Published online Aug 7, 2018. doi: 10.4292/wjgpt.v9.i3.22
Peer-review started: May 19, 2018
First decision: June 14, 2018
Revised: July 3, 2018
Accepted: July 15, 2018
Article in press: July 16, 2018
Published online: August 7, 2018
Processing time: 80 Days and 16.2 Hours
To investigate possible changes of blood glucose levels after oral intake of lactulose in healthy subjects.
The study was performed as prospective, randomized, two-part study with 4-way cross-over design with n = 12 in each study arm. Capillary blood glucose levels were determined over a time period of 180 min after intake of a single dose of 10 g or 20 g lactulose provided as crystal or liquid formulation. During the manufacturing process of lactulose, impurities with sugars (e.g., lactose, fructose, galactose) occur. Water and 20 g glucose were used as control and reference. Because lactulose is used as a functional food ingredient, it may also be consumed by people with impaired glucose tolerance, including diabetics. Therefore, it is of interest to determine whether the described carbohydrate impurities may increase blood glucose levels after ingestion.
The blood glucose concentration-time curves after intake of 10 g lactulose, 20 g lactulose, and water were almost identical. None of the three applications showed any changes in blood glucose levels. After intake of 20 g glucose, blood glucose concentration increased by approximately 3 mmol/L (mean Cmax = 8.3 mmol/L), reaching maximum levels after approximately 30 min and returning to baseline within approximately 90 min, which was significantly different to the corresponding 20 g lactulose formulations (P < 0.0001). Comparing the two lactulose formulations, crystals and liquid, in the dosage of 10 g and 20 g, there was no difference in the blood glucose profile and calculated pharmacokinetic parameters despite the different amounts of carbohydrate impurities (1.5% for crystals and 26.45% for liquid). Anyhow, the absolute amount of single sugars was low with 0.3 g in crystals and 5.29 g in liquid formulation in the 20 g dosages. Lactulose was well tolerated by most volunteers, and only some reported mild to moderate mainly gastrointestinal side effects.
The unchanged blood glucose levels after lactulose intake in healthy subjects suggest its safe use in subjects with impaired glucose tolerance.
Core tip: Lactulose can be used as a functional food ingredient. During manufacturing as liquid or crystalline formulation, impurities with different sugars occur. Lactulose may also be consumed by people with impaired glucose tolerance, including diabetics. For these consumers, it is of interest whether the described carbohydrate impurities may increase blood glucose levels after ingestion. This study was performed to investigate possible changes of blood glucose levels after oral intake of 10 g and 20 g of liquid and crystalline lactulose in healthy subjects. The small amounts of carbohydrate impurities did not influence blood glucose levels, indicating potential applicability to people with impaired glucose tolerance.
- Citation: Steudle J, Schön C, Wargenau M, Pauly L, Schwejda-Güttes S, Gaigg B, Kuchinka-Koch A, Stover JF. Blood glucose response after oral intake of lactulose in healthy volunteers: A randomized, controlled, cross-over study. World J Gastrointest Pharmacol Ther 2018; 9(3): 22-30
- URL: https://www.wjgnet.com/2150-5349/full/v9/i3/22.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v9.i3.22
The undigestible lactulose (galacto-fructose) is a semi-synthetic product made by isomerization from lactose (galacto-glucose). Lactulose does occur in very small amounts in heated milk but is not present in nature. In contrast to its substrate lactose, the β-glycosidic linkage of lactulose can not be split by human digestive enzymes. Reaching the colon, lactulose is mainly metabolized by saccharolytic intestinal bacteria[1]. Lactulose has been used as food ingredient, e.g., as a sugar substitute or functional food ingredient with prebiotic effects for human consumption[2,3]. Changes in gut microbiota composition and health-promoting effects due to its bifidogenic effects were described. Furthermore, the growth of pathogenic bacteria, e.g., Salmonella, could be inhibited. Moreover, lactulose improves the survival of probiotic strains like Lactobacillus rhamnosus and Bifidobacterium bifidum in yogurt[4].
The particularly gentle and natural laxative action of lactulose at dosages of 10 g and more result from its prebiotic, osmotic, and peristalsis-activating effects. This is also reflected in the European Food Safety Authority (EFSA)-approved health claim that “Lactulose contributes to an acceleration of intestinal transit”[5]. Furthermore, lactulose is also marketed as a drug to treat constipation, hepatic encephalopathy and dysbacteria[1]. Besides these health benefits, lactulose has some desirable properties like taste improvement, favorable browning behavior and excellent solubility in water for the development and manufacture of functional food products[4]. Lactulose is produced from lactose. During manufacturing impurities, including epilactose, lactose, galactose, fructose, tagatose and small amounts of unspecified or unknown sugars, occur[4]. Lactulose is available in two formulations: Crystals (powder to be dissolved in water) and liquid syrup (solution). Carbohydrate impurities are found up to 3% in the crystalline and approximately 30% in the liquid form. The amount and the pattern of related substances of the carbohydrate impurities varies depending on the conditions during the manufacturing process used to produce lactulose.
Because lactulose is used as a functional food ingredient, it may also be consumed by people with impaired glucose tolerance, including diabetics. For these consumers, it is of interest whether the described carbohydrate impurities may increase blood glucose levels after ingestion. A case report showed higher blood glucose levels in a diabetic patient after changing the lactulose syrup brand[6]. In previous human studies, only slight or no increases in blood glucose concentrations were observed with single doses of 20-25 g of lactulose[7-9].
In this study, possible changes in blood glucose levels after oral intake of lactulose products from Fresenius Kabi were investigated in healthy volunteers. Because of the small amounts of impurities, particularly in lactulose crystals, no or only small changes in blood glucose levels were expected. In future studies, the results of this study need to be confirmed for a diabetic collective.
The study was performed as a prospective, open, mono-center, randomized, two-part study with 4-way cross-over design in each study arm. BioTeSys GmbH, Esslingen, was charged as a contract research organization (CRO) for study coordination, product supply and data collection. The study was performed in the study center of BioTeSys. The protocol followed the Declaration of Helsinki guidelines and was approved by the Ethics Committee of the Landesärztekammer Baden-Württemberg.
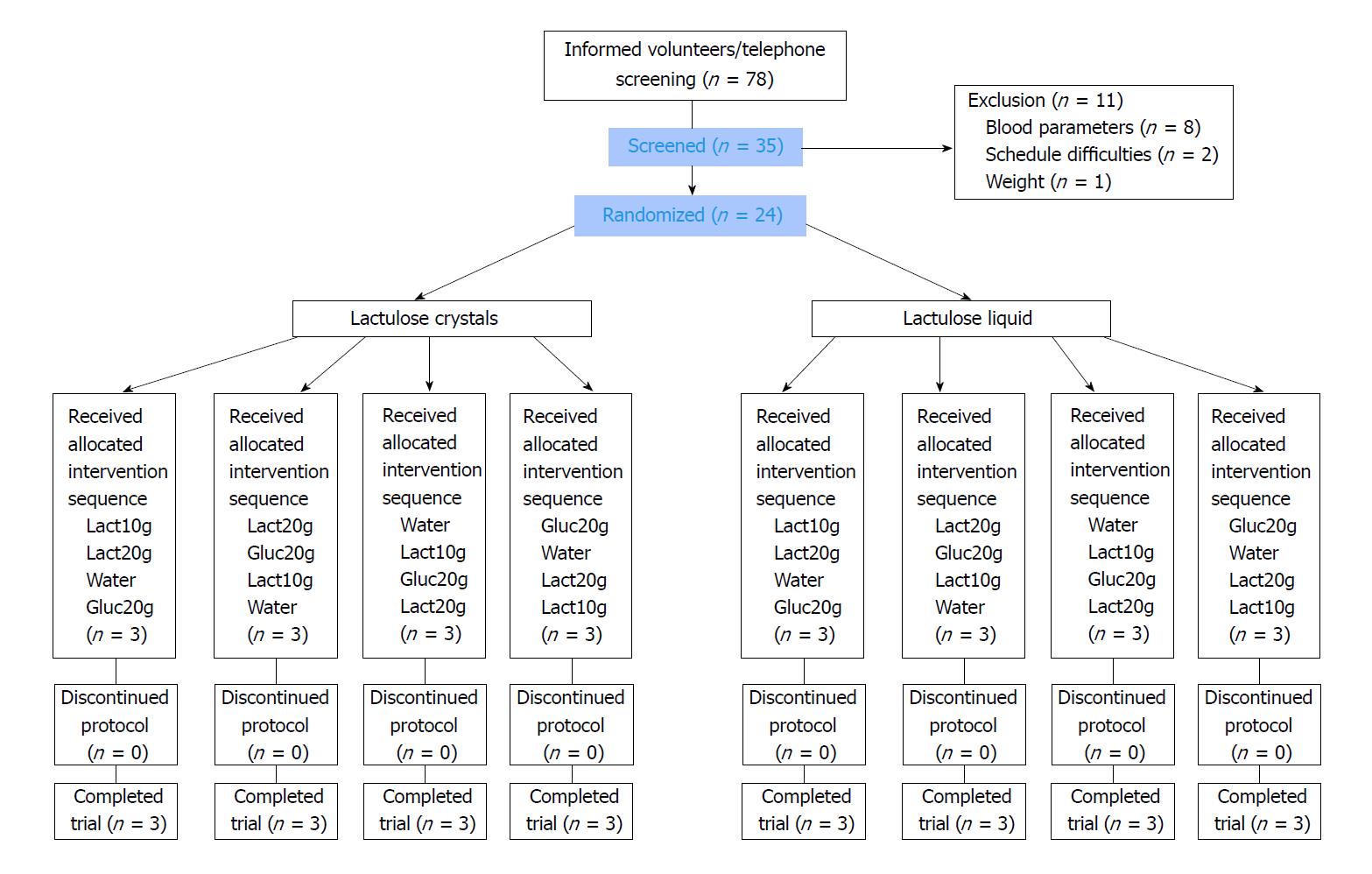
The study consisted of a screening visit and four study visits. After inclusion, subjects, who gave their written informed consent to participate, were stratified by gender and randomized to study arm (intervention with crystals or liquid) and sequence group 1-4 (random order of 10 g or 20 g lactulose either as crystal or liquid, water or 20 g glucose, according to Williams design). Randomization of volunteers was performed by means of the computer program DatInf® RandList, version 1.2.
Volunteers were instructed to have an individually standardized meal composed of farmhouse bread, cream cheese and cucumber in the evening prior to study visit 1-4. Furthermore, volunteers were not allowed to consume food or drink other than water for at least 10 h before the tests. In the morning of study visits, volunteers were instructed to drink 1-2 glasses (minimum 200 mL) of water after sleep before coming to the study site. Consumption of alcohol, as well as intensive exercise, was not allowed 24 h prior to all visits. Furthermore, in the morning of all visits, volunteers were not allowed to use means of transport accompanied with vigorous exercise (e.g., jogging, cycling).
Subjects were recruited by advertisement in local newspapers and a database of the study site. Eligible subjects were healthy Caucasian men and women, aged ≥ 18 and ≤ 65 years, without known (family) history of diabetes mellitus or use of anti-hyperglycemic drugs or insulin, having approximately 3-5 bowel movements per week. The blood routine parameters were determined in venous blood samples at screening and judged by the investigator according to the exclusion criteria. Main criteria for exclusion were clinically relevant renal or hepatic disease, liver enzymes > 10% above reference range, fasting blood glucose > 100 mg/dL or glycated hemoglobin (HbA1c) > 5.7%, total cholesterol > 250 mg/dL or triglycerides > 150 mg/dL, hemoglobin < 11 g/dL (women); < 12.5 g/dL (men), body mass index (BMI) < 19 kg/m² and ≥ 30 kg/m², intentional and unintentional weight loss > 5% in the previous 6 mon, smoker, major medical or surgical event requiring hospitalization within the previous 3 mo, presence of disease or drugs influencing digestion and absorption of nutrients or bowel habits, intake of medications known to affect glucose tolerance, e.g., steroids, protease inhibitors or antipsychotics, chronic intake of substances affecting blood coagulation, which in the investigator’s opinion would impact volunteer safety, hereditary galactose or fructose intolerance, lactase deficiency or glucose-galactose malabsorption.
For sample size estimation, the precision of the estimate for the absolute difference between interventions con-cerning incremental area under the curve (iAUC) was considered, which was expressed as half of the width of the 95% confidence interval (CI) for the mean difference. The precision was regarded as sufficient if half of the width of the 95%CI did not exceed one (intra-subject) standard deviation. Based on this approach, 11 evaluable subjects would have been required. To consider the 4-way cross-over in each group, including four intervention sequences, the final sample size should have been a multiple of four to be able to adjust for potential period effects. Therefore, 12 subjects in each study arm were enrolled (i.e., 24 subjects in total).
Lactulose crystals were produced by S.C.M., Società Chimica Mugello, S.r.l., a Fresenius Kabi Company in Vicchio, Italy, and lactulose liquid was produced by Fresenius Kabi Austria GmbH in Linz, Austria. Maximum carbohydrate impurities of both lactulose formulations according to the European Pharmacopoeia monographs are listed in Table 1. The total impurities of the study products were 1.5% in lactulose crystals and 26.45% in lactulose liquid.
| Lactulose crystals | Lactulose liquid1 | |
| Epilactose | ≤ 0.5% | ≤ 10.0% |
| Galactose | ≤ 0.5% | ≤ 15.0% |
| Lactose | ≤ 3.0% | ≤ 10.0% |
| Fructose | ≤ 0.5% | ≤ 1.0% |
| Tagatose | ≤ 0.5% | ≤ 4.0% |
| 3-Deoxy-D-glyceropentulose | - | ≤ 4.0% |
| Total impurities | ≤ 3.0% | ≤ 12.0% (excluding galactose and lactose) |
Subjects received study products in a single dose at the study site under fasting conditions. Despite the open nature of the study, volunteers were kept blinded on the dosage of study products (10 g or 20 g lactulose or 20 g glucose) as well as on the formulation (lactulose crystals and liquid). Study products were provided by study staff dissolved in 250 mL water, ready for consumption.
Blood glucose levels were measured in capillary whole blood obtained by finger prick. For analysis of glucose levels, the HemoCue Glucose 201+ Analyzer (Ängelholm, Sweden) was used, and glucose was determined photometrically using a modified glucose dehydrogenase method. Blood glucose concentrations were monitored over a period of 180 min at specified time points (0, 15, 30, 45, 60, 90, 120, 150, 180 min post-intake). From the boundary conditions including the methodology of blood glucose device, the study was performed in compliance with ISO 26642:2010 for determination of glycemic index (GI) of foods[10].
Paper CRFs served as source documents and were transferred into an electronic database (ALPHADAS®, an electronic data capturing system from Instem plc, Stone, Staffordshire, United Kingdom, ensuring full audit trail). Trial on-site monitoring verified the accurateness of source data transfer into electronic database as well as Good Clinical Practices compliance. Data were transferred to Biostatistics contract research organization M.A.R.C.O. GmbH and Co. KG, Düsseldorf, for statistical analysis. Statistical analysis was performed with SAS software (Version 9.3).
The primary endpoint was iAUC, i.e., above baseline levels for blood glucose concentration after oral intake of lactulose products compared to water (negative control). Secondary efficacy endpoints were: The maximum blood glucose concentration (Cmax), the time to reach maximum blood glucose concentration (Tmax), the maximum blood glucose concentration minus baseline value (Max_increase), the total area under the curve (AUC) from 0 to 180 min for blood glucose concentration (AUC(0-180min)), the baseline corrected AUC from 0 to 180 min for blood glucose concentration (AUCbase = AUC from 0 to 180 min - baseline*180 min). After glucose intake, the parameter Tbaseline, the first time to reach baseline again after increase in blood was evaluated. The following comparisons were done: (1) lactulose with negative control (water); (2) 20 g lactulose products with 20 g glucose; and (3) lactulose crystals and liquid.
Untransformed endpoints were analyzed separately for the two study arms using a mixed analysis-of-variance model with intervention (4 levels), period (4 levels), and baseline blood glucose level within study periods as fixed effects and subject as random effect. Group means were calculated from the model (“LS Means”). Data are presented for the Intention-to-treat (ITT) population which, however, was identical with the Per-Protocol (PP) population in this study. Due to the 7 d wash-out period, an examination of possible carry-over effects was not necessary. There were only minor time deviations for the wash-out period, which were discussed during data review meetings and judged to have no impact on study results.
Subjects were instructed to document any adverse events (AEs) and concomitant medication in diaries, starting after screening and ending with 24 h tolerability assessment at visit 4. All AEs were followed until resolution. During visits, all AEs were asked and reviewed by an investigator and reported on the CRF.
From November 2016 to January 2017, a total of 24 (of 35 screened) volunteers were included, as shown in Figure 1. The main reasons for non-inclusion were relevant findings of blood routine parameters. All 24 volunteers (12 women and 12 men) completed the study successfully without major protocol deviations. Only small deviations occurred, e.g., in the standardized dinner in the evening or amount of water in the morning before visits, which were judged as minor with no impact on study results. Participants were 20 to 62 years old. Table 2 shows the demographic data of the volunteers. The study product was administered to all subjects under supervision of study staff after at least 10 h of fasting. Despite the open nature of the study, volunteers were kept blinded on the dosage of study products (10 g or 20 g lactulose or 20 g glucose, water) as well as on the formulation (lactulose crystals and liquid). All study products were provided dissolved in a total volume of 250 mL water, equivalent to the negative control. The intake was complete with no residual amounts, resulting in 100% compliance.
| Variable | mean | SD |
| Age (yr) | 35.4 | 13.11 |
| BMI (kg/m²) | 22.71 | 2.202 |
| Venous fasting glucose level (mg/dL) | 85.0 | 5.63 |
| HbA1c (%) | 4.98 | 0.297 |
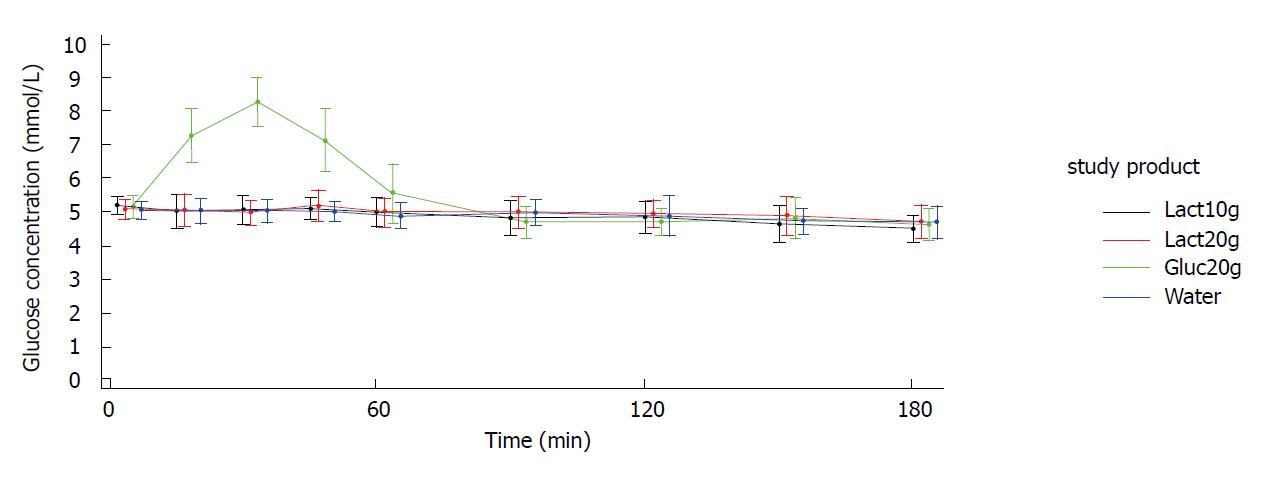
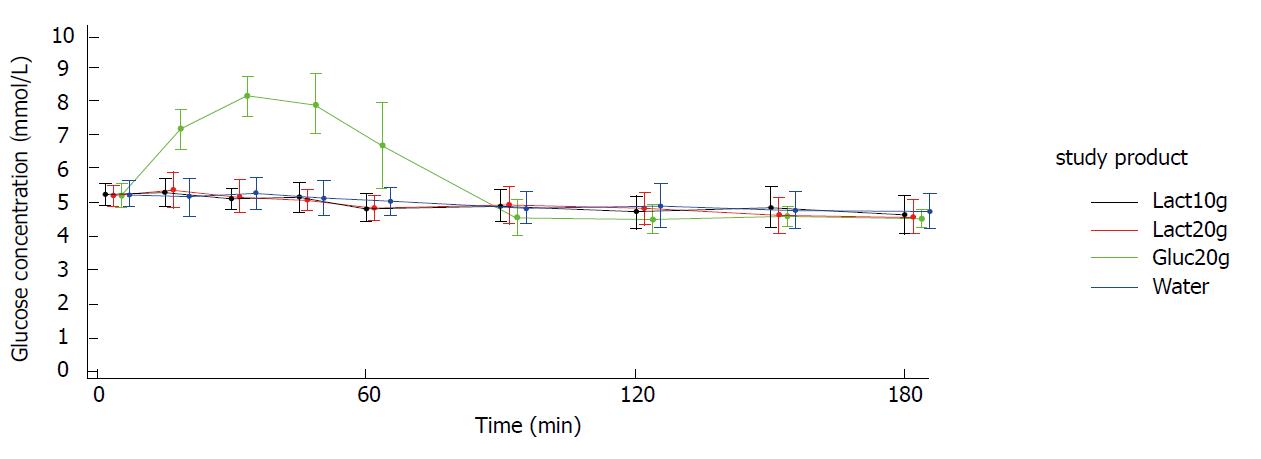
Figures 2 and 3 show the blood glucose concentration-time curves for the different applications. In both study arms (crystals and liquid), the glucose concentration-time curves after intake of 10 g lactulose, 20 g lactulose, and water were almost identical. None of the three applications showed any influence on the blood glucose levels.
As expected, there was a distinct increase in blood glucose concentration of approximately 3 mmol/L [mean Max_increase: 3.2 mmol/L (crystal arm); 3.1 mmol/L (liquid arm)] after intake of 20 g glucose (mean Cmax = 8.3 mmol/L in both study arms), reaching its maximum after approx. 30 min [Tmax: 30.6 min (crystal arm), 37.5 min (liquid arm)] and returning to baseline within approx. 90 min [Tbaseline: 76.8 min (crystal arm), 90.2 min (liquid arm)]. Mean baseline values (i.e., pre-dose values in each study period) were very similar for the four treatments within each study arm.
When taking lactulose as crystals or as liquid up to a dosage of 20 g, no differences on blood glucose concentration were observed over a time period of 180 min in comparison to water. iAUCs after the intake of lactulose were comparable with the control group receiving water. LS means are summarized in Tables 3-6. The findings were also confirmed for the secondary endpoints for which no treatment difference compared to water could be identified. Tmax for lactulose and water were not reported, as there were no glucose concentration peaks, which are necessary to define a distinct time to reach maximum glucose concentration.
| Crystals | Lactulose 10 g | Water 250 mL | Treatment difference | |||||
| Variable | n | LS mean | 95%CI | n | LS mean | 95%CI | Difference | 95%CI |
| iAUC (mmol/L*min) | 12 | 6.91 | (-7.41; 21.24) | 12 | 7.74 | (-6.57; 22.05) | -0.822 | (-19.50; 17.85) |
| AUC (mmol/L*min) | 12 | 873.11 | (841.26; 904.97) | 12 | 896.86 | (865.03; 928.70) | -23.75 | (-51.80; 4.30) |
| AUC_base (mmol/L*min) | 12 | -50.14 | (-81.99; -18.28) | 12 | -26.39 | (-58.22; 5.45) | -23.75 | (-51.80; 4.30) |
| C_max (mmol/L) | 12 | 5.32 | (5.12; 5.53) | 12 | 5.36 | (5.16; 5.57) | -0.04 | (-0.30; 0.22) |
| Max_increase (mmol/L) | 12 | 0.20 | (-0.01; 0.40) | 12 | 0.24 | (0.03; 0.44) | -0.04 | (-0.30; 0.22) |
| Crystals | Lactulose 20 g | Water 250 mL | Treatment difference | |||||
| Variable | n | LS mean | 95%CI | n | LS mean | 95%CI | Difference | 95%CI |
| iAUC (mmol/L*min) | 12 | 12.51 | (-1.74; 26.76) | 12 | 7.74 | (-6.57; 22.05) | 4.772 | (-13.58; 23.12) |
| AUC (mmol/L*min) | 12 | 902.78 | (871.02; 934.53) | 12 | 896.86 | (865.03; 928.70) | 5.912 | (-21.48; 33.30) |
| AUC_base (mmol/L*min) | 12 | -20.47 | (-52.23; 11.28) | 12 | -26.39 | (-58.22; 5.45) | 5.912 | (-21.48; 33.30) |
| C_max (mmol/L) | 12 | 5.41 | (5.21; 5.62) | 12 | 5.36 | (5.16; 5.57) | 0.049 | (-0.20; 0.30) |
| Max_increase (mmol/L) | 12 | 0.28 | (0.08; 0.49) | 12 | 0.24 | (0.03; 0.44) | 0.049 | (-0.20; 0.30) |
| Liquid | Lactulose 10 g | Water 250 mL | Treatment difference | |||||
| Variable | n | LS mean | 95%CI | n | LS mean | 95%CI | Difference | 95%CI |
| iAUC (mmol/L*min) | 12 | 5.89 | (-8.94; 20.72) | 12 | 9.92 | (-4.92; 24.77) | -4.029 | (-20.97; 12.91) |
| AUC (mmol/L*min) | 12 | 886.19 | (852.75; 919.64) | 12 | 890.45 | (856.97; 923.93) | -4.258 | (-33.36; 24.85) |
| AUC_base (mmol/L*min) | 12 | -53.93 | (-87.38; -20.48) | 12 | -49.67 | (-83.15; -16.19) | -4.258 | (-33.36; 24.85) |
| C_max (mmol/L) | 12 | 5.41 | (5.17; 5.66) | 12 | 5.49 | (5.24; 5.73) | -0.073 | (-0.35; 0.20) |
| Max_increase (mmol/L) | 12 | 0.19 | (-0.05; 0.43) | 12 | 0.26 | (0.02; 0.51) | -0.073 | (-0.35; 0.20) |
| Liquid | Lactulose 20 g | Water 250 mL | Treatment difference | |||||
| Variable | n | LS mean | 95%CI | n | LS mean | 95%CI | Difference | 95%CI |
| iAUC (mmol/L*min) | 12 | 11.62 | (-3.21; 26.46) | 12 | 9.92 | (-4.92; 24.77) | 1.701 | (-15.29; 18.69) |
| AUC (mmol/L*min) | 12 | 886.03 | (852.58; 919.49) | 12 | 890.45 | (856.97; 923.93) | -4.419 | (-33.65; 24.82) |
| AUC_base (mmol/L*min) | 12 | -54.09 | (-87.55; -20.64) | 12 | -49.67 | (-83.15; -16.19) | -4.419 | (-33.65; 24.82) |
| C_max (mmol/L) | 12 | 5.57 | (5.33; 5.81) | 12 | 5.49 | (5.24; 5.73) | 0.083 | (-0.19; 0.36) |
| Max_increase (mmol/L) | 12 | 0.35 | (0.10; 0.59) | 12 | 0.26 | (0.02; 0.51) | 0.083 | (-0.19; 0.36) |
The estimated treatment difference of iAUC with 95%CIs for lactulose crystals 20 g vs glucose 20 g was -109.58 (95%CI: -128.03; -91.13). The estimated treatment difference of iAUC with 95%CIs for lactulose liquid 20 g vs glucose 20 g was -130.94 (95%CI: -147.87; -114.00). Thus, a very large difference was observed between glucose and both lactulose formulations (P < 0.0001). The same applies to AUC and AUCbase, which were much smaller for 20 g of both lactulose formulations compared to 20 g glucose (P < 0.0001).
The maximum blood glucose concentrations (Cmax) and the maximum increase of blood glucose (Max_increase) after intake of both lactulose formulations were also significantly lower in comparison to glucose (P < 0.0001).
For the 10 g lactulose dose, the mean iAUC after intake of crystals was 5.12 ± 6.63 mmol/L*min, and after intake of liquid 5.80 ± 8.47 mmol/L*min.
The mean iAUC of blood glucose concentrations after application of 20 g lactulose was 13.56 ± 17.60 mmol/L*min for crystals and 11.74 ± 13.33 mmol/L*min for liquid. As reference, the mean iAUC of water in the crystals study arm was 9.42 ± 8.87 mmol/L*min and 9.76 ± 14.80 mmol/L*min in the liquid study arm. No differences in blood glucose concentration profiles after administration were identified between the two lactulose formulations.
For the AUC and AUCbase of blood glucose concentration after intake of 10 g and 20 g lactulose, very similar results for both formulations were observed (data not shown). As there were no distinct glucose concentration peaks after intake of lactulose secondary endpoints, Cmax and Tmax were not appropriate to add findings next to the AUC data.
In total, 19 AEs were reported by 11 volunteers, of which three AEs occurred between the screening visit and study visit 1. During the intervention phase (study visit 1-4) in each study arm, eight AEs occurred, which were reported by six subjects in the crystals study arm and five subjects in the liquid study arm. Overall, none of the AEs were serious and all AEs resolved at the end of the study. No AE led to discontinuation or modification of study product dosage.
Of these 16 AEs, seven possibly related AEs [digestive system (6 ×), headache (1 ×)] were reported and rated 6 × as mild and 1 × as moderate (heartburn). These AEs occurred after the intake of lactulose. Frequencies of reported possibly related AEs attributed to the digestive system were higher in the liquid study arm (five AEs reported by three subjects) compared to the crystals study arm (one AE); especially stomach ache was reported [3 × liquid study arm (2 × 10 g + 1 × 20 g)]. Furthermore, flatulence (1 × 10 g liquid), diarrhea (1 × 20 g liquid) and heartburn (1 × 20 g crystals) were reported. All other AEs were not related [six AEs: rhinitis, running nose, cough, migraine, headache (2 ×)] or unlikely related [three AEs: one subject after 10 g crystals (headache) and two AEs: one subject after intake of 20 g glucose (nausea and headache)].
Tolerability was assessed directly after the end of the blood glucose concentration assessment period (after 180 min) and 24 h after intake of study products after each intervention phase. The majority of subjects judged the tolerability at the single assessment time points as “well tolerated”. Only twice at the 180 min time point (1 × glucose; 1× lactulose 20 g liquid) and five times 24 h after intake, the product was rated as slightly unpleasant (after 10 g lactulose: 1 × crystal arm, 2 × liquid arm; 2 × after 20 g lactulose liquid). Very unpleasant tolerability was only rated 1 × after 20 g lactulose crystals. The tolerance assessment by subjects reflects the occurrence of AEs.
This study was performed as a prospective, open, mono-center, randomized, two-part study with 4-way cross-over design in each study arm to investigate blood glucose levels after oral intake of lactulose.
Due to the small amounts of carbohydrate impurities, up to 3% in lactulose crystals and approximately 30% in the liquid formulation, no or only small changes in blood glucose levels were expected. These expectations were confirmed by all primary and secondary endpoints. The glucose concentration-time curves showed that there was no difference after intake of lactulose (crystals or liquid) and water. iAUCs, AUCs and AUCbase after intake of lactulose were comparable with the control assessment with water. The intake of the two single lactulose dosages 10 g and 20 g did not affect blood glucose levels. This is in accordance with previous studies showing that 25 g lactulose did not increase blood glucose concentration in female lactose digesters and maldigesters[9] or diabetic subjects[8]. Hoffmann et al[7] showed only a slight increase of blood glucose level after intake of 36.4 g lactulose syrup containing 8.4 g of sugars and 20 g of lactulose. The dose of lactulose and accompanying sugars in the case report published by Kirkman et al[6] was higher compared to this study (daily intake 3 × 30 mL lactulose syrup, containing 24 g of simple sugars). However, the increase in blood glucose after changing the lactulose brand (3 × 30 mL lactulose syrup, containing 27.6 g of simple sugars) could not be explained by sugar impurities because the difference in daily intake was only 3.6 g. Normal food may cause much higher differences in daily sugar intake.
Furthermore, investigating AUCbase indicated an overall negative area for all lactulose administrations and water. This means that blood glucose concentrations tended to decrease over time. Of note, these changes over time during/after lactulose administration occurred within a tight range and within the normal limits of fasting blood glucose and were comparable with water. Consequently, no hypoglycemic values were observed during and after lactulose administration. The decrease expressed as AUCbase was to the same extent as with water and only reflects the normal physiologic metabolism. One should bear in mind that at the end of the intervention phase, subjects were fasting for at least 13 h.
The analysis of blood glucose levels was performed in capillary blood to also enable the determination of minor changes. Capillary measurement reflects changes in blood glucose more readily at finger sites than at the forearm[10,11]. This approach is also used for the determination of the GI. In this context, analysis in capillary finger-prick samples is discussed to be less variable and more sensitive to postprandial changes compared to venous blood samplings[12,13]. As expected, there was a significant difference for iAUC, AUC, AUCbase and Cmax after the intake of lactulose (crystals or liquid) compared to glucose. Interestingly, there was a decrease in blood glucose concentrations below baseline 90 min and 120 min after intake of glucose in both study arms. This minor decrease was counteracted by the body returning to baseline concentrations after 150 and 180 min. This profile reflects the regulation of the body by a normal slight overcompensation in the management of blood glucose concentration and is also described in GI determinations for various foods containing simple sugars[14].
Despite the higher concentration of carbohydrate impurities in the used lactulose liquid formulation with 26.45% in comparison to only 1.5% in crystals, both formulations showed similar blood glucose curves with no differences when evaluating primary and secondary endpoints. In both study arms, a difference between lactulose dosages was not apparent. This can be explained by the low total amount of sugars in both lactulose products (i.e., 0.3 g in crystals and 5.29 g in liquid formulation in the 20 g dosages). Furthermore, not all sugars may affect blood glucose to the same extent as glucose[15]. Even comparable decreases in blood glucose concentrations over time (expressed as baseline corrected AUC) were observed for the 10 g and 20 g dosages as well as for water.
This study adds data for currently marketed lactulose products to available data published between 1964 and 1999. For the first time, two doses of two lactulose formulations (crystals and liquid) were compared in a cross-over design ensuring high credibility and power of the study since expected high variation between subjects is of no concern in cross-over settings. Furthermore, older studies included less subjects[7,8] or women only[9]. The study was performed in compliance with ISO 26642:2010.
One limitation of the study might be the open design. Despite subjects being blinded on the dosage of study products (10 g or 20 g lactulose or 20 g glucose) as well as on the formulation (crystal or liquid), subjects could possibly distinguish between water as control and the study products due to the slight sweet taste of lactulose. Anyhow, it is expected that the impact of such a confounding factor on the objective measure of blood glucose level is rather limited, and placebo effects on blood glucose level were not identified from the study results.
These results clearly demonstrate that the carbohydrate impurities in 10-20 g of lactulose preparations have no impact on the glucose metabolism in healthy adults. As a next step, data should be confirmed in a study collective with impaired glucose tolerance. Overall, tolerability was good. Some volunteers reported mild and one volunteer reported moderate gastrointestinal side effects of lactulose, which are known[16,17].
In summary, a single dose administration of 10 g and 20 g of lactulose as crystals or liquid with their small amounts of carbohydrate impurities had no impact on blood glucose concentration in healthy subjects. Comparable to water, there was no glycemic response. Taken together, these data suggest that lactulose used as a functional food ingredient may also be consumed by people with impaired glucose tolerance.
During the manufacturing process of lactulose, impurities with sugars (e.g., lactose, fructose, galactose) occur. Because lactulose is used as a functional food ingredient, it may also be consumed by people with impaired glucose tolerance, including diabetics. Therefore, it is of interest whether the described carbohydrate impurities may increase blood glucose levels after ingestion.
There is only limited information if lactulose and especially the currently marketed formulations (liquid formulation and crystals) influence the blood glucose level.
The main objective was to investigate possible changes of blood glucose levels after oral intake of lactulose in healthy subjects.
The study was performed as a prospective, randomized, two-part study with a 4-way cross-over design with n = 12 in each study arm. Capillary blood glucose levels were determined over a time period of 180 min after intake of a single dose of 10 g or 20 g lactulose provided as crystal or liquid formulation. Water and 20 g glucose were used as control and reference, respectively.
The blood glucose concentration-time curves after intake of 10 g lactulose, 20 g lactulose, and water were almost identical. The three applications did not show any changes in the blood glucose levels. There was no difference between lactulose liquid and crystals. After intake of 20 g glucose, blood glucose concentration increased by approximately 3 mmol/L (mean Cmax = 8.3 mmol/L), reaching maximum levels after approximately 30 minutes and returning to baseline within approximately 90 minutes, which was significantly different to the corresponding 20 g lactulose formulations (P < 0.0001).
The unchanged blood glucose levels after lactulose intake in healthy subjects suggest its safe use in subjects with impaired glucose tolerance.
As a next step, data should be confirmed in a study collective with impaired glucose tolerance.
The authors thank all subjects who took part in this trial.
| 1. | Schumann C. Medical, nutritional and technological properties of lactulose. An update. Eur J Nutr. 2002;41 Suppl 1:I17-I25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Ballongue J, Schumann C, Quignon P. Effects of lactulose and lactitol on colonic microflora and enzymatic activity. Scand J Gastroenterol Suppl. 1997;222:41-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 137] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Levine MM, Hornick RB. Lactulose therapy in Shigella carrier state and acute dysentery. Antimicrob Agents Chemother. 1975;8:581-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Panesar PS, Kumari S. Lactulose: production, purification and potential applications. Biotechnol Adv. 2011;29:940-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | European Food Safety Authority. Scientific Opinion on the sub-stantiation of health claims related to lactulose and decreasing potentially pathogenic gastro-intestinal microorganisms (ID 806) and reduction in intestinal transit time (ID 807) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2010;8:1806. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Kirkman MS, Zimmerman DR, Filippini SA. Marked deterioration in glycemic control with change in brand of lactulose syrup. South Med J. 1995;88:492-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Hoffmann K, Mossel DA, Korus W, Van de Kamer JH. Investigations on the mode of action of lactulose/beta-galactosido-fructose in the human intestine. Klin Wochensch. 1964;42: 126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Lieberthal M, Conn HO, Bircher J. Management with Lactulose and related Carbohydrates. Hepatic Encephalopathy. Medi-Ed Press, East Lansing Michigan. 1988;145-175. |
| 9. | Teuri U, Vapaatalo H, Korpela R. Fructooligosaccharides and lactulose cause more symptoms in lactose maldigesters and subjects with pseudohypolactasia than in control lactose digesters. Am J Clin Nutr. 1999;69:973-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | ISO 26642:2010: Food products-Determination of the glycaemic index (GI) and recommendation for food classification. 2010. Available from: https://www.iso.org/standard/43633.html. |
| 11. | Ellison JM, Stegmann JM, Colner SL, Michael RH, Sharma MK, Ervin KR, Horwitz DL. Rapid changes in postprandial blood glucose produce concentration differences at finger, forearm, and thigh sampling sites. Diabetes Care. 2002;25:961-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Wolever TM, Vorster HH, Björck I, Brand-Miller J, Brighenti F, Mann JI, Ramdath DD, Granfeldt Y, Holt S, Perry TL. Determination of the glycaemic index of foods: interlaboratory study. Eur J Clin Nutr. 2003;57:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 185] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Hätönen KA, Similä ME, Virtamo JR, Eriksson JG, Hannila ML, Sinkko HK, Sundvall JE, Mykkänen HM, Valsta LM. Methodologic considerations in the measurement of glycemic index: glycemic response to rye bread, oatmeal porridge, and mashed potato. Am J Clin Nutr. 2006;84:1055-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Brand-Miller JC, Stockmann K, Atkinson F, Petocz P, Denyer G. Glycemic index, postprandial glycemia, and the shape of the curve in healthy subjects: analysis of a database of more than 1,000 foods. Am J Clin Nutr. 2009;89:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Ercan N, Nuttall FQ, Gannon MC, Redmon JB, Sheridan KJ. Effects of glucose, galactose, and lactose ingestion on the plasma glucose and insulin response in persons with non-insulin-dependent diabetes mellitus. Metabolism. 1993;42:1560-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Bouhnik Y, Attar A, Joly FA, Riottot M, Dyard F, Flourié B. Lactulose ingestion increases faecal bifidobacterial counts: a randomised double-blind study in healthy humans. Eur J Clin Nutr. 2004;58:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Tuohy KM, Ziemer CJ, Klinder A, Knöbel Y, Pool-Zobel L, Gibson GR. A Human Volunteer Study to Determine the Prebiotic Effects of Lactulose Powder on Human Colonic Microbiota. Microb Ecol Health Dis. 2002;14:165-173. [RCA] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Abbasnezhad A, Gasim GI, Poullis A S- Editor: Ji FF L- Editor: Filipodia E- Editor: Tan WW