a 50% recurrence. Patient satisfaction varied, with a lower satisfaction reported in PIP joint cases. Recurrence exceeding 20 degrees on the total passive extension deficit was observed, indicating a challenge for sustained efficacy. Significant differences were noted between outcomes at the 7-year and 10-year intervals.
CCH demonstrated sustained efficacy when applied to the MCP joint. However, caution is warranted for CCH treatment at the PIP joint due to a high level of recurrence and low patient satisfaction. Re-intervention is needed within a decade of treatment.
Core Tip: Collagenase has shown efficacy in the treatment of Dupuytren’s contracture (DC). While its short-term effectiveness is well-documented in the existing literature, there is an absence of studies addressing the long-term outcomes of collagenase treatment of DC. The objectives of this study were to compare the short-term and long-term (10 years) outcomes and to assess the satisfaction with the treatment in 45 subjects enrolled in a phase 3 study in 2012.
- Citation: Passiatore M, Cilli V, Cannella A, Caruso L, Sassara GM, Taccardo G, De Vitis R. Long-term assessment of collagenase treatment for Dupuytren’s contracture: A 10-year follow-up study. World J Orthop 2024; 15(4): 355-362
- URL: https://www.wjgnet.com/2218-5836/full/v15/i4/355.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i4.355
Patients with Dupuytren’s contracture (DC), also known as palmar fibromatosis, experienced a significant breakthrough for treatment in the early 21st century. This advancement was marked by the introduction of the enzymatic fasciotomy technique, which is a novel approach involving the infiltration of the fibrous cord with collagenase derived from collagenase clostridium histolyticum (CCH)[1-5]. In contrast to traditional surgical procedures, enzymatic fasciotomy is a less invasive alternative[5-11]. However, the long-term outcomes of this innovative technique are unknown due to its recent introduction and the scarcity of studies with extended follow-up periods[6-9,12-18].
There is a growing trend of re-assessing patients who underwent enzyme fasciotomy[5]. Notably, it has been observed that some individuals treated with this technique have not experienced sustained long-term benefits. In 2012, our institution enrolled 45 patients into a phase 3 study to receive CCH for the treatment of DC with palpable cord manifestations. A comprehensive 7-year follow-up revealed a recurrence of the disease, particularly among patients who were treated at the proximal interphalangeal (PIP) joint. Additionally, there was evidence of recurrence in patients who were treated at the metacarpophalangeal (MCP) joint[6]. The aim of this study was to compare the outcomes observed at 12 wk post-treatment with those documented over a 10-year follow-up period.
This study was part of a multicenter trial aligned with the Ministry of Health Decree of May 8, 2003 and was carried out at the Unit of Orthopaedics and Surgery of the Hand at the Fondazione Policlinico Universitario A. Gemelli IRCCS in Rome (Ethics Committee Protocol P/488-857-872-1041-1113/CE/2012)[3]. Initiated in January 2012, the study involved 45 patients receiving CCH injection for the treatment of DC with palpable cord manifestations. The primary focus was to evaluate the long-term (10 years) clinical outcomes following CCH treatment in individuals diagnosed with DC.
The inclusion and exclusion criteria of the prospective study are listed in Table 1. Within the framework of the present investigation, all individuals who had been previously subjected to a comprehensive review during the 7-year follow-up were systematically contacted. Those re-examined at 10 years after treatment underwent assessments encompassing both goniometric and clinical parameters.
| Inclusion criteria | Exclusion criteria |
| DC with a PED of at least 20° at MCPJ and any degree at PIPJ | Breastfeeding or pregnant (or planning to be) during the treatment phase |
| No oral anticoagulant therapy; patient in therapy with anti-platelet drugs (discontinued for at least 7 d before treatment) | Undergoing any treatment of the affected hand up to 90 d prior to commencement of the trial |
| Positive table-top test (a patient fails to lay the palm of the hand and the fingers flat on a table surface) | Known systemic hypersensitivity to collagenase or any of the other components of the product |
| TPED ≥ 45° (that is greater than or equal to the second stage according to the Tubiana-Michon classification) | Presence of other psychiatric or organic conditions that could jeopardize the patient’s compliance |
| Palpable cord | |
| Informed consent from the patient | |
| Consent for examination according to the plan |
The surgery procedure was conducted by experienced hand surgeons injecting the appropriate drug quantity into the affected cords. A sterile dressing was applied, and patients were told to refrain from finger extension. The following day, a forced extension disrupted the pathologic cord, and a thermoplastic splint was applied for 7 d continuously followed by 12 h each day for an additional 7 d. Evaluations were conducted before treatment and 7 d after the procedure by the surgeon and a physiotherapist. The 10-year follow-up was conducted by the same treating surgeon.
Passive extension deficit (PED) and total PED (TPED) measurements were recorded before treatment and 12 wk, 7 years, and 10 years after treatment. Additionally, the recurrence rate of the disease at 7 years after treatment was assessed. Recurrence was characterized as a postoperative angular deformity exceeding 20° in at least one of the treated joints accompanied by the presence of a detectable cord[10,11]. Recurrence could be accompanied by a loss of hand function necessitating further intervention. The overall satisfaction of participants was appraised using a 10-point scale known as the general satisfaction index administered during the 10-year follow-up visit.
In light of recent advancements in patient-reported outcome measures, our patients underwent evaluation utilizing the Michigan Hand Questionnaire (MHQ) and the Unité Rhumatologique des Affections de la Main Scale (URAM Scale)[19,20].
The primary endpoint of the study was assessment of the long-term efficacy and the occurrence of significant disease recurrence at the 10-year follow-up. The secondary outcomes included evaluating sustained functionality at the 7-year follow-up and assessing general satisfaction with the received treatment.
The presented data encompassed mean values and standard deviations, with precision limited to a single decimal digit. Parametric data were subjected to comparative analysis using the Student’s t test, while non-parametric data underwent analysis via the Mann-Whitney test or Wilcoxon test. Significance levels were set at P < 0.05. The statistical analyses were conducted using GraphPad Software Prism 8 for Mac (La Jolla, CA, United States).
For the initial study, 45 patients (38 males and 7 females) were enrolled. At the 7-year follow-up, 3 patients required surgical treatment before completing the established follow-up due to an unsatisfactory clinical result. Two patients died and did not complete the 7-year follow-up assessment. At the 10-year follow-up, an additional 2 patients did not com
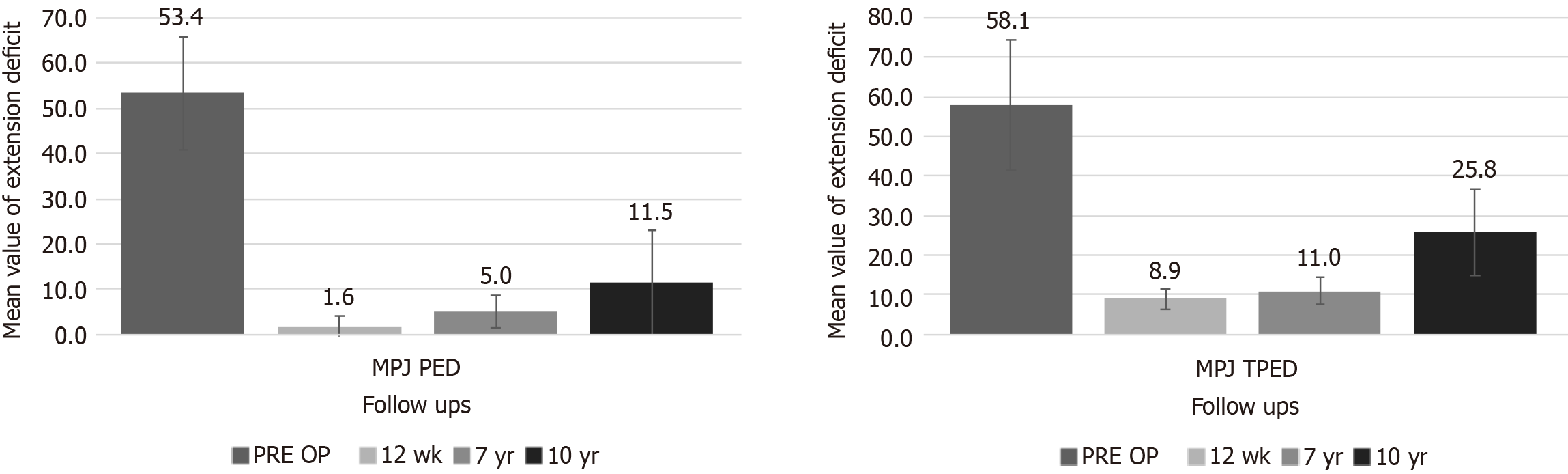
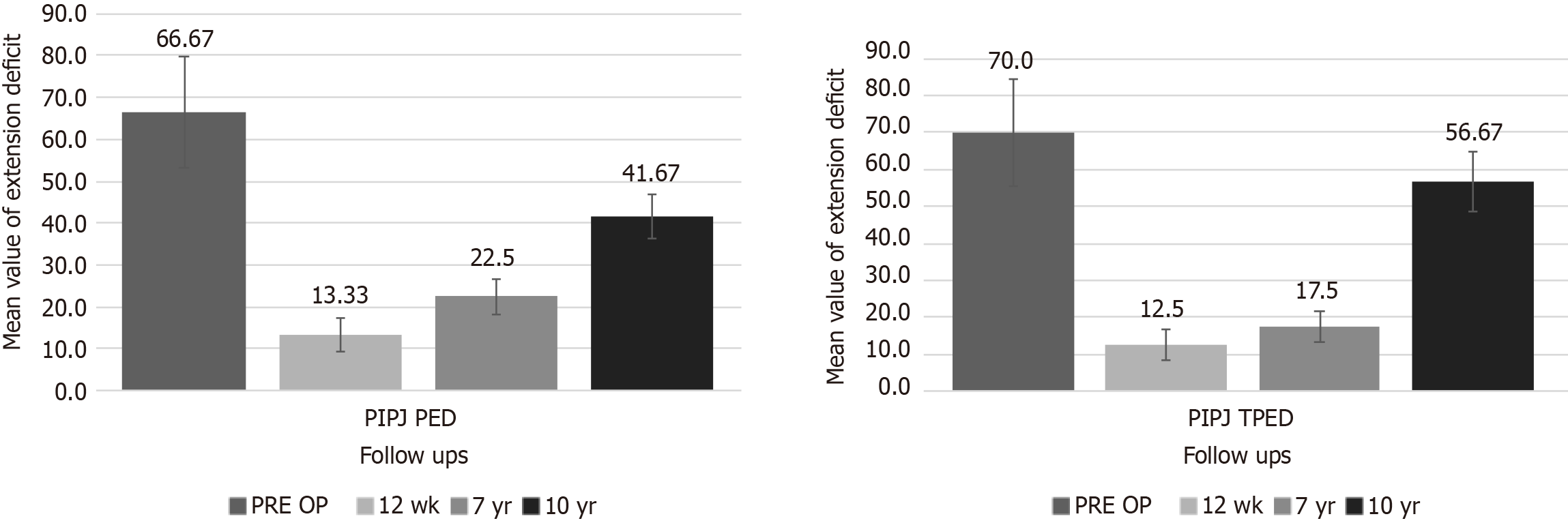
There were 31 patients treated at the MCP joint (10-year PED: 11.5 ± 11.4; range: 0-30). Nine patients (29.0%) had a recurrence on the treated joint (Figure 1 and Table 2). Seventeen patients (54.8%) had a worse TPED due to recurrence of disease by PIP joint involvement (10-year TPED: 25.8 ± 10.9; range: 0-50). Overall patient satisfaction was 6.7 ± 1.7. The mean MHQ score was 80 ± 21. The mean URAM score was 59 ± 19. A statistically significant difference was observed when comparing the outcomes at the 7-year follow-up and at the 10-year follow-up for PED (P = 0.00222) and TPED (P < 0.00001).
| Patient | MCPJ PED in degrees | MCPJ TPED in degrees | 10-yr recurrence | ||||||
| Before | 12 wk | 7 yr | 10 yr | Before | 12 wk | 7 yr | 10 yr | ||
| 1 | 60 | 0 | 0 | 5 | 70 | 10 | 10 | 25 | Yes |
| 2 | 75 | 0 | 5 | 5 | 75 | 10 | 10 | 25 | Yes |
| 3 | 50 | 0 | 5 | 5 | 50 | 5 | 10 | 15 | No |
| 4 | 45 | 0 | 0 | 0 | 60 | 10 | 10 | 20 | No |
| 5 | 45 | 0 | 5 | 5 | 55 | 10 | 10 | 15 | No |
| 6 | 90 | 0 | 5 | 30 | 100 | 10 | 10 | 40 | Yes |
| 7 | 50 | 0 | 5 | 5 | 50 | 5 | 10 | 15 | No |
| 8 | 50 | 0 | 5 | 5 | 50 | 5 | 10 | 15 | No |
| 9 | 45 | 0 | 5 | 10 | 95 | 10 | 10 | 20 | No |
| 10 | 70 | 0 | 5 | 35 | 75 | 10 | 10 | 40 | Yes |
| 11 | 70 | 0 | 0 | 0 | 70 | 10 | 10 | 10 | No |
| 12 | 45 | 5 | 5 | 5 | 50 | 5 | 10 | 15 | No |
| 13 | 45 | 5 | 5 | 10 | 45 | 10 | 10 | 20 | No |
| 14 | 50 | 5 | 5 | 5 | 50 | 10 | 15 | 40 | Yes |
| 15 | 50 | 0 | 5 | 5 | 50 | 5 | 5 | 15 | No |
| 16 | 45 | 5 | 10 | 10 | 45 | 10 | 10 | 20 | No |
| 17 | 45 | 0 | 5 | 5 | 45 | 5 | 10 | 25 | Yes |
| 18 | 70 | 5 | 0 | 0 | 80 | 10 | 10 | 30 | Yes |
| 19 | 50 | 0 | 5 | 25 | 50 | 10 | 15 | 40 | Yes |
| 20 | 45 | 0 | 5 | 25 | 45 | 10 | 10 | 40 | Yes |
| 21 | 80 | 0 | 5 | 5 | 95 | 10 | 10 | 30 | Yes |
| 22 | 45 | 5 | 5 | 25 | 45 | 10 | 20 | 50 | Yes |
| 23 | 50 | 0 | 0 | 0 | 45 | 10 | 10 | 30 | Yes |
| 24 | 45 | 5 | 5 | 5 | 45 | 15 | 10 | 15 | No |
| 25 | 45 | 0 | 5 | 5 | 45 | 5 | 10 | 35 | Yes |
| 26 | 45 | 0 | 5 | 25 | 45 | 10 | 10 | 40 | Yes |
| 27 | 65 | 5 | 10 | 30 | 65 | 10 | 25 | 30 | Yes |
| 28 | 45 | 5 | 15 | 30 | 45 | 10 | 10 | 30 | Yes |
| 29 | 50 | 0 | 0 | 0 | 50 | 5 | 10 | 10 | No |
| 30 | 45 | 5 | 5 | 5 | 55 | 10 | 10 | 15 | No |
| 31 | 45 | 0 | 15 | 30 | 55 | 10 | 10 | 30 | Yes |
| mean ± SD | 60 | 0 | 0 | 5 | 70 | 10 | 10 | 25 | N/A |
| 75 | 0 | 5 | 5 | 75 | 10 | 10 | 25 | N/A | |
There were 6 patients treated at the PIP joint (10-year PED: 41.7 ± 5.2; range: 35-50). All patients experienced recurrence at the treated joint (Figure 2 and Table 3). All patients had a worse TPED due to recurrence of the disease by PIP joint involvement (10-year TPED: 56.7 ± 8.2; range: 50-70). Overall patient satisfaction was 5.0 ± 0.6. The mean MHQ score was 70 ± 15. The mean URAM score was 63 ± 16. The sample size (n = 6) did not meet the criteria for the Wilcoxon test to approximate normality. Therefore, accurate computation of a P value was not feasible.
| Patient | PIPJ PED in degrees | PIPJ TPED in degrees | 10-yr recurrence | ||||||
| Before | 12 wk | 7 yr | 10 yr | Before | 12 wk | 7 yr | 10 yr | ||
| 32 | 50 | 10 | 30 | 40 | 50 | 10 | 15 | 50 | Yes |
| 33 | 65 | 10 | 20 | 40 | 70 | 10 | 15 | 50 | Yes |
| 34 | 70 | 20 | 25 | 50 | 70 | 20 | 25 | 60 | Yes |
| 35 | 65 | 10 | 20 | 40 | 65 | 10 | 20 | 50 | Yes |
| 36 | 60 | 15 | 20 | 45 | 70 | 10 | 15 | 70 | Yes |
| 37 | 90 | 15 | 20 | 35 | 95 | 15 | 15 | 60 | Yes |
| mean ± SD | 66.7 | 13.3 | 22.5 | 41.7 | 70.0 | 12.5 | 17.5 | 56.7 | N/A |
| 13.3 | 4.1 | 4.2 | 5.2 | 14.5 | 4.2 | 4.2 | 8.2 | N/A | |
This investigation represents one of the longest follow-up studies demonstrating the efficacy of enzymatic fasciotomy. It should be noted that during this follow-up study, collagenase was removed from the European market, but not due to safety or efficacy issues. The data from this 10-year follow-up, along with data from the 7-year follow-up[6], has revealed novel findings for the use of collagenase in the treatment of DC. Our results mostly align with trends observed in other studies with shorter follow-up periods[6,12-18].
Previous studies with extended follow-ups have already reported instances of disease recurrence. Zhang et al[12] documented a recurrence rate of 80% and the necessity for re-intervention in 53% of cases after a minimum of 5 years of follow-up. Similarly, Göransson et al[14] reported a 5-year recurrence rate of 50% that was accompanied by high patient satisfaction. Our previous study, evaluating the population 7 years after treatment[6], revealed that 86.7% of PIP joint-treated patients and 65.6% of MCP joint-treated patients experienced recurrence of the contracture. Notably, 86.7% of patients concluded treatment after a single collagenase injection despite subsequent recurrences[6].
Our analysis adhered to the international consensus definition of recurrence[11], which revealed that 54.8% of patients exhibited a deterioration of more than 20 degrees of TPED in the MCP joints. According to this criterion, 100% of patients treated at the PIP joint experienced a recurrence. Additionally, if we included patients with 20 degrees of TPED (the lower limit of recurrence definition), the recurrence rate would reach 67.7%. Notably, no patient exhibited a TPED of zero at the 10-year follow-up. In addition, our evaluation did not account for the potential activation of the disease in untreated fingers.
The recurrence is likely due to DC pathophysiology and the nature of CCH treatment. While CCH enables cord lysis, it does not eliminate a substantial portion of pathological aponeurosis, which allows the persistence of pathological collagen. There is limited evidence suggesting that CCH induces inflammatory stimulation, potentially activating the generation of further pathological collagen.
Despite these challenges, patients generally express satisfaction with the treatment, particularly when applied to the MCP joint. Conversely, patients treated at the PIP joint exhibited lower satisfaction levels, necessitating further treatment in most cases.
Given our findings, we would recommend collagenase treatment for palpable cords at the MCP joint if it were curr
Our study had some limitations, including result disparities between the MCP joint and PIP joint, and a 17.6% loss to follow-up from the initial sample of 45 patients. The deterioration observed in this case series underscores the importance of re-evaluating cases beyond the typical 5-year follow-up.
The use of CCH in treating DC is recommended when applied to palpable cords at the MCP joint. The benefits of the treatment are the non-invasiveness and the rapid postoperative recovery. However, patients should be informed of the risk of recurrence.
Dupuytren’s contracture (DC), also known as palmar fibromatosis, has been shown to be successfully treated with enzymatic fasciotomy. This novel approach involves the injection of collagenase derived from collagenase clostridium histolyticum (CCH) into a fibrous cord causing DC.
In contrast to traditional surgical procedures, enzymatic fasciotomy is a less invasive alternative. However, the long-term outcomes of this innovative technique remain largely unexplored. Recently, there has been a growing trend of re-assessing patients who underwent enzymatic fasciotomy. Notably, it has been observed that not all individuals treated with this technique have experience long-term efficacy.
This study compared the short-term (12 wk) and long-term (10 years) outcomes of CCH treatment of DC.
This was a prospective study that was part of a multicenter trial conducted in a university hospital beginning in 2012. Our institution conducted 45 injections of CCH for the treatment of DC with palpable cord manifestations. A comprehensive 7-year follow-up revealed a recurrence of the disease, particularly among patients injected at the proximal inter
When CCH was injected at the PIP joint, 100% of patients experienced recurrence at 10 years. When CCH was injected at the MCP joint, over 50% of patients experienced recurrence after 10 years. There was a statistically significant difference in passive extension deficit (PED) and total PED when comparing the outcomes at the 7-year follow-up and the 10-year follow-up.
The use of CCH for the treatment of DC is recommended when applied to palpable cords at the MCP joint. However, patients should be informed of the risk of recurrence. We do not recommend CCH for the treatment of DC at the PIP joint due to low patient satisfaction, the high rate of recurrence, and the need for re-intervention within 10 years.
The deterioration observed in our case series underscores the importance of re-evaluating cases beyond the typical 5-year follow-up. Further long-term studies are required to completely evaluate the long-term efficacy of CCH for the treatment of DC.
| 1. | Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FT, Meals RA, Smith TM, Rodzvilla J; CORD I Study Group. Injectable collagenase clostridium histolyticum for Dupuytren's contracture. N Engl J Med. 2009;361:968-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 471] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 2. | Hurst LC, Badalamente MA. Nonoperative treatment of Dupuytren's disease. Hand Clin. 1999;15:97-107, vii. [PubMed] |
| 3. | Badalamente MA, Hurst LC. Development of Collagenase Treatment for Dupuytren Disease. Hand Clin. 2018;34:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Gilpin D, Coleman S, Hall S, Houston A, Karrasch J, Jones N. Injectable collagenase Clostridium histolyticum: a new nonsurgical treatment for Dupuytren's disease. J Hand Surg Am. 2010;35:2027-38.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 230] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 5. | Smeraglia F, Del Buono A, Maffulli N. Collagenase clostridium histolyticum in Dupuytren's contracture: a systematic review. Br Med Bull. 2016;118:149-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | De Vitis R, Passiatore M, Perna A, Careri S, Cilli V, Taccardo G. Seven-year clinical outcomes after collagenase injection in patients with Dupuytren's disease: A prospective study. J Orthop. 2020;21:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Eckerdal D, Lauritzson A, Åkesson A, Atroshi I. Risk Factors for Long-Term Contracture Recurrence after Collagenase Injection for Dupuytren Disease: A Prospective Cohort Study. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | David M, Smith G, Pinder R, Craigen M, Waldram M, Mishra A, Dickson D, Wu F, Brewster M. Outcomes and Early Recurrence Following Enzymatic (Collagenase) Treatment of Moderate and Severe Dupuytren Contractures. J Hand Surg Am. 2020;45:1187.e1-1187.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Simón-Pérez C, Alía-Ortega J, García-Medrano B, Rodríguez-Mateos JI, Brotat-Rodríguez M, Aguado-Hernandez H, Martín-Ferrero MA. Factors influencing recurrence and progression of Dupuytren's disease treated by Collagenase Clostridium histolitycum. Int Orthop. 2018;42:859-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Werker PM, Pess GM, van Rijssen AL, Denkler K. Correction of contracture and recurrence rates of Dupuytren contracture following invasive treatment: the importance of clear definitions. J Hand Surg Am. 2012;37:2095-2105.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Lanfranchi E, Fairplay T, Arcuri P, Lando M, Marinelli F, Pillastrini P, Vanti C. The Italian version of the Unité Rhumatologique des Affections de la Main (URAM) for Dupuytren's disease: The URAM-I(10). Hand Ther. 2021;26:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Zhang D, Earp BE, Benavent KA, Blazar P. Collagenase Treatment of Dupuytren's Disease with Minimum 5-Year Follow-Up: Recurrence, Reintervention, and Satisfaction. Plast Reconstr Surg. 2020;146:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Räisänen MP, Karjalainen T, Göransson H, Reito A, Kautiainen H, Malmivaara A, Leppänen OV. DupuytrEn Treatment EffeCtiveness Trial (DETECT): a protocol for prospective, randomised, controlled, outcome assessor-blinded, three-armed parallel 1:1:1, multicentre trial comparing the effectiveness and cost of collagenase clostridium histolyticum, percutaneous needle fasciotomy and limited fasciectomy as short-term and long-term treatment strategies in Dupuytren's contracture. BMJ Open. 2018;8:e019054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 14. | Göransson I, Brudin L, Irbe A, Turesson C. Hand function 5 years after treatment with collagenase Clostridium histolyticum injection for Dupuytren's disease. J Hand Surg Eur Vol. 2021;46:985-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Watt AJ, Curtin CM, Hentz VR. Collagenase injection as nonsurgical treatment of Dupuytren's disease: 8-year follow-up. J Hand Surg Am. 2010;35:534-539, 539.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Werlinrud JC, Hansen KL, Larsen S, Lauritsen J. Five-year results after collagenase treatment of Dupuytren disease. J Hand Surg Eur Vol. 2018;43:841-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Hwee YK, Park D, Vinas M, Litts C, Friedman D. Outcome of Dupuytren Contractures After Collagenase Clostridium Histolyticum Injection: A Single-institution Experience. Ann Plast Surg. 2017;79:145-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Bradley J, Warwick D. Patient Satisfaction With Collagenase. J Hand Surg Am. 2016;41:689-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Felici N, Marcoccio I, Giunta R, Haerle M, Leclercq C, Pajardi G, Wilbrand S, Georgescu AV, Pess G. Dupuytren contracture recurrence project: reaching consensus on a definition of recurrence. Handchir Mikrochir Plast Chir. 2014;46:350-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Passiatore M, De Vitis R, Cilli V, Milano G, Saccomanno MF, Cotroneo C, Brozzini E, Vigliarolo D, Taccardo G. The Italian Version of the Michigan Hand Outcomes Questionnaire (MHQ): Translation, Cross-Cultural Adaptation and Validation. J Hand Surg Asian Pac Vol. 2021;26:666-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sanjuan-Cervero R, Spain S-Editor: Luo ML L-Editor: A P-Editor: Zhao YQ