Published online Apr 18, 2024. doi: 10.5312/wjo.v15.i4.346
Peer-review started: November 10, 2023
First decision: January 12, 2024
Revised: February 7, 2024
Accepted: March 19, 2024
Article in press: March 19, 2024
Published online: April 18, 2024
Processing time: 157 Days and 8.9 Hours
Tranexamic acid (TXA), a synthetic antifibrinolytic drug, effectively reduces blood loss by inhibiting plasmin-induced fibrin breakdown. This is the first study in the United Kingdom to investigate the effectiveness of TXA in the surgical mana
To assess the safety of TXA in isolated spine trauma. The primary and secondary outcomes are to assess the rate of thromboembolic events and to evaluate blood loss and the incidence of blood transfusion, respectively.
This prospective observational study included patients aged ≥ 17 years with isolated spine trauma requiring surgical intervention over a 6-month period at two major trauma centers in the United Kingdom.
We identified 67 patients: 26 (39%) and 41 (61%) received and did not receive TXA, respectively. Both groups were matched in terms of age, gender, American Society of Anesthesiologists grade, and mechanism of injury. A higher proportion of patients who received TXA had a subaxial cervical spine injury classification or thoracolumbar injury classification score > 4 (74% vs 56%). All patients in the TXA group underwent an open approach with a mean of 5 spinal levels involved and an average operative time of 203 min, compared with 24 patients (58%) in the non-TXA group who underwent an open approach with an average of 3 spinal levels involved and a mean operative time of 159 min. Among patients who received TXA, blood loss was < 150 and 150–300 mL in 8 (31%) and 15 (58%) patients, respectively. There were no cases of thromboembolic events in any patient who received TXA.
Our study demonstrated that TXA is safe for isolated spine trauma. It is challenging to determine whether TXA effectively reduces blood loss because most surgeons prefer TXA for open or multilevel cases. Further, larger studies are necessary to explore the rate, dosage, and mode of administration of TXA.
Core Tip: Since the introduction of tranexamic acid (TXA), it has been used for reducing blood loss in various surgical specialties such as Urology, general surgery, trauma and orthopedics. TXA use for elective spine surgery is well documented but there is scarce literature to explain the safety of TXA in isolated whole spine trauma. This study looks at the clinical practice of spine surgeons in two major United Kingdom trauma centres and explore the safety of TXA. The study sets the foundation for future research with larger number of patients and to improve the clinical practice.
- Citation: Zahra W, Nayar SK, Bhadresha A, Jasani V, Aftab S. Safety of tranexamic acid in surgically treated isolated spine trauma. World J Orthop 2024; 15(4): 346-354
- URL: https://www.wjgnet.com/2218-5836/full/v15/i4/346.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i4.346
Trauma is the foremost cause of mortality globally among individuals under the age of 45 years[1]. On a worldwide scale, traumatic spine injuries (TSI) are estimated to occur at a rate of 10.5 cases per 100000 people, leading to 768473 new TSI cases each year. Almost half of them (48.8%) require surgical intervention[2]. In the United Kingdom, traumatic spinal cord injuries manifest in 16 new cases per million people[3,4], with an annual report of over 1200 new cases[5].
Tranexamic acid (TXA), a synthetic antifibrinolytic drug, effectively reduces blood loss by inhibiting the plasmin-induced breakdown of fibrin[6]. Its initial application dates back to the early 1960s for managing postpartum hemorrhage[7]. Extensive randomized controlled trials (RCTs) and meta-analyses have consistently validated the safety and efficacy of TXA in various medical domains, including cardiac surgery, obstetrics, urology, orthopedics, and trauma[8-14].
Although there exists supporting evidence for the safety of TXA in elective spine procedures and complex deformity operations[15-18], the literature on the use of TXA in spinal operations due to trauma remains limited[19]. Furthermore, there is no consensus on the use and dosage of TXA in isolated spine trauma[10,9,20]. Hence, this prospective observational study marks a pioneering effort in the United Kingdom to investigate the efficacy of TXA in treating isolated spine trauma.
The primary objective of this study was to establish whether TXA administration increases the risk of thromboembolic events in patients with isolated spine trauma who underwent surgical intervention. The secondary objective was to assess the safety of TXA by evaluating blood loss and the incidence of perioperative blood transfusions.
This was a prospective observational investigation conducted at two major trauma centers in United Kingdom. This study was registered with the local audit and quality improvement teams as a service evaluation and adhered to the principles of the Declaration of Helsinki, revised in 2013. The need for formal ethical review was determined using the National Health Service Research Ethics Committee decision tool.
We included patients aged ≥ 17 years who had isolated spine trauma at any level requiring surgical intervention between January 1 and June 31, 2022, across the two sites. Patients < 16 years of age and those with polytrauma and isolated spine trauma managed nonoperatively were excluded.
The sample size was calculated for two independent groups TXA and Non-TXA by using G*power software. The estimated sample size obtained from the power analysis was at least 50 respondents for group 1 and 50 respondents for group 2 respectively.
Data collection was prospectively conducted using Google Forms with a standardized proforma divided into three sections. Section 1 captured baseline characteristics, including demographic data, comorbidities, preoperative hemoglobin (Hb) levels, mechanism of injury, level of spine involvement, subaxial cervical spine injury classification (SLIC) or thoracolumbar injury classification (TLIC) scores, and regular medications, including antithrombotic (antiplatelet or anticoagulant). Section 2 focused on surgical specifics, including the type of surgery, approach, technique (open vs minimally invasive), administration, dosage and regimen of TXA, intraoperative blood loss, use of cell salvage, use of a drain, and the number of units of blood transfused intraoperatively. Section 3 assessed postoperative factors, such as postoperative Hb levels, length of hospital stays, and complications such as wound breakdown, venous thromboembolism (VTE), acute kidney injury, and mortality.
Preoperative Hb levels were recorded on the basis of the most recent preoperative laboratory full blood count, whereas postoperative Hb levels were documented from the first laboratory full blood count measured postoperatively. Intraoperative blood loss (IBL) was defined as the total blood collected through suction, surgical sponges, and drapes at the end of the operation. It was categorized into four groups: < 150, 150–300, 300–500, and < 500 mL.
Quantitative data are presented as mean and standard deviation, whereas qualitative data are summarized as frequencies and percentages. Group comparisons used chi-squared or Fisher’s exact test and Student’s t-test, with statistical significance set at P value < 0.05.
Between January 1 and June 31, 2022, 67 patients with isolated spine injuries underwent surgical intervention across both sites. These patients were categorized into two groups: those who received TXA were placed in the TXA group (n = 26, 39%), and the remaining patients were assigned to the non-TXA group (n = 41, 61%). The average age of patients in the TXA and non-TXA groups was 57 and 56 years, respectively. Both groups were similar in terms of age (P = 0.9), gender (P = 0.2), antithrombotic usage (P = 0.6), spine pathology (P = 0.45), SLIC or TLIC score (P = 0.7), and American Society of Anesthesiologists grade (P = 0.48) (Table 1).
| Groups | TXA group (n = 26) | Non-TXA group (n = 41) | P value |
| Demographics | |||
| Number of patients | 26 | 41 | |
| Mean age (yr) | 57 | 56 | 0.9 |
| Male | 15 (58) | 26 (63) | 0.2 |
| Female | 11 (42) | 15 (36) | |
| Regular use of antithrombotic | |||
| Yes | 6 (23) | 3 (7) | 0.6 |
| No | 20 (77) | 38 (93) | |
| Mechanism of injury | |||
| RTA | 5 (19) | 4 (10) | 0.45 |
| Fall | 18 (69) | 34 (83) | |
| Other (eizures, trauma) | 3 (7) | 3 (7) | |
| Spine pathology | |||
| Spinal fracture | 23 (88) | 37 (90) | 0.45 |
| Spinal cord injury | 3 (11) | 3 (10) | |
| SLIC/TLIC score | |||
| 0-3 | 2 (7.6) | 3 (7) | 0.7 |
| 4 | 3 (11) | 14 (34) | |
| > 4 | 19 (74) | 23 (56) | |
| Not applicable | 2 (7.6) | 1 (2) | |
| ASA grades | |||
| Grade 4 | 1 (4) | 3 (7) | 0.48 |
| Grade 3 | 9 (35) | 8 (19) | |
| Grade 2 | 8 (30.6) | 21 (51) | |
| Grade 1 | 8 (30.6) | 9 (22) | |
Mechanical falls were the most frequent cause of injury in both groups. Two individuals in the non-TXA group were hospitalized because of epileptic episodes resulting in spinal fractures. Spinal fractures were the primary underlying pathology in both groups. In the TXA group, four patients were regularly taking anticoagulants (apixaban), one was on clopidogrel, and one was on aspirin. Among the patients in the non-TXA group, three were using regular aspirin. An SLIC or TLIC score of > 4 was prevalent in both groups, with a higher proportion observed in the TXA group (74% vs 56%, P = 0.7) (Table 1).
Operative details for both groups are compared in Table 2. TXA use was relatively common (22%) during stabilization and fusion procedures. In the TXA group, all patients underwent open surgery, with a posterior approach used in 17 cases (65%). Meanwhile, 17 patients (41%) in the non-TXA group underwent minimally invasive surgery. Regarding the number of spine levels involved, the TXA group ranged from 2 to 9 levels, with an average of 5, whereas the non-TXA group averaged 3.4 levels (P = 0.04). When comparing the TXA and non-TXA groups, the TXA group had significantly longer operation times (P = 0.03).
| Surgery characteristics | TXA group (n = 26) | Non-TXA group (n = 41) | P value |
| Type of operation | |||
| Corpectomy + fusion | 2 (7.6) | 1 (2) | |
| Stabilization | 6 (23) | 24 (58) | |
| Decompression | 1 (4) | 0 | |
| Fusion | 1 (4) | 1 (2) | |
| Stabilization + fusion | 6 (23) | 0 | |
| Decompression + fusion | 7 (27) | 9 (22) | |
| Decompression + stabilization | 3 (11) | 6 (14) | |
| Approach | |||
| Open | 26 (100) | 24 (58) | 0.04 |
| Minimally invasive | 0 | 17 (41) | |
| Type of approach | |||
| Anterior | 8 (31) | 8 (19) | 0.4 |
| Posterior | 17 (65) | 32 (78) | |
| Both | 1 (4) | 1 (2) | |
| Levels involved | 4.6 (2-9) | 1.4 (1–7) | 0.02 |
| Duration of surgery (min) | 203 (120–428) | 159 (48–540) | 0.03 |
In the TXA group, 15 patients (58%) experienced blood loss between 150 and 300 mL, compared with only four patients (10%) in the non-TXA group (Table 3). The administration of TXA was based on the surgeons’ preferences. In the non-TXA group, the majority (88%) of patients had blood loss of <150 mL. Cell salvage was performed twice in the TXA group and only once in the non-TXA group. Two patients in the non-TXA group and one in the TXA group received two units of platelet transfusion during surgery, following consultations with the hematology team. These patients were on aspirin and had low platelet counts upon admission. Regarding drain placement, 21 patients (81%) in the TXA group had drains inserted postoperatively, compared with 19 patients (46%) in the non-TXA group. Drains were removed within 24 h for most patients because of minimal blood loss.
| Outcomes | TXA group (n = 26) | Non-TXA group (n = 41) | P value |
| Intraoperative blood loss | |||
| < 150 mL | 8 (31) | 36 (88) | 0.03 |
| 150-300 mL | 15 (58) | 4 (10) | |
| 300-500 mL | 3 (11) | 1 (2) | |
| Intraoperative cell salvage | 2 (7.6) | 1 (2) | |
| Intra-operative transfusion | 1 (4) | 2 (5) | |
| Drain inserted | 21 (81) | 19 (46) | |
| Time of drain removal | |||
| 24 h | 11 (42) | 14 (34) | 0.002 |
| 48 h | 9 (35) | 5 (12) | |
| > 48 h | 1 (4) | 0 | |
| Admittance to discharge (d) | 10 | 3 | 0.7 |
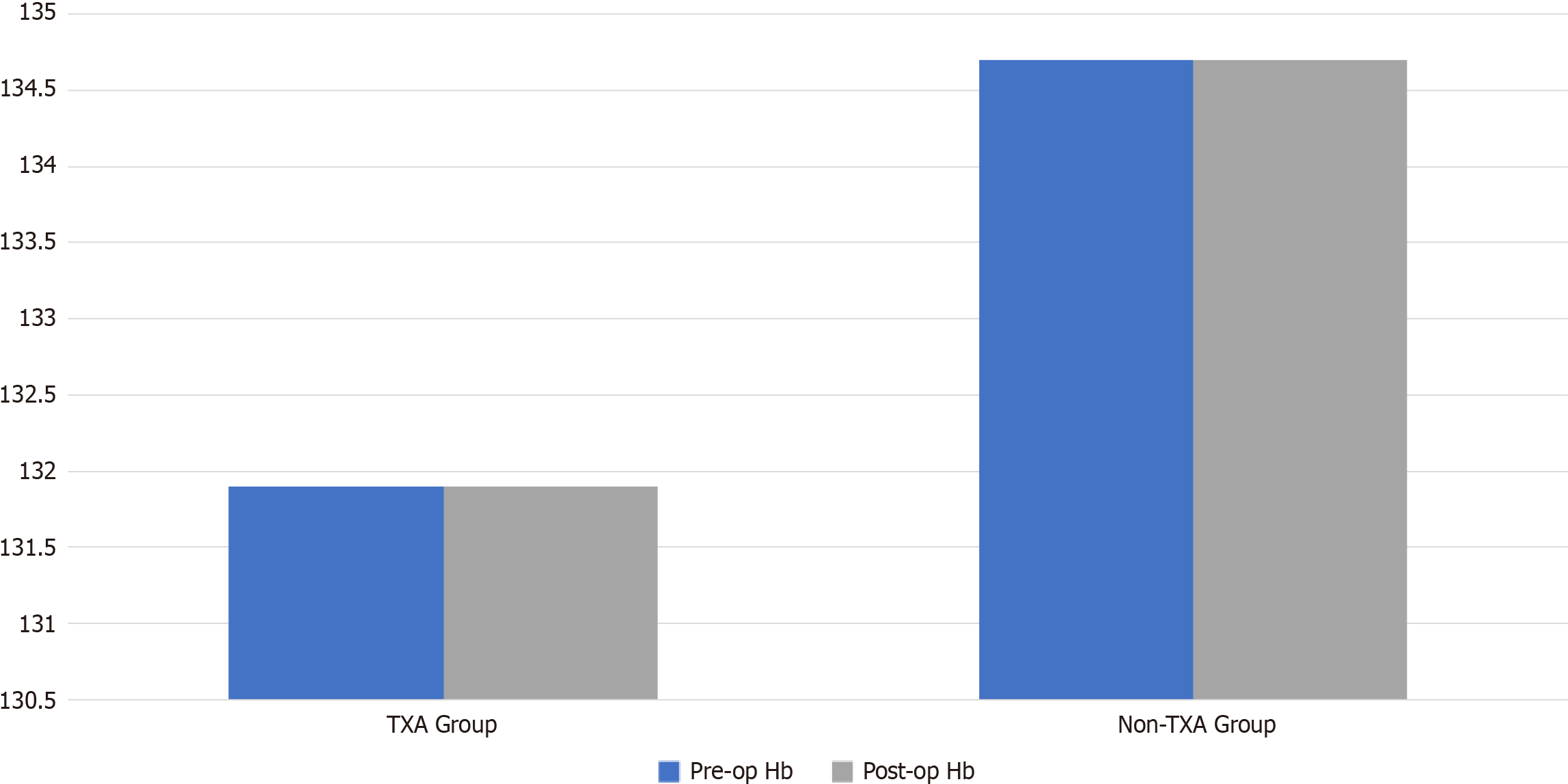
The average hospital stay was 10 d, with no significant difference between the two groups (3 d in the non-TXA group vs 10 d in the TXA group, P = 0.7). This variation was primarily attributed to patients with spinal cord injuries who required extended hospitalization while awaiting a bed in the spine injury units (Table 3). Preoperative Hb levels showed no significant differences between the TXA and non-TXA groups (127.5 mL vs 136.7 mL; range 8–15.9 g/dL vs 9–18 g/dL, P = 0.5) (Figure 1).
Table 4 shows regression model investigating the association between the dependent variable and three predictors: Estimated intraoperative blood loss, blood loss in drain, and TXA. The total model has a modest positive correlation (R = 0.338) and accounts for roughly 11.4% of the variability in the dependent variable, as shown by R square. The adjusted R square, accounting for the number of predictors, is 0.072. The estimate's standard error is 1.337, indicating the model's forecast precision.
| Model | R | R square | Adjusted R square | Std. Error of the estimate |
| 1 | 0.3381 | 0.114 | 0.072 | 1.337 |
Only one patient in the TXA group received a postoperative blood transfusion because of a substantial drop in Hb from 112 to 76. This patient underwent a three-level decompression and stabilization procedure, received TXA on induction, and was not on any antithrombotic medication. In the non-TXA group, one patient experienced a posto
In the TXA group, 14 patients (53.8%) did not experience any complications. The most common complication in this group was chest infection. Two patients tested positive for coronavirus disease 2019 (COVID-19) during their hospital stay. One patient later developed hospital-acquired pneumonia (HAP). Four other patients developed HAP, one of whom required intensive care support for noninvasive ventilation. Additionally, one patient developed type 2 respiratory failure but was deemed unfit for further interventions. Wound infections were observed in three patients, two of whom were managed using antibiotics and regular monitoring, whereas one required further wound washout in the operating theater. One patient experienced unstable liver function tests postoperatively, and the cause remained unidentified. No thromboembolic events were reported in the TXA group. However, one patient with an incomplete spine injury passed away postoperatively because of cardiovascular complications.
In the non-TXA group, two patients were diagnosed with pulmonary embolisms confirmed by computed tomography pulmonary angiograms, and one patient experienced non-ST segment elevation myocardial infarction during their hospital stay. Two patients in this group tested positive for COVID-19, and three developed pneumonia during admi
This study represents the first reported investigation into the effectiveness of TXA in cases of isolated whole-spine trau
This study serves as a preliminary exploration of the potential use of TXA in isolated spine injuries. Multiple RCTs have previously demonstrated the efficacy of TXA in elective spine surgery[9] and thoracolumbar burst fractures[21]. These trials have shown a reduction in intraoperative and total blood loss in patients with TXA. However, there remains a lack of consensus in the literature regarding the optimal TXA administration regimen[15]. The literature indicates that TXA can be administered in various forms, including intravenously and topically or as an infusion. A meta-analysis by Xiong et al[16] reported no significant difference in outcomes between intravenous and topical TXA administration. In this study, the choice of TXA administration regimen was left to the surgeons’ preferences, with 1 g of intravenous TXA administered upon induction in the TXA group.
Interestingly, in our study, we observed that surgeons did not use TXA for two-level cervical decompressions and fusions, which could be due to the expectation of lower blood loss in cervical procedures and aligns with the findings of an RCT by Elwatidy et al[22], who found no significant difference in blood loss with TXA use in cervical operations. However, in our study, the TXA group included cases involving multiple cervical spine levels (3–6) for fusion and decompression, where TXA was deemed necessary because of a higher risk of bleeding when multiple levels were involved. There are limited available data on the use of TXA in isolated cervical spine trauma. A prospective, randomized study on three cervical spine levels reported a significant reduction in blood loss during cervical laminoplasty surgery using TXA[23]. Our study found no significant difference in the number of levels operated on between the two groups, which contradicts the findings of Colomina et al[9] in elective settings. Individual data indicate that surgeons tend to prefer an open technique for decompression and stabilization surgeries involving more than three levels. There is also limited literature available regarding the use of TXA in open vs minimally invasive spine surgery approaches. In our study, TXA was administered for cases performed via an open approach but not for minimally invasive surgery, consistent with cases where blood loss was < 150 mL in two-level fracture fixations, and patients did not require a drain or experience postoperative issues. Despite the variability in levels involved in the TXA group, our investigation identified a significant difference (P = 0.03) in the duration of surgery between the two groups, aligning with the findings of Shen et al[21] for thoracolumbar burst fractures and elective spine operations[9].
An increasing number of meta-analyses of RCTs have emphasized the blood-saving effects of TXA in spine surgery[24]. Shen et al[21] demonstrated a significant reduction in blood loss in the TXA group of 39 patients undergoing surgery for thoracolumbar burst fractures compared with the placebo group. They reported reductions in total blood loss, IBL, and postoperative blood loss in the TXA group (P = 0.001), whereas hidden blood loss (HBL) remained similar between the TXA and placebo groups (P = 0.08). HBL was calculated using a formula rather than a direct measurement[21]. These findings contrast with an earlier study by Sudprasert et al[19], who found no significant difference (P = 0.8) in a cohort of 29 patients with thoracolumbar trauma undergoing posterior fusion and receiving topical TXA. Our findings align with the results of Sudprasert et al[19] because we found no significant reduction in IBL in the two groups. Zhang et al[25] conducted a meta-analysis of 11 studies involving multilevel elective spine surgery in 2019 and reported that intravenous TXA effectively reduced IBL. In contrast, Elmose et al[26] found that intravenous TXA had no statistically significant impact on IBL, operative time, or complications during minor lumbar spine surgery. We understand that all these studies show variation in results and lack consensus on the usage of TXA in spinal patients. Additional research is warranted to better understand the use of TXA in spine trauma cases.
In our study, two patients in the TXA group received cell salvage, and platelets were transfused intraoperatively. One patient also required a blood transfusion during surgery. These findings are consistent with a meta-analysis of seven studies conducted by Yang et al[15] and the results reported by Sudprasert et al[19] for thoracolumbar trauma. Multiple studies[15,19,21,27] have shown a significant decrease in drain blood loss and the average time to drain removal. However, our study did not identify any difference in the time for drain removal, which may be attributed to the fact that patients in the TXA group underwent multilevel spine surgery, leading to the surgeon’s preference for keeping the drain. To date, there has been limited research on postoperative Hb levels. Our study found no significant difference in Hb levels before the operation and on day 1 Hb level postoperative.
This study has subject to several limitations. Data were collected over 6 months, resulting in a sample size of 67 patients. The exclusion of patients with missing data introduced some heterogeneity into the study, including variations in spine levels and diverse surgical techniques performed by approximately 15 different surgeons across two distinct centers. As an observational study, it carries inherent confounding risks when patients who received TXA are compared with those who did not, given the absence of randomization. Factors such as the type of surgery, surgical approach, levels, preo
There could be a potential for inaccuracy of the first postoperative Hb value; the results may be affected by intravenous crystalloid-induced hemodilution during the intraoperative period. Instead of precise IBL values, we categorized the data into four distinct ranges, accounting for blood loss through surgical sponges and drapes. Data on blood loss in the drain were not collected for all patients, which contributed to the limitation.
When assessing the postoperative risks of VTE during the hospital stay, we considered the intraoperative administration of both mechanical and chemical thromboprophylaxis. This study included patients who regularly used antithrombotic medications but did not provide information regarding the duration of medication use or whether it was dis
Our study indicates that TXA can be safely used in cases of isolated spine trauma, with no evidence of an elevated risk of blood transfusion or VTE in either group. However, it is challenging to definitively conclude from this study whether TXA effectively reduces blood loss because TXA was predominantly used in open or multilevel surgical cases. To establish the full extent of the efficacy of TXA in isolated spine trauma, further research is warranted to compare its effectiveness across open vs minimally invasive techniques and in cases involving single vs multilevel procedures.
There is no data looking at safety of the tranexamic acid (TXA) in the surgical management of isolated whole spine trauma. This study sets the foundation for the future research work.
This is the only study looking at the safety of TXA in surgically treated isolated whole spine trauma. There is no consensus on the administration, dosage and route of TXA delivered for these injuries.
The overall objective of this study is to look at the safety of the TXA in surgically treated isolated whole spine trauma.
This prospective observational study included patients aged ≥ 17 years with isolated spine trauma requiring surgical intervention over a 6-month period at two major trauma centers in the United Kingdom. We used SPSS for statistical analysis.
We identified 67 patients: 26 (39%) and 41 (61%) received and did not receive TXA, respectively. Both groups were matched in terms of age, gender, American Society of Anesthesiologists grade, and mechanism of injury. A higher proportion of patients who received TXA had a subaxial cervical spine injury classification or thoracolumbar injury classification score > 4 (74% vs 56%). All patients in the TXA group underwent an open approach with a mean of 5 spinal levels involved and an average operative time of 203 min, compared with 24 patients (58%) in the non-TXA group who underwent an open approach with an average of 3 spinal levels involved and a mean operative time of 159 min. Among patients who received TXA, blood loss was < 150 and 150–300 mL in 8 (31%) and 15 (58%) patients, respectively. There were no cases of thromboembolic events in any patient who received TXA.
We concluded that TXA is safe for isolated spine trauma. It is challenging to determine whether TXA effectively reduces blood loss because most surgeons prefer TXA for open or multilevel cases.
This study sets the foundation for further research trials in patients with isolated spine trauma managed with surgical intervention.
| 1. | Sakran JV, Greer SE, Werlin E, McCunn M. Care of the injured worldwide: trauma still the neglected disease of modern society. Scand J Trauma Resusc Emerg Med. 2012;20:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Kumar R, Lim J, Mekary RA, Rattani A, Dewan MC, Sharif SY, Osorio-Fonseca E, Park KB. Traumatic Spinal Injury: Global Epidemiology and Worldwide Volume. World Neurosurg. 2018;113:e345-e363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 425] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 3. | McCaughey EJ, Purcell M, McLean AN, Fraser MH, Bewick A, Borotkanics RJ, Allan DB. Changing demographics of spinal cord injury over a 20-year period: a longitudinal population-based study in Scotland. Spinal Cord. 2016;54:270-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | NICE. Spinal injury: assessment and initial management. Clinical guideline. London: National Institute for Health and Care Excellence; 2015. |
| 5. | McDaid D, Park AL, Gall A, Purcell M, Bacon M. Understanding and modelling the economic impact of spinal cord injuries in the United Kingdom. Spinal Cord. 2019;57:778-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Mannucci PM. Hemostatic drugs. N Engl J Med. 1998;339:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 419] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 7. | Tengborn L, Blombäck M, Berntorp E. Tranexamic acid--an old drug still going strong and making a revival. Thromb Res. 2015;135:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 8. | Henry DA, Carless PA, Moxey AJ, O'Connell D, Stokes BJ, Fergusson DA, Ker K. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;2011:CD001886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Colomina MJ, Koo M, Basora M, Pizones J, Mora L, Bagó J. Intraoperative tranexamic acid use in major spine surgery in adults: a multicentre, randomized, placebo-controlled trial†. Br J Anaesth. 2017;118:380-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Zufferey P, Merquiol F, Laporte S, Decousus H, Mismetti P, Auboyer C, Samama CM, Molliex S. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105:1034-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 232] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 12. | Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184:880-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, Cook L, Kawahara T, Perel P, Prieto-Merino D, Ramos M, Cairns J, Guerriero C. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17:1-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 439] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 14. | CRASH-3 trial collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet. 2019;394:1713-1723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 521] [Cited by in RCA: 614] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 15. | Yang B, Li H, Wang D, He X, Zhang C, Yang P. Systematic review and meta-analysis of perioperative intravenous tranexamic acid use in spinal surgery. PLoS One. 2013;8:e55436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Xiong Z, Liu J, Yi P, Wang H, Tan M. Comparison of Intravenous versus Topical Tranexamic Acid in Nondeformity Spine Surgery: A Meta-Analysis. Biomed Res Int. 2020;2020:7403034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Walterscheid Z, O'Neill C, Carmouche J. Tranexamic acid in adult elective orthopaedic and complex spinal surgery: A review. Surg Rehabil. 2017;1. [DOI] [Full Text] |
| 18. | Slattery C, Kark J, Wagner T, Verma K. The Use of Tranexamic Acid to Reduce Surgical Blood Loss: A Review Basic Science, Subspecialty Studies, and The Evolution of Use in Spine Deformity Surgery. Clin Spine Surg. 2019;32:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Sudprasert W, Tanaviriyachai T, Choovongkomol K, Jongkittanakul S, Piyapromdee U. A Randomized Controlled Trial of Topical Application of Tranexamic Acid in Patients with Thoracolumbar Spine Trauma Undergoing Long-Segment Instrumented Posterior Spinal Fusion. Asian Spine J. 2019;13:146-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Yu CC, Fidai M, Washington T, Bartol S, Graziano G. Oral Is as Effective as Intravenous Tranexamic Acid at Reducing Blood Loss in Thoracolumbar Spinal Fusions: A Prospective Randomized Trial. Spine (Phila Pa 1976). 2022;47:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Shen J, Yang Z, Fu M, Hao J, Jiang W. The influence of topical use of tranexamic acid in reducing blood loss on early operation for thoracolumbar burst fracture: a randomized double-blinded controlled study. Eur Spine J. 2021;30:3074-3080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Elwatidy S, Jamjoom Z, Elgamal E, Zakaria A, Turkistani A, El-Dawlatly A. Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: a prospective, randomized, double-blind, placebo-controlled study. Spine (Phila Pa 1976). 2008;33:2577-2580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Tsutsumimoto T, Shimogata M, Ohta H, Yui M, Yoda I, Misawa H. Tranexamic acid reduces perioperative blood loss in cervical laminoplasty: a prospective randomized study. Spine (Phila Pa 1976). 2011;36:1913-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Yoo JS, Ahn J, Karmarkar SS, Lamoutte EH, Singh K. The use of tranexamic acid in spine surgery. Ann Transl Med. 2019;7:S172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Zhang Y, Liu H, He F, Chen A, Yang H, Pi B. Does Tranexamic Acid Improve Bleeding, Transfusion, and Hemoglobin Level in Patients Undergoing Multilevel Spine Surgery? A Systematic Review and Meta-Analysis. World Neurosurg. 2019;127:289-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Elmose S, Andersen MØ, Andresen EB, Carreon LY. Double-blind, randomized controlled trial of tranexamic acid in minor lumbar spine surgery: no effect on operative time, intraoperative blood loss, or complications. J Neurosurg Spine. 2019;1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Yan L, Yang H, Jiang H, Yu M, Tan J, Su T, Xu G. Impact of the Tranexamic Acid on Bleeding Amount of Surgical Patient With Degenerative Spinal Disease: A Randomized Blinded Study. Front Surg. 2021;8:655692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Han J, China; Papadopoulos VP, Greece S-Editor: Zhang H L-Editor: A P-Editor: Zhao YQ