Published online Feb 6, 2017. doi: 10.4292/wjgpt.v8.i1.39
Peer-review started: October 22, 2016
First decision: December 1, 2016
Revised: December 13, 2016
Accepted: January 2, 2017
Article in press: January 3, 2017
Published online: February 6, 2017
Processing time: 93 Days and 20.7 Hours
Gastrointestinal (GI) diseases comprise a large spectrum of clinical conditions ranging from indigestion to inflammatory bowel diseases (IBDs) and carcinomas. Endoscopy is the usual method employed to diagnose these condition. Another noninvasive way to assess and diagnose GI conditions are fecal biomarkers. Fecal biomarkers provide information regarding a specific disease process and are perhaps more acceptable to clinicians and patients alike because of their non-invasivity compared to endoscopy. Aim of this review was to evaluate the current status of the fecal biomarkers in clinical and research for in GI diseases. Multiple types of fecal biomarkers are discussed in this review including; markers to assess IBD, which are released as a results of an inflammatory insults to intestinal epithelia such as antimicrobial peptides (lactoferrin) or inflammation related proteins (calprotectin). While markers related to function of digestion are primarily related to partially digested food or mucosal proteins such as abnormal amount of fecal fat α1-antitrypsin, elastase and secretary IgA. The upcoming fecal biomarker like M2 pyruvate kinase and neutrophil gelatinase associated lipocalin are discussed as well. Apart from above mention, the fecal biomarkers under exploration for possible clinical use in future are also discussed. These include cathelicidins, osteoprotegerin, β-glucuronidase, Eosinophil proteins, etc.
Core tip: There is a general inclination of clinicians as well as pathologists’ to consider fecal biomarkers due to its non-invasivity. There are multiple types of fecal biomarkers in clinical use and under exploration for potential clinical use in future. It includes biomarkers for evaluating inflammatory bowel disease (e.g., calprotectin, lactoferrin), for evaluating colorectal cancer, malabsorption and eosinophilic protein for allergic gastrointestinal diseases. In this review we have analyzed the current status in terms of their practical utilization of fecal biomarkers with established indications and those which are under various stages of investigation.
- Citation: Siddiqui I, Majid H, Abid S. Update on clinical and research application of fecal biomarkers for gastrointestinal diseases. World J Gastrointest Pharmacol Ther 2017; 8(1): 39-46
- URL: https://www.wjgnet.com/2150-5349/full/v8/i1/39.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v8.i1.39
A biomarker is an endogenous or exogenous substance measured in blood, plasma or urine whose levels correlate with disease occurrence or severity. Biomarkers are used to distinct a pathological condition from a physiological state and also monitoring treatment and disease progression. There is a general inclination of clinicians as well as pathologists’ to consider fecal biomarkers due to its non-invasive nature with likely acceptability to the patient. Fecal biomarkers can be subdivided into following types based on their clinical application.
These include inflammation related proteins, released during an inflammatory process in the gastrointestinal (GI) tract (e.g., calprotectin) or antimicrobial peptides (e.g., lactoferrin).
These are found in undifferentiated tissues and cells with increased expression in rapid turnover of such cells, e.g., M2-pyruvate kinase.
Multiple markers are identified; most are undigested food particles like fecal fat globules, enzymes like α1-antitrypsin and elastase for malabsorption assessment.
Eosinophil related proteins are either released by or related to eosinophils and have application in assessing allergic and parasitic infestation of GI tract.
This is an interesting group of biomarkers which with point toward the overall health of the gut mucosa. These biomarkers assess the integrity of gut barrier proteins and microbial fermentation products which are produced while fermentation of dietary particles by bacteria produces various chemicals, few of which such as short chain fatty acids, are used as biomarkers.
Different types of fecal biomarkers for GI diseases in clinical use and under investigations are shown in Tables 1 and 2. Although evidence for the newer markers is growing, currently only few fecal biomarkers have achieved a place in routine clinical practice notably calprotectin is on top of that list. Out of the multiple fecal biomarkers with emerging roles in clinical use for GI diseases, only some are extensively studied for their clinical and diagnostic utilities. In this review we aim to provide an overview of current status of fecal biomarkers for GI diseases with established value in clinical and potential for future use.
| S# | Name | Indication | Limitations | Sensitivity | Specificity |
| Biomarkers of IBD | |||||
| 1 | Calprotectin | Distinguishing functional from organic bowel disease and predicting relapse in IBD | Disease nonspecific Affected by age, comorbidities, NSAIDs use Day to day variations Miss low level inflammatory activity | 70%-100% | 70%-100% |
| 2 | S100 proteins | Inflammatory marker for IBD | 60%-67% | 70%-90% | |
| 3 | Lactoferrin | Markers of inflammation, Distinguish between IBS and IBD | Nonspecific marker of inflammation Raised in breastfeeding infants Cannot predict low level inflammation | 67%-87% | 90%-100% |
| Biomarker of cell turnover | |||||
| 4 | M2-PK | Screening of gastrointestinal tract cancers | Also raised in inflammation | 67%-93% | 88%-92% |
| Biomarkers of digestion and malabsorption | |||||
| 5 | Elastase-1 (e1) | Pancreatic insufficiency | Low specificity, also affected by other intestinal disorders | 100% | 96% |
| 6 | Fecal fat | Liver damage, hypolipidemic drugs, impaired gallbladder function, Celiac disease, Small bowel bacterial overgrowth | Cannot predict severity of disease Cannot be performed in diarrhea Not accurate or specific test | 70%-94% | 80%-99% |
| 7 | Α1-antitrypsin | Protein-Losing Enteropathy, Whipple lipodystrophy, gastric carcinoma, intestinal lymphangiectasia | Nonspecific marker. Levels affected by inflammation | 60%-78% | 80%-85% |
| S# | Name | Source | Function | Indication | Limitations |
| Biomarkers of inflammatory bowel disease | |||||
| 1 | Cathelicidins | Secreted by Neutrophils, keratinocytes and epithelial cells of gastrointestinal tract, respiratory tract, urogenital tract | Antibacterial activity, modulate inflammation by altering cytokine response, chemoattraction of inflammatory cells in diseased tissues | Marker of inflammation (IBD) and Shigellosis | Antimicrobial peptides so also increased in GI infections |
| 2 | Osteoprotegerin | Member of the TNF receptor superfamily | Binds to RANKL and blocks its interaction with RANK | Marker of inflammation (IBD) | Plays a role in bone metabolism so levels are increased in bone diseases |
| 3 | Beta-glucuronidase | Produced by colonocytes Also produced by anaerobic gut bacteria (particularly E. coli) | Enzyme that breaks down complex carbohydrates Deconjugate glucuronide molecules from a variety of toxins, carcinogens, hormones, and drugs | Marker of inflammation (IBD) | False results in cases of GI bacterial infection |
| 4 | Neutrophil Gelatinase Associated lipocalin | Member of the lipocalin family, secreted by neutrophils | Immunomodulation. Attaches to and neutralizes bacterial formylpepetides | Marker of inflammation (IBD) | Also increased in GI infections like enterocolitis |
| Eosinophil related proteins | |||||
| 5 | Eosinophil Protein X | When lamina propria is damaged, eosinophils migrate into the gut lumen Released by eosinophil; contribute to ongoing inflammation and tissue destruction | Marker of Eosinophil activity, Allergic and Parasitic influences | IgE-mediated food allergy Intestinal parasitic infection IBD | Also increased in GI inflammation |
| Biomarker of cell turnover | |||||
| 6 | Defensins | Expressed by neutrophils, epithelial and mucosal lining cell in small and large intestine | Antimicrobial peptide | Markers of colorectal cancer | Also raised in inflammation |
| Biomarkers of gut health | |||||
| 7 | Fecal secretory IgA | Secreted from mucosal surfaces | Gut epithelial barrier; Defense against the entry of enteric toxins and pathogenic organisms Development of immune tolerance of normal commensal gut organisms | Evaluate immunological response to intestinal pathogens Colorectal cancer | Cannot be used in subjects with immunoglobulin deficiency |
| 8 | SCFAs | Products of fermentation by colonic microbial flora; common ones are propionate, acetate, and butyrate | Provides 60%-70% of colonocytes energy requirements Lower colonic pH | Marker of inflammation (IBD) | < 5% of SCFA produced is excreted in stool Also levels altered by diet and rate of transit |
A search of databases PubMed, MEDLINE and Google Scholars was performed using the search terms “fecal biomarkers” and “gastrointestinal disease biomarkers”. We selected articles written in English, published since 1990 in peer-reviewed journals, excluding reviews. The articles were then reviewed by two pathologists and one gastroenterologist keeping in view the ideology behind this review and relevant articles selected. This review is divided in two parts, in part one we have aimed to include diagnostic accuracies for the established markers in clinical use and in second part the clinical applications of fecal biomarkers under investigation for GI diseases are discussed.
Calprotectin (S100A8/S100A9) and S100 A12 proteins: The S100 proteins are a family of calcium-binding proteins specifically linked to innate immune functions by their expression in phagocytes, monocytes, macrophages and granulocytes. The calprotectin is a heterodimer of S100A8 and S100A9. These proteins are released by cells of innate immunity and GI epithelial cells in condition of inflammation. They limit the growth of bacteria and fungi by sequestering manganese and zinc.
Calprotectin is a marker to diagnose or monitor inflammatory bowel disease (IBD), presently considered a gold standard and also included in clinical practice guidelines. It is reported to perform better than S100A12 in diagnosing IBD and its levels correlate with the severity of IBD. Calprotectin is observed to perform better in predicting ulcerative colitis then Chron’s disease[1,2]. Meta-analysis have reported that calprotectin perform better in adults (sensitivity 93% and specificity 96%) than children (sensitivity 92% and specificity 76%)[3]. In contrast there are conflicting studies regarding diagnostic utility of the S100A12 as an inflammatory marker and it have moderate performance compared to other inflammatory markers[4-6].
Calprotectin is resistant to bacterial degradation in the gut and is stable in stool for up to one week at room temperature and is readily measured using immunochemical techniques. Limitations and diagnostic accuracies are conversed in Table 1.
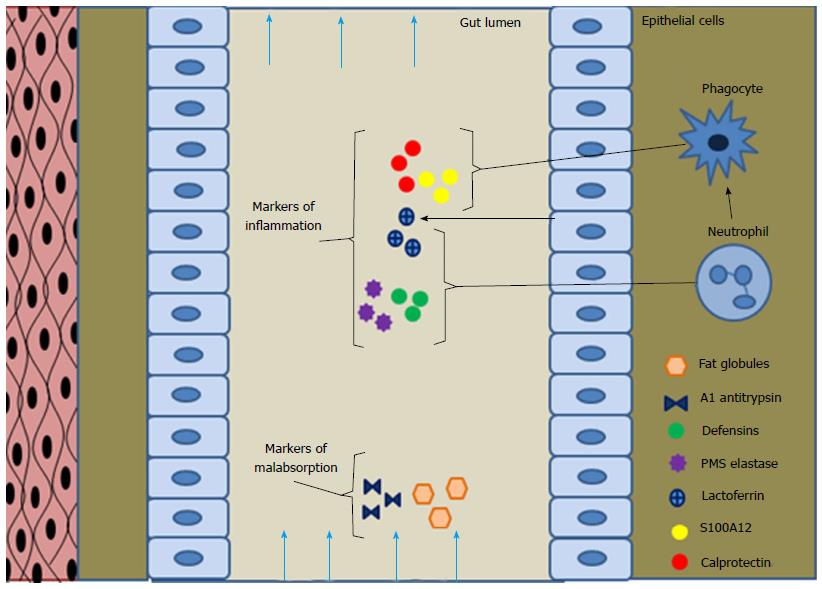
Lactoferrin: Lactoferrin, an iron binding glycoprotein secreted in body fluids and produced by neutrophils, mononuclear phagocytes and epithelial cells, Figure 1. It limits the growth of bacteria by limited availability of iron and causes direct damage to bacterial cell membrane leading to its bactericidal activity. This glycoprotein is stable in feces as it is resistant to proteolysis and can be measured by immunochemical methods. These glycoproteins are released in excess amounts by neutrophils and phagocytic cells after inflammation, making it a unique marker of inflammation[7]. Commercial assay for fecal lactoferrin measurement are now available based on immunochemical methods.
This is considered a good marker for evaluating IBD subjects, while evaluation as marker to distinguish between IBD and irritable bowel syndrome (IBS) there remains question marks, due to differences in results reported by different studies[8-11].
Cathelicidins: These are small cationic antimicrobial peptides like defensins and are produced by neutrophils and epithelial cells of GI tract, released upon stimulation of these cells during infection. These peptides exhibit antimicrobial activity against GI pathogens, gram-negative and positive bacteria by disrupting microbial membrane integrity, Table 2. These peptides play a vital role in maintaining the balance between the GI luminal bacteria and antibacterial peptides, which is crucial for a healthy GI tract. Studies have reported this balance is disturbed in various disease states. However the role of these peptides as the cause or consequence of disease state is still unknown. They could participate in the development of different disorders ranging from inflammation to cancer.
Schauber et al[12] reported that colonic expression of cathelicidin is increased in ulcerative colitis but not Crohn’s disease. A study looking at cathelicidin role in Escherichia coli O157:H7 infection in mice and subsequent renal damage found that its deficiency was associated with severe infection and renal damage[13]. Another study by Sarker et al[14] reported that antimicrobial peptides cathelicidins expressions were decreased in rectal and colonic epithelia in shigellosis infection in rabbits along with decreased expression in epithelia of lung and trachea, a sign of systemic infection. They also observed the treatment with phenylbutyrate counteracted the decreased expression of cathelicidins in such patients offering a potential antimicrobial activity against shigella infection[14].
Osteoprotegerin: Osteoprotegerin is member of the tumor necrosis factor receptor superfamily. It binds to the receptor activator of nuclear factor kappa B ligand (RANKL), which in turn has pro-inflammatory properties, Table 2. A study by Nahidi et al[15] examining the role of osteoprotegerin in pathogenesis of IBD reported that it induced gut barrier deformities; increased permeability and decreased integrity of cell membrane along with loss of tight junctions; indicating that osteoprotegerin has pro-inflammatory effect and may contribute in pathogenesis of IBD[15]. However the complete understanding of its function in IBDs needs further evaluation.
Beta-glucuronidase: Beta-glucuronidases enzymes secreted by lysosomes of colonocytes and certain bacteria, e.g., E. coli, belong to glycosidase family of enzymes. This enzyme catalyzes the complex dietary carbohydrates, like glycosaminoglycansheparan sulfate. They also deconjugate variety of drugs, toxins, hormones and also bilirubin in gut and are considered culprit of breast milk related jaundice in neonates, Table 2.
A study by Mroczynska et al[16], done on IBD and healthy children, reported that beta-glucuronidase activity was decreased by two times in children with IBD compared to healthy group. While in another study Manoj et al[17] reported that reduction in activity of intestinal as well as decreased levels of fecal beta-glucuronidase by using dietary fibers isolated from coconut or black gram may potentially play a role in preventing the formation of colon tumors induced by the carcinogen 1,2-dimethylhydrazine. These debatable findings warrant that further researchs is needed to completely understand chemical basis of beta-glucuronidase function.
Neutrophil gelatinase associated lipocalin: These proteins are released by neutrophils in response to some bacterial peptides (formylpeptides), which initiate bacterial protein synthesis. The neutrophil gelatinase associated lipocalin (NGAL) released into the gut lumen then binds with bacterial peptide and neutralizes it, stopping bacterial protein synthesis[18].
NGAL is another important inflammation related fecal marker under investigation for potential clinical utility. Serum and urinary NGAL are considered established markers for acute kidney injury and few studies have shown its levels are elevated in IBD but the levels don't correlate with disease severity[19]. Recently multiple studies have shown that fecal NGAL levels are raised in subjects with IBD and its levels are significantly associated with disease activity and severity[20-22].
Pyruvate kinase: M2-pyruvate kinase is a dimer of pyruvate kinase; an enzyme involved in glycolysis pathway and plays an important role in tumor metabolism. It has increased expression in undifferentiated tissues and cells with in rapid turnover cells.
Its main role is in predicting GI cancers, both bleeding and non-bleeding types. Studies have reported it to be a marker in predicting colorectal cancers with good diagnostic accuracy, sensitivity and specificity of 93% and 97% respectively, Table 1[23]. Currently it is being used for monitoring colorectal cancer subjects after treatment. Along with it, levels of M2-Pk are also elevated in breast, lung, ovarian, and thyroid cancers[24]. One of its important limitations is that its levels are also increased in inflammation, so it should be used with caution in inflammatory conditions[25].
Defensins: Defensins are small cationic antimicrobial peptides, classified into alpha and beta defensins on basis of their disulfide bond and sizes. These are expressed by neutrophils, epithelial and mucosal lining cell in small and large intestine, Figure 1. They play an important role in innate immunity; antimicrobial activity against bacteria, fungi and some enveloped viruses and the expression is induced by the pro-inflammatory cytokines and also through microorganisms.
As the name implies they were considered as markers of infectious and inflammatory GI diseases. However there is now accumulating evidence suggesting defensins as an evolving marker for evaluating colorectal cancers but there are controversial findings[26-28]. Studies have also reported it to be an important marker for colorectal cancer. A study by Layton et al[29] presented at American Association of Cancer Research 104th Annual Meeting in 2013 reported that β-defensins 1 was expressed in colon tissue samples of normal subjects while this expression was lost in subjects with colorectal cancer. While another study by Melle et al[30], reported that α-defensins are expressed more in colonic epithelium of patients with colorectal cancer than in normal epithelium, establishing defensins potential role as a tumor markers[30]. So there remains a question mark regarding utility of this biomarker for evaluating colorectal cancer.
Elastase-1(e1) and PMN elastase: Serum elastase is a protease present in pancreatic secretion reaches the colon without being metabolized and is not affected by intestinal transit times or pancreatic enzyme replacement therapy, Figure 1. Elastase hydrolyzes denatured hemoglobin, casein, fibrin and albumin. It is a known biomarker for assessing exocrine pancreatic insufficiency, been in use for more than 3 decades. While its deficiency is associated with development of pulmonary emphysema and excess release results in hemorrhage due to vascular injury of acute pancreatic necrosis. Serum Elastase e1 levels are used for the diagnosis of acute or chronic pancreatitis, pancreatic insufficiency with good diagnostic accuracy[31], Table 1.
Another type of elastase enzymes, the polymorphonuclear elastase (PMN-elastase) is secreted by neutrophils in response to inflammation[32]. A study assessed the performance of calprotectin, lactoferrin and PMN-elastase in assessing IBD severity and differentiating between IBS and IBD, found that all these markers were able to differentiate active IBD from inactive IBD as well as from IBS with diagnostic accuracies for lactoferrin, calprotectin and PMN-elastase of 80%, 80% and 74%[33].
Fecal fat: Excess fat in the stool (steatorrhea) is often the first sign of fat malabsorption. This can be due to a number of factors, including chronic pancreatitis with or without stone obstruction, cystic fibrosis, neoplasia, Whipple disease, regional enteritis, tuberculous enteritis, celiac disease, or the atrophy of malnutrition, Table 1.
The fecal fat assessment is done by microscopy after sudan stain and is largely considered non-specific as it is affected by diet, discrepancies in sample collection, qualitative reporting and assay variation leading to lower diagnostic accuracy. To overcome these hurdles a new quantitative fecal fat microscopic method was introduced by Fine et al[34] in 2000, reported to have improve diagnostic accuracy; sensitivity of 94% and a specificity of 95% compared to the traditional method sensitivity and specificity of 76% and 99%, respectively. In this method they microscopically counted the fat globules of different diameter ranges (0-5 μm, 6-10 μm, 11-20 μm, 21-40 μm, 41-80 μm, and > 80 μm) in five high-power fields and the average number of each size range fat globules present were multiplied by the size-range midpoint. All products were then added to get a single fecal fat droplet total size number product. They reported that results obtained by this method correlates well with chemically measured fecal fat output and has a high diagnostic accuracy.
Αlpha-1-antitrypsin: Alpha-1-antitrypsin a protease inhibitor is produced by the liver, macrophages, and intestinal epithelium and is resistant to degradation by digestive enzymes. Therefore offers utility for use as a biomarker in assessing the proteins loss distal to the pylorus. Protein loss is associated in certain GI conditions such as gastroenteritis and sprue. Alpha-1-antitrypsin can readily be measured by using commercially available assays, Table 1.
Fecal alpha-1-antitrypsin clearance has been a marker of clinical disease severity in IBDs for many years[35]. Although α1-antitrypsin deficiency is more often associated with lung and liver pathologies, α1-antitrypsin deficient patients with concomitant IBD have been shown to develop more aggressive disease and rapid progression requiring surgery[36]. In a study by Becker et al[37] it was found that individual fecal α1-antitrypsin can predict prognosis in IBD patients.
Eosinophil protein X: When lamina propria is damaged, eosinophils migrate into the gut lumen and multiple eosinophil granules related proteins are released, Table 2. These proteins contribute towards ongoing inflammation and tissue destruction associated with eosinophil related diseases like allergic diseases esophagitis, colitis, celiac disease, intestinal parasitic infections and IgE-mediated food allergy[38]. There is multiple eosinophil proteins including major basic protein, eosinophil cationic protein, eosinophil derived neurotoxin and eosinophil peroxidase associated with eosinophilic activity during inflammation[39,40]. This biomarker is however nonspecific and requires further studies to understand its role in eosinophil related disease pathology.
Short-chain fatty acids: These are fatty acids with 1-6 carbon atoms, common ones are propionate, acetate, and butyrate produced as a results of metabolism of polysaccharides, oligosaccharides, peptides and glycoproteins by bacterial fermentation, absorbed by portal circulation and are an important energy source for colonic cells[41,42]. They lower the gut pH by regulating fluid and electrolyte uptake via activation of apical Na+/H+ exchange receptor[43]. These Short-chain fatty acids (SCFAs) are considered markers of colonic health and are known to have anti-inflammatory properties, Table 2. A study by Ohigashi et al[44], comparing colorectal carcinoma, adenoma and non-adenomatous subjects reported that compared to rest, subjects with carcinoma had decreased SCFA levels and altered microbial environment and pH.
Fecal secretory IgA: Immunoglobulin-A (IgA) are secreted by mucous membranes and as the name implies are antibodies important for mucosal immunity. These antibodies only form 15% for all immunoglobulins and in dimeric form called secretory IgA. This immunoglobin forms a defense against enteric toxins and pathogenic organisms. Secretory IgA is mainly secreted in mucosal secretions like tears, saliva, sweat, genitourinary tract, GI tract, prostate and respiratory epithelium.
Fecal secretory IgA is a part of mucosal barrier against infections and is also know to inhibit inflammation playing a protective role; therefore it is considered as a marker of gut health[45]. This biomarker is used to assess intestinal infections, coeliac disease and food allergies (Table 2)[46,47]. Few studies have also evaluated its clinical utility as an alternate marker of IBD but its use for these diseases is limited due to non-specific nature of this molecule.
The currently used diagnostic tools for identifying GI diseases are endoscopic procedures. Endoscopies are costly, invasive, time consuming, and also require patient preparation. Most of the time endoscopic procedure also required sedation especially in pediatrics patients. Interpretation of an endoscopic report is also subjective and opinions of two experts can differ at time. Generally speaking non-invasive approaches like serological test, urinary, fecal or salivary biomarkers are logically more acceptable to patients. Fecal biomarkers are now increasingly being used and the development of sensitive and specific immunochemical techniques have led to its increased utility.
Newer biomarkers with established diagnostic utilities in clinical use include lactoferrin, defensins and S100 proteins especially calprotectin. Calprotectin and lactoferrin are now also included in clinical practice guidelines in the management of IBD. However the clinical application of these biomarkers is well established for IBD but validation studies are still needed to understand their role in other GI pathologies. Also the reference cut offs used by each study is different, so there is need to standardize the assays and reference cutoffs of the established markers to clearly distinct diseased from non-diseased states. With more research to increase our understanding regarding roles of these biomarkers in GI health and disease, there is the potential for few more markers such as cathelicidins to be incorporated into clinical practice in near future.
Currently, apart from the fecal markers of inflammation there is not enough literature regarding fecal biomarkers clinical utility in other GI diseases or health. For example eosinophilic proteins have the potential to be used as disease markers for allergic states and parasitic infestations; very common in developing country. But these markers require more studies to better understand their roles in diseased states. Another advantage of these markers will be that they will provide more insight into the cause of disease. Furthermore as we are in an era of preventive medicine markers which can pick early changes in gut health are required so the patients screened out before developing a diseased state. In conclusion development of fecal biomarker and establishment of their clinical and diagnostic utilities is a developing field with a lot of promise, but we still need more research to validate these findings.
| 1. | Mao R, Xiao YL, Gao X, Chen BL, He Y, Yang L, Hu PJ, Chen MH. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis. 2012;18:1894-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 2. | Lin JF, Chen JM, Zuo JH, Yu A, Xiao ZJ, Deng FH, Nie B, Jiang B. Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis. 2014;20:1407-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 3. | van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 500] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 4. | Manolakis AC, Kapsoritakis AN, Georgoulias P, Tzavara C, Valotassiou V, Kapsoritaki A, Potamianos SP. Moderate performance of serum S100A12, in distinguishing inflammatory bowel disease from irritable bowel syndrome. BMC Gastroenterol. 2010;10:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Däbritz J, Langhorst J, Lügering A, Heidemann J, Mohr M, Wittkowski H, Krummenerl T, Foell D. Improving relapse prediction in inflammatory bowel disease by neutrophil-derived S100A12. Inflamm Bowel Dis. 2013;19:1130-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 370] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 7. | Borkowska A, Liberek A, Łuczak G, Jankowska A, Plata-Nazar K, Korzon M, Kamińska B. Fecal lactoferrin, a marker of intestinal inflammation in children with inflammatory bowel disease. Acta Biochim Pol. 2015;62:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Schoepfer AM, Trummler M, Seeholzer P, Seibold-Schmid B, Seibold F. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis. 2008;14:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 9. | Sidhu R, Wilson P, Wright A, Yau CW, D’Cruz FA, Foye L, Morley S, Lobo AJ, McAlindon ME, Sanders DS. Faecal lactoferrin--a novel test to differentiate between the irritable and inflamed bowel? Aliment Pharmacol Ther. 2010;31:1365-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Wang Y, Pei F, Wang X, Sun Z, Hu C, Dou H. Diagnostic accuracy of fecal lactoferrin for inflammatory bowel disease: a meta-analysis. Int J Clin Exp Pathol. 2015;8:12319-12332. [PubMed] |
| 11. | Menees SB, Powell C, Kurlander J, Goel A, Chey WD. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol. 2015;110:444-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 267] [Article Influence: 24.3] [Reference Citation Analysis (1)] |
| 12. | Schauber J, Rieger D, Weiler F, Wehkamp J, Eck M, Fellermann K, Scheppach W, Gallo RL, Stange EF. Heterogeneous expression of human cathelicidin hCAP18/LL-37 in inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 2006;18:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Chromek M, Arvidsson I, Karpman D. The antimicrobial peptide cathelicidin protects mice from Escherichia coli O157: H7-mediated disease. PLoS One. 2012;7:e46476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Sarker P, Ahmed S, Tiash S, Rekha RS, Stromberg R, Andersson J, Bergman P, Gudmundsson GH, Agerberth B, Raqib R. Phenylbutyrate counteracts Shigella mediated downregulation of cathelicidin in rabbit lung and intestinal epithelia: a potential therapeutic strategy. PLoS One. 2011;6:e20637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Nahidi L, Leach ST, Lemberg DA, Day AS. Osteoprotegerin exerts its pro-inflammatory effects through nuclear factor-κB activation. Dig Dis Sci. 2013;58:3144-3155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Mroczyńska M, Galecka M, Szachta P, Kamoda D, Libudzisz Z, Roszak D. Beta-glucuronidase and Beta-glucosidase activity in stool specimens of children with inflammatory bowel disease. Pol J Microbiol. 2013;62:319-325. [PubMed] |
| 17. | Manoj G, Thampi BS, Leelamma S, Menon PV. Effect of dietary fiber on the activity of intestinal and fecal beta-glucuronidase activity during 1,2-dimethylhydrazine induced colon carcinogenesis. Plant Foods Hum Nutr. 2001;56:13-21. [PubMed] |
| 18. | Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 336] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 19. | Nielsen OH, Gionchetti P, Ainsworth M, Vainer B, Campieri M, Borregaard N, Kjeldsen L. Rectal dialysate and fecal concentrations of neutrophil gelatinase-associated lipocalin, interleukin-8, and tumor necrosis factor-alpha in ulcerative colitis. Am J Gastroenterol. 1999;94:2923-2928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Thorsvik S, Damås JK, Granlund AV, Flo TH, Bergh K, Østvik AE, Sandvik AK. Fecal neutrophil gelatinase-associated lipocalin as a biomarker for inflammatory bowel disease. J Gastroenterol Hepatol. 2017;32:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Zinkevich OD, Mukhametova DD, Abdulganieva DI, Safina NA, Koporulina MO, Odintsova AKh. Fecal neutrophil gelatinase-associated lipocalin is a surrogate marker of inflammation in inflammatory bowel disease. Eksp Klin Gastroenterol. 2014;22-27. [PubMed] |
| 22. | Yeşil A, Gönen C, Senateş E, Paker N, Gökden Y, Koçhan K, Erdem ED, Gündüz F. Relationship between neutrophil gelatinase-associated lipocalin (NGAL) levels and inflammatory bowel disease type and activity. Dig Dis Sci. 2013;58:2587-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Sithambaram S, Hilmi I, Goh KL. The Diagnostic Accuracy of the M2 Pyruvate Kinase Quick Stool Test--A Rapid Office Based Assay Test for the Detection of Colorectal Cancer. PLoS One. 2015;10:e0131616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 24. | Muñoz-Colmenero A, Fernández-Suárez A, Fatela-Cantillo D, Ocaña-Pérez E, Domínguez-Jiménez JL, Díaz-Iglesias JM. Plasma Tumor M2-Pyruvate Kinase Levels in Different Cancer Types. Anticancer Res. 2015;35:4271-4276. [PubMed] |
| 25. | Jeffery J, Lewis SJ, Ayling RM. Fecal dimeric M2-pyruvate kinase (tumor M2-PK) in the differential diagnosis of functional and organic bowel disorders. Inflamm Bowel Dis. 2009;15:1630-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | O’Neil DA, Porter EM, Elewaut D, Anderson GM, Eckmann L, Ganz T, Kagnoff MF. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718-6724. [PubMed] |
| 27. | Wehkamp J, Schauber J, Stange EF. Defensins and cathelicidins in gastrointestinal infections. Curr Opin Gastroenterol. 2007;23:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Albrethsen J, Møller CH, Olsen J, Raskov H, Gammeltoft S. Human neutrophil peptides 1, 2 and 3 are biochemical markers for metastatic colorectal cancer. Eur J Cancer. 2006;42:3057-3064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Layton A, Bohanes PO, Zhang W, Lenz HJ, Labonte MJ. Evaluation of the novel tumor suppressor gene, β-Defensin-1, in colorectal cancer cell line models. Cancer Res. 2013;73:1526-1526. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Melle C, Ernst G, Schimmel B, Bleul A, Thieme H, Kaufmann R, Mothes H, Settmacher U, Claussen U, Halbhuber KJ. Discovery and identification of alpha-defensins as low abundant, tumor-derived serum markers in colorectal cancer. Gastroenterology. 2005;129:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Soldan W, Henker J, Sprössig C. Sensitivity and specificity of quantitative determination of pancreatic elastase 1 in feces of children. J Pediatr Gastroenterol Nutr. 1997;24:53-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Silberer H, Küppers B, Mickisch O, Baniewicz W, Drescher M, Traber L, Kempf A, Schmidt-Gayk H. Fecal leukocyte proteins in inflammatory bowel disease and irritable bowel syndrome. Clin Lab. 2005;51:117-126. [PubMed] |
| 33. | Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Fine KD, Ogunji F. A new method of quantitative fecal fat microscopy and its correlation with chemically measured fecal fat output. Am J Clin Pathol. 2000;113:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Biancone L, Fantini M, Tosti C, Bozzi R, Vavassori P, Pallone F. Fecal alpha 1-antitrypsin clearance as a marker of clinical relapse in patients with Crohn’s disease of the distal ileum. Eur J Gastroenterol Hepatol. 2003;15:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 36. | Collins CB, Aherne CM, Ehrentraut SF, Gerich ME, McNamee EN, McManus MC, Lebsack MD, Jedlicka P, Azam T, de Zoeten EF. Alpha-1-antitrypsin therapy ameliorates acute colitis and chronic murine ileitis. Inflamm Bowel Dis. 2013;19:1964-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Becker K, Berger M, Niederau C, Frieling T. Individual fecal alpha 1-antitrypsin excretion reflects clinical activity in Crohn’s disease but not in ulcerative colitis. Hepatogastroenterology. 1999;46:2309-2314. [PubMed] |
| 38. | Kapel N, Matarazzo P, Haouchine D, Abiola N, Guérin S, Magne D, Gobert JG, Dupont C. Fecal tumor necrosis factor alpha, eosinophil cationic protein and IgE levels in infants with cow’s milk allergy and gastrointestinal manifestations. Clin Chem Lab Med. 1999;37:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Wagner M, Peterson CG, Stolt I, Sangfelt P, Agnarsdottir M, Lampinen M, Carlson M. Fecal eosinophil cationic protein as a marker of active disease and treatment outcome in collagenous colitis: a pilot study. Scand J Gastroenterol. 2011;46:849-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Wedemeyer J, Vosskuhl K. Role of gastrointestinal eosinophils in inflammatory bowel disease and intestinal tumours. Best Pract Res Clin Gastroenterol. 2008;22:537-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Pang T, Leach ST, Katz T, Jaffe A, Day AS, Ooi CY. Elevated fecal M2-pyruvate kinase in children with cystic fibrosis: a clue to the increased risk of intestinal malignancy in adulthood? J Gastroenterol Hepatol. 2015;30:866-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Campbell JM, Fahey GC, Wolf BW. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr. 1997;127:130-136. [PubMed] |
| 43. | Mortensen PB, Clausen MR. Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand J Gastroenterol Suppl. 1996;216:132-148. [PubMed] |
| 44. | Ohigashi S, Sudo K, Kobayashi D, Takahashi O, Takahashi T, Asahara T, Nomoto K, Onodera H. Changes of the intestinal microbiota, short chain fatty acids, and fecal pH in patients with colorectal cancer. Dig Dis Sci. 2013;58:1717-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 45. | Bridgman SL, Konya T, Azad MB, Sears MR, Becker AB, Turvey SE, Mandhane PJ, Subbarao P, Scott JA, Field CJ. Infant gut immunity: a preliminary study of IgA associations with breastfeeding. J Dev Orig Health Dis. 2016;7:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Faria AM, Gomes-Santos AC, Gonçalves JL, Moreira TG, Medeiros SR, Dourado LP, Cara DC. Food components and the immune system: from tonic agents to allergens. Front Immunol. 2013;4:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Chalkias A, Nikotian G, Koutsovasilis A, Bramis J, Manouras A, Mystrioti D, Katergiannakis V. Patients with colorectal cancer are characterized by increased concentration of fecal hb-hp complex, myeloperoxidase, and secretory IgA. Am J Clin Oncol. 2011;34:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Pakistan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen JQ, Wang WH, Yu B S- Editor: Ji FF L- Editor: A E- Editor: Wu HL