Published online Nov 6, 2016. doi: 10.4292/wjgpt.v7.i4.540
Peer-review started: May 2, 2016
First decision: July 4, 2016
Revised: July 14, 2016
Accepted: August 15, 2016
Article in press: August 17, 2016
Published online: November 6, 2016
Processing time: 182 Days and 17.7 Hours
To examine the role of A20 in the regulation of intestinal epithelial cells (IECs) inflammation.
Using gene transfection, both stable overexpression and knockdown A20-expressed HT-29 cell lines were established. Accordingly, the cells were divided into the following groups: The control group, the A20 overexpression group, the A20 knockdown group and the respective controls. A20 was stimulated with lipopolysaccharide (LPS) in a dose- and time-dependent manner and was detected using western blotting and real-time polymerase chain reaction (PCR) analyses. Immunofluorescence and western blotting analyses were performed to investigate the role of A20 in the regulation of nuclear factor (NF)-κB activation and translocation into the nucleus. ELISA and real-time PCR were performed to examine A20 in regulating the release of the following inflammatory cytokines: Tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and IL-8.
The expression of A20 in IECs was inducible. When intestinal epithelial cells were subjected to the stimulation of LPS, the expression of A20 was increased, and the expression of A20 was induced in a dose- and time-dependent manner. The expression of A20 was very low in HT-29 cells without LPS stimulation but rapidly increased and was maintained at a high level 2-4 h after stimulation with LPS. These levels gradually declined with a change in time-course, and the expression of A20 increased with increasing LPS stimulation. Western blotting and immunofluorescence revealed that overexpression of A20 can inhibit NF-κB activation and its translocation to the nucleus. The overexpression of A20 can reduce the levels of proinflammatory cytokines involved in the pathophysiology of inflammatory bowel disease. There was no significant difference in the expression of IL-8 mRNA in the control group, A20 overexpression group or A20 knockdown group without LPS stimulation (P > 0.05); however, while after 2 h, 4 h and 8 h stimulation with LPS, the expression of IL-8 in the A20 overexpression group was lower than the control group and the A20 knockdown group (P < 0.05 or P < 0.01). The expression of TNF-α was different at different time points after 8 h of LPS stimulation (F = 31.33, DF = 5, P < 0.001), and the expression of TNF-α increased as the LPS stimulation time increased. Upon LPS stimulation, lower levels of TNF-α were detected in the A20 overexpression cell lines (P < 0.05). There were no significant differences in the induction of IL-6 and IL-1β among the control group, A20 overexpression group and A20 knockdown group (P > 0.05).
A20 plays an important role in limiting inflammation by inhibiting LPS-induced NF-κB responses in the gut luminal. A20 may be a potential therapeutic tool for inflammatory diseases.
Core tip: The use of A20-deficient mice and RNA interference technologies has revealed that mice or enterocytes lacking A20 showed hyper-responsiveness to stimulation. However, studies on whether the overexpression of A20 can extenuate enterocyte inflammation are limited. Our present results demonstrated that the expression of A20 was increased in a dose- and time-dependent manner upon lipopolysaccharide (LPS) stimulation in intestinal epithelial cells. More importantly, the overexpression of A20 suppressed the activation of nuclear factor-κB and the induction of pro-inflammatory molecules, such as Tumor necrosis factor-α and IL-8. Taken together, these findings indicate that A20 plays a critical role in limiting LPS-induced inflammation in the gut luminal and may be a potential therapeutic tool for immune and inflammatory diseases.
- Citation: Zheng CF, Shi JR, Huang Y, Wang SN. A20 inhibits lipopolysaccharide-induced inflammation in enterocytes. World J Gastrointest Pharmacol Ther 2016; 7(4): 540-549
- URL: https://www.wjgnet.com/2150-5349/full/v7/i4/540.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i4.540
Inflammatory bowel disease (IBD) has been proposed to be caused by an inappropriate inflammatory response to normal components of the intestinal micro-biota in genetically predisposed individuals[1-3]. Bacterial wall products play an important role in the activation of the immune and non-immune cells of the intestinal mucosa, and intestinal epithelial cells (IECs) represent a unique population of cells that exist in direct contact with a dense and complex milieu of commensal microorganisms. As a primary interface between pathogens and the intestinal tract, the epithelial cells lining the gut luminal play a key role in defence against microbial pathogens[4]. Rather than functioning as a passive barrier, IECs are an active participant in the mucosal immune response. The interaction between IECs and intestinal micro-biota results in the activation of multiple intracellular signalling events, including the activation of nuclear factor (NF)-κB. The inappropriate activation of NF-κB is central to the pathogenesis of IBD. On the one hand, NF-κB regulates the expression of various cytokines and other modulators of the inflammatory processes in IBD[5,6]. On the other hand, the inhibition of NF-κB activity has been suggested as a major component of the anti-inflammatory activity of glucocorticoids that are frequently used for treatment of IBD[7,8]. Thus, a tight regulation of the NF-κB signalling pathway and the genes induced is an absolute requirement. A20 (also known as Tumour Necrosis Factor Alpha-Induced Protein 3 or TNFAIP3) is a widely expressed and inducible cytoplasmic protein that plays a key role in the negative regulation of inflammation and immunity[9,10]. Several studies have shown that the ubiquitin-editing protein A20 is a key player in the negative feedback regulation of NF-κB signalling in response to multiple stimulants[11]. An essential role of A20 in the regulation of NF-κB signalling was clearly demonstrated with the generation of A20-deficient mice and RNA interference technologies. Mice deficient for A20 are extremely susceptible to sub-lethal doses of TNF and die prematurely due to severe multi-organ inflammation and cachexia[12].
More specifically related to IBD, a recent genome-wide association study identified A20 as a Crohn’s disease (CD) susceptibility gene[13]. Specific deletion of A20 in enterocytes increased the susceptibility of mice to dextran sodium sulphate (DSS)-induced colitis and prevented the recovery from acute DSS-induced inflammation[14]. Finally, mucosal biopsies from 69 CD patients were analysed and confirmed a consistent down-regulation of mucosal A20 expression[15]. Our previous work found that there is an excessive inflammatory response but insufficient up-regulation of A20 expression in IBD patients[16]. These studies indicate that defective A20 expression or activity could be a risk factor for IBD.
The use of A20-deficient mice and RNA interference technologies has revealed that mice or enterocytes lacking A20 showed hyper-responsiveness to stimulation. Based on the knowledge that A20 plays a central role in inflammation and immunity, this study aimed to determine whether A20 has a potential therapeutic value and whether overexpression of A20 could extenuate enterocyte inflammation. Previous studies have confirmed that the overexpression of A20 can attenuate allergic airway inflammation in mice[17] and protect kidneys from ischaemia/reperfusion injury[18]. However, studies on whether the overexpression of A20 may reduce intestinal inflammation are limited. Thus, we performed this study to examine the effectiveness of A20 in reducing IECs inflammatory reaction and NF-κB activation. In this study, we recapitulated the response of epithelial cells to LPS to mimic the in vivo response of intestinal epithelial cells to infection of pathogenic and/or commensal microbes, so as to explore the role of A20 in the regulation of intestinal epithelial cell inflammation.
The main reagents and antibodies used in our experiments are as follows: Unpurified LPS from Escherichia coli 0127:B8 (Sigma, St. Louis, MO, United States, L4516), anti-A20 polyclonal antibody (Abcam, Cambridge, United Kingdom, ab45366), anti-NF-κB p65 monoclonal antibody (Epitomics, California, United States, E379), anti-β-actin antibody (Sigma, St. Louis, MO, United States), TNF-α and IL-1β ELISA kit (Senxiong Technology Industrial Company, Shanghai, China), TRIzol (Invitrogen, Carlsbad, CA, United States, 15596-018), SYBR green PCR reagent kits (Toyobo Co, Osaka, Japan, QPK-201), and Lipofectamine 2000 (Invitrogen, Carlsbad, CA, United States).
The human intestinal epithelial cell line HT-29 was purchased from the Chinese Academy of Sciences (Shang hai) and grown in Dulbecco’s Modified Eagle’s Medium (DMEM, Invitrogen, C11965), supplemented with 10% foetal bovine serum (Invitrogen) and maintained at 37 °C in a humidified incubator under an atmosphere of 5% CO2.
Human A20 cDNA was amplified with the following primers: F1: 5’-AGAGGTGTTGGAGAGCACAATGG-3’ and R1: 5’-CACCTGTTTCCGGTTAGCCATACA-3’, and HA-tagged A20 was cloned into the lentiviral vector pWPI. Lentiviral particles were produced by transfecting HEK-293T cells with pWPI.1-HA-A20, psPAX2 and pMD2.G. After incubation with HT-29 cells for 1 wk, the cells were screened using a flow cytometer.
To silence A20, we employed the lentiviral silencing system. ShRNA oligos against A20 were cloned into the lentiviral vector pLKO.1-TRC cloning vector (Addgene Plasmid 10878). The 21-bp target sequences of A20 were CACTGGAAGAAATACACATAT and GCACCGATACACACTGGAAAT. To produce lentiviral particles, the shRNA construct was co-transfected with psPAX2 and pMD2.G into HEK-293T cells. The media was harvested to obtain lentiviral particles. The target cells with lentiviral particles were infected for 24 h and selected with puromycin at a concentration of 2 μg/mL. The day before transfection, the cells were plated in growth medium at a density of approximately 70%. The shRNA was transfected at a concentration of 50 nmol/L using Lipofectamine 2000 according to the manufacturer’s instructions.
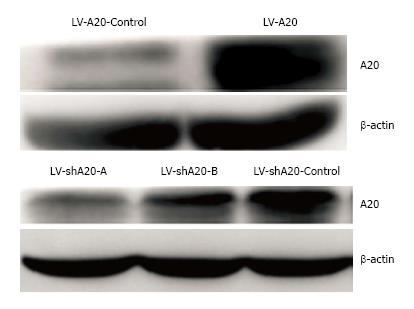
HT-29 cells were transduced with LV-A20, LV-shA20, or LV-controls, respectively. Accordingly, the cells were divided into the following groups: The control group (HT-29 cells without transfection), A20 overexpression group, A20 knockdown group, A20 overexpression-control group and A20 knockdown-control group. Overexpression and silencing of A20 were validated by Western blotting analysis prior to the experimentation. The presence of the 90-kDa bands of extracts from transfected cell populations were significantly higher or lower than the controls (Figure 1). We have constructed two shRNA (A and B) sequences to silence A20, as shown in Figure 1. The silencing effect of A was more pronounced than B. Thus, sequence A was selected for subsequent experiments.
Total RNA was isolated from cells using TRIzol reagent, and equal amounts of RNA were reverse-transcribed into cDNA using a quantitative PCR cDNA kit (TaKaRa, Biotechnology, Dalian, China, DRR037Ab). Specific primers (Table 1) used in the real-time PCR studies were designed and generated by Takara Biotechnology Company (Dalian, China). Quantitative real-time PCR was performed using SYBR green PCR reagent kits according to the manufacturer’s instructions. Data were recorded and analysed using the real-time PCR analysis software Bio-Rad iQ5. The A20, IL-6 and IL-8 mRNA levels were normalized against GAPDH levels in the cells. The relative gene expression was calculated by comparing the number of thermal cycles that were necessary to generate the threshold amounts of product (CT).
| Gene | Sequences(5’-3’) |
| A20 | Forward: AAAGCCCTCATCGACAGAAA |
| Reverse: CAGTTGCCAGCGGAATTTA | |
| IL-6 | Forward: AAGCCAGAGCTGTGCAGATGAGTA |
| Reverse: TGTCCTGCAGCCACTGGTTC | |
| IL-8 | Forward: ACACTGCGCCAACACAGAAATTA |
| Reverse: TTTGCTTGAAGTTTCACTGGCATC | |
| GAPDH | Forward: GCACCGTCAAGGCTGAGAAC |
| Reverse: TGGTGAAGACGCCAGTGGA |
HT-29 cells were homogenized with protease and phosphatase inhibitors and prepared in protein extraction solution (Thermo, 78835). Protein lysates, quantified using a BCA assay (Sangon Biotech Company, Ltd., Shanghai, China), were separated on reducing SDS-polyacrylamide gels and transferred onto polyvinyldifluoride membranes (PVDF, Millipore). The membranes were blocked with 5% non-fat milk TBS buffer for 2 h at room temperature and incubated with primary antibodies overnight at 4 °C. β-actin levels were used to normalize loading. The first antibody exposure was followed by incubation with an anti-rabbit IgG, HRP-linked secondary antibody (Cell Signaling, Beverly, MA, United States). Antigen-antibody complexes were visualized using the enhanced chemiluminescence detection method (ECL kit, Thermo, Waltham, MA, United States).
The concentrations of TNF-α and IL-1β in the culture supernatants were measured using a commercially available enzyme-linked immunosorbent assay kit (Sen-xiong Technology Industrial Company, Shanghai, China), according to the manufacturer’s instructions.
The immunofluorescence of cultured cells was performed as recommended by the antibody manufacturers. Horseradish peroxidase-conjugated anti-rabbit IgG was used as a secondary antibody. Images were obtained on a BX51 microscope equipped with a colour camera using Picture Frame software (Olympus).
For all statistical analysis, data were expressed as the mean ± standard deviations (SD) or standard error of the mean. Student’s t-test was used for comparisons between groups for continuous variables. One-way analysis of variance (ANOVA) was used to compare differences between groups. P values < 0.05 were accepted as significant.
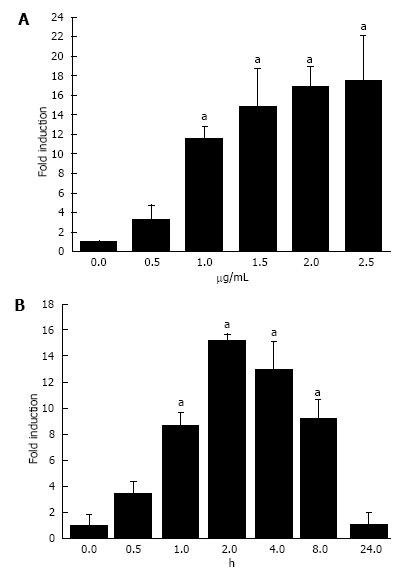
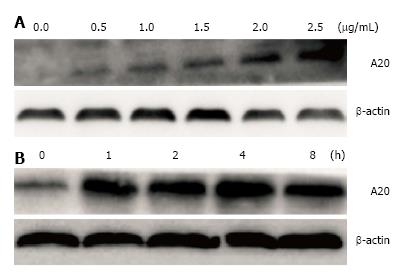
Initially, we aimed to determine whether LPS could induce the expression of A20 in a dose-dependent manner. Cultured HT-29 cells were stimulated with LPS at different doses for 1 h and harvested for the analysis of A20 using real-time PCR and Western blotting analyses. As shown in Figures 2A and 3A, the expression of A20 increased with increasing LPS stimulation. Furthermore, to gain insight into the time-course changes of A20 levels when enterocytes were subjected to infection, the expression of A20 protein and mRNA was detected at various time points after 1 μg/mL LPS stimulation using Western blotting and real-time PCR analyses. We found that the expression of A20 increased in a time-dependent manner after LPS stimulation. The expression of A20 was very low in HT-29 cells without LPS stimulation but rapidly increased and maintained a high level at 2-4 h after stimulation with LPS. These levels then declined gradually with a change in time-course (Figures 2B and 3B).
Overexpression of A20 inhibited NF-κB translocation to the nucleus
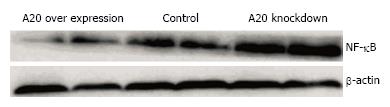
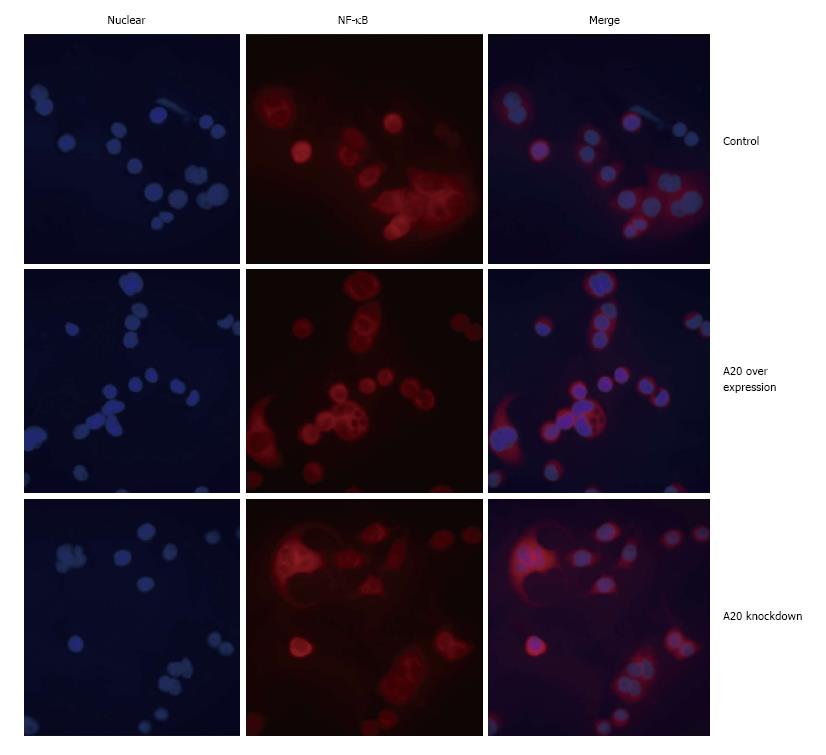
It is known that NF-κB is kept inactive by binding to the inhibitor of κB (IκB) proteins in resting cells. Upon stimulation with a wide variety of agonists, IκB is phosphorylated and subsequently polyubiquitinated and degraded by the proteasome, thereby releasing NF-κB, which then accumulates in the nucleus and activates the transcription of its target genes. As a negative regulator of NF-κB signalling pathway, we confirmed whether overexpression of A20 will alter the expression of NF-κB and its translocation to the nucleus in HT-29 cells. After a 2-h stimulation with LPS (1 μg/mL), Western blotting was performed to detect the expression of NF-κB p65 in the control group, A20 overexpression group and A20 knockdown group. As shown in Figure 4, the expression of NF-κB p65 in A20 overexpression cell lines was much lower than the control group and A20 knockdown group. In addition, immunofluorescence was performed to detect the location of NF-κB p65 in the different groups, and the results demonstrated that overexpression of A20 reduced NF-κB p65 translocation to the nucleus (Figure 5). Taken together, these data indicated that A20 inhibits the translocation of NF-κB to the nucleus in intestinal epithelial cells.
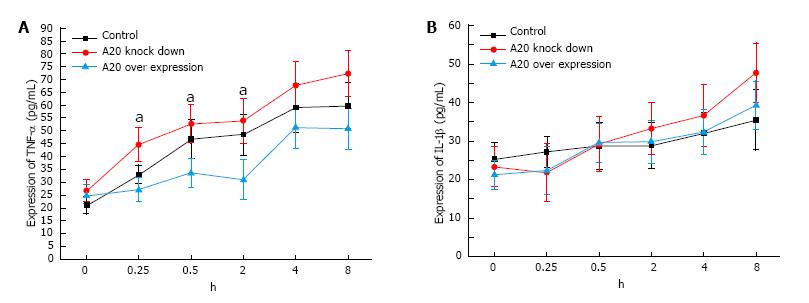
To determine whether the overexpression of A20 would decrease proinflammatory cytokines, such as TNF-α, IL-1β, IL-6 and IL-8, in IECs via modulation of NF-κB activation, the total RNA was isolated from IECs that were subjected to LPS. IL-6 and IL-8 expression were analysed using RT-PCR, while the expression of TNF-α and IL-1βin the culture supernatants were assayed by ELISA. The expression of TNF-α was different at different time points during an 8 h stimulation with LPS (F = 31.33, DF = 5, P < 0.001), and the expression of TNF-α was increased as the LPS stimulation time increased (F = 111.435, DF = 1, P < 0.001). At different time points during the 8 h stimulation with LPS, there was a significant difference in the expression of TNF-α between the A20 knockdown and the A20 overexpression (P < 0.05). In addition, there was a significant difference in the expression of TNF-α between the control group and the A20 knockdown group during the 8 h stimulation (P < 0.05, Figure 6A). Similar to the expression of TNF-α, the expression of IL-1β was different at different time points during the 8 h stimulation with LPS (F = 9.216, DF = 5, P < 0.001), and the expression of IL-1β increased with increasing LPS stimulation time (F = 80.829, DF = 1, P < 0.001). However, there was no significant difference in the expression of IL-1β in the control group, A20 knockdown group or A20 overexpression group during the 8 h stimulation of LPS (F = 2.456, DF = 2, P = 0.166, Figure 6B).
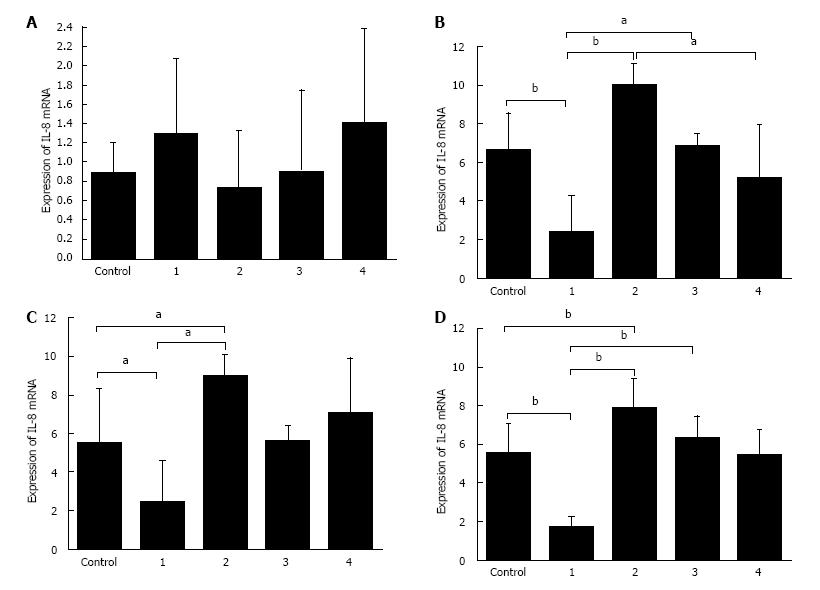
As shown in Figure 7, there was no significant difference in the expression of IL-8 mRNA in the control group, A20 overexpression-control group and A20 knockdown-control group. After 2 h, 4 h and 8 h stimulation with LPS, the expression of IL-8 mRNA in the A20 overexpression group was lower than the control group and A20 knockdown group, and the expression of IL-8 in the A20 knockdown group was higher than both the control group and the A20 overexpression group. However, there was no significant difference in the expression of IL-6 mRNA in the control group, A20 knockdown group or A20 overexpression group after 8 h of stimulation with LPS. These results demonstrated that the overexpression of A20 can significantly reduce the levels of pro-inflammatory cytokines.
TNF-α is regarded as a critical contributor to IBD. Its functions include the recruitment of circulating inflammatory cells to local tissue sites of inflammation, induction of oedema, the activation of the coagulation cascade, and the initiation of the formation of granuloma[19]. Clinical trials demonstrating symptomatic improvement and remission of IBD by suppressing TNF-α have provided additional evidence of the role of TNF-α in the pathogenesis of IBD[20,21]. Thus, TNF-α is considered an attractive target for the treatment of IBD. The introduction of anti-TNF-α agents (e.g., Infliximab) has greatly advanced the therapeutic armamentarium of IBD, but the clinical application of infliximab is limited due to the occurrence of neutralizing antibodies[22,23], which is associated with allergic reactions and a loss of response. Thus, the development of novel therapeutic strategies for IBD is needed.
There is accumulating evidence to support the therapeutic potential of A20 in autoimmune diseases. The specific deletion of A20 in enterocytes increased the susceptibility of mice to dextran sodium sulphate (DSS)-induced colitis and prevented recovery from DSS-induced inflammation[14], whereas the expression of A20 by dendritic cells protects mice from LPS-induced mortality and DSS-induced colitis[24,25]. Not limited to IBD, A20 was also identified to be associated with numerous autoimmune diseases[11,26]. It was previously reported that prior injection with adenovirus containing A20 cDNA significantly diminished OVA challenge-mediated TNF-α production and attenuated allergic airway inflammation in mice[17]. In this study, we showed that the NF-κB responsive gene A20 is a major protective factor in intestinal epithelium cells, and the overexpression of A20 can significantly reduce the level of TNF-α in IECs. Taken together, these findings may provide some useful clues towards the development of novel anti- TNF-α strategies.
A20 has been described as a central gatekeeper in inflammation and immunity[9]. Emerging evidence indicates that LPS is a pathogenic factor that induces several inflammatory disorders, including necrotizing enterocolitis and IBD[27]. The tendency of A20-deficient mice to develop severe inflammation and their hyper-responsive nature to LPS[12] suggests that A20 may act as an endogenous regulatory system in controlling the inflammatory response to gram-negative bacteria. Oshima et al[28] showed that the gene expression of A20 is rapidly increased after ligand stimulation. In the present study, our results also confirmed that A20 expression was markedly up-regulated at both the mRNA and protein level upon stimulation with LPS in IECs. In addition, our experiments also found that LPS-induced A20 expression not only in a time dependent manner but also in a dose-dependent manner. The expression of A20 was very low in HT-29 cells without LPS stimulation but rapidly increased and was maintained at a high level 2-4 h after stimulation with LPS. This expression gradually declined with a change in time-course. Furthermore, the expression of A20 increased with increasing LPS stimulation.
Wang et al[29] found that A20 is necessary and sufficient for the development of LPS tolerance in enterocytes. Given that the human gut harbours a large collection of commensal bacteria, LPS released by gut microbes has a large effect on gut homeostasis, and the expression of A20 was rapidly increased in a dose- and time-dependent manner upon LPS stimulation. Taken together, these findings indicate the importance of A20 in the IEC response to inflammatory stimuli.
The transcription factor NF-κB has served as a standard for inducible transcription factors for more than 20 years. NF-κB can be found in the cytoplasm of most cells as an inactive complex with unprocessed precursor proteins (e.g., p105) or IκB (e.g., IκBa) proteins. Upon activation, NF-κB translocates into the nucleus and binds to DNA. The initiation of NF-κB signalling is tightly regulated because prolonged and excessive activation of NF-κB can lead to uncontrolled inflammation that is detrimental to the host, and thus, the inducible regulation of gene expression is a central element of normal physiology and is key to the ability of multicellular organisms to adapt to environmental, mechanical, chemical, and microbiological stresses[30]. In this study, RNA interference technology was utilized to down-regulate the expression of A20. Consistent with these findings, our results proved that the lack of A20 increased LPS-induced activation of NF-κB and its translocation to the nucleus. Furthermore, we also showed that overexpression of A20 resulted in a dramatic decrease in LPS-induced activation of NF-κB. Immunofluorescence results revealed that NF-κB translocation to the nucleus was reduced in the A20 overexpression cell group. These data indicated that the overexpression of A20 can inhibit LPS-induced NF-κB activation and translocation to the nucleus.
By far, the gastrointestinal tract is the most susceptible tissue to inflammatory responses due to its constant exposure to various antigenic, mutagenic and toxic factors. Deregulated cytokine production and signalling mechanisms by epithelial cells, mucosal lymphocytes and macrophages have been implicated in the pathogenesis of IBD. Numerous studies have identified altered proinflammatory cytokines in IBD[31]. A previous study showed that the overexpression of A20 significantly inhibited the activation of IL-8 in airway epithelial cells[32]. Furthermore, the present study demonstrates that overexpression of A20 can significantly reduce LPS-induced expression of TNF-α, IL-6 and IL-8, while the lack of A20 increased the level of TNF-α, IL-6 and IL-8 in IECs. These results demonstrated that A20 plays an important role in ameliorating the production of inflammatory cytokines.
Taken together, these findings underscore the importance of A20 in controlling inflammatory responses and indicate that A20 may be a potential therapeutic tool for the treatment of inflammatory diseases. However, its function remains poorly understood and additional investigations are necessary to elucidate the precise role of A20 in inflammatory diseases.
We wish to express our deepest gratitude to the team of Professor Qing-Hai Ye, Zhongshan Hospital, the Liver Cancer Institute, Fudan University, Shanghai, for their generous help throughout the entire course of this project, without which it would not have been possible for us to complete this work.
A20 is regarded as the central gatekeeper in inflammation and immunity and is associated with numerous autoimmune diseases. The use of A20-deficient mice and RNA interference technologies has revealed that mice or enterocytes lacking A20 showed hyper-responsiveness to stimulations. Previous studies have confirmed that overexpression of A20 can attenuate allergic airway inflammation in mice and protects kidneys from ischaemia/reperfusion injury. However, studies on whether the overexpression of A20 extenuates enterocyte inflammation are rare.
The inappropriate activation of nuclear factor (NF)-κB is central to the pathogenesis of inflammatory bowel disease (IBD). Thus, tight regulation of the NF-κB signalling pathway and the genes induced is an absolute requirement. Several studies have shown that the ubiquitin-editing protein A20 is a key player in the negative feedback regulation of NF-κB signalling in response to multiple stimulants.
A20 may be a potential therapeutic tool for immune and inflammatory diseases. Previous studies have identified that defective A20 expression or activity could be a risk factor for IBD. However, research studies related to the protective effect of A20 on intestinal epithelial cells are limited. These results demonstrated that the overexpression of A20 suppressed the activation of NF-κB and induction of proinflammatory molecules, such as TNF-α and IL-8. These data indicate that A20 plays an important role in limiting lipopolysaccharide (LPS)-induced inflammation in the gut luminal.
The introduction of infliximab has greatly advanced the therapeutic armamentarium of inflammatory bowel diseases, but the clinical application of infliximab is limited due to the occurrence of neutralizing antibodies. Thus, the development of novel therapeutic strategies for IBD is always needed. The experiments showed that A20 is critical for the inhibition of LPS-induced inflammation in enterocytes. These findings underscore the importance of A20 in controlling inflammatory responses and indicate that A20 may be a potential therapeutic tool for the treatment of inflammatory diseases.
LPS is the major component of the outer membrane of gram-negative bacteria. The lentiviral vector is a type of retroviral vector that has become an ideal vector for target gene transfer due to its high efficiency of transfection, ability of transfection into dividing or non-dividing cells and its capacity for large target gene fragments. RNA interference technology is a type of technology that can be used to eliminate a specific gene or to suppress the expression of a specific gene.
This paper in interesting and clear way shows the negative regulation of inflammation under the influence of bacterial lipopolysaccharide. The experiments are well designed and the results demonstrate that A20 is able to limit the intestinal inflammation associated to NF-κB.
| 1. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3414] [Article Influence: 179.7] [Reference Citation Analysis (12)] |
| 2. | Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 799] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 3. | Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3922] [Cited by in RCA: 3599] [Article Influence: 211.7] [Reference Citation Analysis (4)] |
| 4. | Cario E. Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut. 2005;54:1182-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 237] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 666] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 6. | Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med (Berl). 2004;82:434-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 718] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 7. | Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1763] [Cited by in RCA: 1736] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 8. | Thiele K, Bierhaus A, Autschbach F, Hofmann M, Stremmel W, Thiele H, Ziegler R, Nawroth PP. Cell specific effects of glucocorticoid treatment on the NF-kappaBp65/IkappaBalpha system in patients with Crohn’s disease. Gut. 1999;45:693-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Coornaert B, Carpentier I, Beyaert R. A20: central gatekeeper in inflammation and immunity. J Biol Chem. 2009;284:8217-8221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 253] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 10. | Verstrepen L, Verhelst K, van Loo G, Carpentier I, Ley SC, Beyaert R. Expression, biological activities and mechanisms of action of A20 (TNFAIP3). Biochem Pharmacol. 2010;80:2009-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Vereecke L, Beyaert R, van Loo G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 2009;30:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 385] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 12. | Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1202] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 13. | Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7741] [Cited by in RCA: 7221] [Article Influence: 380.1] [Reference Citation Analysis (0)] |
| 14. | Vereecke L, Sze M, Mc Guire C, Rogiers B, Chu Y, Schmidt-Supprian M, Pasparakis M, Beyaert R, van Loo G. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J Exp Med. 2010;207:1513-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 251] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 15. | Arsenescu R, Bruno ME, Rogier EW, Stefka AT, McMahan AE, Wright TB, Nasser MS, de Villiers WJ, Kaetzel CS. Signature biomarkers in Crohn’s disease: toward a molecular classification. Mucosal Immunol. 2008;1:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Zheng CF, Huang Y. [Expression of zinc finger protein A20 in pediatric inflammatory bowel disease]. Zhonghua Erke Zazhi. 2011;49:261-265. [PubMed] |
| 17. | Kang NI, Yoon HY, Lee YR, Won M, Chung MJ, Park JW, Hur GM, Lee HK, Park BH. A20 attenuates allergic airway inflammation in mice. J Immunol. 2009;183:1488-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Lutz J, Luong le A, Strobl M, Deng M, Huang H, Anton M, Zakkar M, Enesa K, Chaudhury H, Haskard DO. The A20 gene protects kidneys from ischaemia/reperfusion injury by suppressing pro-inflammatory activation. J Mol Med (Berl). 2008;86:1329-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Van Deventer SJ. Tumour necrosis factor and Crohn’s disease. Gut. 1997;40:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 394] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Nakamura K, Honda K, Mizutani T, Akiho H, Harada N. Novel strategies for the treatment of inflammatory bowel disease: Selective inhibition of cytokines and adhesion molecules. World J Gastroenterol. 2006;12:4628-4635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 77] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, Liu G, Travers S, Heuschkel R, Markowitz J. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology. 2007;132:863-873; quiz 1165-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 651] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 22. | Vande Casteele N, Khanna R, Levesque BG, Stitt L, Zou GY, Singh S, Lockton S, Hauenstein S, Ohrmund L, Greenberg GR. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut. 2015;64:1539-1545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 224] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 23. | O’Meara S, Nanda KS, Moss AC. Antibodies to infliximab and risk of infusion reactions in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2014;20:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | Hammer GE, Turer EE, Taylor KE, Fang CJ, Advincula R, Oshima S, Barrera J, Huang EJ, Hou B, Malynn BA. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat Immunol. 2011;12:1184-1193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 25. | Xuan NT, Wang X, Nishanth G, Waisman A, Borucki K, Isermann B, Naumann M, Deckert M, Schlüter D. A20 expression in dendritic cells protects mice from LPS-induced mortality. Eur J Immunol. 2015;45:818-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Zhang M, Peng LL, Wang Y, Wang JS, Liu J, Liu MM, Hu J, Song B, Yang HB. Roles of A20 in autoimmune diseases. Immunol Res. 2016;64:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Rhee SH. Lipopolysaccharide: basic biochemistry, intracellular signaling, and physiological impacts in the gut. Intest Res. 2014;12:90-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 28. | Oshima N, Ishihara S, Rumi MA, Aziz MM, Mishima Y, Kadota C, Moriyama I, Ishimura N, Amano Y, Kinoshita Y. A20 is an early responding negative regulator of Toll-like receptor 5 signalling in intestinal epithelial cells during inflammation. Clin Exp Immunol. 2010;159:185-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Wang J, Ouyang Y, Guner Y, Ford HR, Grishin AV. Ubiquitin-editing enzyme A20 promotes tolerance to lipopolysaccharide in enterocytes. J Immunol. 2009;183:1384-1392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3245] [Cited by in RCA: 3915] [Article Influence: 217.5] [Reference Citation Analysis (0)] |
| 31. | Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 2083] [Article Influence: 173.6] [Reference Citation Analysis (1)] |
| 32. | Gon Y, Asai Y, Hashimoto S, Mizumura K, Jibiki I, Machino T, Ra C, Horie T. A20 inhibits toll-like receptor 2- and 4-mediated interleukin-8 synthesis in airway epithelial cells. Am J Respir Cell Mol Biol. 2004;31:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: China
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Futoma-Koloch B, Kamada N, Vivas S S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ