Published online Aug 6, 2016. doi: 10.4292/wjgpt.v7.i3.434
Peer-review started: February 12, 2016
First decision: March 18, 2016
Revised: March 18, 2016
Accepted: May 10, 2016
Article in press: May 11, 2016
Published online: August 6, 2016
Processing time: 139 Days and 11.1 Hours
AIM: To investigate the clinical impact of post-hyperthermic intraperitoneal chemotherapy (HIPEC) leukopenia, intraperitoneal and combined intravenous/intraperitoneal drug administrations were compared.
METHODS: Two patient cohorts were retrospectively analyzed regarding the incidence of postoperative leukopenia. The first cohort (n = 32) received Mitomycin C (MMC)-based HIPEC intraperitoneally (35 mg/m² for 90 min) and the second cohort (n = 10) received a bi-directional therapy consisting of oxaliplatin (OX) (300 mg/m2 for 30 min) intraperitoneally and 5-fluorouracil (5-FU) 400 mg/m² plus folinic acid 20 mg/m² intravenously. The following data were collected retrospectively: Age, sex, length of operation, length of hospital stay, amount of resection including extent of peritonectomy, peritoneal cancer index, CC (completeness of cytoreduction)-status and leukocyte-count before cytoreductive surgery (CRS) and HIPEC, on days 3, 7 and 14 after CRS and HIPEC. HIPEC leukopenia was defined as < 4000 cells/m³.
RESULTS: Leukopenia occurred statistically more often in the MMC than in the OX/5-FU-group (10/32 vs 0/10; P = 0.042). Leukopenia set-on was on day 7 after CRS and MMC-HIPEC and lasted for two to three days. Three patients (33%) required medical treatment. Patients affected by leukopenia were predominantly female (7/10 patients) and older than 50 years (8/10 patients). The length of hospital stay tended to be higher in the MMC-group without reaching statistical significance (22.5 ± 11 vs 16.5 ± 3.5 d). Length of operation (08:54 ± 01:44 vs 09:48 ± 02:28 h) were comparable between patients with and without postoperative leukopenia. Prior history of systemic chemotherapy did not trigger post-HIPEC leukopenia. Occurrence of leucopenia did not trigger surgical site infections, intraabdominal abscess formations, hospital-acquired pneumonia or anastomotic insufficiencies.
CONCLUSION: Surgeons must be aware that there is a higher incidence of postoperative leukopenia in MMC-based HIPEC protocols primarily affecting females and older patients.
Core tip: Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) are considered the therapy of choice for patients with pseudomyxoma peritonei. Nevertheless this treatment is a major undertaking associated with elevated morbidity. The occurrence of postoperative leukopenia can deteriorate the patient’s outcome by triggering complications like anastomotic insufficiencies or intraabdominal abscess formations so that surgeons must be aware that special patient subsets (primarily older patients and females) exist that are at a higher risk for developing post-HIPEC leukopenia.
- Citation: Horvath P, Beckert S, Struller F, Königsrainer A, Königsrainer I. Incidence of leukopenia after intraperitoneal vs combined intravenous/intraperitoneal chemotherapy in pseudomyxoma peritonei. World J Gastrointest Pharmacol Ther 2016; 7(3): 434-439
- URL: https://www.wjgnet.com/2150-5349/full/v7/i3/434.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i3.434
Pseudomyxoma peritonei (PMP) is a rare clinical condition generally associated with perforated appendiceal neoplasmas. It is characterized by huge amounts of intraabdominal gelatinous fluid collections accompanied by mucinous implants on the peritoneum. Preferred areas for these implants are areas of reduced persistaltic movement such as the ileocoecal region, sigmoid colon and ligament of Treitz. Despite controversy regarding the pathological classification, PMP is nowadays classified as low-grade or high-grade disease.
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) are the treatment of choice in patients with PMP, creating 5-year-survival rates of 62% to 100% for low-grade, and 0% to 65% for high-grade PMP[1]. Also for many other gastrointestinal and gynecological tumors this dual-approach was able to achieve a survival benefit[1]. A part from its clinical benefits, morbidity rates after CRS and HIPEC remain high (up to 70%). Reasons are long operations, multivisceral resections including stripping of peritoneum thus creating large wound areas and the side-effects of HIPEC itself. Frequently used chemotherapeutic agents for HIPEC in PMP are Mitomycin C (MMC) und oxaliplatin (OX). These two combine beneficial properties for intraperitoneal administration. A high molecular weight resulting in decelerated systemic absorption and prolonged local toxicity makes these agents attractive for intraabdominal administration. Furthermore, enhanced toxicity is achieved by hyperthermia of the dialysate.
Systemic absorption of the chemotherapeutic agents and consecutive leukopenia can account for a variety of postoperative complications and might depend on the agent used and partly on the amount of stripped peritoneum, leading to a larger area of exposed subperitoneal veins, thus facilitating systemic absorption.
This study investigates the incidence of postoperative leukopenia in patients either treated with MMC only intraperitoneally or with OX/5-fluorouracil (5-FU) intravenously/intraperitoneally.
From 2007 to 2015, 42 patients diagnosed with PMP originating from mucinous appendiceal tumors were included. All patients gave informed consent prior to their inclusion. Thirty-two patients were treated with an MMC-based HIPEC protocol (35 mg/m²) for 90 min in an open-closed technique at 42.5 °C as described elsewhere[2]. Ten patients were treated according to an OX-based HIPEC protocol (300 mg/m²) for 30 min in the same way and additionally 5-FU (400 mg/m²) and folinic acid (20 mg/m²) were administered intravenously prior to HIPEC.
The following data were collected retrospectively: age, sex, length of operation, length of hospital stay, amount of resection including extent of peritonectomy, peritoneal cancer index (PCI), completeness of cytoreduction (CC)-status and leukocyte-count before CRS and HIPEC, on days 3, 7 and 14 after CRS and HIPEC. HIPEC leukopenia was defined as < 4000 cells/m³.
All complications were graded using the Clavien-Dindo classification of surgical complications[2].
Prior to CRS and HIPEC all patients underwent clinical examinations and blood tests to guarantee adequate performance status and computed tomography was performed to rule out extraabdominal disease.
CRS was conducted according to a standardized procedure consisting of midline laparotomy and screening of the abdomen for peritoneal tumor implants in order to define the PCI-score as described by Königsrainer et al[3]. After maximal cytoreduction, achieving a CC-0/1 status, HIPEC was administered. All anastomoses were completed before HIPEC started. Total peritonectomy was defined as complete removal of the parietal peritoneum. In total six tubes were transcutaneously inserted into the abdomen to guarantee high-volume influx and efflux of the dialysate. Temperature was monitored to ensure an influx-temperature of 42.5 °C. After HIPEC and removal of all chemotherapy-containing fluid, the abdomen was lavaged again and then closed.
SPSS ver. 12.0 (SPSS Inc. Chicago, IL, United States) was used for statistical analysis and data are written as mean ± SD. A P < 0.05 was considered statistically significant when using the chi-square test and the t-test. The χ2 test was used for nominal variables and the t-test for continuous variables.
In total 40 patients diagnosed with PMP underwent CRS and HIPEC. Complete data were available on all patients. Table 1 shows patients and treatment characteristics. Of the patients 53 (53%) were female. Mean age and PCI-score were comparable in the MMC- and OX/5-FU-groups without being statistically significant (50 ± 14 years vs 54 ± 10 years; PCI 20 ± 11 vs 25 ± 10). In 58% a CC-0 status and in 42% a CC-1 status was achieved. Three patients in the MMC- and one patient in the OX/5-FU-group received systemic chemotherapy prior to CRS and HIPEC. These 4 patients underwent a FOLFOX regimen. Only one patient (MMC-group) with prior history of systemic chemotherapy developed postoperative leukopenia. The length of hospital stay tended to be greater in the MMC-group without reaching statistical significance (22.5 ± 11 d vs 16.5 ± 3.5 d). Total peritonectomy was conducted in 30 of 32 patients in the MMC-group and in all patients in the OX/5-FU-group. Splenectomy was necessary due to tumor involvement in 14 of 40 patients (12 in the MMC-group and 2 in the OX/5-FU-group). All anastomoses were performed prior to HIPEC and no anastomotic insufficiencies occurred. No statistically significant differences were observed between the MMC- and the OX/5-FU-group regarding occurrence of hospital-acquired pneumonia (HAP) (2 vs 0 patients), pleural effusion (PE) (2 vs 2 patients) surgical site infections (SSI) (one vs one patient), intraabdominal abscess formations (IAA) (0 vs 1 patient) and urinary tract infections (UTI) (1 vs 0 patient). Patients with HAP required antibiotic treatment (Clavien-Dindo grade II). Patients with SSI required bed-side wound treatment but no antibiotic treatment (Clavien-Dindo grade I). Two of four patients with PE needed pleural drainage (Clavien-Dindo grade IIIa). One patient with IAA required antibiotic treatment (Clavien-Dindo grade II) and one patient with a UTI also required antibiotic treatment (Clavien-Dindo grade II).
| MMC | OX/5-FU | |
| No. | 32 | 10 |
| Gender | ||
| Male (n = 20; 47%) | 15 | 5 |
| Female (n = 22; 53%) | 17 | 5 |
| Age (yr) | 50 ± 14 | 54 ± 10 |
| Length of operation (h) | 09:41 ± 2:27 | 09:07 ± 1:44 |
| CC-status | ||
| CC-0 | 21 | 3 |
| CC-1 | 11 | 7 |
| PCI | 20 ± 11 | 25 ± 10 |
| Length of hospital stay (d) | 22.5 ± 11 | 16.5 ± 3.5 |
| Resections | ||
| Total peritonectomy | 30 | 10 |
| Splenectomy | 11 | 3 |
| Omentectomy | 25 | 3 |
| Cholecystectomy | 19 | 7 |
| Colon | 18 | 3 |
| Small bowel | 4 | 1 |
| Anastomotic insufficiencies | 0 | 0 |
| Leucopenia | 10 | 0 |
| G-CSF treatment | 3 | 0 |
| HAP | 2 | 0 |
| SSI | 1 | 1 |
| PE | 2 | 2 |
| IAA | 0 | 1 |
| UTI | 1 | 0 |
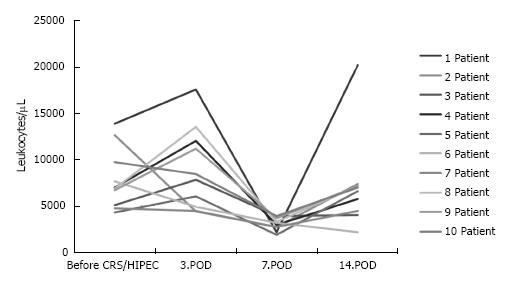
Leukopenia occurred in ten of 32 patients (31%) in the MMC-group. Of these ten patients with MMC-associated leukopenia seven were female. No patient in the OX/5-FU-group developed postoperative leukopenia. Figure 1 shows the postoperative course of the leukocyte count. Leukopenia occurred in all patients between day 6 and day 7 after CRS and HIPEC was administered for 2 to 3 d. Three patients required medical treatment with filgastrim till leukocyte counts were in normal range. Length of operation (08:54 ± 01:44 h vs 09:48 ± 02:28 h) and of hospital stay (25 ± 12 d vs 20 ± 10 d) were comparable and not statistically significant in patients with or without postoperative leukopenia (Table 2). Splenectomy was necessary in two (20%) of 10 patients with leukopenia and in twelve (40%) of 32 patients without leukopenia. Of 10 patients with leukopenia eight were older than 50 years which was statistically significant (60 ± 16 years vs 48.5 ± 11 years; P = 0.01).
| Leukopenia | No leukopenia | |
| No. | 10 | 32 |
| Gender | ||
| Male | 3 | 16 |
| Female | 7 | 16 |
| Age (yr) | 60 ± 16 | 48.5 ± 11 |
| MMC | 10 | 22 |
| OX/5-FU | 0 | 10 |
| Splenectomy | 2 | 12 |
| Hospital stay (d) | 25 ± 12 | 20 ± 10 |
| Length of operation (h) | 08:54 ± 01:44 | 09:48 ± 02:28 |
| HAP | 2 | 0 |
| SSI | 1 | 1 |
| PE | 2 | 2 |
| IAA | 0 | 1 |
| UTI | 1 | 0 |
Occurrence of postoperative leukopenia did not trigger surgical site infections, intraabdominal abscess formations, anastomotic insufficiencies or urinary tract infections.
PMP is a rare clinical condition arising in the vast majority of cases from ruptured appendiceal malignancies. In the past debulking surgery accompanied by systemic chemotherapy was applied. Due to the efforts of Sugarbaker PH this aforementioned strategy has been widely abandoned and superseded by a dual-approach therapy consisting of CRS and HIPEC, resulting in a 15-year survival rate of up to 60%[1,4-8]. Furthermore Chua et al[1] were able to demonstrate an impressive median progression-free survival rate of 8.2 years after CRS and HIPEC thus once more emphasizing the efficacy of this dual-approach in disease control. Nevertheless, every surgeon dealing with CRS and HIPEC has to be aware that this therapy is a major undertaking accompanied by long operating times and multivisceral resections and is thus associated with high morbidity rates of up to 70% and mortality rates of up to 11%[9-15]. MMC and OX are the most frequently used intraperitoneally administered drugs in PMP-patients. Both are alkylating chemotherapeutics, interfering with DNA and DNA-synthesis without being cell-cycle dependent[16]. In order to potentiate the activity of the intraperitoneally administered OX, patients are given intravenous 5-FU and folinic acid 30 min prior to HIPEC. Because of a pH incompatibility 5-FU cannot be mixed with OX for intraperitoneal use[17,18]. Despite its advantageous pharmacokinetic properties MMC-induced leukopenia due to bone marrow toxicity after HIPEC is a known and frequently encountered side-effect of this treatment[19]. In our study the incidence of MMP-induced leukopenia was 31% (n = 10/32 patients), which was lower than in other reports in the literature[20,21]. None of the patients in the OX/5-FU-group developed postoperative leukopenia. This might be related to the different route of drug elimination. Oxaliplatin is predominantly excreted via urine, by tissue-binding and by renal elimination, whereas MMC undergoes hepatic metabolism which might contribute to systemic accumulation thus promoting occurrence of leukopenia. In accordance with other studies[20,21], prior systemic chemotherapy was not associated with a higher risk of leukopenia. Only four patients (10%) in our study population were not chemo-naïve and one patient, having received MMC-HIPEC, developed postoperative leukopenia. Patients who received systemic chemotherapy prior to CRS and HIPEC and had a history of chemotherapy-associated leukopenia are at greater risk for further episodes of leukopenia. This is the main reason why some authors recommend a dose reduction in MMC-HIPEC in this special patient subgroup[21]. In our analysis female sex and age (> 50 years) were associated with the occurrence of MMC-induced leukopenia. The phenomenon of female gender being associated with a greater risk for chemotherapy-induced leukopenia has been reported previously, but its reasons are still unknown. Bécouarn et al[17] provide an explanation for the association between leukopenia and female gender. The authors speculate that women harbor a relatively large surface area of the peritoneum combined with a smaller plasma volume as compared with men of equal weight[22]. As it is a body-surface-area (BSA)-based MMC dose, women with equal weight and a smaller plasma volume have higher MMC-plasma concentrations than do males in the case of equal absorption, thus explaining a higher cytotoxic effect of MMC. Due to these circumstances some HIPEC-centers introduced lower doses of chemotherapeutics in HIPEC for female patients[23].
Our data show that splenectomy was not associated with a higher incidence of MMC-induced leukopenia. Only two (20%) of ten patients suffered from leukopenia after splenectomy whereas twelve (40%) of 30 patients without postoperative leukopenia were splenectomized. These data might suggest a protective effect of splenectomy on leukopenia due to post-splenectomy leukocytosis. As far as this is concerned the literature presents controversial results. Bécouarn et al[17] found a higher, although not significant, incidence of neutropenia in the splenectomized patients after MMC-HIPEC, whereas Bidus et al[24] reported also a potentially protective effect of splenectomy on leukopenia in patients receiving adjuvant systemic chemotherapy. The time when chemotherapy was administered could be the decisive variable for these conflicting results.
The role of peritonectomy in the pathophysiology of post-HIPEC leukopenia was negligible in our study because 38 out of 40 patients received total peritonectomy, so that we could not evaluate the definite effect of total peritonectomy on the incidence of post-HIPEC leukopenia.
In our study patients with post-HIPEC leukopenia tended to have a longer hospital stay (25 d vs 20 d) without reaching statistical significance. In accordance with the study by Hompes et al[16] MMC-induced leukopenia neither elevated the risk of postoperative infections and anastomotic insufficiencies nor prolonged the patient’s hospital stay. A larger study population might have found a higher global infection risks in patients with MMC-induced leukopenia. Nonetheless, this clinical condition should not be underestimated and especially in females, older patients and patients with a prior history of chemotherapy-induced leukopenia the possibility that a balance between optimal oncological treatment and systemic cytotoxicity, maybe achieved by a dose reduction in HIPEC, should be taken into account.
Hyperthermic intraperitoneal chemotherapy (HIPEC) followed by complete cytoreductive surgery is the therapy of choice for patients with pseudomyxoma peritonei (PMP). In the vast majority of cases ruptured appendiceal neoplasms are causal for PMP. HIPEC protocols for the treatment of PMP after complete cytoreduction include only intraperitoneal or concomitant intravenous/intraperitoneal drug administration. Mitomycin C (MMC) and oxaliplatin (OX)/ fluorouracil (5-FU) are the most frequently used agents. The aim of the study was to find out the incidence of postoperative leukopenia depending on the chemotherapy regimen used.
The bi-directional therapy consisting of HIPEC and complete cytoreduction is used in the vast majority of patients with PMP. Nevertheless this treatment is associated with high morbidity rates due to long operative times, multivisceral resections and by the HIPEC itself. HIPEC-associated leukopenia can further contribute to postoperative morbidity. The results show that especially in MMC-based HIPEC protocols and in female and in elderly patients post-HIPEC leukopenia can occur.
In this study the author demonstrated that MMC-HIPEC protocols provoke post-HIPEC leukopenia between day six and seven after operation. Every surgeon dealing with this therapy should be aware of the fact and especially in women and older patients the incidence of post-HIPEC leukopenia is elevated, thus this special patient subset should be even more monitored in the postoperative course. Previous studies reported similar results and some of them also suggested a dose reduction in MMC-HIPEC in women and older patients.
This study suggests that in women and elderly patients a-priori a dose reduction should be taken into account in order to decreased the incidence of post-HIPEC leukopenia in MMC-protocols.
HIPEC: Hyperthermic intraperitoneal chemotherapy: Combined with complete cytoreduction it is the treatment of choice for pseudomyxoma peritonei.
This paper presents an essential and interesting data. In my opinion this is a professional report of an important and currently still discussed in a lack number of papers problematic leukopenia incidence occurring after HIPEC. For me as a person who is working scientifically and clinically on HIPEC method this is a brief but very professional work which is very worthy. The properly presented data and good quality of English are a very strong plus points of this paper.
| 1. | Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, Baratti D, Deraco M, Elias D, Sardi A. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449-2456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 814] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 2. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 9183] [Article Influence: 540.2] [Reference Citation Analysis (1)] |
| 3. | Königsrainer I, Horvath P, Struller F, Forkl V, Königsrainer A, Beckert S. Risk factors for recurrence following complete cytoreductive surgery and HIPEC in colorectal cancer-derived peritoneal surface malignancies. Langenbecks Arch Surg. 2013;398:745-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Mazzei MA, Khader L, Cirigliano A, Cioffi Squitieri N, Guerrini S, Forzoni B, Marrelli D, Roviello F, Mazzei FG, Volterrani L. Accuracy of MDCT in the preoperative definition of Peritoneal Cancer Index (PCI) in patients with advanced ovarian cancer who underwent peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC). Abdom Imaging. 2013;38:1422-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Gough DB, Donohue JH, Schutt AJ, Gonchoroff N, Goellner JR, Wilson TO, Naessens JM, O’Brien PC, van Heerden JA. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg. 1994;219:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 266] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Miner TJ, Shia J, Jaques DP, Klimstra DS, Brennan MF, Coit DG. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg. 2005;241:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 257] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | Youssef H, Newman C, Chandrakumaran K, Mohamed F, Cecil TD, Moran BJ. Operative findings, early complications, and long-term survival in 456 patients with pseudomyxoma peritonei syndrome of appendiceal origin. Dis Colon Rectum. 2011;54:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Yan TD, Bijelic L, Sugarbaker PH. Critical analysis of treatment failure after complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from appendiceal mucinous neoplasms. Ann Surg Oncol. 2007;14:2289-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Jacquet P, Stephens AD, Averbach AM, Chang D, Ettinghausen SE, Dalton RR, Steves MA, Sugarbaker PH. Analysis of morbidity and mortality in 60 patients with peritoneal carcinomatosis treated by cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy. Cancer. 1996;77:2622-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Loggie BW, Fleming RA. Complications of heated intraperitioneal chemotherapy and strategies for prevention. Cancer Treat Res. 1996;82:221-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Witkamp AJ, de Bree E, Kaag MM, van Slooten GW, van Coevorden F, Zoetmulder FA. Extensive surgical cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in patients with pseudomyxoma peritonei. Br J Surg. 2001;88:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Loungnarath R, Causeret S, Bossard N, Faheez M, Sayag-Beaujard AC, Brigand C, Gilly F, Glehen O. Cytoreductive surgery with intraperitoneal chemohyperthermia for the treatment of pseudomyxoma peritonei: a prospective study. Dis Colon Rectum. 2005;48:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Smeenk RM, Verwaal VJ, Zoetmulder FA. Toxicity and mortality of cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei--a report of 103 procedures. Eur J Surg Oncol. 2006;32:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Butterworth SA, Panton ON, Klaassen DJ, Shah AM, McGregor GI. Morbidity and mortality associated with intraperitoneal chemotherapy for Pseudomyxoma peritonei. Am J Surg. 2002;183:529-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Deraco M, Baratti D, Inglese MG, Allaria B, Andreola S, Gavazzi C, Kusamura S. Peritonectomy and intraperitoneal hyperthermic perfusion (IPHP): a strategy that has confirmed its efficacy in patients with pseudomyxoma peritonei. Ann Surg Oncol. 2004;11:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Hompes D, D’Hoore A, Wolthuis A, Fieuws S, Mirck B, Bruin S, Verwaal V. The use of Oxaliplatin or Mitomycin C in HIPEC treatment for peritoneal carcinomatosis from colorectal cancer: a comparative study. J Surg Oncol. 2014;109:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Bécouarn Y, Ychou M, Ducreux M, Borel C, Bertheault-Cvitkovic F, Seitz JF, Nasca S, Nguyen TD, Paillot B, Raoul JL. Phase II trial of oxaliplatin as first-line chemotherapy in metastatic colorectal cancer patients. Digestive Group of French Federation of Cancer Centers. J Clin Oncol. 1998;16:2739-2744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 103] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Elias D, Benizri E, Di Pietrantonio D, Menegon P, Malka D, Raynard B. Comparison of two kinds of intraperitoneal chemotherapy following complete cytoreductive surgery of colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2007;14:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Schnake KJ, Sugarbaker PH, Yoo D. Neutropenia following perioperative intraperitoneal chemotherapy. Tumori. 1999;85:41-46. [PubMed] |
| 20. | Sugarbaker PH, Alderman R, Edwards G, Marquardt CE, Gushchin V, Esquivel J, Chang D. Prospective morbidity and mortality assessment of cytoreductive surgery plus perioperative intraperitoneal chemotherapy to treat peritoneal dissemination of appendiceal mucinous malignancy. Ann Surg Oncol. 2006;13:635-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 21. | Lambert LA, Armstrong TS, Lee JJ, Liu S, Katz MH, Eng C, Wolff RA, Tortorice ML, Tansey P, Gonzalez-Moreno S. Incidence, risk factors, and impact of severe neutropenia after hyperthermic intraperitoneal mitomycin C. Ann Surg Oncol. 2009;16:2181-2187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Sugarbaker PH. Management of peritoneal surface malignancies using intraperitoneal chemotherapy and cytoreductive surgery. A manual for physicians and nurses. Grand rapids; MI: Ludann Co 1998; . |
| 23. | Sugarbaker PH, Stuart OA, Carmignani CP. Pharmacokinetic changes induced by the volume of chemotherapy solution in patients treated with hyperthermic intraperitoneal mitomycin C. Cancer Chemother Pharmacol. 2006;57:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Bidus MA, Krivak TC, Howard R, Rose GS, Cosin J, Dainty L, Elkas JC. Hematologic changes after splenectomy for cytoreduction: implications for predicting infection and effects on chemotherapy. Int J Gynecol Cancer. 2006;16:1957-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript Source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: Germany
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Alzahrani NA, Ihemelandu C, Nowacki M S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ