Published online Nov 6, 2016. doi: 10.4292/wjgpt.v7.i4.524
Peer-review started: July 21, 2016
First decision: September 5, 2016
Revised: September 12, 2016
Accepted: October 5, 2016
Article in press: October 7, 2016
Published online: November 6, 2016
Processing time: 101 Days and 15.4 Hours
Thiopurines are essential drugs to maintain remission in patients with inflammatory bowel disease (IBD). Thiopurines used in IBD are azathioprine (2.0-2.5 mg/kg), mercaptopurine (1.0-1.5 mg/kg) and thioguanine (0.2-0.3 mg/kg). However, mainly due to numerous adverse events associated with thiopurine use, almost 50% of the patients have to discontinue conventional thiopurine treatment. Extensive monitoring and the application of several treatment strategies, such as split-dose administration, co-administration with allopurinol or dose reduction/increase, may increase the chance of successful therapy. With this review, we provide practical information on how thiopurines are initiated and maintained in two thiopurine research centers in The Netherlands. We provide clinical information concerning safety issues, indications and management of therapy that may serve as a guide for the administration of thiopurines in IBD patients in daily practice.
Core tip: Conventional thiopurine therapy with azathioprine and mercaptopurine in inflammatory bowel disease is associated with several adverse events causing cessation of therapy in up to half of the patients. On the contrary, thiopurine therapy is often unnecessarily discontinued. In this practical review, we provide information on how thiopurine therapy is initiated and maintained using periodical laboratory tests and the application of various treatment strategies (including the administration of a third thiopurine; thioguanine), based on the experience in the two expert thiopurine centers in The Netherlands.
- Citation: Meijer B, Mulder CJ, van Bodegraven AA, de Boer NKH. How I treat my inflammatory bowel disease-patients with thiopurines? World J Gastrointest Pharmacol Ther 2016; 7(4): 524-530
- URL: https://www.wjgnet.com/2150-5349/full/v7/i4/524.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i4.524
Inflammatory bowel disease (IBD) is a chronic condition of the gastrointestinal tract which is characterized by episodes of remission and relapses and encompasses both Crohn’s disease (CD) and ulcerative colitis (UC). In the management of IBD, thiopurines [i.e., azathioprine (AZA), mercaptopurine (MP) and thioguanine (TG)] play an important role in clinical practice, mainly in order to maintain remission[1-4]. Over the past decades, extensive research has been performed to elucidate the complex metabolism of thiopurine derivatives[5-7]. In this article, we demonstrate and discuss the way we use thiopurine therapy in the treatment of adult IBD patients in two referral centers in The Netherlands in daily practice. For information about thiopurine therapy in pediatric IBD, we refer to reviews focused on this patient group[8-10].
Thiopurines were firstly described in the early 1950s by Gertrude Elion and George Hitchings, primarily as antimetabolite therapy[11]. Initially, thiopurines were used in the treatment of acute lymphatic leukemia in children and in the prevention of organ transplant rejection. The first IBD patient treated with thiopurines has been described by Dr. Bean[12] in 1962. At this moment, thiopurines are used in a variety of autoimmune disorders and hematologic malignancies[13,14].
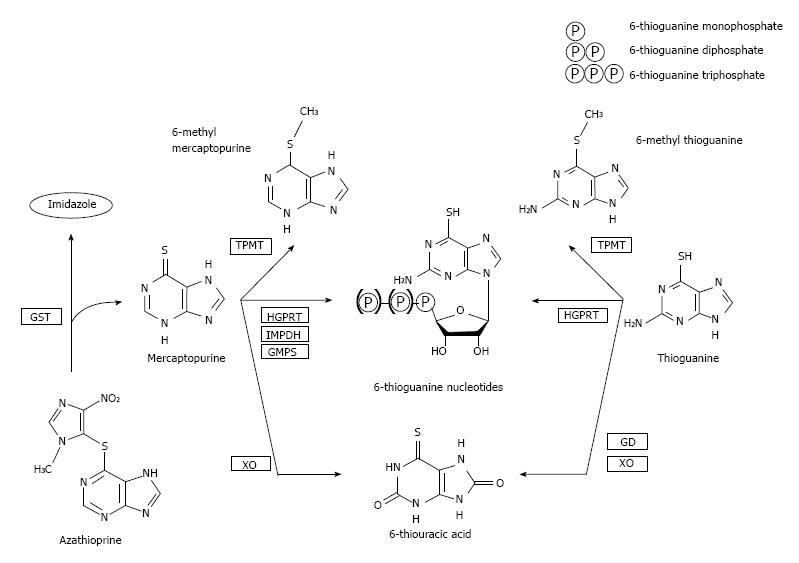
The thiopurine derivatives AZA, MP and TG are all pro-drugs which are subsequently converted into the allegedly most important pharmacologically active end-metabolites, 6-thioguanine nucleotides (6-TGN)[5]. AZA is converted into MP by the enzyme glutathione S-transferase, after which MP is metabolized by three competing enzymatic systems. First, a part of the concentration MP is withdrawn from bioavailability by xanthine oxidase (XO) and thiopurine-S-methyltransferase (TPMT), converting MP into 6-thiouric acid (6-TUA) and 6-methylmercaptopurine (6-MMP), respectively. The remaining concentration of MP is metabolized via the purine salvage pathway into 6-TGN by a cascade of hypoxanthine-guanine phosphoribosyl transferase (HGPRT), inosine monophosphate dehydrogenase (IMPD) and guanosine monophosphate synthetase (GMPS). The 6-TGN can be incorporated in the DNA (thus achieving an anti-metabolic effect), but also account for inhibition of anti-apoptotic effects and down-regulation of pro-inflammatory cytokines. In daily practice, we measure 6-TGN and 6-MMP in red blood cells (RBC), which is mainly due to the fact that in patients with leukemia, the original indication for thiopurine therapy, successful treatment with thiopurines leads to the unavailability of leukocytes[11,15].
In contrast to AZA and MP, the metabolism of TG is less extensive as TG is directly converted into 6-TGN by HGPRT. Whether TG is also withdrawn from bioavailability by the effect of TPMT and XO, this effect is relatively smaller than in AZA and MP, leaving a larger portion of TG available for (direct) conversion into 6-TGN (Figure 1)[5,7,16,17].
When treating IBD patients in our centers, we apply the therapeutic approach known as accelerated step up care in both CD and UC[18]. Conventional thiopurines (i.e., AZA and MP) do not play a standard role in the active phase of CD and UC as such, however it may be added to induction course therapy with corticosteroids in those patients who are suspected of having a more severe or prolonged disease course. In patients with only mildly active disease with good reaction on initial induction therapy, thiopurines do not have to be initiated straight away[19]. However, in those patients with a relapse of disease despite two induction courses of corticosteroids, thiopurines are required to maintain remission[20]. Furthermore, thiopurines are co-administered as a routine to treatment with anti-TNF therapy in our centers, in line with recent observations from the SONIC trial[2-4,21]. In those patients receiving vedolizumab (Entyvio®) evidence is scarce whether to (dis)continue simultaneous thiopurine therapy[22,23]. In our centers, we continue thiopurine therapy in the majority of patients, since patients receiving vedolizumab are likely to have highly complex disease in which vedolizumab is often initiated as rescue drug. Additionally, in line with the observations in the SONIC trial in patients receiving infliximab and adalimumab, we presume that thiopurines might have a protecting effect on the development of antibodies against vedolizumab[24,25]. Finally, thiopurines are administered in surgical CD patients to prevent post-surgical recurrence, especially in complex patients with fistulizing disease or multiple surgical interventions[3].
Thiopurine therapy is initiated in a dosage of 2.0-2.5 mg/kg for AZA or 1.0-1.5 mg/kg for MP, starting with 50 mg/d in the first week and increasing to full-dose when patients experience no adverse effects on low-dose therapy[1]. In those patients in whom thiopurines were co-administered next to induction corticosteroid therapy, the steroids are tapered down in 2-3 mo.
In our center, we prefer to initiate thiopurine therapy using MP, based on results of several rechallenge studies[26-31]. Furthermore, in those patients with mild adverse events (i.e., no severe myelotoxicity or pancreatitis) on MP therapy, we rechallenge these patients with MP with low threshold.
As thiopurines are associated with a broad spectrum of adverse events (i.e., flu-like symptoms, arthralgia, gastro-intestinal complaints, rash, pancreatitis, hepatotoxicity and myelotoxicity), one of the applied strategies to reduce the risk of developing adverse events is to measure TPMT activity before initiating thiopurine therapy, to identify patients at risk of developing adverse events based on an aberrant thiopurine metabolism[7,16,32,33]. Literature reports show that 1:300 (Caucasian) individuals have TPMT deficiency, making them at risk for developing (severe) myelosuppression due to preferential 6-TGN formation[34]. In our centers, however, TPMT activity is not determined as we initiate thiopurine therapy with a low-dose start up scheme. Furthermore, many patients with normal TPMT activity could still develop adverse events of thiopurine therapy[32,35,36]. For this reason amongst others, we choose to extensively monitor laboratory and clinical parameters in the first three months after initiation of thiopurine therapy. At week 0, 1, 2, 4, 8 and 12, hematologic and hepatic parameters are being measured, as well as creatinine and C-reactive protein (Table 1). After initiation, these parameters are determined each 3-4 mo during thiopurine maintenance therapy.
| Hematologic parameters |
| Hemoglobin |
| White blood cell count |
| Platelet count |
| Hepatic parameters |
| Alkalic phosphatase |
| Gamma glutamyl transpeptidase |
| Alanine aminotransferase |
| Other parameters |
| Creatinine |
| C-reactive protein |
Measurement of thiopurine metabolites (6-TGN and 6-MMP) is not performed routinely in clinical practice at our centers. In those patients who experience adverse events or non-response to treatment, metabolite levels may explain why these patients are intolerant or resistant to therapy (Table 2). Based on these results, individual treatment strategies (i.e., split-dose administration, dose reduction/increase, the addition of mesalazine or allopurinol to a 25%-33% dose of the original thiopurine in patients with an altered thiopurine metabolism, so-called “skewers”) may be applied[37-39].
| 6-TGN (pmol/8 × 108 RBC) | 6-MMP (pmol/8 × 108 RBC) | Non-response | Adverse event (dose-dependent) | Recommendation |
| << 230 | << 5700 | Non-compliance | Not expected | Gain compliance |
| < 230 | < 5700 | Non-compliance/under dosing | Not expected | Gain compliance/increase dose1 |
| 230-400 | < 5700 | Possible resistance to thiopurine therapy | Not expected | Increase dose1 or change therapy2 |
| > 400 | < 5700 | Therapy resistance | Myelotoxicity | Change therapy2 |
| < 230 | >> 5700 | Shunting | Myelotoxicity | Consider allopurinol3 or switch to TG4 |
| < 230 | > 5700 | Shunting | Hepatotoxicity | Consider allopurinol, 5-ASA or switch to TG |
| 230-400 | > 5700 | Possible resistance to thiopurine therapy | Hepatotoxicity | Consider allopurinol3 or 5-ASA |
| > 400 | > 5700 | Therapy resistance | Hepatotoxicity | Change therapy2 |
| Myelotoxicity | Decrease dose5 |
In those patients with either idiosyncratic (e.g., pancreatitis) adverse events on conventional thiopurine therapy or adverse events based on elevated 6-MMP concentrations, these patients can be switched to TG. In some countries, TG is considered as rescue drug when conventional therapy fails, however, in The Netherlands this drug is officially registered as treatment option for IBD since March 2016. One of the feared complications of TG treatment is the development of nodular regenerative hyperplasia (NRH), a condition of the liver in which patients might develop non-cirrhotic portal hypertension. However, in contrary to earlier observations[40-42], the development of NRH is seldomly witnessed in those patients treated with low-dose TG therapy (i.e., 0.3 mg/kg)[43-47] and furthermore not associated with clinically relevant liver disease[48]. In our patients treated with TG, liver biopsies are only performed in patients with symptoms of portal hypertension or persisting liver test abnormalities and no longer as a routine[48].
The use of thiopurines is associated with a three- to fivefold higher risk of the development of lymphoproliferative disorders, in particular non-Hodgkin lymphoma, as well as hepatosplenic T-cell lymphoma, especially in patients without prior Epstein-Barr virus (EBV) exposure[49]. We do not systematically test EBV seroprevalence in patients starting with thiopurines, since over 90% of Dutch inhabitants are exposed to EBV during childhood[50].
Furthermore, there is a clinically significant elevated risk of developing non-melanoma skin cancer, such as squamous cell carcinoma and basal cell carcinoma[49,51]. In our centers, we inform our patients of this higher risk and instruct them to, for example, apply sunscreen to unprotected skin and mention newly developed skin lesions directly to the treating physician or IBD-nurse. However, since the absolute incidence of these malignancies in thiopurine-using IBD patients is still low, we do not systematically screen our patients for the existence of these tumors.
According to recent literature reviews, conventional thiopurine use during pregnancy is not associated with a higher risk of preterm birth, congenital disorders or children with low birth weight[52-54]. For this reason amongst others, we do not cessate thiopurine therapy in patients that become pregnant, but we refer patients during pregnancy to a dedicated team of gynecologists with interest in IBD. Furthermore, after a successful pregnancy, there is insufficient evidence to discourage patients to give breastfeeding; however, this should always be adjusted to the individual patient wishes[55-57]. Evidence concerning the use of TG during pregnancy is scarce and further prospective trials are needed to confirm the safety of this thiopurine derivative in pregnant women[58].
Whether patients achieving a deep remission may successfully stop thiopurines is not known[19,59,60]. In our centers, we continue thiopurine therapy with low threshold, especially in patients with a predicted complex course (i.e., severe or difficult to manage disease). An exception is the patient with deep prolonged (i.e., ≥ 2-3 years) remission on thiopurine therapy with no signs of active disease on clinical, biochemical, endoscopic, histological and radiologic evaluation. In these patients, thiopurine therapy could be ceased with a good probability of relapse-free disease.
Whereas treatment with thiopurine therapy in IBD patients is hampered by a high number of discontinuations, mostly due to adverse events, several treatment strategies may be applied to maximize effectiveness and optimize safety. With this article, we provided a practical overview on how thiopurine therapy is being prescribed in two of the thiopurine research expert centers in Europe. We provided information concerning pharmacotherapy, indications of thiopurine treatment, toxicity of thiopurines and how to optimize treatment in individual patients using different treatment strategies.
| 1. | Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel JF, Allez M, D’Haens G, D’Hoore A, Mantzaris G, Novacek G. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 708] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 2. | Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, Danese S, D’Hoore A, Gassull M, Gomollón F. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis. 2010;4:28-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1041] [Article Influence: 65.1] [Reference Citation Analysis (1)] |
| 3. | Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2015;10:CD000067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Chande N, Tsoulis DJ, MacDonald JK. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2013;4:CD000545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Van Asseldonk DP, de Boer NK, Peters GJ, Veldkamp AI, Mulder CJ, Van Bodegraven AA. On therapeutic drug monitoring of thiopurines in inflammatory bowel disease; pharmacology, pharmacogenomics, drug intolerance and clinical relevance. Curr Drug Metab. 2009;10:981-997. [PubMed] |
| 6. | Al Hadithy AF, de Boer NK, Derijks LJ, Escher JC, Mulder CJ, Brouwers JR. Thiopurines in inflammatory bowel disease: pharmacogenetics, therapeutic drug monitoring and clinical recommendations. Dig Liver Dis. 2005;37:282-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | de Boer NK, van Bodegraven AA, Jharap B, de Graaf P, Mulder CJ. Drug Insight: pharmacology and toxicity of thiopurine therapy in patients with IBD. Nat Clin Pract Gastroenterol Hepatol. 2007;4:686-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Ruemmele FM, Turner D. Differences in the management of pediatric and adult onset ulcerative colitis--lessons from the joint ECCO and ESPGHAN consensus guidelines for the management of pediatric ulcerative colitis. J Crohns Colitis. 2014;8:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, Amil Dias J, Barabino A, Braegger CP, Bronsky J. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8:1179-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 870] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 10. | Rosen MJ, Dhawan A, Saeed SA. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015;169:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 561] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 12. | Bean RH. The treatment of chronic ulcerative colitis with 6-mercaptopurine. Med J Aust. 1962;49:592-593. [PubMed] |
| 13. | Karran P, Attard N. Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nat Rev Cancer. 2008;8:24-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 368] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 14. | Fong SC, Blaker PA, Arenas-Hernandez M, Marinaki AM, Sanderson JD. Getting the best out of thiopurine therapy: thiopurine S-methyltransferase and beyond. Biomark Med. 2015;9:51-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Dervieux T, Chu Y, Su Y, Pui CH, Evans WE, Relling MV. HPLC determination of thiopurine nucleosides and nucleotides in vivo in lymphoblasts following mercaptopurine therapy. Clin Chem. 2002;48:61-68. [PubMed] |
| 16. | Teml A, Schaeffeler E, Herrlinger KR, Klotz U, Schwab M. Thiopurine treatment in inflammatory bowel disease: clinical pharmacology and implication of pharmacogenetically guided dosing. Clin Pharmacokinet. 2007;46:187-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Zaza G, Cheok M, Krynetskaia N, Thorn C, Stocco G, Hebert JM, McLeod H, Weinshilboum RM, Relling MV, Evans WE. Thiopurine pathway. Pharmacogenet Genomics. 2010;20:573-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | van Bodegraven AA, van Everdingen JJ, Dijkstra G, de Jong DJ, Oldenburg B, Hommes DW. [Guideline ‘Diagnosis and treatment of inflammatory bowel disease in adults’. I. Diagnosis and treatment]. Ned Tijdschr Geneeskd. 2010;154:A1899. [PubMed] |
| 19. | Frei P, Biedermann L, Nielsen OH, Rogler G. Use of thiopurines in inflammatory bowel disease. World J Gastroenterol. 2013;19:1040-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 20. | Goel RM, Blaker P, Mentzer A, Fong SC, Marinaki AM, Sanderson JD. Optimizing the use of thiopurines in inflammatory bowel disease. Ther Adv Chronic Dis. 2015;6:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Colombel JF, Reinisch W, Mantzaris GJ, Kornbluth A, Rutgeerts P, Tang KL, Oortwijn A, Bevelander GS, Cornillie FJ, Sandborn WJ. Randomised clinical trial: deep remission in biologic and immunomodulator naïve patients with Crohn’s disease - a SONIC post hoc analysis. Aliment Pharmacol Ther. 2015;41:734-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 22. | Bressler B, Marshall JK, Bernstein CN, Bitton A, Jones J, Leontiadis GI, Panaccione R, Steinhart AH, Tse F, Feagan B. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology. 2015;148:1035-1058 .e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 311] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 23. | Côté-Daigneault J, Bouin M, Lahaie R, Colombel JF, Poitras P. Biologics in inflammatory bowel disease: what are the data? United European Gastroenterol J. 2015;3:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2451] [Article Influence: 153.2] [Reference Citation Analysis (1)] |
| 25. | Lam MC, Bressler B. Vedolizumab for ulcerative colitis and Crohn’s disease: results and implications of GEMINI studies. Immunotherapy. 2014;6:963-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Meijer B, Seinen ML, Leijte NN, Mulder CJ, van Bodegraven AA, de Boer NK. Clinical Value of Mercaptopurine After Failing Azathioprine Therapy in Patients With Inflammatory Bowel Disease. Ther Drug Monit. 2016;38:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Bowen DG, Selby WS. Use of 6-mercaptopurine in patients with inflammatory bowel disease previously intolerant of azathioprine. Dig Dis Sci. 2000;45:1810-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Boulton-Jones JR, Pritchard K, Mahmoud AA. The use of 6-mercaptopurine in patients with inflammatory bowel disease after failure of azathioprine therapy. Aliment Pharmacol Ther. 2000;14:1561-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Actis GC, Pellicano R, Rosina F. 6-Mercaptopurine for Azathioprine Intolerant Inflammatory Bowel Disease: Literature Search and Reappraisal of Own Data. Inflamm Allergy Drug Targets. 2015;14:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Domènech E, Nos P, Papo M, López-San Román A, Garcia-Planella E, Gassull MA. 6-mercaptopurine in patients with inflammatory bowel disease and previous digestive intolerance of azathioprine. Scand J Gastroenterol. 2005;40:52-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Hindorf U, Johansson M, Eriksson A, Kvifors E, Almer SH. Mercaptopurine treatment should be considered in azathioprine intolerant patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2009;29:654-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Chouchana L, Narjoz C, Roche D, Golmard JL, Pineau B, Chatellier G, Beaune P, Loriot MA. Interindividual variability in TPMT enzyme activity: 10 years of experience with thiopurine pharmacogenetics and therapeutic drug monitoring. Pharmacogenomics. 2014;15:745-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Gearry RB, Barclay ML. Azathioprine and 6-mercaptopurine pharmacogenetics and metabolite monitoring in inflammatory bowel disease. J Gastroenterol Hepatol. 2005;20:1149-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 178] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 34. | Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980;32:651-662. [PubMed] |
| 35. | Higgs JE, Payne K, Roberts C, Newman WG. Are patients with intermediate TPMT activity at increased risk of myelosuppression when taking thiopurine medications? Pharmacogenomics. 2010;11:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | McGovern DP, Travis SP, Duley J, Shobowale-Bakre el M, Dalton HR. Azathioprine intolerance in patients with IBD may be imidazole-related and is independent of TPMT activity. Gastroenterology. 2002;122:838-839. [PubMed] |
| 37. | Seinen ML, de Boer NK, Smid K, van Asseldonk DP, Bouma G, van Bodegraven AA, Peters GJ. Allopurinol enhances the activity of hypoxanthine-guanine phosphoribosyltransferase in inflammatory bowel disease patients during low-dose thiopurine therapy: preliminary data of an ongoing series. Nucleosides Nucleotides Nucleic Acids. 2011;30:1085-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Seinen ML, van Asseldonk DP, de Boer NK, Losekoot N, Smid K, Mulder CJ, Bouma G, Peters GJ, van Bodegraven AA. The effect of allopurinol and low-dose thiopurine combination therapy on the activity of three pivotal thiopurine metabolizing enzymes: results from a prospective pharmacological study. J Crohns Colitis. 2013;7:812-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Meijer B, Bouma G, de Boer NK. Optimize Thiopurine Therapy in Autoimmune Hepatitis. Clin Gastroenterol Hepatol. 2016;14:1062-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 40. | Dubinsky MC, Vasiliauskas EA, Singh H, Abreu MT, Papadakis KA, Tran T, Martin P, Vierling JM, Geller SA, Targan SR. 6-thioguanine can cause serious liver injury in inflammatory bowel disease patients. Gastroenterology. 2003;125:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 191] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 41. | Seiderer J, Zech CJ, Reinisch W, Lukas M, Diebold J, Wrba F, Teml A, Chalupna P, Stritesky J, Schoenberg SO. A multicenter assessment of liver toxicity by MRI and biopsy in IBD patients on 6-thioguanine. J Hepatol. 2005;43:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Teml A, Schwab M, Hommes DW, Almer S, Lukas M, Feichtenschlager T, Florin T, Seiderer J, Petritsch W, Bokemeyer B. A systematic survey evaluating 6-thioguanine-related hepatotoxicity in patients with inflammatory bowel disease. Wien Klin Wochenschr. 2007;119:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Ansari A, Elliott T, Fong F, Arenas-Hernandez M, Rottenberg G, Portmann B, Lucas S, Marinaki A, Sanderson J. Further experience with the use of 6-thioguanine in patients with Crohn’s disease. Inflamm Bowel Dis. 2008;14:1399-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | van Asseldonk DP, Jharap B, Kuik DJ, de Boer NK, Westerveld BD, Russel MG, Kubben FJ, van Bodegraven AA, Mulder CJ. Prolonged thioguanine therapy is well tolerated and safe in the treatment of ulcerative colitis. Dig Liver Dis. 2011;43:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | de Boer NK, Zondervan PE, Gilissen LP, den Hartog G, Westerveld BD, Derijks LJ, Bloemena E, Engels LG, van Bodegraven AA, Mulder CJ. Absence of nodular regenerative hyperplasia after low-dose 6-thioguanine maintenance therapy in inflammatory bowel disease patients. Dig Liver Dis. 2008;40:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Gilissen LP, Derijks LJ, Driessen A, Bos LP, Hooymans PM, Stockbrügger RW, Engels LG. Toxicity of 6-thioguanine: no hepatotoxicity in a series of IBD patients treated with long-term, low dose 6-thioguanine. Some evidence for dose or metabolite level dependent effects? Dig Liver Dis. 2007;39:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Seinen ML, van Asseldonk DP, Mulder CJ, de Boer NK. Dosing 6-thioguanine in inflammatory bowel disease: expert-based guidelines for daily practice. J Gastrointestin Liver Dis. 2010;19:291-294. [PubMed] |
| 48. | van Asseldonk DP, Jharap B, Verheij J, den Hartog G, Westerveld DB, Becx MC, Russel MG, Engels LG, de Jong DJ, Witte BI. The Prevalence of Nodular Regenerative Hyperplasia in Inflammatory Bowel Disease Patients Treated with Thioguanine Is Not Associated with Clinically Significant Liver Disease. Inflamm Bowel Dis. 2016;22:2112-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Magro F, Peyrin-Biroulet L, Sokol H, Aldeger X, Costa A, Higgins PD, Joyce JC, Katsanos KH, Lopez A, de Xaxars TM. Extra-intestinal malignancies in inflammatory bowel disease: results of the 3rd ECCO Pathogenesis Scientific Workshop (III). J Crohns Colitis. 2014;8:31-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 50. | National Institute for Public Health and the Environment (RIVM). LCI-guideline Pfeiffer’s disease, 2011. [accessed. 2016;Sept 12] Available from: http://www.rivm.nl/Documenten_en_publicaties/Professioneel_Praktisch/Richtlijnen/Infectieziekten/LCI_richtlijnen/LCI_richtlijn_Pfeiffer_ziekte_van. |
| 51. | Biancone L, Onali S, Petruzziello C, Calabrese E, Pallone F. Cancer and immunomodulators in inflammatory bowel diseases. Inflamm Bowel Dis. 2015;21:674-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 52. | van der Woude CJ, Ardizzone S, Bengtson MB, Fiorino G, Fraser G, Katsanos K, Kolacek S, Juillerat P, Mulders AG, Pedersen N. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015;9:107-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 341] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 53. | Damas OM, Deshpande AR, Avalos DJ, Abreu MT. Treating Inflammatory Bowel Disease in Pregnancy: The Issues We Face Today. J Crohns Colitis. 2015;9:928-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Jharap B, de Boer NK, Stokkers P, Hommes DW, Oldenburg B, Dijkstra G, van der Woude CJ, de Jong DJ, Mulder CJ, van Elburg RM. Intrauterine exposure and pharmacology of conventional thiopurine therapy in pregnant patients with inflammatory bowel disease. Gut. 2014;63:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 55. | de Meij TG, Jharap B, Kneepkens CM, van Bodegraven AA, de Boer NK. Long-term follow-up of children exposed intrauterine to maternal thiopurine therapy during pregnancy in females with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 56. | Gardiner SJ, Gearry RB, Roberts RL, Zhang M, Barclay ML, Begg EJ. Exposure to thiopurine drugs through breast milk is low based on metabolite concentrations in mother-infant pairs. Br J Clin Pharmacol. 2006;62:453-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 57. | Van Assche G, Dignass A, Reinisch W, van der Woude CJ, Sturm A, De Vos M, Guslandi M, Oldenburg B, Dotan I, Marteau P. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Special situations. J Crohns Colitis. 2010;4:63-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 549] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 58. | de Boer NK, Van Elburg RM, Wilhelm AJ, Remmink AJ, Van Vugt JM, Mulder CJ, Van Bodegraven AA. 6-Thioguanine for Crohn’s disease during pregnancy: thiopurine metabolite measurements in both mother and child. Scand J Gastroenterol. 2005;40:1374-1377. [PubMed] |
| 59. | Clarke K, Regueiro M. Stopping immunomodulators and biologics in inflammatory bowel disease patients in remission. Inflamm Bowel Dis. 2012;18:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: The Netherlands
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Actis GC, Capasso R, Serban DE S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ