Published online Oct 18, 2024. doi: 10.5312/wjo.v15.i10.918
Revised: August 25, 2024
Accepted: September 9, 2024
Published online: October 18, 2024
Processing time: 169 Days and 18.3 Hours
Urine-derived stem cells (USCs) are derived from urine and harbor the potential of proliferation and multidirectional differentiation. Moreover, USCs could be reprogrammed into pluripotent stem cells [namely urine-derived induced pluripotent stem cells (UiPSCs)] through transcription factors, such as octamer binding transcription factor 4, sex determining region Y-box 2, kruppel-like factor 4, myelocytomatosis oncogene, and Nanog homeobox and protein lin-28, in which the first four are known as Yamanaka factors. Mounting evidence supports that USCs and UiPSCs possess high potential of neurogenic, myogenic, and osteogenic differentiation, indicating that they may play a crucial role in the treatment of neurological and musculoskeletal diseases. Therefore, we summarized the origin and physiological characteristics of USCs and UiPSCs and their therapeutic application in neurological and musculoskeletal disorders in this review, which not only contributes to deepen our understanding of hallmarks of USCs and UiPSCs but also provides the theoretical basis for the treatment of neurological and musculoskeletal disorders with USCs and UiPSCs.
Core Tip: Urine-derived stem cells and urine-derived induced pluripotent stem cells possess high potential of neurogenic, myogenic, and osteogenic differentiation, which are able to be used for the treatment of neurological and musculoskeletal disorders.
- Citation: Yang HS, Zheng YX, Bai X, He XY, Wang TH. Application prospects of urine-derived stem cells in neurological and musculoskeletal diseases. World J Orthop 2024; 15(10): 918-931
- URL: https://www.wjgnet.com/2218-5836/full/v15/i10/918.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i10.918
Neurological and musculoskeletal diseases are a large class of complex and heterogeneous disorders. Neurological diseases, including traumatic injuries, cerebrovascular diseases, and neurodegenerative diseases, typically lead to loss of motor function, sensory dysfunction, and memory and cognitive impairment, which seriously threaten the life and health of the patients[1]. Additionally, the muscle and skeletal systems accomplish the daily activities under the control of the nervous system. The disorders in these systems (abbreviated as musculoskeletal diseases) result in local pain, my
Stem cells are a class of cells with unlimited proliferation and differentiation potential. According to the developmental stage, stem cells could be divided into embryonic stem cells and adult stem cells. In the light of the differentiation potential, they are divided into totipotent stem cells, pluripotent stem cells, oligopotent stem cells, and unipotent stem cells[3]. Urine-derived stem cells (USCs), obtained from urine, are a kind of pluripotent stem cells with high proliferative ability and multidirectional differentiation potential. Studies have shown that under the stimulation of different inducing factors, USCs could differentiate into neurocytes, osteoblasts, chondrocytes, muscle cells, adipocytes, vascular endothelial cells, rod cells, cone cells, alveolar type II epithelial cells, hepatocytes, etc[4-8]. This evidence suggests that USCs might play an important role in the treatment of the diseases induced by damage or loss of these cells. Moreover, USCs could also be reprogrammed into urine-derived induced pluripotent stem cells (UiPSCs) to further expand its application. In fact, USCs and UiPSCs have been reported to hold considerable therapeutic value in urologic diseases[9], cardiac diseases[10], stroke[11], spinal cord injury[12], muscular dystrophy[13], osteoarthrosis, and osteoporosis[14].
Thanks to the great potential of neurogenic, myogenic, and osteogenic differentiation[15-17], the role of USCs and UiPSCs in the treatment of neurological and musculoskeletal diseases should not be ignored. In this review, we summarized the origin and physiological characteristics of USCs and UiPSC, and further elaborated their therapeutic potential in neurological and musculoskeletal diseases.
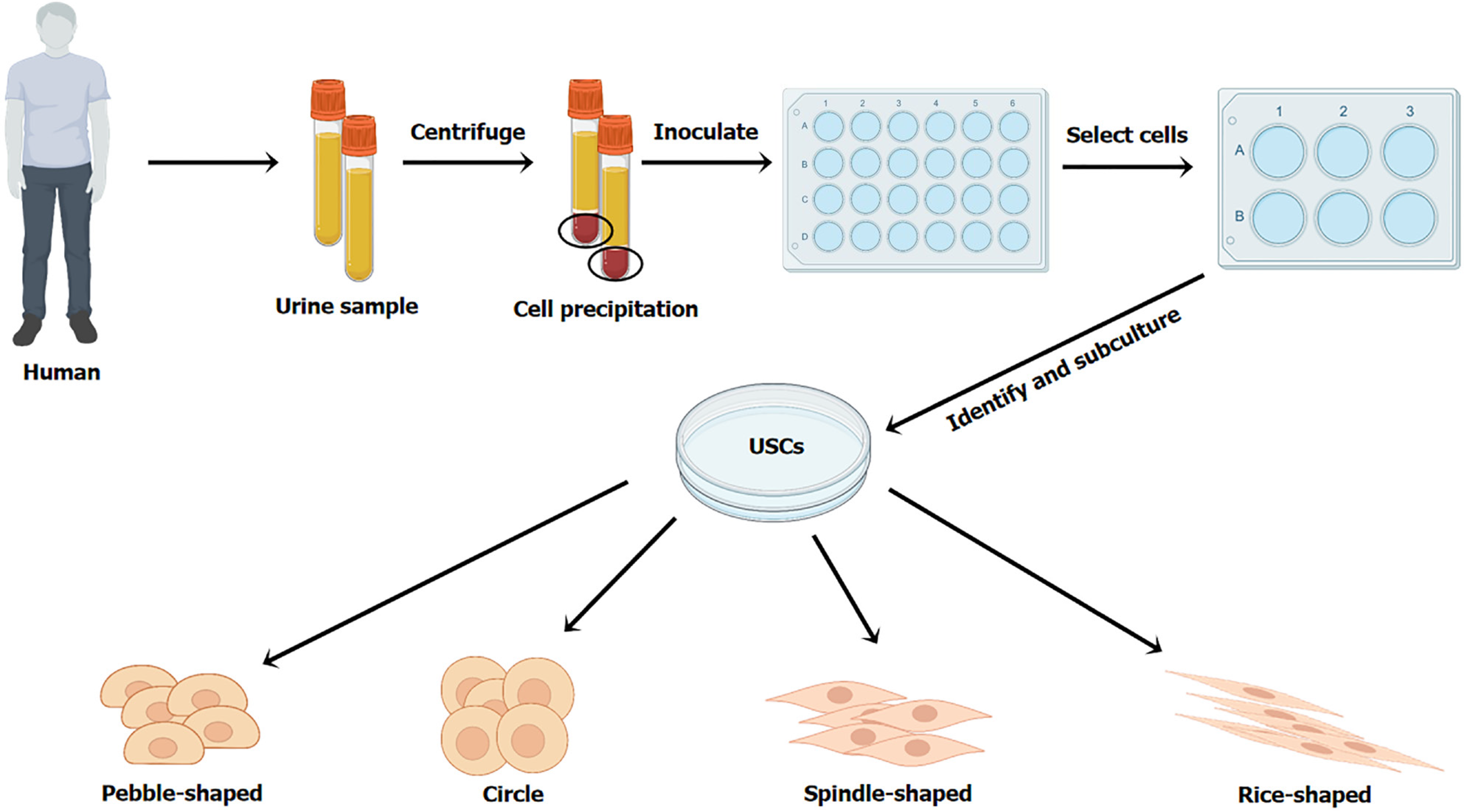
USCs are isolated from urine. The specific steps to obtain USCs are as follows: (1) Collect urine samples (USCs could survive in the urine for 24 h)[18]; (2) Centrifuge the urine samples at a low speed for 10 min to obtain cell precipitation; (3) Resuspend the cell precipitation by a specific medium (keratinocyte serum-free medium: Dulbecco vs modified eagle medium = 1:1, with 5% fetal bovine serum), and then inoculate the cell suspension in culture plates to obtain cell clone spheres; and (4) Select appropriate cell clone spheres for subculture and identification[5,19-22] (Figure 1). Due to the high proliferative activity, a considerable number of USCs can be obtained from a single urine sample[4,23]. Evidence showed that USCs from the upper urinary tract urine had a greater proliferative activity than that from the voided urine[24]. Additionally, matrigel and Y-27632, an inhibitor of rho-associated protein kinase, facilitate the isolation, proliferation, and osteogenic or chondrogenic differentiation of USCs[15].
Additionally, USCs may originate from multiple tissues of the urinary system. Firstly, USCs express the specific markers of podocytes and glomerular parietal cells (paired box 2 and paired box 8)[23,25,26], suggesting that USCs originate from kidney tissues. Secondly, USCs express the marker of uroepithelial basal cells and pericytes [cluster of differentiation (CD) 146][4]. Furthermore, evidence demonstrated that spindle-shaped USCs (SS-USCs) might be of renal interstitial origin, and rice-shaped USCs (RS-USCs) might be of renal tubular origin[27]. However, USCs poorly express the markers of hematopoietic stem cells, such as CD34 and CD45, suggesting that they are not derived from hema
Primary cultured USCs grow adherent to the wall in the shape of rice, spindle, pebble, or circle[13,22,24,29] (Figure 1). Chen et al[27] believed that RS-USCs were similar to SS-USCs in terms of colony formation efficiency. The cultured USCs have typical stem cell ultramorphology and can release extracellular vesicles[4]. After passage, USCs are elongated[30] and show fibroblast-like morphology[19,31].
Isolated USCs hold high proliferative activity in vitro. The macroscopic colonies of USCs form on the third to fifth day of culture[5,20,24,30]. During the first ten passages, USCs still show accelerated growth and stem cell characteristics in vitro[4,20]. After the tenth passage, cell proliferation is significantly weakened[5]. Evidence suggested that the proliferation ability of stem cells is positively correlated with the activity and length of telomere[23]. Of course, this evidence is also extended to USCs. Firstly, as for the USCs from the same donor, the telomerase-positive ones show higher proliferation ability and more stable cell morphology than telomerase-negative ones[32]. Secondly, for the telomerase-positive USCs, their proliferation ability is closely related to the activity and length of telomerase.
It is well known that the telomerase activity is age-related, which decreases with age. Without doubt, USCs derived from the younger population hold higher proliferative potential. Compared with USCs derived from people over 50 years old, USCs isolated from middle-aged people (< 50 years old) harbor longer lengths of telomeres and stronger proliferation ability[33]. Moreover, the USCs in children (5 to 14 years old) show higher proliferative ability than that in adults[30]. Conversely, the USCs from the elderly population (70 to 94 years old) hold much lower proliferative activity[34]. Taken together, the proliferative ability (quality) of the isolated USCs is negatively correlated with age. Unfortunately, there is no evidence to support the correlation between the yield and proliferation activity of USCs and gender. In addition, compared with other pluripotent stem cells [such as basal-derived mesenchymal stem cells (BMSCs) and placenta decidual BMSCs], USCs possess superior proliferation activity[22].
Fortunately, the tumorigenicity of USCs has not been identified[23,24]. For example, researchers found that the USCs that were injected into subcutaneous tissues and the subcapsular space of mice kidneys did not form tumors[23,24,35]. Even the USCs from clear cell renal cell carcinoma patients did not show tumorigenicity[36]. In order to further inve
Like other stem cells, USCs also hold the potential of multidirectional differentiation. Numerous studies proved that USCs could differentiate into neurocytes, osteoblasts, chondrocytes, muscle cells, adipocytes, endothelial cells, and hepatocytes[4,23,38]. Firstly, USCs highly express mesenchymal stem cell (MSC)-like surface markers, including CD29, CD44, CD54, CD73, CD90, CD105, CD166, stromal cell antigen-1, CD24, and CD146[4,23,39] (Table 1), indicating that USCs might hold MSC-like functions (like self-renewal and differentiation into a variety of cell types)[40]. Moreover, USCs harbored better potential to differentiate into adipose, endothelial, and vascular cells than BMSCs and placenta decidual MSCs[22].
| Surface markers in USCs | Ref. | |
| MSC-like markers | CD73, CD90, CD29, CD44, CD146 | |
| CD73, CD29, CD44 | ||
| CD73, CD90, CD44, CD146 | ||
| CD73, CD90, CD105 (high), CD44, CD146 | ||
| CD73, CD90, CD146 | ||
| CD73, CD90, CD105, CD29, CD24, CD146 | ||
| CD73, CD90, CD29 | ||
| CD73, CD90, CD29, CD44, CD54, CD166, CD146 | ||
| CD73, CD90, CD105, CD29, CD44 | ||
| CD90, CD29, CD44 | ||
| CD73, CD90, CD105, CD29, CD44, CD166, STRO-1, CD54, CD146 | ||
| CD73, CD90, CD105, CD44 | ||
| CD73, CD90, CD105, STRO-1 (low), CD146 | ||
| CD73, CD90, CD105, CD24, CD29, CD44, CD117, STRO-1, CD146 | ||
| CD29, CD44, CD73, CD90, CD105 (low) | ||
| CD24, CD29, CD34, CD44, CD73, CD90 | ||
| Other pluripotency markers | TRA-1-60, TRA-1-81, SSEA-4, SOX2, OCT4, MYC, KLF4 | |
| SSEA-4 | ||
| OCT4, MYC | ||
| OCT4, NANOG | [20] | |
| CD133 | ||
| Urinary system markers | Nephrin, WT1 | [20] |
| PAX2, PAX8 | ||
| Uroplakin Ia, cytokeratin 7, cytokeratin 19 | ||
| MP markers | PAX7, MYF5, MOYD, desmin, dystrophin, myogenin, α-smooth muscle actin | [24] |
Secondly, USCs express other pluripotency markers, such as tumor resistance antigen-1-60, tumor resistance antigen-1-81, stage-specific embryonic antigen-4, octamer binding transcription factor 4 (OCT4), sex determining region Y-box 2 (SOX2), kruppel-like factor 4 (KLF4), myelocytomatosis oncogene (MYC), Nanog homeobox (NANOG), and CD133[23,24,27,38] (Table 1). Thirdly, USCs express urinary system markers. Specifically, USCs express specific renal markers including nephrin and Wilms’ tumor-1[20], and urothelium and smooth muscle markers including uroplakin Ia, cytokeratin 7, and cytokeratin 19 of urothelial lineage[24] (Table 1).
At the same time, USCs express markers of myogenic lineage in various stages, including the early paired-box 7, myogenic factor 5, and myogenic differentiation 1, the intermediate (desmin and dystrophin), and the late (myogenin and α-smooth muscle actin) (Table 1), demonstrating that USCs hold myocyte differentiation potential[30]. Fourthly, USCs could be induced into neural stem cells (NSCs) or other cells of the neural lineage[28]. Expect for that, when co-cultured with hepatic progenitor cells, approximately 10% of USCs undergo hepatocyte differentiation[5].
In addition, USCs with different morphologies hold slightly different differentiation potential. SS-USCs originating from renal interstitium possess higher osteogenic and adipogenic differentiation potential, while RS-USCs originating from renal tubules show greater chondrogenic differentiation potential[27]. Taken together, USCs possess great potential for osteogenic, myogenic, and neurogenic and endothelial differentiation[33]. However, overexpansion and predifferentiation of USCs in vitro damage its survival and the capability of tissue repairing[41].
The emergence of iPSCs makes it possible to obtain the desired cells by using cells from various sources. Urine-derived cells (UCs) or USCs could be easily collected from excreted urine, so they might serve as an ideal cell source for obtaining iPSCs[42]. It is attractive to establish a non-invasive method to generate iPSCs. Mounting evidence suggested that iPSCs could be acquired by delivering reprogramming factors to UCs or USCs through retroviruses, lentiviruses, Sendai virus (SeV), exogenous plasmids, mRNA, self-replicating RNA (srRNA), small molecules, etc (Table 2). Among these, SeV and exogenous plasmids are most commonly used to generate iPSCs.
| Reprogramming method | Vectors | Factors | Ref. |
| SeV | SeV | OSKM | [15,43,44,48,49,58,60,63,71] |
| OCT4, SOX2, NANOG, LIN28 | |||
| JDP2, JHDM1B, MKK6, GLIS1, NANOG, ESSRB, SALL4 | |||
| Exogenous plasmids | Epi | KLD4, MYC, OCT4, SOX2, LIN28, shP53 | |
| OCT4, SOX2, KLF4, miR-302-367 | |||
| OCT4, SOX2, NANOG, LIN28, KLF4 and MYC | [85,86] | ||
| OCT4, SOX2, SV40 LT, KLF4 and miR302-367 | |||
| OCT4, SOX2, NANOG, MYC, KLF4, LIN28 | |||
| OCT4, SOX2, KLF4, and miR-302-367 | |||
| pCXLE-hOCT4-shP53-F, pCXLE-hSK, pCXLE-h UL | OSKM, shP53, LIN28 | ||
| TET-ON-induced expression vector | OSKM | ||
| pEP4EO2SET2K | OCT4, SOX2, SV40 LT and KLF4, miR-302b, c, a, d and miR-367 | ||
| SM | Cyclic pifithrin-a, A-83-01, CHIR99021, thiazovivin, NaB, PD0325901 | ||
| mRNA | OSKM LNg | ||
| srRNA | OSKM, NSP 1-4 | ||
| Lentivirus | Lentiviral vectors | OSKM |
Currently, the reprogramming factors delivered by SeV mainly consists of four transcription factors including OCT4, SOX2, KLF4 and MYC (the four factors are abbreviated as OSKM)[43,44]. Except the classic OSKM factors, studies also provided other alternative combinations of transcription factors (Table 2). For example, Yu et al[45] found that OCT4, SOX2, NANOG, and protein lin-28 induced UCs to generate human UiPSCs. Wang et al[46] demonstrated that the com
Of note, cell death and timely elimination of SeVs are two issues that cannot be ignored in the reprogramming process. Some researchers have applied the autologous USC feeder culture method to overcome the massive cell death[47]. Also, evidence showed that SeVs usually disappeared on their own after more than five passages, but a method to quickly eliminate them has not been found[43,48,49]. It was advocated that UiPSCs obtained by this reprogramming method should be used as early as possible, although their proliferation potential could last up to five passages after being frozen in liquid nitrogen[50].
As another major reprogramming method, exogenous plasmid vectors carrying reprogramming factors also could induce USCs into UiPSCs. These reprogramming factors included OCT4, SOX2, KLF4, MYC, NANOG, LIN28, Kelch domain-containing protein 4, simian virus 40 large T antigen, etc (Table 2). For example, Ncube et al[51] successfully induced urine-derived renal progenitor cells into UiPSCs by electro transfecting exogenous plasmids expressing OCT4, SOX2, NANOG, MYC, KLF4, and LIN28. Kibschull et al[52] selected Kelch domain-containing protein 4, LIN28, MYC, OCT4, SOX2, and P53 short-hairpin RNA as reprogramming factors to reprogram UCs into UiPSCs. Among the exogenous plasmids, episomal plasmid (Epi) was frequently used. Particularly, owing to the replication of Epstein-Barr virus during the process of cell proliferation, the Epstein-Barr virus-carrying Epi had an extended ability to express reprogramming factors[53]. Moreover, the methods that combined the above reprogramming factors with exogenous plasmids (especially Epi) for cell reprogramming have been commercialized[54].
Apart from the two main programming methods mentioned above, researchers have also discovered other rep
Using the patient’s autologous cells as the cell sources, the obtained UiPSCs do not pose a risk of immune rejection and can differentiate into various required cell types[55]. Moreover, UiPSCs induced by autologous cells have the same ge
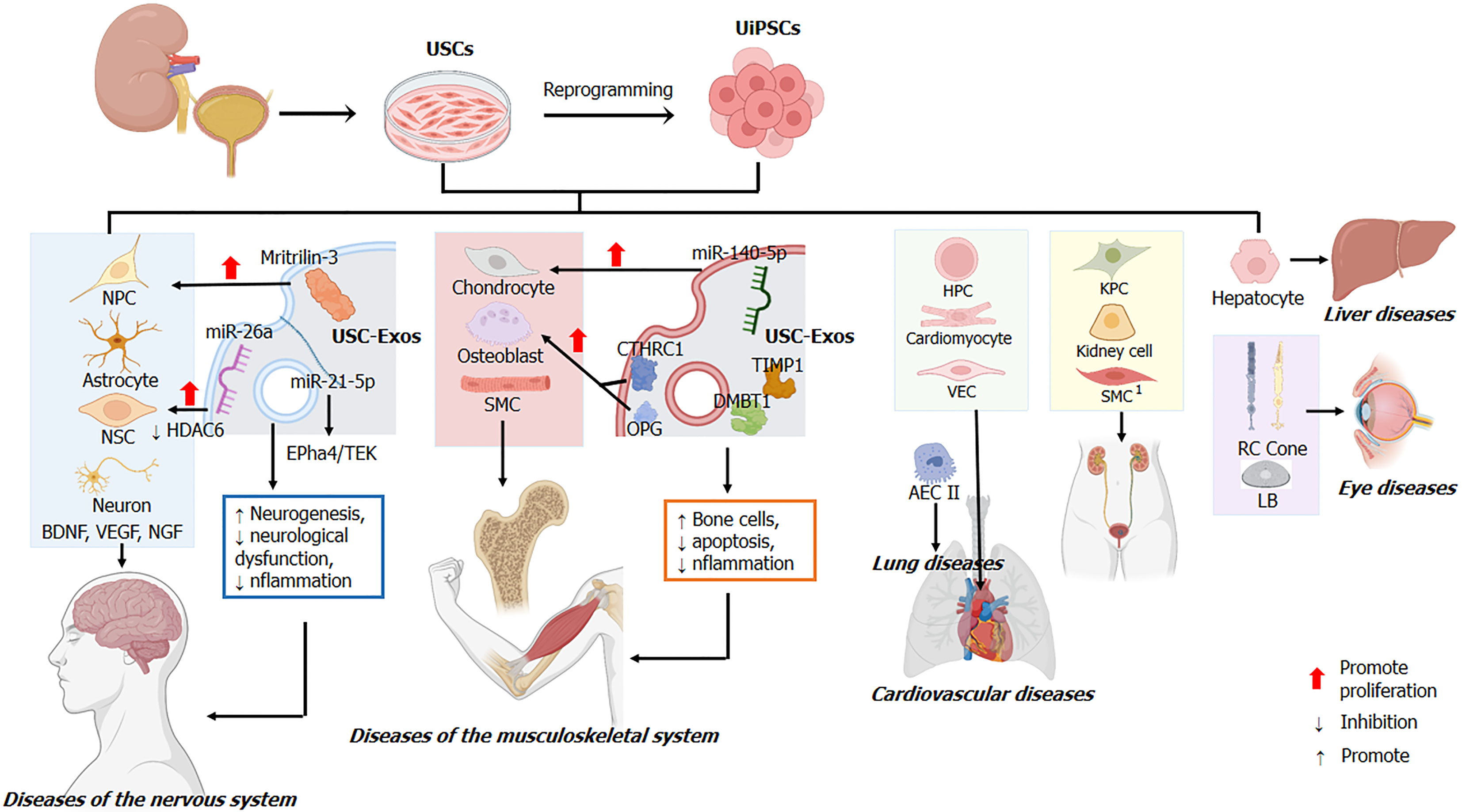
It is reported that USCs and UiPSCs exert the therapeutic effects mainly through the targeted differentiation of desired cells and their exosomes (Figure 2). Firstly, USCs and UiPSCs possess the potential of multidirectional differentiation and can be developed into therapeutic stem cells. As stated above, USCs show high neurogenic, myogenic, and osteogenic differentiation potential[33]. Similar to USCs, UiPSCs could also differentiate into nephrocytes, neurocytes, osteoblasts, chondrocytes, and muscle cells[65-68]. Based on this, USCs/UiPSCs were used for neurological, musculoskeletal, and circulatory diseases (shown in Figure 2). For example, UiPSCs in patients with acute kidney injury could be induced to differentiate into kidney progenitor cells, which could be a new therapeutic strategy for acute kidney injury (Table 3)[69].
| Cells | Differentiation | Factors | Diseases/animal model | Ref. |
| USCs | NPCs | hEGF, bFGF | [28] | |
| Neural lineage cells (astrocytes) | Transplantation | CCI model (rat) | [28] | |
| Neurons | Laminin, PDGF-BB | |||
| VPA, CHIR99021, repsox, forskolin, SP600625, GO6983, Y27632 | ||||
| NSCs | Cocktail (P, F, V, N) and srRNA (with OSKG) | |||
| Osteoblasts | Silver nanoparticles | Bone defect model | ||
| Y-27632 | ||||
| Calcium silicate ionic, BMP2 | ||||
| BMP2, FAK | ||||
| BCP-CS | ||||
| Surface mineralized BCP | [19] | |||
| BMP2-CSM/Col I hydrogel | ||||
| Chondrocytes | Hyaluronic acid with USCs | |||
| Y-27632 | ||||
| ACM scaffolds | ||||
| Muscle cells | VEGF, IGF-1, FGF-1, PDGF-BB, HGF, NGF | |||
| HD myogenic differentiation medium | [103] | |||
| Lentiviral with MYOD | ||||
| hp-HA (IGF-1, HGF, PDGF-BB) | ||||
| Hepatocytes | Co-culture with HPCs | [5] | ||
| UiPSCs | Cardiac myocytes | Activin A, bFGF, BMP4, DKK1, VEGF | [56] | |
| Cardiomyocyte differentiation kit (Gibco, catA2921201) | ||||
| CHIR99021, BIO, KY02111, IWP2 | ||||
| GIWI2 | ||||
| HIR99021, IWP2 | MI (rat) | |||
| KPCs | AKI | [69] | ||
| Kidney organoids | Y-27632, APEL-2, CHIR99021, hFGF9, heparin | |||
| Neurons, astrocytes | Transplantation | SCI model (rat) | ||
| NPCs | N2B27, dorsomorphin, SB4315242 | |||
| HPCs | Flavonoid | |||
| Muscle cells | CHIR99021 | [52] | ||
| Neurons, astrocytes, VECs | Simulate the brain microenvironment of diabetes mellitus | |||
| RCs, CCs | RDM | |||
| LB | BMP, BMP4, BMP7, bFGF, WNT3A | |||
| AECs II | ||||
| Hepatocytes |
Additionally, UiPSCs were induced into functional cardiomyocytes by temporal modulation of canonical wingless/integrated (WNT) signaling with small molecules for the treatment of myocardial infarction[56,70]. Evidence indicated that the combination of WNT activators (CHIR99021 and 6-bromoindole-3-oxime) and WNT inhibitors (KY02111 and inhibitor WNT protein 2) were feasible to induce UiPSCs into cardiomyocytes[71]. Moreover, albumin-free GIWI also induced UiPSCs to differentiate into functional cardiomyocytes (Table 3)[44]. Functionally, transplanting these induced cardiomyocytes improved cardiac function by enhancing glucose metabolism of cardiomyocytes and promoting angiogenesis in the infarction sites[10].
Secondly, USCs/UiPSCs can secrete functional exosomes to achieve therapeutic effects. Exosomes are a class of extracellular vesicles with diameters ranging from 40 nm to 160 nm (average of about 100 nm) and contain a variety of small molecules such as nucleic acids, proteins, lipids, cytokines, and others. Evidence suggested that exosomes play an important role in cell-to-cell communication[72,73]. Moreover, it has been demonstrated that USCs/UiPSCs-derived exosomes (USC-Exos) also promote tissue repair and regeneration in various diseases, including neurological and musculoskeletal diseases. The application of USCs and UiPSCs and the underlying mechanisms in neurological and musculoskeletal diseases are discussed in detail below.
Human NSCs have great prospects for the treatment of neurological diseases. They could differentiate into neurons to repair neurologic impairment[74]. A growing body of evidence indicates that both USCs and UiPSCs could be induced into NSCs and other neural lineages. Firstly, Guan et al[28] induced USCs into neural progenitor cells with human epidermal growth factor, basic fibroblast growth factor, and B27 in vitro. Secondly, laminin and platelet-derived growth factor BB (PDGF-BB) could promote neuronal differentiation of USCs in vitro[75].
Thirdly, the mixture of valproic acid, CHIR99021, repsox, forskolin, SP600625, GO6983, and Y27632 can induce USCs into neuron-like cells[76]. Fourthly, USCs can be transformed into NSCs by combining a cocktail of small molecules (including puromorphamine, folliculin, vitamin C, and sodium butyrate) with self-replicable mRNA delivery encoding the reprogramming factors including OCT4, SOX2, KLF4, and GLIS1 (the four factors are abbreviated as OSKG) (Table 3). The advantage of this method was that the obtained NSCs did not contain impurity genes and held no tumorigenicity[74]. Furthermore, USCs also harbor great potential for neural differentiation in vivo[28]. In summary, USCs and UiPSCs hold considerable therapeutic prospects in neurological diseases.
In neurological diseases, USC therapy facilitates neurological recovery. After sudden cardiac arrest and cardiopulmonary resuscitation, transplanted USCs secreted brain-derived neurotrophic factor and vascular endothelial growth factor to suppress cerebral edema and reduce the expression level of serum S100 calcium-binding protein B in the hippocampus and temporal lobe cortex of rats[77]. In addition, USC-Exos also improved ischemia-induced neurological dysfunction. Intravenous injection of USC-Exos resulted in enhanced neurogenesis and alleviated neurological impairment in stroke rats[11]. Moreover, USC-Exos promoted the proliferation and neuronal differentiation of NSCs after oxygen-glucose deprivation/reoxygenation in vitro. Mechanistically, the pro-neurogenesis effect of USC-Exos might be attributed to the inhibition of histone deacetylase 6 function by exosomal microRNA-26a[11]. Inhibiting histone deacetylase 6 restored microtubule-dependent transport and facilitated neural recovery in neurons[78]. Additionally, USC-Exos-derived miR-21-5p also promoted early neurogenesis via the ephrin receptor A4/TEK receptor tyrosine kinase axis[79].
Beyond that, USC transplantation plays a neuroprotective role in spinal cord injury. Evidence suggested that a considerable proportion of transplanted UiPSCs survived and migrated toward the lesion center of spinal cord injury. They differentiated into neurons and astrocytes to reduce the inflammation and the size of the lesion cavity[80]. In addition, USC transplantation inhibited neuronal apoptosis by promoting the expression of neurotrophic factors (including nerve growth factor and brain-derived neurotrophic factor)[12].
As a frequently occurring disease, lumbar disc herniation is the most common cause of lumbocrural pain[81]. Research showed that USC-Exos inhibited the apoptosis of nucleus pulposus cells and the degeneration of intervertebral disc by controlling protein kinase B and extracellular signal-regulated kinases[82]. Moreover, matrilin-3 carried by USC-Exos also alleviated the degeneration of intervertebral disc and promoted proliferation of nucleus pulposus cells and synthesis of extracellular matrix[83]. In short, USCs-Exos can suppress degenerative changes of the intervertebral disc and promote its structural remodeling.
In addition, since USCs and UiPSCs retain the genetic background of the donor, they are also induced as cell models for neurological diseases and used for pathogenesis study and drug screening. The cell models of paroxysmal kinesigenic dyskinesia[84], diabetic encephalopathy[80], Down’s syndrome[85,86], and neurofibromatosis type 1[87] have been developed.
Mounting evidence demonstrated that USCs could be induced to differentiate into osteoblasts and chondrocytes. Firstly, silver nanoparticles promoted osteogenic differentiation of USCs by activating RhoA protein, inducing actin polymerization and increasing cytoskeletal tension[17]. Secondly, the Rho-associated protein kinase inhibitor Y-27632 promoted USC differentiation into osteoblasts and chondrocytes[15]. Thirdly, calcium silicate ionic extract enhanced osteogenic differentiation of USCs through activating WNT/β-catenin signaling. However, the WNT/β-catenin signaling inhibitor myristin held the opposite effect[88]. Finally, hyaluronic acid was confirmed to facilitate the differentiation of USCs into chondrocytes[89]. Of note, the CD133-positive USC subsets possessed a stronger ability to differentiate into the chon
Bone morphogenetic protein-2 (BMP2), a member of transforming growth factors B superfamily, plays an essential role in skeletal development and osteoblast differentiation[91]. Actually, whether increasing BMP2 expression through lentivirus or co-culturing with BMP2, USCs can be induced to differentiate into the osteogenic lineage[88,92]. Moreover, focal adhesion kinase could enhance BMP2-induced osteogenic differentiation of USCs by activating adenosine 5’-monophosphate-activated protein kinase and WNT signaling[92].
Clinical trials corroborated the osteogenic differentiation potential of USCs. Furthermore, the differentiation ability of USCs was related to the age of the donors. Compared with the elderly group, the USCs from children held a stronger proliferative activity and osteogenic differentiation capacity[30].
Owing to the high osteogenic and chondrogenic differentiation, USCs and UiPSCs are also utilized for the treatment of skeletal diseases. To improve the survival and differentiation ability of USCs in vivo, USCs are generally combined with a biological scaffold or biomaterial and implanted into skeletal lesions[19]. At the defective femur sites, the transplantation of USCs wrapped with β-tricalcium phosphate scaffold induced new bone formation[88]. Moreover, USCs loaded with biphasic calcium phosphate bioceramic ornamented with chitosan sponge could differentiate into osteoblasts and promote bone regeneration[93]. Additionally, USCs loaded with acellular cartilage extracellular matrix scaffolds repaired the defects of the knee articular cartilage, and the nascent tissues were mainly hyaline cartilage[94]. Furthermore, BMP2 enhanced the osteogenic differentiation of USCs in vivo. Note that the high concentration of BMP2 led to ectopic bone formation or inflammation[95], so it was particularly important to control the amount of BMP2. For instance, the inje
As a key messenger of cell-cell interactions, exosomes derived from USCs could promote tissue repair and regeneration[72,73]. In the mouse model of osteoporosis and cranial osteolysis, USC-Exos enhanced osteogenesis and inhibited os
USCs can differentiate myogenic lineage cells in vitro and in vivo under the stimulation of growth factors (including vascular endothelial growth factor, insulin-like growth factor-1, fibroblast growth factor, PDGF, hepatocyte growth factor, nerve growth factor, PDGF-BB, and myogenic differentiation 1)[16,41,101,102], HD myogenic medium[103], and the small molecule CHIR99021[52] (Table 3). One study has demonstrated that subcutaneous injection of the mixture of USCs, alginate microbeads (containing growth factors), and the collagen gel type 1 enhanced revascularization and innervation and stimulated resident cell growth in vivo[16]. Moreover, USCs could be efficiently reprogrammed into skeletal muscle cells by transducing lentiviral vectors that expressed myogenic P.
USCs are a class of stem cells with high proliferation and multidirectional differentiation potential. More importantly, they are easy to obtain and cost-effective. Current evidence suggests that USCs are derived from the kidneys, mainly from glomerular wall epithelial cells, and isolated from urine. The cultured USCs are usually rice-like with the typical morphology of stem cells. Of course, they are also spindle, circle, and pebble-like in shape. Moreover, USCs could be reprogrammed into UiPSCs (shown in Table 2), further expanding the application. Evidence indicates that USCs and UiPSCs express MSC-like markers, including CD29, CD44, CD54, CD73, CD90, CD105, CD166, stromal cell antigen-1, CD24, and CD146 (Table 1). Moreover, the surface markers of USCs and UiPSCs are not limited to MSC-like markers (see Table 1).
Under the stimulation of inducing factors, USCs and UiPSCs can differentiate into neurocytes, osteoblasts, chon
However, the current investigations on the physiological effect of USCs and UiPSCs are insufficient. Firstly, the source of USCs needs to be further determined. There is no direct and strong evidence to prove that it comes from glomerular wall epithelial cells. Identifying the exact source of USCs would facilitate its isolation and application selection. Secondly, the therapeutic value of USCs is evaluated by its proliferative ability. Except for age-related telomerase activity and length, could the proliferation ability of USCs be evaluated by other methods? Moreover, further exploration is needed to determine whether the quantity and quality of USCs obtained are related to gender. Thirdly, it is necessary to explore the potential of USCs and UiPSCs to differentiate into other cells. Fourthly, the effectiveness and safety of the existing reprogramming methods to induce USCs or UCs into UiPSCs need to be further assessed. For instance, the risk of infecting host genes and tumorigenicity needs to be eliminated. Of course, more efficient and secure methods need to be explored. Finally, the current investigation on the treatment of USCs and UiPSCs in neurological and musculoskeletal diseases is still in its infancy. Before USCs or UiPSCs enter clinical application, the effectiveness and safety of them must be determined in vivo, especially in the human body.
We would like to express our gratitude to Professor Mauki for his assistance in language editing of the manuscript.
| 1. | Fiani B, Covarrubias C, Wong A, Doan T, Reardon T, Nikolaidis D, Sarno E. Cerebrolysin for stroke, neurodegeneration, and traumatic brain injury: review of the literature and outcomes. Neurol Sci. 2021;42:1345-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A. Complications of Cushing's syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4:611-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 426] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 3. | Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 937] [Cited by in RCA: 1047] [Article Influence: 149.6] [Reference Citation Analysis (35)] |
| 4. | Culenova M, Nicodemou A, Novakova ZV, Debreova M, Smolinská V, Bernatova S, Ivanisova D, Novotna O, Vasicek J, Varga I, Ziaran S, Danisovic L. Isolation, Culture and Comprehensive Characterization of Biological Properties of Human Urine-Derived Stem Cells. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Hu C, He Y, Fang S, Tian N, Gong M, Xu X, Zhao L, Wang Y, He T, Zhang Y, Bi Y. Urine-derived stem cells accelerate the recovery of injured mouse hepatic tissue. Am J Transl Res. 2020;12:5131-5150. [PubMed] |
| 6. | Li G, Xie B, He L, Zhou T, Gao G, Liu S, Pan G, Ge J, Peng F, Zhong X. Generation of Retinal Organoids with Mature Rods and Cones from Urine-Derived Human Induced Pluripotent Stem Cells. Stem Cells Int. 2018;2018:4968658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Fu Q, Qin Z, Jin X, Zhang L, Chen Z, He J, Ji J, Yao K. Generation of Functional Lentoid Bodies From Human Induced Pluripotent Stem Cells Derived From Urinary Cells. Invest Ophthalmol Vis Sci. 2017;58:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Wang C, Hei F, Ju Z, Yu J, Yang S, Chen M. Differentiation of Urine-Derived Human Induced Pluripotent Stem Cells to Alveolar Type II Epithelial Cells. Cell Reprogram. 2016;18:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Abbas TO, Ali TA, Uddin S. Urine as a Main Effector in Urological Tissue Engineering-A Double-Edged Sword. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Gao Y, Wu S, Pan J, Zhang K, Li X, Xu Y, Jin C, He X, Shi J, Ma L, Wu F, Yao Y, Wang P, He Q, Lan F, Zhang H, Tian M. CRISPR/Cas9-edited triple-fusion reporter gene imaging of dynamics and function of transplanted human urinary-induced pluripotent stem cell-derived cardiomyocytes. Eur J Nucl Med Mol Imaging. 2021;48:708-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Ling X, Zhang G, Xia Y, Zhu Q, Zhang J, Li Q, Niu X, Hu G, Yang Y, Wang Y, Deng Z. Exosomes from human urine-derived stem cells enhanced neurogenesis via miR-26a/HDAC6 axis after ischaemic stroke. J Cell Mol Med. 2020;24:640-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Li Z, Wu H. [Effects of human urine-derived stem cells combined with chondroitinase ABC on the expressions of nerve growth factor and brain-derived neurotrophic factor in the spinal cord injury]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017;31:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Ghori FF, Wahid M. Induced pluripotent stem cells from urine of Duchenne muscular dystrophy patients. Pediatr Int. 2021;63:1038-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Chen CY, Rao SS, Tan YJ, Luo MJ, Hu XK, Yin H, Huang J, Hu Y, Luo ZW, Liu ZZ, Wang ZX, Cao J, Liu YW, Li HM, Chen Y, Du W, Liu JH, Zhang Y, Chen TH, Liu HM, Wu B, Yue T, Wang YY, Xia K, Lei PF, Tang SY, Xie H. Extracellular vesicles from human urine-derived stem cells prevent osteoporosis by transferring CTHRC1 and OPG. Bone Res. 2019;7:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 15. | Kim K, Gil M, Dayem AA, Choi S, Kang GH, Yang GM, Cho S, Jeong Y, Kim SJ, Seok J, Kwak HJ, Saha SK, Kim A, Cho SG. Improved Isolation and Culture of Urine-Derived Stem Cells (USCs) and Enhanced Production of Immune Cells from the USC-Derived Induced Pluripotent Stem Cells. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Liu G, Pareta RA, Wu R, Shi Y, Zhou X, Liu H, Deng C, Sun X, Atala A, Opara EC, Zhang Y. Skeletal myogenic differentiation of urine-derived stem cells and angiogenesis using microbeads loaded with growth factors. Biomaterials. 2013;34:1311-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Qin H, Zhu C, An Z, Jiang Y, Zhao Y, Wang J, Liu X, Hui B, Zhang X, Wang Y. Silver nanoparticles promote osteogenic differentiation of human urine-derived stem cells at noncytotoxic concentrations. Int J Nanomedicine. 2014;9:2469-2478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Lang R, Liu G, Shi Y, Bharadwaj S, Leng X, Zhou X, Liu H, Atala A, Zhang Y. Self-renewal and differentiation capacity of urine-derived stem cells after urine preservation for 24 hours. PLoS One. 2013;8:e53980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Xing F, Li L, Sun J, Liu G, Duan X, Chen J, Liu M, Long Y, Xiang Z. Surface mineralized biphasic calcium phosphate ceramics loaded with urine-derived stem cells are effective in bone regeneration. J Orthop Surg Res. 2019;14:419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Li X, Liao J, Su X, Li W, Bi Z, Wang J, Su Q, Huang H, Wei Y, Gao Y, Li J, Liu L, Wang C. Human urine-derived stem cells protect against renal ischemia/reperfusion injury in a rat model via exosomal miR-146a-5p which targets IRAK1. Theranostics. 2020;10:9561-9578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 21. | Wu R, Soland M, Liu G, Shi Y, Zhang C, Tang Y, Almeida-Porada G, Zhang Y. Functional characterization of the immunomodulatory properties of human urine-derived stem cells. Transl Androl Urol. 2021;10:3566-3578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Wu C, Chen L, Huang YZ, Huang Y, Parolini O, Zhong Q, Tian X, Deng L. Comparison of the Proliferation and Differentiation Potential of Human Urine-, Placenta Decidua Basalis-, and Bone Marrow-Derived Stem Cells. Stem Cells Int. 2018;2018:7131532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Bharadwaj S, Liu G, Shi Y, Wu R, Yang B, He T, Fan Y, Lu X, Zhou X, Liu H, Atala A, Rohozinski J, Zhang Y. Multipotential differentiation of human urine-derived stem cells: potential for therapeutic applications in urology. Stem Cells. 2013;31:1840-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 234] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 24. | Chun SY, Kim HT, Lee JS, Kim MJ, Kim BS, Kim BW, Kwon TG. Characterization of urine-derived cells from upper urinary tract in patients with bladder cancer. Urology. 2012;79:1186.e1-1186.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Qin D, Long T, Deng J, Zhang Y. Urine-derived stem cells for potential use in bladder repair. Stem Cell Res Ther. 2014;5:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Zhang D, Wei G, Li P, Zhou X, Zhang Y. Urine-derived stem cells: A novel and versatile progenitor source for cell-based therapy and regenerative medicine. Genes Dis. 2014;1:8-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 27. | Chen AJ, Pi JK, Hu JG, Huang YZ, Gao HW, Li SF, Li-Ling J, Xie HQ. Identification and characterization of two morphologically distinct stem cell subpopulations from human urine samples. Sci China Life Sci. 2020;63:712-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Guan JJ, Niu X, Gong FX, Hu B, Guo SC, Lou YL, Zhang CQ, Deng ZF, Wang Y. Biological characteristics of human-urine-derived stem cells: potential for cell-based therapy in neurology. Tissue Eng Part A. 2014;20:1794-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Zhang N, Zhao L, Liu D, Hu C, Wang Y, He T, Bi Y, He Y. Characterization of Urine-Derived Stem Cells from Patients with End-Stage Liver Diseases and Application to Induced Acute and Chronic Liver Injury of Nude Mice Model. Stem Cells Dev. 2021;30:1126-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Gao P, Han P, Jiang D, Yang S, Cui Q, Li Z. Effects of the donor age on proliferation, senescence and osteogenic capacity of human urine-derived stem cells. Cytotechnology. 2017;69:751-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Yang X, Xiong X, Zhou W, Feng G, Zhang Y, Dai H, Zhou J. Effects of human urine-derived stem cells on the cementogenic differentiation of indirectly-cocultured periodontal ligament stem cells. Am J Transl Res. 2020;12:361-378. [PubMed] |
| 32. | Shi Y, Liu G, Wu R, Mack DL, Sun XS, Maxwell J, Guan X, Atala A, Zhang Y. Differentiation Capacity of Human Urine-Derived Stem Cells to Retain Telomerase Activity. Front Cell Dev Biol. 2022;10:890574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Kang HS, Choi SH, Kim BS, Choi JY, Park GB, Kwon TG, Chun SY. Advanced Properties of Urine Derived Stem Cells Compared to Adipose Tissue Derived Stem Cells in Terms of Cell Proliferation, Immune Modulation and Multi Differentiation. J Korean Med Sci. 2015;30:1764-1776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Ferreiro E, Monteiro M, Pereira F, Barroso C, Egas C, Macedo P, Valero J, Sardão VA, Oliveira PJ. Age-dependent energy metabolism and transcriptome changes in urine-derived stem cells. Mech Ageing Dev. 2024;218:111912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 35. | Chun SY, Kim HT, Kwon SY, Kim J, Kim BS, Yoo ES, Kwon TG. The efficacy and safety of Collagen-I and hypoxic conditions in urine-derived stem cell ex vivo culture. Tissue Eng Regen Med. 2016;13:403-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Luo S, Shao L, Geng R, Liu Q, Jiang W, Gong M, Zhang Y, He Y. Identification and biological characteristics of clear cell renal cell carcinoma associated urine-derived stem cells. Am J Transl Res. 2021;13:2143-2162. [PubMed] |
| 37. | Kim SH, Lee SH, Jin JA, So HJ, Lee JU, Ji MJ, Kwon EJ, Han PS, Lee HK, Kang TW. In vivo safety and biodistribution profile of Klotho-enhanced human urine-derived stem cells for clinical application. Stem Cell Res Ther. 2023;14:355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 38. | Hwang Y, Cha SH, Hong Y, Jung AR, Jun HS. Direct differentiation of insulin-producing cells from human urine-derived stem cells. Int J Med Sci. 2019;16:1668-1676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Kenigsberg Z, Welch RC, Bejoy J, Williams FM, Veach RA, Jarrett I, Thompson TK, Wilson MH, Woodard LE. Genome Engineering of Human Urine-Derived Stem Cells to Express Lactoferrin and Deoxyribonuclease. Tissue Eng Part A. 2023;29:372-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal Stem Cell Migration and Tissue Repair. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 719] [Article Influence: 102.7] [Reference Citation Analysis (36)] |
| 41. | Liu G, Wu R, Yang B, Shi Y, Deng C, Atala A, Mou S, Criswell T, Zhang Y. A cocktail of growth factors released from a heparin hyaluronic-acid hydrogel promotes the myogenic potential of human urine-derived stem cells in vivo. Acta Biomater. 2020;107:50-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 42. | Lu D, Xue Y, Song B, Liu N, Xie Y, Cheng Y, Chen B, He L, Chen D, Zeng J, Yang Y, Guo X, Li Y, Sun X. Generation of induced pluripotent stem cell GZLSL-i001-A derived from urine-derived cells of Hemophilia A patient with Inv22 mutation. Stem Cell Res. 2020;49:102053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 43. | Boonkaew B, Thummavichit W, Netsrithong R, Vatanashevanopakorn C, Pattanapanyasat K, Wattanapanitch M. Establishment of an integration-free induced pluripotent stem cell line (MUSIi005-A) from exfoliated renal epithelial cells. Stem Cell Res. 2018;30:34-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Huang P, Zhu J, Liu Y, Liu G, Zhang R, Li D, Pei D, Zhu P. Identification of New Transcription Factors that Can Promote Pluripotent Reprogramming. Stem Cell Rev Rep. 2021;17:2223-2234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 45. | Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7589] [Cited by in RCA: 7333] [Article Influence: 385.9] [Reference Citation Analysis (0)] |
| 46. | Wang B, Wu L, Li D, Liu Y, Guo J, Li C, Yao Y, Wang Y, Zhao G, Wang X, Fu M, Liu H, Cao S, Wu C, Yu S, Zhou C, Qin Y, Kuang J, Ming J, Chu S, Yang X, Zhu P, Pan G, Chen J, Liu J, Pei D. Induction of Pluripotent Stem Cells from Mouse Embryonic Fibroblasts by Jdp2-Jhdm1b-Mkk6-Glis1-Nanog-Essrb-Sall4. Cell Rep. 2019;27:3473-3485.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Li D, Wang L, Hou J, Shen Q, Chen Q, Wang X, Du J, Cai X, Shan Y, Zhang T, Zhou T, Shi X, Li Y, Zhang H, Pan G. Optimized Approaches for Generation of Integration-free iPSCs from Human Urine-Derived Cells with Small Molecules and Autologous Feeder. Stem Cell Reports. 2016;6:717-728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 48. | Afzal MZ, Strande JL. Generation of induced pluripotent stem cells from muscular dystrophy patients: efficient integration-free reprogramming of urine derived cells. J Vis Exp. 2015;52032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Geuder J, Wange LE, Janjic A, Radmer J, Janssen P, Bagnoli JW, Müller S, Kaul A, Ohnuki M, Enard W. A non-invasive method to generate induced pluripotent stem cells from primate urine. Sci Rep. 2021;11:3516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 50. | Afzal MZ, Gartz M, Klyachko EA, Khan SS, Shah SJ, Gupta S, Shapiro AD, Vaughan DE, Strande JL. Generation of human iPSCs from urine derived cells of a non-affected control subject. Stem Cell Res. 2017;18:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Ncube A, Bewersdorf L, Spitzhorn LS, Loerch C, Bohndorf M, Graffmann N, May L, Amzou S, Fromme M, Wruck W, Strnad P, Adjaye J. Generation of two Alpha-I antitrypsin deficiency patient-derived induced pluripotent stem cell lines ISRM-AATD-iPSC-1 (HHUUKDi011-A) and ISRM-AATD-iPSC-2 (HHUUKDi012-A). Stem Cell Res. 2023;71:103171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 52. | Kibschull M, Nguyen TTN, Chow T, Alarab M, Lye SJ, Rogers I, Shynlova O. Differentiation of patient-specific void urine-derived human induced pluripotent stem cells to fibroblasts and skeletal muscle myocytes. Sci Rep. 2023;13:4746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 53. | Gaignerie A, Lefort N, Rousselle M, Forest-Choquet V, Flippe L, Francois-Campion V, Girardeau A, Caillaud A, Chariau C, Francheteau Q, Derevier A, Chaubron F, Knöbel S, Gaborit N, Si-Tayeb K, David L. Urine-derived cells provide a readily accessible cell type for feeder-free mRNA reprogramming. Sci Rep. 2018;8:14363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 54. | Wang L, Chen Y, Guan C, Zhao Z, Li Q, Yang J, Mo J, Wang B, Wu W, Yang X, Song L, Li J. Using low-risk factors to generate non-integrated human induced pluripotent stem cells from urine-derived cells. Stem Cell Res Ther. 2017;8:245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Steinle H, Weber M, Behring A, Mau-Holzmann U, von Ohle C, Popov AF, Schlensak C, Wendel HP, Avci-Adali M. Reprogramming of Urine-Derived Renal Epithelial Cells into iPSCs Using srRNA and Consecutive Differentiation into Beating Cardiomyocytes. Mol Ther Nucleic Acids. 2019;17:907-921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Jiang YF, Chen M, Zhang NN, Yang HJ, Rui Q, Zhou YF. In vitro and in vivo differentiation of induced pluripotent stem cells generated from urine-derived cells into cardiomyocytes. Biol Open. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Qi Z, Cui Y, Shi L, Luan J, Zhou X, Han J. Generation of urine-derived induced pluripotent stem cells from a patient with phenylketonuria. Intractable Rare Dis Res. 2018;7:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Baliña-Sánchez C, Aguilera Y, Adán N, Sierra-Párraga JM, Olmedo-Moreno L, Panadero-Morón C, Cabello-Laureano R, Márquez-Vega C, Martín-Montalvo A, Capilla-González V. Generation of mesenchymal stromal cells from urine-derived iPSCs of pediatric brain tumor patients. Front Immunol. 2023;14:1022676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 59. | Yang X, Zhou T, Zhang H, Li Y, Dong R, Liu N, Pan G, Liu Y, Gai Z. Generation of an induced pluripotent stem cell line (SDQLCHi008-A) from a patient with ASD and DD carrying an 830 kb de novo deletion at chr7q11.22 including the exon 1 of AUTS2 gene. Stem Cell Res. 2019;40:101557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 60. | Guo X, Ji W, Niu C, Ding Y, Chen Z, Chen C, Tong H, Han Z, Chu M. Generation of an urine-derived induced pluripotent stem cell line from a 5-year old X-linked Alport syndrome (X-LAS) patient. Stem Cell Res. 2020;49:102085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Wang L, Gao B, Mo X, Guo X, Huang J. Generation of an urine-derived induced pluripotent stem cell line from a 6-year old X-linked adrenoleukodystrophy (X-ALD) patient. Stem Cell Res. 2021;51:102170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 62. | Zhou Y, Cui Z, Jing Y, Mao S, Chen D, Ding C, Gu J, Chan HF, Tang S, Chen J. Establishment of non-integrate induced pluripotent stem cell line CSUASOi006-A, from urine-derived cells of a PRPF8-related dominant retinitis pigmentosa patient. Stem Cell Res. 2020;49:102041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 63. | Yan X, Guo Y, Chen J, Cui Z, Gu J, Wang Y, Mao S, Ding C, Chen J, Tang S. Establishment of CSUASOi001-A, a non-integrated induced pluripotent stem cell line from urine-derived cells of a Chinese patient carrying RS1 gene mutation. Stem Cell Res. 2019;38:101466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | Qi Z, Cui Y, Shi L, Wang J, Zhao Q, Luan J, Han J. Generation of a non-integrated induced pluripotent stem cell line (SMBCi009-A) from urine-derived cells of a Chinese Familial hypercholesterolemia patient. Stem Cell Res. 2022;59:102624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Zhou T, Benda C, Dunzinger S, Huang Y, Ho JC, Yang J, Wang Y, Zhang Y, Zhuang Q, Li Y, Bao X, Tse HF, Grillari J, Grillari-Voglauer R, Pei D, Esteban MA. Generation of human induced pluripotent stem cells from urine samples. Nat Protoc. 2012;7:2080-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 448] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 66. | Mulder J, Sharmin S, Chow T, Rodrigues DC, Hildebrandt MR, D'Cruz R, Rogers I, Ellis J, Rosenblum ND. Generation of infant- and pediatric-derived urinary induced pluripotent stem cells competent to form kidney organoids. Pediatr Res. 2020;87:647-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 67. | Liu A, Kang S, Yu P, Shi L, Zhou L. Transplantation of human urine-derived neural progenitor cells after spinal cord injury in rats. Neurosci Lett. 2020;735:135201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Shi L, Cui Y, Zhou X, Luan J, Wang L, Han J. Comparative transcriptomic analysis identifies reprogramming and differentiation genes differentially expressed in UiPSCs and ESCs. Biosci Trends. 2017;11:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 69. | Jin Y, Zhang M, Li M, Zhang H, Zhang F, Zhang H, Yin Z, Zhou M, Wan X, Li R, Cao C. Generation of Urine-Derived Induced Pluripotent Stem Cell Line from Patients with Acute Kidney Injury. Cell Reprogram. 2021;23:290-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 70. | Ghori FF, Wahid M. Induced pluripotent stem cells derived cardiomyocytes from Duchenne Muscular Dystrophy patients in vitro. Pak J Med Sci. 2021;37:1376-1381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 71. | Cao Y, Xu J, Wen J, Ma X, Liu F, Li Y, Chen W, Sun L, Wu Y, Li S, Li J, Huang G. Generation of a Urine-Derived Ips Cell Line from a Patient with a Ventricular Septal Defect and Heart Failure and the Robust Differentiation of These Cells to Cardiomyocytes via Small Molecules. Cell Physiol Biochem. 2018;50:538-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Xing F, Liu G, Duan X, Xiang Z. [The application of urine derived stem cells in regeneration of musculoskeletal system]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018;32:1477-1482. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 73. | Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6920] [Cited by in RCA: 7471] [Article Influence: 1245.2] [Reference Citation Analysis (4)] |
| 74. | Kang PJ, Son D, Ko TH, Hong W, Yun W, Jang J, Choi JI, Song G, Lee J, Kim IY, You S. mRNA-Driven Generation of Transgene-Free Neural Stem Cells from Human Urine-Derived Cells. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Kim JY, Chun SY, Park JS, Chung JW, Ha YS, Lee JN, Kwon TG. Laminin and Platelet-Derived Growth Factor-BB Promote Neuronal Differentiation of Human Urine-Derived Stem Cells. Tissue Eng Regen Med. 2018;15:195-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Liu D, Rychkov G, Al-Hawwas M, Manaph NPA, Zhou F, Bobrovskaya L, Liao H, Zhou XF. Conversion of human urine-derived cells into neuron-like cells by small molecules. Mol Biol Rep. 2020;47:2713-2722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 77. | Pan C, Zheng X, Wang L, Chen Q, Lin Q. Pretreatment with human urine-derived stem cells protects neurological function in rats following cardiopulmonary resuscitation after cardiac arrest. Exp Ther Med. 2020;20:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 78. | Richter-Landsberg C, Leyk J. Inclusion body formation, macroautophagy, and the role of HDAC6 in neurodegeneration. Acta Neuropathol. 2013;126:793-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 79. | Pan W, Xu X, Zhang M, Song X. Human urine-derived stem cell-derived exosomal miR-21-5p promotes neurogenesis to attenuate Rett syndrome via the EPha4/TEK axis. Lab Invest. 2021;101:824-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 80. | Liu W, Zhang P, Tan J, Lin Y. Differentiation of Urine-Derived Induced Pluripotent Stem Cells to Neurons, Astrocytes, and Microvascular Endothelial Cells from a Diabetic Patient. Cell Reprogram. 2020;22:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Yu P, Mao F, Chen J, Ma X, Dai Y, Liu G, Dai F, Liu J. Characteristics and mechanisms of resorption in lumbar disc herniation. Arthritis Res Ther. 2022;24:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 91] [Reference Citation Analysis (0)] |
| 82. | Xiang H, Su W, Wu X, Chen W, Cong W, Yang S, Liu C, Qiu C, Yang SY, Wang Y, Zhang G, Guo Z, Xing D, Chen B. Exosomes Derived from Human Urine-Derived Stem Cells Inhibit Intervertebral Disc Degeneration by Ameliorating Endoplasmic Reticulum Stress. Oxid Med Cell Longev. 2020;2020:6697577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 83. | Guo Z, Su W, Zhou R, Zhang G, Yang S, Wu X, Qiu C, Cong W, Shen N, Guo J, Liu C, Yang SY, Xing D, Wang Y, Chen B, Xiang H. Exosomal MATN3 of Urine-Derived Stem Cells Ameliorates Intervertebral Disc Degeneration by Antisenescence Effects and Promotes NPC Proliferation and ECM Synthesis by Activating TGF-β. Oxid Med Cell Longev. 2021;2021:5542241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 84. | Zhang SZ, Li HF, Ma LX, Qian WJ, Wang ZF, Wu ZY. Urine-derived induced pluripotent stem cells as a modeling tool for paroxysmal kinesigenic dyskinesia. Biol Open. 2015;4:1744-1752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 85. | M Lee Y, Zampieri BL, Scott-McKean JJ, Johnson MW, Costa ACS. Generation of Integration-Free Induced Pluripotent Stem Cells from Urine-Derived Cells Isolated from Individuals with Down Syndrome. Stem Cells Transl Med. 2017;6:1465-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 86. | Teles E Silva AL, Yokota BY, Sertié AL, Zampieri BL. Generation of Urine-Derived Induced Pluripotent Stem Cells and Cerebral Organoids for Modeling Down Syndrome. Stem Cell Rev Rep. 2023;19:1116-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 87. | Shi L, Cui Y, Qi Z, Zhou X, Luan J, Han J. Generation of two non-integrated induced pluripotent stem cell lines from urine-derived cells of a Chinese patient carrying NF1 gene mutation. Stem Cell Res. 2020;46:101842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 88. | Guan J, Zhang J, Li H, Zhu Z, Guo S, Niu X, Wang Y, Zhang C. Human Urine Derived Stem Cells in Combination with β-TCP Can Be Applied for Bone Regeneration. PLoS One. 2015;10:e0125253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 89. | Chen L, Li L, Xing F, Peng J, Peng K, Wang Y, Xiang Z. Human Urine-Derived Stem Cells: Potential for Cell-Based Therapy of Cartilage Defects. Stem Cells Int. 2018;2018:4686259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 90. | Tong X, Xu Y, Zhang T, Deng C, Xun J, Sun D, Xu D. Exosomes from CD133(+) human urine-derived stem cells combined adhesive hydrogel facilitate rotator cuff healing by mediating bone marrow mesenchymal stem cells. J Orthop Translat. 2023;39:100-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 91. | Reyes R, Rodríguez JA, Orbe J, Arnau MR, Évora C, Delgado A. Combined sustained release of BMP2 and MMP10 accelerates bone formation and mineralization of calvaria critical size defect in mice. Drug Deliv. 2018;25:750-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 92. | Sun X, Zheng W, Qian C, Wu Q, Hao Y, Lu G. Focal adhesion kinase promotes BMP2-induced osteogenic differentiation of human urinary stem cells via AMPK and Wnt signaling pathways. J Cell Physiol. 2020;235:4954-4964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 93. | Liu G, Sun J, Gong M, Xing F, Wu S, Xiang Z. Urine-derived stem cells loaded onto a chitosan-optimized biphasic calcium-phosphate scaffold for repairing large segmental bone defects in rabbits. J Biomed Mater Res B Appl Biomater. 2021;109:2014-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 94. | Sun J, Xing F, Zou M, Gong M, Li L, Xiang Z. Comparison of chondrogenesis-related biological behaviors between human urine-derived stem cells and human bone marrow mesenchymal stem cells from the same individual. Stem Cell Res Ther. 2021;12:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 95. | Zara JN, Siu RK, Zhang X, Shen J, Ngo R, Lee M, Li W, Chiang M, Chung J, Kwak J, Wu BM, Ting K, Soo C. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A. 2011;17:1389-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 469] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 96. | Wu S, Chen Z, Yu X, Duan X, Chen J, Liu G, Gong M, Xing F, Sun J, Huang S, Xiang Z. A sustained release of BMP2 in urine-derived stem cells enhances the osteogenic differentiation and the potential of bone regeneration. Regen Biomater. 2022;9:rbac015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 97. | Li H, Fan XL, Wang YN, Lu W, Wang H, Liao R, Zeng M, Yang JX, Hu Y, Xie J. Extracellular Vesicles from Human Urine-Derived Stem Cells Ameliorate Particulate Polyethylene-Induced Osteolysis. Int J Nanomedicine. 2021;16:7479-7494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | Xie J, Hu Y, Li H, Wang Y, Fan X, Lu W, Liao R, Wang H, Cheng Y, Yang Y, Wang J, Liang S, Ma T, Su W. Targeted therapy for peri-prosthetic osteolysis using macrophage membrane-encapsulated human urine-derived stem cell extracellular vesicles. Acta Biomater. 2023;160:297-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 99. | Chen CY, Du W, Rao SS, Tan YJ, Hu XK, Luo MJ, Ou QF, Wu PF, Qing LM, Cao ZM, Yin H, Yue T, Zhan CH, Huang J, Zhang Y, Liu YW, Wang ZX, Liu ZZ, Cao J, Liu JH, Hong CG, He ZH, Yang JX, Tang SY, Tang JY, Xie H. Extracellular vesicles from human urine-derived stem cells inhibit glucocorticoid-induced osteonecrosis of the femoral head by transporting and releasing pro-angiogenic DMBT1 and anti-apoptotic TIMP1. Acta Biomater. 2020;111:208-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 100. | Liu Y, Zeng Y, Si HB, Tang L, Xie HQ, Shen B. Exosomes Derived From Human Urine-Derived Stem Cells Overexpressing miR-140-5p Alleviate Knee Osteoarthritis Through Downregulation of VEGFA in a Rat Model. Am J Sports Med. 2022;50:1088-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 101. | Kim EY, Page P, Dellefave-Castillo LM, McNally EM, Wyatt EJ. Direct reprogramming of urine-derived cells with inducible MyoD for modeling human muscle disease. Skelet Muscle. 2016;6:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 102. | Talmon M, Massara E, Pruonto G, Quaregna M, Boccafoschi F, Riva B, Fresu LG. Characterization of a functional Ca(2+) toolkit in urine-derived stem cells and derived skeletal muscle cells. Cell Calcium. 2022;103:102548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 103. | Chen W, Xie M, Yang B, Bharadwaj S, Song L, Liu G, Yi S, Ye G, Atala A, Zhang Y. Skeletal myogenic differentiation of human urine-derived cells as a potential source for skeletal muscle regeneration. J Tissue Eng Regen Med. 2017;11:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/