Published online Dec 18, 2019. doi: 10.5312/wjo.v10.i12.434
Peer-review started: June 11, 2019
First decision: July 30, 2019
Revised: August 27, 2019
Accepted: September 13, 2019
Article in press: September 13, 2019
Published online: December 18, 2019
Processing time: 184 Days and 5.9 Hours
Clopidogrel is a widely prescribed drug for prevention of myocardial infarction and stroke in patients at risk. It inhibits thrombus formation via inhibition of the P2Y12 purinergic receptor on platelets, which is important in their activation by ADP. However, the P2Y12 receptor has also been found to be expressed in both osteoblasts and osteoclasts. Accumulated evidence suggests that purinergic receptors regulate important functions of bone turnover. Previous studies on the effect of clopidogrel on bone metabolism indicated potential harmful effects, but their results remain conflicting. Thus, clopidogrel treatment may affect bone healing, but it has not yet been studied.
To evaluate if continuous perioperative clopidogrel treatment has any negative effect on bone healing in the rabbit calvarial defect model.
Sixteen male white New Zealand rabbits were randomly assigned in two groups: One group received daily 3 mg/kg of clopidogrel per os and the other group received the vehicle alone for a week prior to the surgical procedures; the treatments were continued for another 6 wk postoperatively. The surgical procedures included generation of two circular calvarial defects 11 mm in diameter in every animal. After the 6-wk period of healing, postmortem radiographic and histomorphometric evaluation of the defects was performed.
Both the surgical procedures and the postoperative period were uneventful and well tolerated by all the animals, without any surgical wound dehiscence, signs of infection or other complication. New bone was formed either inwards from the defect margins or in the central portion of the defect as separated bony islets. While defect healing was still incomplete in both groups, the clopidogrel group had significantly improved radiographic healing scores. Moreover, the histomorphometric analysis showed that bone regeneration (%) was 28.07 ± 7.7 for the clopidogrel group and 19.47 ± 4.9 for the control group, showing a statistically significant difference between them (P = 0.018). Statistically significant difference was also found in the defect bridging (%), i.e. 72.17 ± 21.2 for the clopidogrel group and 41.17 ± 8.5 for the control group, respectively (P = 0.004), whereas there was no statistical difference in bone tissue density between the groups.
Our results indicate that maintenance of perioperative clopidogrel treatment does not negatively affect bone healing but rather promotes it. Further research is needed in order to find useful applications of this finding.
Core tip: To our knowledge, the present study is the first to evaluate the effect of clopidogrel treatment on bone healing. Clopidogrel is an antithrombotic drug that inhibits platelet aggregation through inhibition of the P2Y12 purinergic receptor on their surface. The P2Y12 is also expressed in osteoblasts and osteoclasts, and previous studies have raised concerns about clopidogrel’s possible effect on bone metabolism. We report our results on the effect of clopidogrel on bone healing using the rabbit calvarial defect model. Our results indicate that clopidogrel treatment does not negatively affect bone healing but rather promotes it.
- Citation: Lillis T, Veis A, Sakellaridis N, Tsirlis A, Dailiana Z. Effect of clopidogrel in bone healing-experimental study in rabbits. World J Orthop 2019; 10(12): 434-445
- URL: https://www.wjgnet.com/2218-5836/full/v10/i12/434.htm
- DOI: https://dx.doi.org/10.5312/wjo.v10.i12.434
Clopidogrel is a thienopyridine antiplatelet drug widely prescribed for the prevention of thrombotic events such as myocardial infarction and stroke[1]. Following metabolic transformation in liver, clopidogrel active metabolite (CAM) inhibits the platelet purinergic receptor P2Y12 and, therefore, blocks ADP-induced platelet aggregation[2].
The purinergic signaling system is found in almost all tissues and is involved in several important cellular functions such as migration, proliferation, apoptosis and cytokine secretion[3]. The purinergic system is an autocrine and paracrine signaling system, where extracellular purines and pyrimidines act as extracellular signaling molecules, affecting several receptors subtypes[4]. Purinergic receptors are classified into two groups: P1 (further subdivided into A1, A2A, A2B and A3) and P2 (further subdivided into P2X ligand-gated ion channel receptors and P2YG-protein-coupled receptors). P1 receptors are activated by adenosine and P2 receptors by ATP, UDP and their breakdown products[5]. Research on purinergic signaling in bone has gained a lot of attention in recent years, but its role in bone healing and turnover has not been fully elucidated[6]. In particular, the expression of the P2Y12 receptor on osteoblasts and osteoclasts has been demonstrated as well[7-9]. The few existing in vivo studies on the effect of clopidogrel on bone remodeling or osteoporosis show contrasting results, with some of them indicating positive[9,10] and others indicating negative[8,11] impact. For example, Su et al[9] report that clopidogrel treatment increased trabecular bone volume in adult ovariectomized mice, while Syberg et al[8] reported that it decreased the same parameter. Moreover, the effect of the P2Y12 inhibitor clopidogrel on bone healing, when administered systematically, has not been studied yet.
Over the past years, a large body of medical literature in various specialties dealing with bone surgery suggests perioperative maintenance of antiplatelet therapy, whenever possible, in order to avert any thrombotic risk caused by temporary antiplatelet discontinuation[12-15]. On the other hand, when dealing with skeletal surgery, clopidogrel may affect bone healing either directly, by acting on osteoblasts and/or osteoclasts[7,9], or indirectly, by acting on platelets that are known to have an important role in early stages of bone healing[16]. With this background, we undertook this study to evaluate if perioperative systemic administration of clopidogrel produces any negative effect on spontaneous healing of rabbit calvarial defects, which model clinical scenarios of bone defect healing in patients receiving clopidogrel for cardiovascular indications.
The present study was performed on 16 male New Zealand white rabbits that were housed at the institutional animal center. The animals were 6 mo old (mean body weight of 4.8 kg) and were acclimated for at least 1 wk before the experimental procedures, housed in individual cages, and fed with a standard laboratory ad libitum diet. The animal protocol was designed to minimize pain or discomfort to the animals, and it was approved by the Institutional Project Evaluation Committee and authorized by the local Prefectural Veterinary Service according to Directive 2010/63/EU and national law. The animals were randomly assigned in two groups of eight rabbits: An experimental (clopidogrel) group and a control group. Two calvarial defects were created in each animal, and, therefore, each group included 16 defects. The rabbits of clopidogrel group received a daily dose of 3 mg/kg, which has been shown to cause similar level of platelet aggregation inhibition with that of 75 mg in humans[17]. Clopidogrel was added to fruit juice and was administered orally to the rabbits via syringe daily, for 1 preoperative week and 6 postoperative weeks, while the rabbits of the control group received fruit juice without clopidogrel.
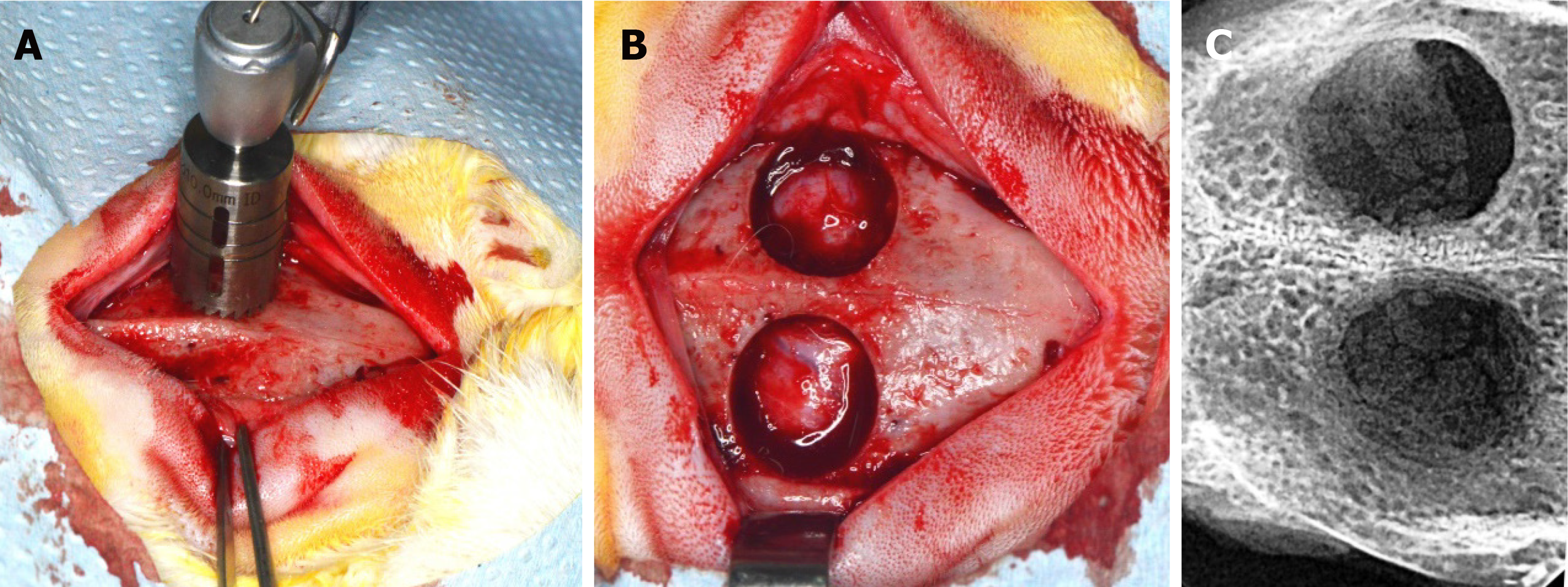
The surgical procedures were performed 1 wk after the beginning of fruit juice and drug administration. Every animal received antibiotic prophylaxis (enrofloxacin 10 mg/kg subcutaneously) 1 hour before general anesthesia (ketamine 20-30 mg/kg intramuscularly and xylazine2-5 mg/kg intramuscularly) and surgery. The surgical procedures were performed under proper aseptic technique, and the skull of every rabbit was shaved, while the skin was disinfected with povidone iodine solution. The parietal bones were exposed through an incision along the sagittal midline of the cranium. Two circular cranial defects were created using a trephine burr, 11 mm in diameter, on both sides of the sagittal suture (Figure 1). Special care was taken in order to avoid damage of their dura matter. This size of defect is considered to be critical in the 6-wk healing period[18,19]. The flaps were then sutured back in place layer by layer with resorbable suture to cover the defects with intact periosteum, in order to heal spontaneously without placing any barrier membrane or bone substitute. After 6 wk, the animals were euthanized by anesthesia overdose followed by whole body perfusion fixation with 10% neutral buffered formalin. Following euthanasia, the portions of cranial bones containing the defects were block-sectioned, and radiographic and histologic evaluation followed.
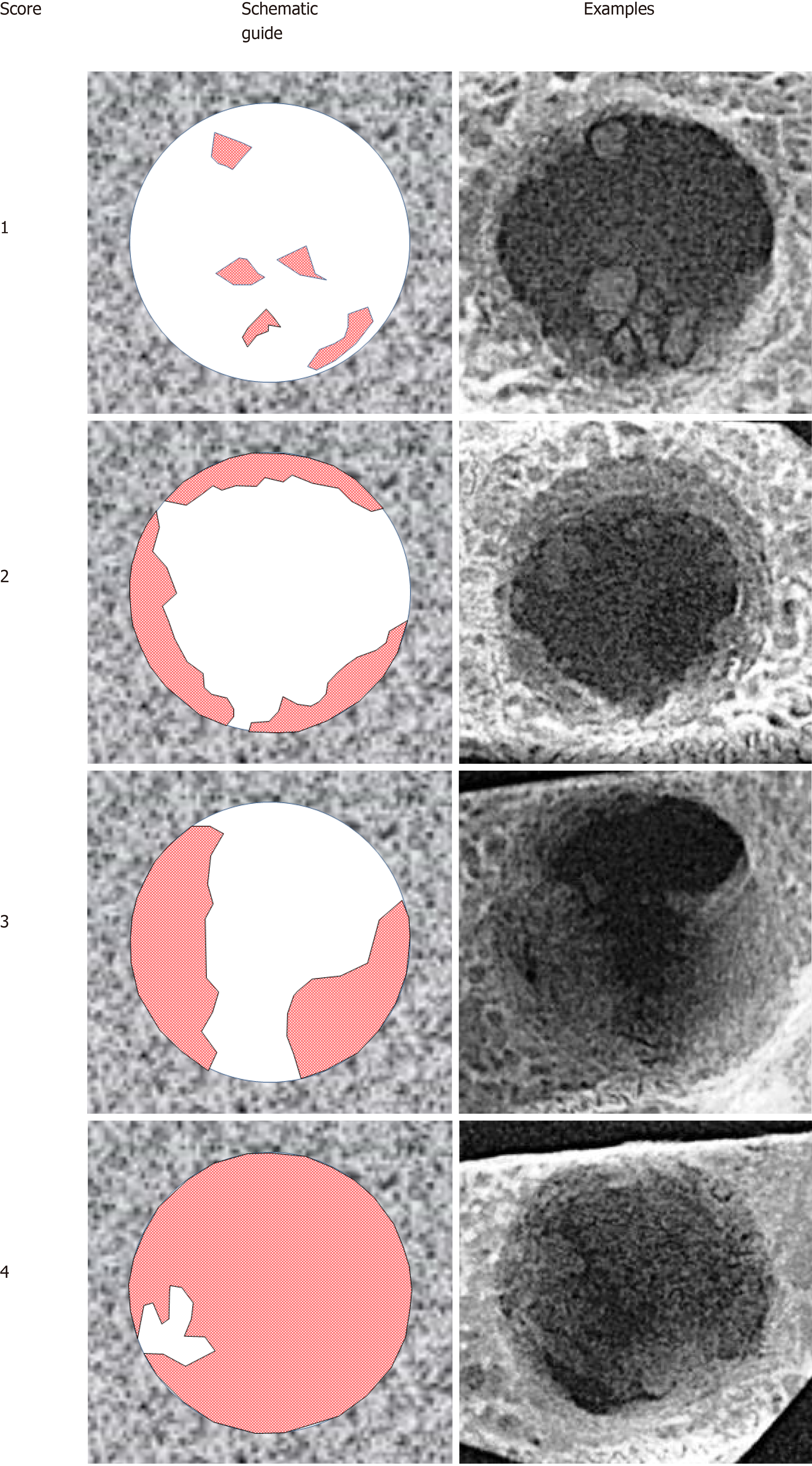
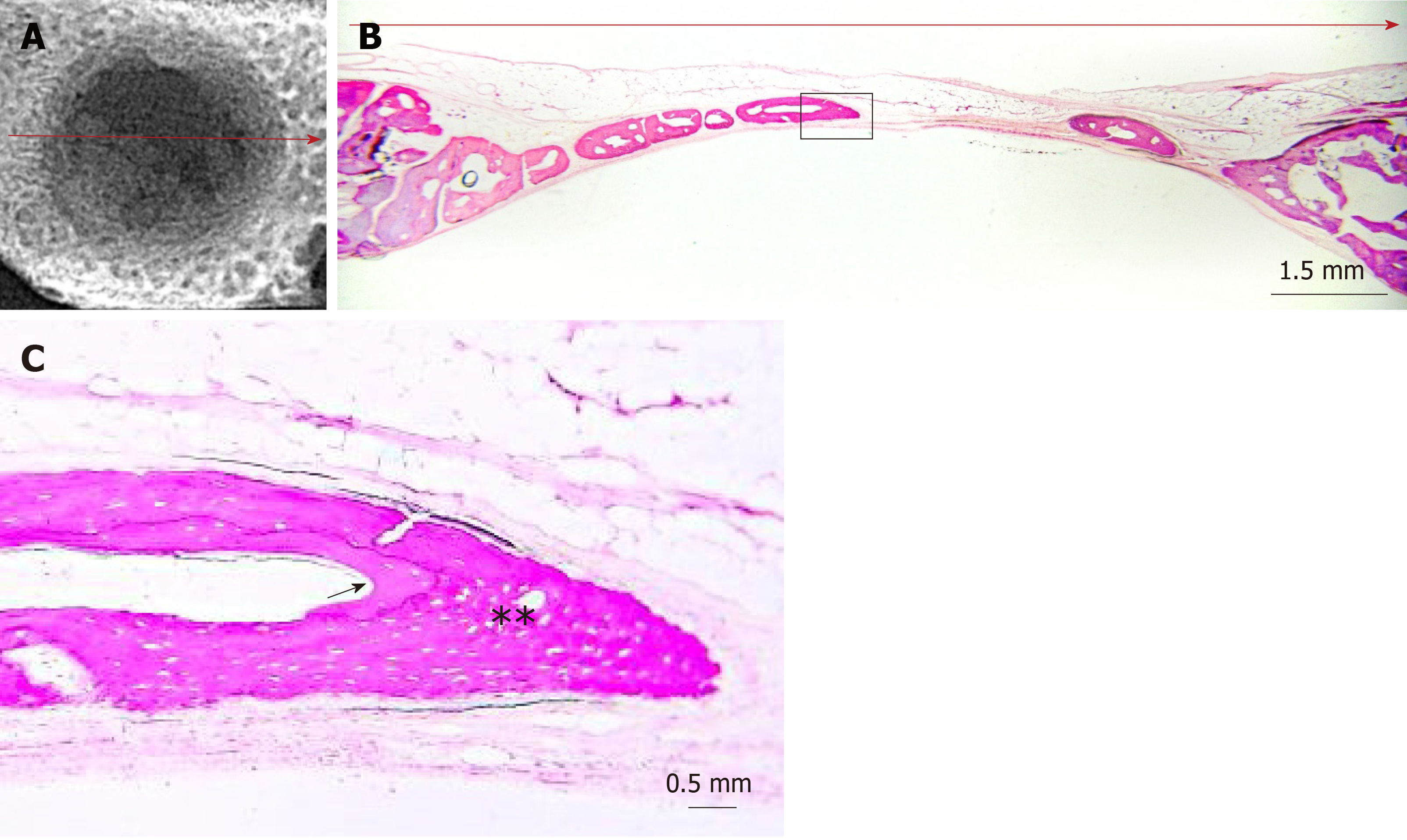
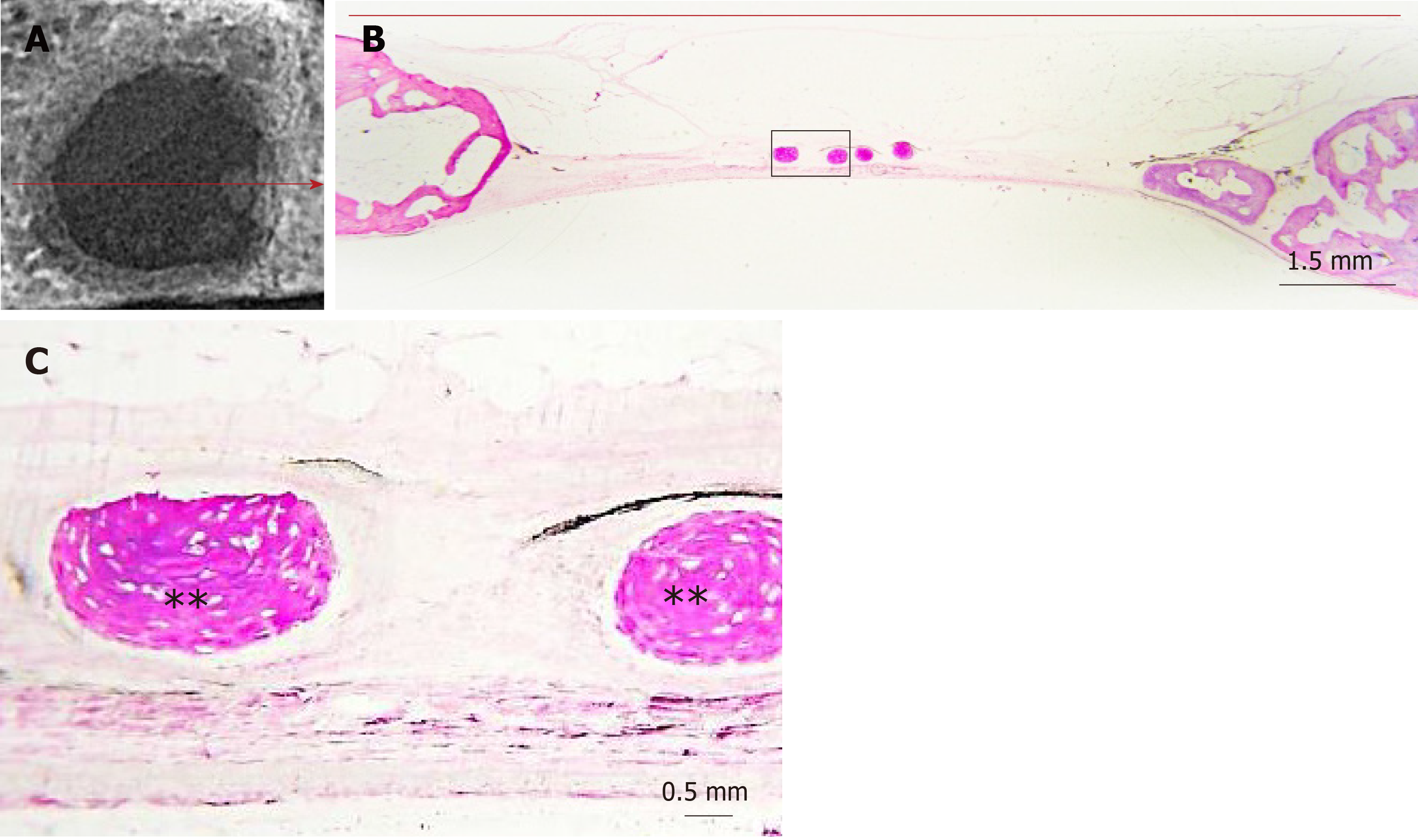
Digital radiographs of all specimens were taken using a dental x-ray unit (X-Mind AC, Satelec Acteon Group, Merignac, France) and a dental CCD sensor (Sopix2, Sopro Acteon Imaging, La Ciota, France), under the same operating parameters (70 kV, 8 mA, 0.125 s exposure time). Bridging and union within the defects were assessed blindly by two examiners (one of the authors and an independent evaluator, both dentists) using the scoring guide described by Patel et al[20]. This scoring system consists of the following five-grade scale: (0) No bone formation within defect; (1) Few bony spicules dispersed through defect; (2) Bony bridging only at defect borders; (3) Bony bridging over partial length of defect; and (4) Bony bridging entire span of defect at longest point (11 mm in the present study) (Figure 2).
Dissected fragments containing the defects were dehydrated by sequential immersion of ascending concentrations of alcohol (50%, 70%, 90%, 100%), every 2 d. Then, the specimens were immersed in alcoholic solutions of increasing concentrations (50%, 70%, 90%) of methyl-methacrylate, also every 2 d (Techonit 7200, Heraeus Kulzer GmbH, Wehrheim, Germany). Next, the specimens were kept in 100% methyl-methacrylate for 10 d, in order to achieve optimum resin infiltration, before they were incubated in fresh 100% methyl-methacrylate and were polymerized for 12 hours, using an appropriate light-curing device. Finally, the polymerized specimens were cut with a diamond band-saw microtome, bonded on glass slides, grinded, and polished as appropriate to create approximately 80 μm thin histological sections. All the above procedures were carried out by using the EXAKT system (Advanced Technologies GmbH, Norderstedt, Germany). The specimens were cut vertically and histological sections were duly oriented to coincide with the direction of maximum defect bridging, as indicated from the corresponding radiographs. The sections were then stained with Toluidine blue/Basic Fuchsin.
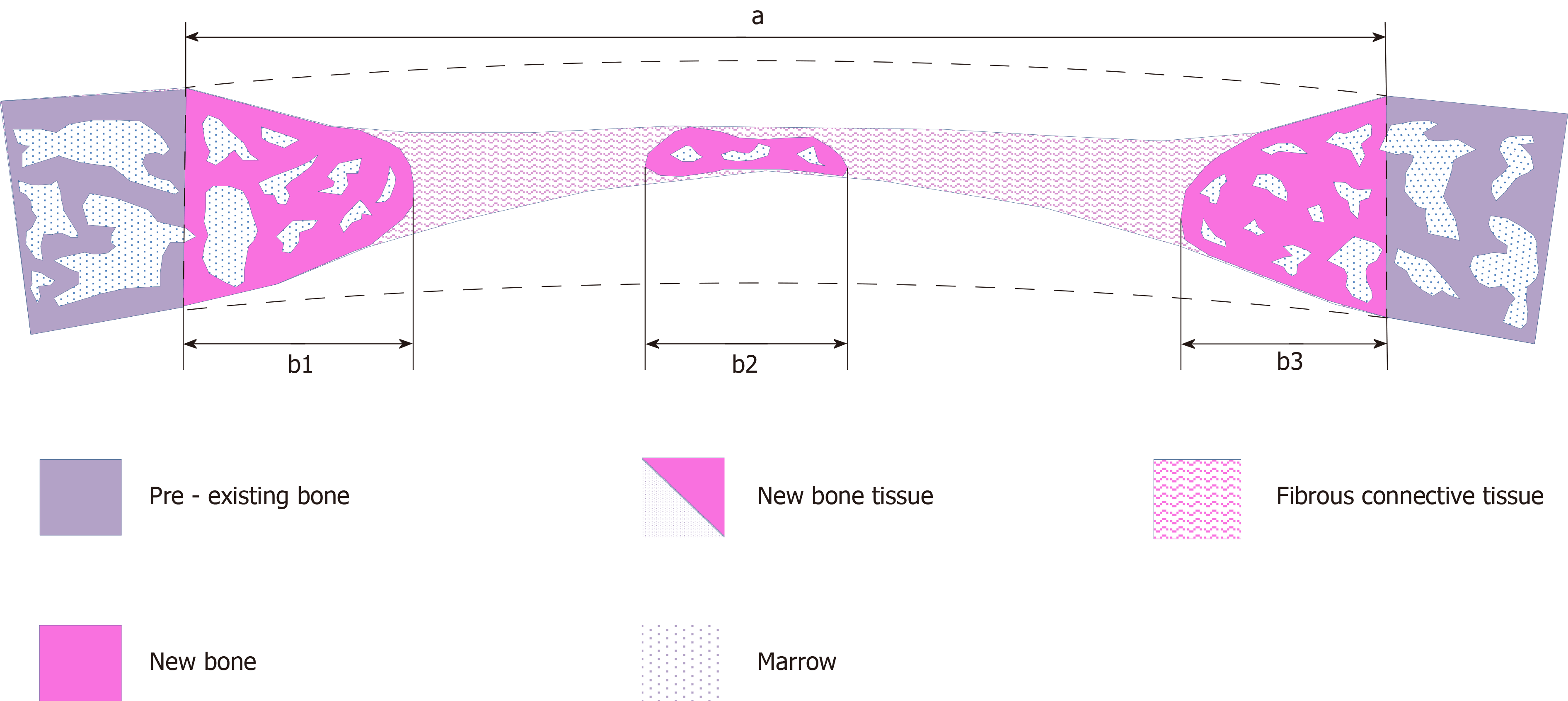
The slides were viewed under light microscope (Axiostar Plus®, Zeiss, Gottingen, Germany) and digital images were captured (AxioCam ICc3, Carl Zeiss), so as to perform histomorphometric measurements (Figure 3) with the appropriate software, Image Pro Plus (Media Cybernetics Inc., Rockville, MD, United States). The following primary histomorphometric parameters were measured: (1) Defect area: Defined by connecting the margins of the defect through appropriate extrapolated curvatures that represent the inner and outer contour of the pre-existing cranial bone, which was removed with the trephine burr; (2) Bone tissue area: Defined as the new bone tissue that was formed within the borders of the determined defect area, including new mineralized bone and associated non-mineralized tissue (osteoid, content of osteonal canals and bone marrow); (3) Bone area: Defined as the new bone matrix (mineralized bone and osteoid) that was formed within the borders of the determined defect area; (4) Defect horizontal dimension: Defined as the length of the line connecting the original margins of the defect; and (5) Total bone tissue horizontal dimension: Defined as the linear extent of the new bone tissue that was regenerated from the defect margins and the new bone tissue islands across the defect horizontal dimension. The following secondary parameters were calculated: (1) Defect regeneration (%): (Bone tissue area)/(Defect area) x 100%; (2) Defect bridging (%): (Total bone tissue horizontal dimension)/(Defect horizontal dimension) x 100%; and (3) Bone tissue density (%): (Bone area)/(Bone tissue area) x 100%. Both the nomenclature and the definitions of primary and secondary parameters are based on the 2012 updated report of the American Society for Bone and Mineral Research Histomorphometry Nomenclature Committee[21].
Based on our pilot study and previous studies[18,19,22,23], we assumed that the primary outcome (defect regeneration %, as defined above) in the control group would be approximately 20% ± 5%. Thus, in order to detect a difference of ± 10% between the groups, we calculated that at least eight animals would be needed per group, when α = 0.05 and (1-β) = 0.95. The sample size was calculated with G*Power v.3.1.9.2 (Frantz Faul, Univerisität Kiel, Germany).
Statistical analyses were performed using SPSS v.18.0 (SPSS, Inc., Chicago, IL, United States). The average radiographic score and the average histomorphometric parameters were initially calculated from the two defects for each animal. The radiographic scoring and histomorphometric parameters are presented as mean ± standard deviation within the groups. Inter-examiner agreement in radiographic scoring was evaluated by Cohen's kappa statistic. The significance of differences between the groups, in relation to radiographic scoring, was determined by the Mann-Whiney U test, since the data did not meet the criteria for normal distribution, as indicated by the Shapiro-Wilk test. The significance of differences between the groups, in relation to secondary histomorphometric parameters, was determined by the t-test, since the data were distributed normally, as indicated by the Shapiro-Wilk test. Statistical significance was determined at P < 0.05 level. The statistical methods of this study were reviewed by Ms Eirini Pagkalidou, Mathematician, Biomedical Statistician, MSc of Public Health in Comparative Effectiveness Research.
Both the surgical procedures and the post-operative period were uneventful and well tolerated by all the animals, without any surgical wound dehiscence, signs of infection or other complication.
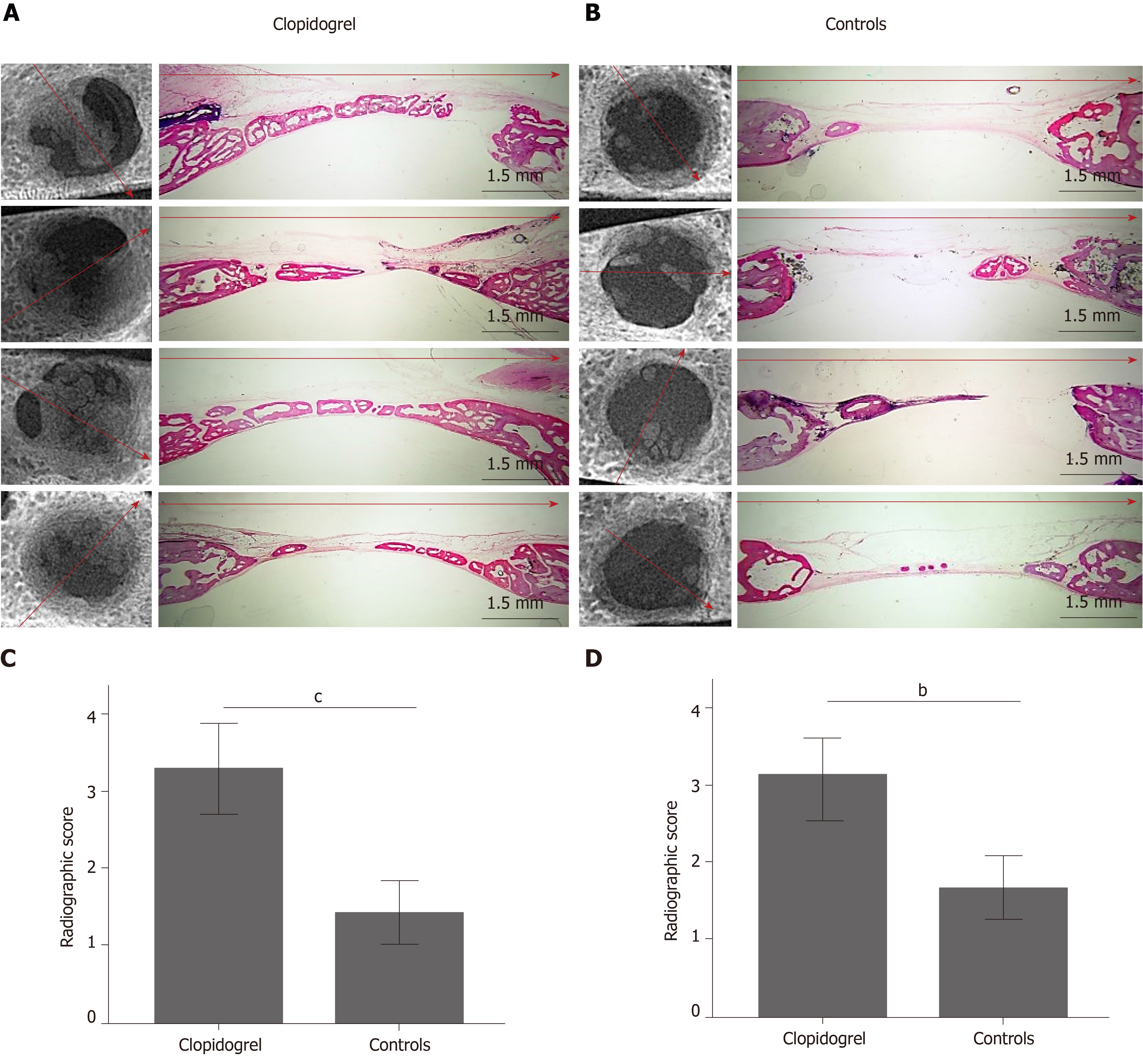
The radiographic imaging showed radiopaque areas within the defects, indicating new bone formation in various extents (Figure 4). New bone was either formed inwards from the defect margins or located in the central portion of the defect as separated bony islets. The mean radiographic score was significantly higher in the clopidogrel group than in the control group, for both examiners [3.31 ± 0.2 vs 1.43 ± 0.2 for examiner A (P < 0.001) and 3.12 ± 0.3 vs 1.88 ± 0.2 for examiner B (P = 0.007); inter-examiner agreement: k = 0.441, P < 0.001].
In both groups, the defect area was partially filled with thin new bone tissue in various extents, and none of the defects was completely regenerated. New bone tissue was evident either as wedge-shaped protrusions from the defect margins or as separated bone tissue islets within the defect area (Figure 4). The area within the new bone tissue was filled with fibrous connective tissue. New bone was mainly woven, but lamellar bone was also evident in various extents (Figures 5 and 6). The lamellar bone was more pronounced in the new bone extending from the defect margins rather than in the islets. Complete bony bridging between the margins of the defect was indicated in one defect of the control group and in three defects of the clopidogrel group. Defect regeneration (%) and defect bridging (%), as assessed by histomorphometry, were significantly greater in the clopidogrel group as compared to the control group, whereas bone tissue density had no statistical difference (Table 1).
| Clopidogrel group, n = 8 | Control group, n = 8 | P value | |
| Defect regeneration, % | 28.07 ± 7.7 | 19.47 ± 4.9 | 0.018 |
| Defect bridging, % | 72.17 ± 21.2 | 41.17 ± 8.5 | 0.004 |
| Bone tissue density, % | 72.13 ± 8.5 | 64.82 ± 11.9 | 0.179 |
Identification of pharmacological agents that may affect bone healing is a challenging issue. Clopidogrel is a widely prescribed antiplatelet drug, and several in vivo studies have indicated that it may affect bone metabolism. To our knowledge, the present study is the first to assess the effect of systemic clopidogrel administration on bone healing. Our study was initially conducted in order to evaluate if clopidogrel received perioperatively for cardiovascular reasons had any negative effect on bone healing following skeletal surgical procedures. Interestingly, our results showed that clopidogrel enhanced new bone formation and bridging in the rabbit calvarial defect model. Our results agree with one previous in vivo study[24] on the subject, where the effect of local application of the active metabolite of clopidogrel (CAM) and ticagrelor in rat calvarial defect healing was evaluated. After implanting collagen sponge or 3D printed resorbable calcium-triphosphate/hydroxyapatite scaffolds saturated with CAM in rat calvarial defects, they study showed that CAM promoted significantly bone regeneration compared to BMP-2 application.
Previous results on the effect of clopidogrel on bone metabolism and turnover, however, are contrasting, as it seems that both dosage and duration of treatment with clopidogrel are important factors for its effects on bone. Syberg et al[8] found in adult ovariectomized mice that treatment with clopidogrel (1 mg/kg/d) for 4 wk resulted in significant reduction of trabecular bone volume, as well as to the reduction in trabecular number in tibia and femur compared with controls. In contrast, Su et al[9] reported that adult ovariectomized mice, treated with high clopidogrel dose (30 mg/kg/d) for 2 or 5 wk, showed significant increase in trabecular bone volume in tibia and significantly decreased serum levels of osteoclast activity marker (CTX), as compared to vehicle-treated mice. Interestingly, the same investigator[9] found that a 30 mg/kg/d clopidogrel treatment for 9 d in young mice, in which bone turnover is high due to skeletal growth, resulted in significant increase in the trabecular bone volume and trabecular bone mineral density of tibia, whereas there was no effect in adult normal mice. Moreover, Su et al[9] demonstrated that a 30 mg/kg/d clopidogrel treatment for 9 d protected mice from tumor-associated bone loss in a mouse model of tumor metastasis in the tibia. Also, in support of a “trophic” role, Yamaguchi et al[10] found that 5 mg/kg/d clopidogrel for 4 wk significantly reduced the incidence of steroid-associated osteonecrosis in a rabbit model. Yet, in a large scale clinical retrospective cohort study, Jørgensen et al[11] showed that clopidogrel therapy is associated with increased risk of osteoporotic fractures. They studied 77503 Danish patients who were prescribed clopidogrel for any indication, dosing and duration of treatment, during the years 1996-2008 and 232510 matched nonusers, and they found that patients on clopidogrel therapy had up to 50% increase in risk of osteoporotic fractures (hip, forearm and spine), especially those in treatment of more than 1 year. However, patients receiving less than 0.01 of the defined daily dose of clopidogrel apparently had a lower risk of fracture than nonusers[11]. Interestingly, in 2017[25], the same group of researchers studied patients taking clopidogrel for stroke alone from the same cohort and found that while these patients run an increased risk of osteoporotic fractures, clopidogrel was not responsible for this. In contrast, they indicated that patients less adherent to the treatment run a lower risk than nonusers and patients with high adherence.
At the cellular level, the available studies indicate that clopidogrel should result in suppression of both bone formation and resorption in vivo and, thus, impairment of bone healing. For instance, CAM inhibited both osteoblast and osteoclast differentiation from bone marrow cells in culture[24]. Moreover, the application of clopidogrel in osteoblast cultures slowed osteoblast proliferation, decreased cell viability of mature osteoblasts and inhibited mineralized bone nodule formation, while the application in osteoclast cultures decreased their number, viability and resorptive activity[8]. Therefore, it appears that inhibition of the P2Y12 receptor on bone cells alone may not explain our results showing bone regeneration at least at 6 wk postoperatively.
Taking into account the foregoing, it is tempting to speculate that clopidogrel may have indirectly enhanced bone regeneration in our study by interfering with various levels of the complex cascades that take place during tissue repair. Firstly, the P2Y12 receptor is also expressed in other cellular players involved in bone healing such as platelets, leukocytes and endothelial cells[26]. Clopidogrel is known to affect various processes occurring in the early stages of tissue healing such as improving endothelial nitric oxide bioavailability and reducing platelet degranulation, platelet-leukocyte aggregate formation, expression of inflammatory cytokines and C-reactive protein[27]. Coimbra et al[28] found that high clopidogrel dose (75 mg/kg/d) increased the number of osteoblasts and mesenchymal stem cell proliferation in the areas of bone remodeling during the initial phase of inflammation resolution, following periodontitis. Moreover, the purinergic signaling system is known to regulate long-term (trophic) effects in tissue regeneration, and its components are highly interdependent and, occasionally, have opposite effects on cellular functions[29-32]. Thus, it is not unreasonable to assume that continuous inhibition of the P2Y12 receptor in the perioperative period by clopidogrel may affect the functions of other purinoreceptor subtypes, resulting in a cumulative effect that favors bone healing, which cannot be fully explained yet.
Admittedly, our study bears some limitations. For example, due to enforced limitations on the number of experimental animals by the local Veterinary Service, the evaluation of bone healing at different time intervals was not possible nor was the administration of different doses of clopidogrel for different perioperative periods, which would have provided better metrics on the positive influence of clopidogrel on bone healing. In the future, comparison with other antiplatelet drugs, such as aspirin or ticagrelor, would provide valuable data for extensively used drugs. Moreover, our study would have benefited from the use of micro-computed tomography in order to obtain 3-D quantitative data of the defect regeneration, although our method (combination of radiographic and histologic interpretation) is also acceptable in the literature[33]. Finally, it should be considered that the present study is experimental, on an animal model, and its results cannot fully be translated to humans.
Despite such limitations, our results indicate that perioperative administration of clopidogrel does not negatively affect bone healing but rather enhances it. Clopidogrel treatment may promote bone healing in vivo by influencing complex mechanisms that involve purinergic signaling on a number of cell types participating in the process. Considering the high clinical impact of the subject due to the wide use of clopidogrel, its role in bone healing and remodeling remains to be further elucidated. In addition, expansion of the present research may provide useful information for future clinical applications on the acceleration of bone healing and the prevention/management of bone loss.
Clopidogrel is a widely prescribed drug that inhibits platelet aggregation and, therefore, prevents thromboembolic events such as myocardial infarction and stroke. Clopidogrel acts by binding on the P2Y12 purinergic receptor on the platelet surface. Purinergic receptors also play an important role in bone homeostasis, and P2Y12, in particular, is expressed in osteoblasts and osteoclasts as well. The exact role of the P2Y12 receptor and the effect of clopidogrel treatment in bone metabolism have not been elucidated. The few existing studies demonstrate contrasting results, with some of them indicating a negative impact on bone turnover. The effect of clopidogrel treatment in bone healing has not yet been studied.
The presence of a drug that may negatively affect bone healing during the perioperative period when dealing with skeletal surgery raised our concerns and motivated us to conduct this study.
The main objective of the present study was to evaluate bone healing during continuous perioperative clopidogrel treatment.
Our study used the well-described critical sized calvarial defect model. Sixteen male New Zealand rabbits were used and randomly divided into two groups; an experimental group taking clopidogrel 3 mg/kg/d per os and a control group taking the vehicle alone. The treatment began 1 wk before the surgical procedures and continued for 6 wk postoperatively. Surgical procedures were conducted to create two circular bony defects on the cranium of every animal. After a 6-wk postoperative period, the animals were euthanized, and postmortem radiographical and histological evaluation was conducted. Radiological evaluation was conducted using a five grade qualitative scale. Histological evaluation included measurements of the percentages of the defect regeneration, bridging and bone density.
The postoperative period was uneventful and without any complication for all animals. The radiological examination showed that the clopidogrel group had a statistically significant improved radiographic score in bone bridging and union. The histomorphometric analyses also revealed significantly greater percentage of bone regeneration and bridging in the clopidogrel group than in the control group. However, bone density was not statistically different between the groups.
The present study results indicate that continuous perioperative clopidogrel treatment does not impair bone healing; instead, it promotes new bone formation. This finding is important when dealing with skeletal surgery in patients who use this drug chronically for cardiovascular indications.
Future research may involve evaluation of the effect of other antiplatelet drugs of the same category, such as ticagrelor or prasugrel, and at different dosing and treatment duration. Moreover, further research is needed in order to evaluate if our findings have useful implications in bone healing improvement such as topically drug releasing vehicles or drug eluting orthopedic implants.
We would like to thank Professor Ioannis Taitzoglou, DVM, PhD and Dr. Ioannis Margaritis DVM, MSc for their assistance in animal welfare, Dr. Ioanna Kiriakaki, DDS, MSc for her contribution as second interpreter in the scoring of radiographic images of the defects, and Mrs. Styliani Voziki for comprehensive English language editing.
| 1. | World Health Organization. 20th WHO Model List of Essential Medicines. March 2017 [Cited 1 March 2018]. Available from: http://www.who.int/medicines/publications/essentialmedicines/20th_EML2017_FINAL_amendedAug2017.pdf?ua=1. |
| 2. | Cattaneo M. Response variability to clopidogrel: Is tailored treatment, based on laboratory testing, the right solution? J Thromb Haemost. 2012;10:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Burnstock G. Purinergic signalling: From discovery to current developments. Exp Physiol. 2014;99:16-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 4. | North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2205] [Cited by in RCA: 2412] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 5. | Orriss IR, Burnstock G, Arnett TR. Purinergic signalling and bone remodelling. Curr Opin Pharmacol. 2010;10:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Rumney RM, Wang N, Agrawal A, Gartland A. Purinergic signalling in bone. Front Endocrinol (Lausanne). 2012;3:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Buckley KA, Golding SL, Rice JM, Dillon JP, Gallagher JA. Release and interconversion of P2 receptor agonists by human osteoblast-like cells. FASEB J. 2003;17:1401-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Syberg S, Brandao-Burch A, Patel JJ, Hajjawi M, Arnett TR, Schwarz P, Jorgensen NR, Orriss IR. Clopidogrel (Plavix), a P2Y12 receptor antagonist, inhibits bone cell function in vitro and decreases trabecular bone in vivo. J Bone Miner Res. 2012;27:2373-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Su X, Floyd DH, Hughes A, Xiang J, Schneider JG, Uluckan O, Heller E, Deng H, Zou W, Craft CS, Wu K, Hirbe AC, Grabowska D, Eagleton MC, Townsley S, Collins L, Piwnica-Worms D, Steinberg TH, Novack DV, Conley PB, Hurchla MA, Rogers M, Weilbaecher KN. The ADP receptor P2RY12 regulates osteoclast function and pathologic bone remodeling. J Clin Invest. 2012;122:3579-3592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Yamaguchi R, Yamamoto T, Motomura G, Ikemura S, Iwasaki K, Zhao G, Iwamoto Y. Effects of an anti-platelet drug on the prevention of steroid-induced osteonecrosis in rabbits. Rheumatology (Oxford). 2012;51:789-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Jørgensen NR, Grove EL, Schwarz P, Vestergaard P. Clopidogrel and the risk of osteoporotic fractures: a nationwide cohort study. J Intern Med. 2012;272:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Rossini R, Tarantini G, Musumeci G, Masiero G, Barbato E, Calabrò P, Capodanno D, Leonardi S, Lettino M, Limbruno U, Menozzi A, Marchese UOA, Saia F, Valgimigli M, Ageno W, Falanga A, Corcione A, Locatelli A, Montorsi M, Piazza D, Stella A, Bozzani A, Parolari A, Carone R, Angiolillo DJ; Italian Society of Interventional Cardiology (SICI-GISE); Italian Society for the Study of Haemostasis and Thrombosis (SISET); Italian Society of Anesthesia and Intensive Care Medicine (SIAARTI); Italian Society of Surgery (SIC); Italian Society for Cardiac Surgery (SICCH); Italian Society of Vascular and Endovascular Surgery (SICVE); Italian Society of Urology (SIU); Italian Orthopaedic Society (SIOT); Italian Society of Thoracic Surgeons (SICT); Italian Federation of Scientific Societies of Digestive System Diseases (FISMAD); Italian Society of Digestive Endoscopy (SIED); Italian Association of Hospital Gastroenterology and Digestive Endoscopy (AIGO); Italian Association of Gastroenterology and Digestive Endoscopy (SIGE); Italian Society of Maxillofacial Surgery (SICMF); Italian Society of Reconstructive Plastic Surgery and Aesthetics (SICPRE); Italian Society of Gynecology and Obstetrics (SIGO); Italian Society of Neurosurgery (SINch); Italian Association of Hospital Pulmonologist (AIPO); Italian Society of Periodontology (SIdP); Italian Society of Ophthalmology (SOI); Italian Association of Hospital Otorhinolaryngologist (AOOI); Italian Association of Hospital Surgeons (ACOI); Association of Obstetricians Gynecologists Italian Hospital (AOGOI). A Multidisciplinary Approach on the Perioperative Antithrombotic Management of Patients With Coronary Stents Undergoing Surgery: Surgery After Stenting 2. JACC Cardiovasc Interv. 2018;11:417-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 13. | Steele MJ, Fox JS, Fletcher JP, Grigg LE, Bell G. Clopidogrel dilemma for orthopaedic surgeons. ANZ J Surg. 2011;81:774-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Ghantous AE, Ferneini EM. Aspirin, Plavix, and Other Antiplatelet Medications: What the Oral and Maxillofacial Surgeon Needs to Know. Oral Maxillofac Surg Clin North Am. 2016;28:497-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Lillis T, Ziakas A, Koskinas K, Tsirlis A, Giannoglou G. Safety of dental extractions during uninterrupted single or dual antiplatelet treatment. Am J Cardiol. 2011;108:964-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105 Suppl 1:S13-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 485] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 17. | Wong PC, Crain EJ, Watson CA, Jiang X, Hua J, Bostwick JS, Ogletree ML, Schumacher WA, Rehfuss R. Platelet aggregometry and receptor binding to predict the magnitude of antithrombotic and bleeding time effects of clopidogrel in rabbits. J Cardiovasc Pharmacol. 2007;49:316-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Delgado-Ruiz RA, Calvo-Guirado JL, Romanos GE. Critical size defects for bone regeneration experiments in rabbit calvariae: systematic review and quality evaluation using ARRIVE guidelines. Clin Oral Implants Res. 2015;26:915-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Sohn JY, Park JC, Um YJ, Jung UW, Kim CS, Cho KS, Choi SH. Spontaneous healing capacity of rabbit cranial defects of various sizes. J Periodontal Implant Sci. 2010;40:180-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931-940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 447] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 21. | Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1872] [Cited by in RCA: 2113] [Article Influence: 162.5] [Reference Citation Analysis (0)] |
| 22. | Calvo-Guirado JL, Garces M, Delgado-Ruiz RA, Ramirez Fernandez MP, Ferres-Amat E, Romanos GE. Biphasic β-TCP mixed with silicon increases bone formation in critical site defects in rabbit calvaria. Clin Oral Implants Res. 2015;26:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Pelegrine AA, Aloise AC, Zimmermann A, de Mello E Oliveira R, Ferreira LM. Repair of critical-size bone defects using bone marrow stromal cells: a histomorphometric study in rabbit calvaria. Part I: use of fresh bone marrow or bone marrow mononuclear fraction. Clin Oral Implants Res. 2014;25:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Mediero A, Wilder T, Reddy VS, Cheng Q, Tovar N, Coelho PG, Witek L, Whatling C, Cronstein BN. Ticagrelor regulates osteoblast and osteoclast function and promotes bone formation in vivo via an adenosine-dependent mechanism. FASEB J. 2016;30:3887-3900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (6)] |
| 25. | Jørgensen NR, Schwarz P, Iversen HK, Vestergaard P. P2Y12 Receptor Antagonist, Clopidogrel, Does Not Contribute to Risk of Osteoporotic Fractures in Stroke Patients. Front Pharmacol. 2017;8:821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Gachet C. P2Y(12) receptors in platelets and other hematopoietic and non-hematopoietic cells. Purinergic Signal. 2012;8:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Schrottmaier WC, Kral JB, Badrnya S, Assinger A. Aspirin and P2Y12 Inhibitors in platelet-mediated activation of neutrophils and monocytes. Thromb Haemost. 2015;114:478-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 28. | Coimbra LS, Steffens JP, Alsadun S, Albiero ML, Rossa C, Pignolo RJ, Spolidorio LC, Graves DT. Clopidogrel Enhances Mesenchymal Stem Cell Proliferation Following Periodontitis. J Dent Res. 2015;94:1691-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Burnstock G, Verkhratsky A. Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell Death Dis. 2010;1:e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 30. | Noronha-Matos JB, Correia-de-Sá P. Mesenchymal Stem Cells Ageing: Targeting the “Purinome” to Promote Osteogenic Differentiation and Bone Repair. J Cell Physiol. 2016;231:1852-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Burnstock G, Arnett TR, Orriss IR. Purinergic signalling in the musculoskeletal system. Purinergic Signal. 2013;9:541-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Novak I. ATP as a signaling molecule: the exocrine focus. News Physiol Sci. 2003;18:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Spicer PP, Kretlow JD, Young S, Jansen JA, Kasper FK, Mikos AG. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat Protoc. 2012;7:1918-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 483] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grawish ME, Katuchova J, Soriano-Ursúa MA, Tanabe S, Peng BG S-Editor: Wang J L-Editor: Filipodia E-Editor: Liu MY