Published online May 6, 2016. doi: 10.4292/wjgpt.v7.i2.334
Peer-review started: July 2, 2015
First decision: September 17, 2015
Revised: November 29, 2015
Accepted: December 17, 2015
Article in press: December 18, 2015
Published online: May 6, 2016
Processing time: 296 Days and 15.3 Hours
AIM: To study the efficacy and safety of pharmacological treatment of constipation in geriatrics.
METHODS: PubMed, MEDLINE, google scholar, and Ovid were searched to identify human studies performed on the use of laxatives in elderly with constipation, which were conducted between January 1990 and January 2013 using the specified keywords. Controlled studies that enrolled geriatric patients with a diagnosis of constipation and addressed the efficacy and/or the safety of pharmacological treatments were included. Studies were excluded from this review if they were non-controlled trials, case series, or case reports.
RESULTS: Out of twenty three studies we initially retrieved in our search, only nine studies met the eligibility criteria of being controlled trials within geriatrics. The laxatives examined in the nine studies were senna, lactulose, sorbital, polyethylene glycol (PEG), lubiprostone, linaclotide, and prucalopride. In those studies, senna combinations had a higher efficacy than sorbitol or lactulose as well as, a very good adverse effect profile. PEG was also shown to be safe and effective in geriatric population. Furthermore, it has been shown that PEG is as safe in geriatrics as in general population. New agents like lubiprostone and prucalopride show promising results but the data about these agents in geriatrics are still limited which warrants further investigation.
CONCLUSION: Senna combinations and PEG appear to have a more favorable profile over the other traditionally used laxatives in elderly patients with constipation.
Core tip: Laxatives are among the most commonly prescribed medications for elderly patients, however, data about safety and efficacy of laxatives in this patient population are limited. We show in this paper, based on reviewing geriatric studies, that senna combinations and polyethylene glycol appear to have better outcomes in this population than other classic laxatives. We also discuss here the promising results of the new agents, lubiprostone, linaclotide, and prucalopride, which can be helpful in treating geriatric populations in the near future.
- Citation: Izzy M, Malieckal A, Little E, Anand S. Review of efficacy and safety of laxatives use in geriatrics. World J Gastrointest Pharmacol Ther 2016; 7(2): 334-342
- URL: https://www.wjgnet.com/2150-5349/full/v7/i2/334.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i2.334
A common complaint amongst the elderly is constipation. The prevalence of constipation increases with age and some statistics estimate that around 50% of the population of adults who are 80 years old and greater will suffer from this condition at some point in time[1]. Recent estimates from the United States Census Bureau showed that the population aged 65 and greater will rise to an estimated 88.5 million in 2050 making this a growing health care concern[2]. Interestingly, the increased prevalence of constipation as patients advance in age is more pronounced amongst male than female patients[3]. The consequences of constipation are important as they do not only negatively impact quality of life but also impact the cost of care. Therefore, it is important that health care practitioners have a well-rounded understanding of the efficacy of these medications and their safety while trying to combat this growing issue in the geriatric population, defined as greater than 65 years. Chronic constipation is routinely defined as no more than three spontaneous bowel movements a week with one or more of the following symptoms for at least twelve weeks during the past year: (1) straining in greater than one-fourth of defecations; (2) lumpy or hard stool in more than one-fourth of defecations; (3) sensation of incomplete evacuation in more than one-fourth of defecations; or (4) no loose or watery bowel movements, Bristol stool form scale score 6-7[4]. Chronic constipation should be distinguished from irritable bowel syndrome-constipation which is characterized by recurrent abdominal discomfort with two or more of the following: (1) improvement with defecation; (2) onset associated with a change in frequency of stool; and (3) onset associated with a change in stool form[5]. Furthermore, there are many conditions, both physiological and iatrogenic which may contribute to the increased prevalence in the geriatric population. Commonly used medications such as antihypertensive, diuretics, pain medications and iron supplements can cause constipation[6]. Possible psychosocial and behavioral factors may also contribute to the elderly developing constipation such as dehydration, decreased mobility and inadequate caloric intake. Anorectal sensation changes may also participate when patients ignore the call to defecate, which can lead to fecal retention. Suppression of rectal sensation will lead to only large stools being perceived and can eventually lead to difficulty defecating. Elderly patients possibly have physiological changes such as failure of recto-anal coordination or pelvic floor dysfunction, which also impact their ability to defecate[7]. In one study, age related neurodegenerative changes in the enteric nervous system were observed. There was shown to be a loss of 37% of enteric neurons in the geriatric subjects when compared to the age group of 20-35 years old. However, it is important to note that these studies do not find whether the difference in quantity of neurons is due to aging or caused by changes in behavior or the chronic use of laxatives in constipated patients[8]. Management strategies differ depending on the etiology, however this article will focus on the pharmacological treatments available for management of chronic idiopathic constipation. The oral pharmacological agents used for treatment of constipation are historically classified into bulking agents, osmotic or secretive agents, and stool softeners. Recently, chloride channel blockers and selective serotonin receptor agonists, represented by lubiprostone and prucalopride, respectively, were approved for treatment of specific cases of constipation. This review will investigate both the efficacy and safety of the aforementioned medications based on trials that included elderly patients with chronic constipation.
A systematic review of the published literature that discussed the clinical effectiveness and safety of laxatives in the management of chronic constipation in the elderly was conducted. The searched databases included PubMed, MEDLINE, Ovid, and google scholar for published literature between January 1990 and January 2013.
The following keywords were used in the search process: Constipation, chronic constipation, elderly, geriatrics, laxatives, bulking agents, senna, lactulose, sorbital, polyethylene glycol (PEG), lubiprostone, linaclotid, and prucalopride. The following filters were applied: English language, human studies, and research support (United States, Non United States, governmental and nongovernmental). Case reports have been excluded from this review. The retrieved studies were screened for inclusion and exclusion criteria. Controlled studies that investigated the use of laxatives in geriatrics with chronic constipation were included. Exclusion criteria were non controlled studies, or case series. Trials on the use of the aforementioned agents in irritable bowel syndrome patients were not included.
A total of 23 articles were found and manually reviewed by a team of two researchers. Out of 23 studies retrieved by the search, nine met the eligibility criteria of being controlled trials with a geriatric population or subpopulation that was diagnosed with chronic constipation and therefore included in our review. The laxatives examined in the nine studies were senna, lactulose, sorbital, PEG, lubiprostone, linaclotide, and prucalopride. The studies included in the article are summarized in Table 1.
| Ref. | Agent | Efficacy | Safety |
| Kinnunen et al[12] | Bulk + senna vs lactulose (20 mL bulk + senna, 30 mL lactulose) | Bulk with senna greater than lactulose | No drug related side effects |
| Passmore et al[13] | Senna + fibre vs lactulose (10 mL senna + fibre, 15 mL lactulose) | Senna - fibre greater than lactulose | No difference (most common urgency and flatulence) |
| Lederle et al[16] | Sorbitol vs lactulose (0 to 60 mL) | Lactulose greater than sorbital | Increase nausea in lactulose |
| DiPalma et al[17] | PEG vs placebo (17 g PEG) | PEG greater than placebo | Increased gastrointestinal complaints |
| Seinelä et al[18] | Isotonic PEG vs hypotonic PEG (12 g isotonic and hypotonic PEG) | Same for hypotonic and isotonic PEG | Hyponatremia in hypotonic PEG (Not clinically significant) |
| Ueno et al[20,21] | Lubiprostone vs placebo (24 mcg bid lubiprostone) | Lubiprostone greater than placebo | Increase nausea in placebo and general population |
| Muller-Lissner et al[25] | Prucalopride vs placebo (0.5, 1, 2 mg prucalopride) | Prucalopride greater than placebo | Increased diarrhea with dosage of Prucalopride |
| Camilleri et al[26] | Prucalopride vs placebo (1, 2, or 4 mg prucalopride) | Efficacy not studied | Increased diarrhea with increase dosage of prucalopride |
| Lembo et al[24] | Linaclotide vs placebo (75, 150, 300, 600 mcg) | Linaclotide greater than placebo | Increased GI adverse effects with increasing Linaclotide dosage |
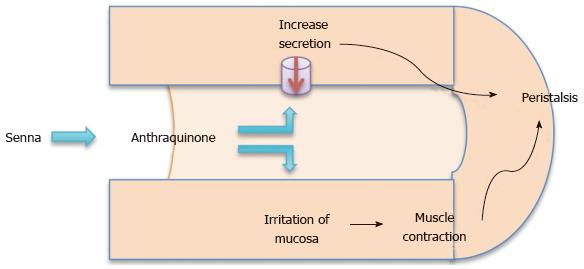
Classification: An anthracine glycoside, senna is manufactured from either the Cassia acutifolia or Cassia angustifolia plant. The dried leaflets or legumes are hydrolyzed in the colon by bacteria into anthraquinones. These free anthraquinone alter the electrolyte transportation of the colon increasing the intraluminal fluids[9,10] as well as acting as irritants on the mucosa. The result is an increase in peristalsis producing mass peristalsis stimulation in the colon which leads to defecation (Figure 1)[11].
Clinical efficacy and safety: In two studies, a senna combination laxative was compared to the commonly used laxative, lactulose, in the treatment of constipation in geriatric patients. In the first trial, thirty patients aged 65-94 years participated in the open, randomized and controlled cross over study. One week run in without laxatives, called a wash out, was followed up with a 5 wk period of a daily dose of 20 mL of bulk laxative with senna (plantago ovata, isphagula, senna pods; Agiolax) or 30 mL lactulose. This 5 wk period was followed with a week’s wash out then followed with another 5 wk period with cross over medications. The results showed that bowel habits were more frequent when treated with the bulk containing senna laxative. The bulk + senna had 4.5 bowel movements per week in both 5 wk periods compared to lactulose which had 2.2 and 1.9 movements. In terms of safety, all side effects were noted on a questionnaire while blood count and serum concentrations of calcium, magnesium, potassium, sodium, albumin and creatinine were measured and analyzed by t test. There were no changes in laboratory measurements or complications which could be considered drug related or statistically significant in either group. Therefore, both were considered safe to use in geriatric patients[12].
In a second trial comparing senna with lactulose, Passmore et al[13], used a senna fibre combination (ispaghula 54%-2%, senna 12%-4%; 10 mL Manevac) or lactulose (15 mL twice daily) with matching placebo for two 14 d periods. There was a 3 to 5 d wash out period before as well as in between treatments. This trial had 77 elderly subjects with a history of constipation. Efficacy wise, results showed that mean daily bowel frequency was greater with the senna-fibre combination than lactulose. Senna-fibre had daily frequency of 0-8, with a 95%CI of 0-7 to 0-9 while lactulose had a frequency of 0-6, 0-5 to 0-7 and a P < 0.001. As with the previous trial, safety showed no significant statistical difference between the two treatments. Most common adverse effects in both treatments were an increased in urgency and flatulence[13].
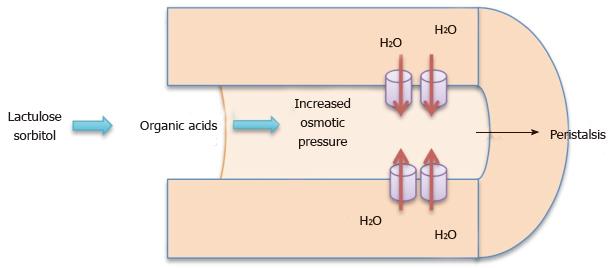
Classification: Lactulose and sorbitol are both non-absorbable disaccharides which pass unchanged into the colon where they are metabolized by bacteria into formic, acetic and lactic acids. The organic acids produced increase intraluminal fluid in the colon (Figure 2)[7]. PEG is an osmotic laxative that is minimally absorbed in the colonic tract. PEG softens stool and increases stool volume which lead to increased peristalsis[14]. PEG is often used in bowel preparations for colonoscopy in the elderly, as well as in treatment of constipation[15].
Clinical efficacy and safety: Lactulose was compared to sorbitol in 30 patients aged 65 to 86 in one trial which was randomized, double blind, cross over trial conducted by Lederle et al[16]. Patients were given either lactulose or 70% sorbitol for 4 wk after a 2-wk washout period and crossed over to take another 4 wk period of the other medication. The results showed an average number of bowel movements per week of 6.71 with sorbitol and 7.02 for patients on lactulose with a 95%CI of -0.43 to 1.06. Common side effects of osmotic laxatives are bloating, flatulence and diarrhea. In this trial, adverse symptoms were recorded by participants in a daily diary. The only difference between the two treatments was nausea. The score for nausea was significantly high in lactulose treatment than in sorbitol with a P value of < 0.05. However, overall the most common adverse effect reported was flatulence with 23 participants suffering from it at some point during the trial. Overall, sorbitol and lactulose had no difference in effect but sorbitol appears to be not only safer to use in the elderly but is also more cost effective[16].
This trial by DiPalma et al[17] was designed using PEG to evaluate its safety and efficacy compared to a placebo (maltodextrin) over a six-month period. A total of 304 patients were enrolled in the double-blind, placebo-controlled, parallel, multicenter study to receive PEG laxative as a single daily dose of 17 g or placebo for 6 mo. In the trial, there were 75 subjects older than 65 years. A baseline for constipation status was established during a 14-d observation period. Success was defined as relief of criteria for constipation in 50% or more of their weeks of treatment. This long term trial showed that use of PEG was better at achieving success in comparison to the placebo at the 6 mo mark in both the total subject population (52.0% of PEG and 11% of placebo subjects; P < 0.001) and the geriatric subpopulation (61% of PEG treatment weeks vs 22% of the placebo weeks; P < 0.001). Throughout the trial there were no statistically significant differences in adverse effects except in gastrointestinal issues (40% vs 25%, P = 0.015), which included nausea, diarrhea and flatulence but were mild and self-limiting. There were also no clinically significant laboratory changes observed during the trial. Similar results were observed for the elderly subpopulation[17].
In a trial completed by Seinelä et al[18], the use of PEG with and without electrolytes was compared in terms of both efficacy and safety. This trial focused on the geriatric population with 62 participants receiving isotonic PEG for one week before patients were randomly assigned to either the hypotonic PEG or isotonic PEG group for the next 4 wk. At the end of 4 wk, the results showed a mean weekly stool frequency of 8.5 in the hypotonic and 8.4 in the isotonic PEG groups. The mean stool frequency ratio was calculated to be 0.90 with a 95%CI (0.74-1.10). Therefore, both isotonic and hypotonic PEG can be considered equal in efficacy. While there was no difference between groups in terms of straining or gastrointestinal complaints, plasma sodium levels were statistically significantly lower in the hypotonic PEG group (137.7 mmol/L vs 138.9 mmol/L, P = 0.012). However there are no clinical differences detected between testing groups and no intervention was needed[18].
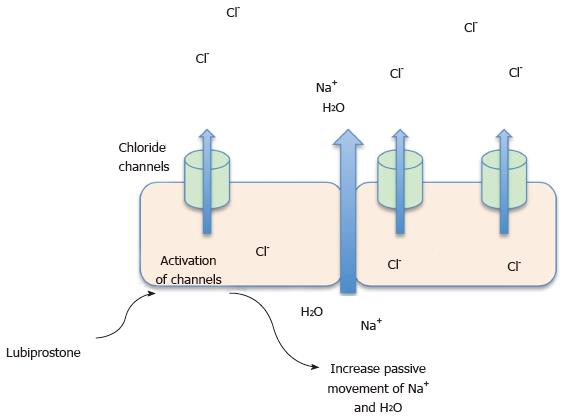
Classification: Lubiprostone is a bicyclic fatty acid compound classified as a prostone. It is derived from a prostaglandin E1 metabolite. It acts by inducing secretion of both electrolytes and fluid through the activation of type 2 chloride channels in the small intestine. It also appears to reduce gastric emptying and increase gastric volume during fasting time (Figure 3)[19].
Clinical efficacy and safety: Two abstracts were presented by Ueno et al[20], which looked at the safety and efficacy of lubiprostone in the elderly. The first abstract was sub-analysis from a number of controlled trials that included a subgroup of elderly patients, 57 patients aged > 65 years who were randomized to lubiprostone 48 mcg/d or placebo for 4 wk. Spontaneous bowel movements significantly improved amongst the lubiprostone elderly group compared to the placebo elderly group (P≤ 0.0286). Increase in frequency of weekly bowel movements ranged from 4.6 to 5.4 bowel movements per week for elderly lubiprostone subjects compared to 1.29 to 2.27 bowel movements for elderly placebo subjects. Also, it is important to note was that fewer adverse effects were reported in the lubiprostone group vs subjects treated with the placebo (46% vs 61%, P not reported)[20]. The second abstract was pooled analysis of elderly population of open labeled trials, which included 163 elderly participants (> 65 years) and 715 non-elderly subjects. Fewer elderly patients reported adverse effects at 74.2% vs 80.1% in the non-elderly subjects. The most common reported side effect was nausea throughout the trial. Improvement in constipation severity and abdominal bloating or discomfort were significantly better in patients who received lubiprostone compared to placebo group of both elderly and non elderly patients[21]. Of note, another abstract presented by the same group about the safety of lubiprostone in general, regardless of the elderly status, showed that the side effects encountered with this medication which are mainly nausea, headache, and diarrhea are generally mild to moderate in severity, intermittent, and limited in duration[22].
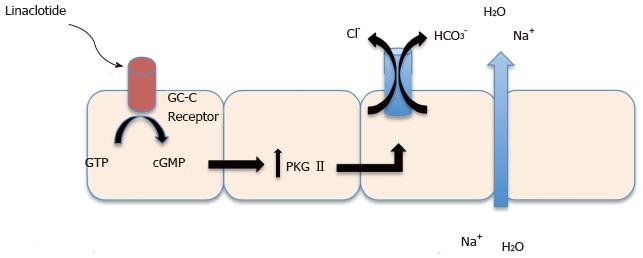
Classification: Linaclotide is a 14 amino acid peptide that acts as a guanylate cyclase C (GC-C) agonist. Linaclotide binds to GC-C receptor on the surface on intestinal enterocytes which increases guanosine monophosphate triggering a signal transduction cascade resulting in the activation of cystic fibrosis transmembrane conductance regulator (Figure 4). This activation will result in chloride and fluid secretion into the lumen of the intestines as well as acceleration of intestinal transit[23].
Clinical efficacy and safety: One study for linaclotide which looked at a geriatric subgroup was completed by Lembo et al[24]. A total of 310 patients with a mean age of 47.3 years participated in the study with a subpopulation of 30 geriatric patients. The patients were randomly assigned to receive 75, 150, 300, or 600 g oral linaclotide or placebo once daily for 4 wk. The efficacy of linaclotide generally improved with increasing doses from 75 to 600 μg per day. Spontaneous bowel movement frequency in the 4-wk treatment period showed a linear dose-response with increases of 2.6, 3.3, 3.6, and 4.3 for linaclotide doses of 75, 150, 300, and 600 μg, respectively, compared to 1.5 for placebo with a P valve of less than 0.05 for each pairwise comparison. There was also a change in stool consistency. There was a 0.50 mean change for patients that received the placebo in comparison to 1.35, 1.57, 1.68 and 2.00 for linaclotide doses of 75, 150, 300 and 600 μg (P≤ 0.0005 for each dose of linaclotide). Linaclotide appears to be equally effective in the geriatric subpopulation as the general study population. Linaclotide was overall well-tolerated in this study population. Adverse effects occurred in 33.8% of patients receiving linaclotide while only 31.9% of patients receiving a placebo reported an adverse effect. Most of the adverse effects in this study were related to the GI tract. Diarrhea, mild to moderate in severity was the most commonly reported effect which is an expected result of linaclotide’s pharmacology. The rate of adverse effects is slightly greater in patients receiving 600 μg linaclotide (38.1%) compared with the other linaclotide groups (29.0% to 35.0%)[24].
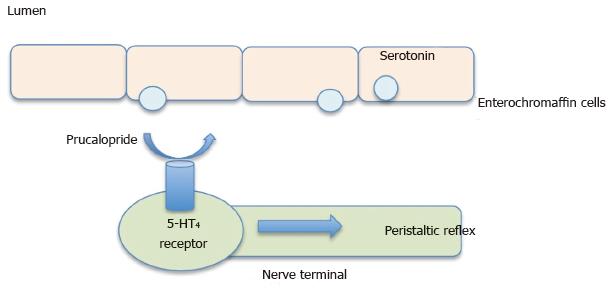
Classification: Prucalopride, a selective 5-HT4 receptor agonist has strong enterokinetic activity. It is believed that patients with constipation have decreased frequency and duration of the giant migrating contractions in their colons. These high amplitude contractions stimulate the urge to defecate. Control of these contractions is suggested to involve serotonin release and its action on 5-HT4 receptors. Therefore, it is believed that selective stimulation of these receptors would elicit strong enterokinetic activity in the colon and help restore the physiologic colonic motility (Figure 5)[25].
Clinical efficacy and safety: In a study with patients aged > 65 years, a 2 wk wash out period was followed by 4 wk of either 1, 2, 4 mg prucalopride or placebo daily with no change in diet or lifestyle. Efficacy was measured through patient’s global assessment. It was found that patients on prucalopride in 1 or 4 mg doses reported a mean improvement in severity of constipation significantly higher that what was reported in the placebo group. At the end of the 4th week, 42% of patients receiving 1 mg, 24% of those receiving 2 mg, and 39% of those receiving 4 mg of prucalopride considered the treatment either moderately effective or extremely effective. However, only 16% of the placebo group believed their treatment was successful (P < 0.001 for 1 mg prucalopride vs placebo, P < 0.05 for both 2 and 4 mg prucalopride vs placebo)[25]. A second study looked at prucalopride in 89 elderly patients using dosages of 0.5, 1, and 2 mg vs placebo in order to compare adverse effects. This trial focused only on the safety of prucalopride in the geriatric population considering the notorious safety profile of other less selective serotonergic prokinetic agents such as tegaserod and cisapride. These drugs have been associated with serious cardiovascular side effects. Tegaserod was withdrawn for the United States market due to the number of patients that reported serious side effects, mainly ischemic cardiovascular events. Therefore the question was asked if prucalopride would also have adverse cardiovascular effects especially in a high-risk population like the elderly. This trial recorded not only reported adverse effects by the participants but also laboratory studies and cardiovascular parameters such as vital signs, EKGs, and Holter monitors. There were no clinically relevant or dose-related effects measured in laboratory. Similarly, no changes were noted in vital signs between prucalopride and the placebo. Extensive ECG and Holter monitoring confirmed that prucalopride does not cause the induction of ventricular arrhythmias, QT prolongation or torsade de pointes. The majority of adverse effects were gastrointestinal related, diarrhea and abdominal pain being the most common. Diarrhea increased with the dose of prucalopride with none being reported in the placebo group[26].
Chenodeoxycholic acid (CDCA) is one of the major bile acids in the human biliary system. A small percentage of bile acids are not absorbed in the terminal ileum, rather they move into the proximal colon where they undergo modification by colonic bacteria to form secondary bile acids which induce colonic secretion[27]. Furthermore, there is evidence that bile acids are able to modify colonic motility separately from its secretory effects[28]. When used at pharmacological dosages, CDCA helps to reduce cholesterol saturation in bile thereby leading to the eventual dissolving of cholesterol gallstones. One of the most common side effects of using CDCA is diarrhea[29]. In one study using high doses of CDCA (750-1000 mg/d) in order to observe bile-lipid composition and common side effects, 40% of patients had diarrhea[30]. A more recent study conducted by Rao et al[31], showed accelerated colonic transit and improved bowel function in 36 females with Irritable Bowel Syndrome - constipation subtype[31]. However, larger studies with a geriatric population will need to be completed to determine whether these benefits can be replicated and maintained over a longer time period.
Elobixiblat (A3309) is an oral agent that decreases the reabsorption of bile acids in the terminal ileum by inhibiting bile acid transporters thereby increasing the concentration of bile acids that enter the colon. Studies of A3309 have shown acceleration in colon transit with relief of constipation related symptoms in patients[32]. One study of 36 women with functional chronic constipation in a double blind placebo controlled study focused on colonic transit at 24 then 48 h compared to placebo after 14 d of treatment. At both dosages of 15 and 20 mg, colonic transit was accelerated compared to the placebo. Improvement in stool consistency and straining by participants was also reported[33].
Colchicine is an anti-inflammatory medication used in the treatment of gout. It works by inhibiting microtubule assembly in white blood cells but has been shown to cause diarrhea when taken in higher dose. It is believed that colchicine increases the production of prostaglandins, increases gastrointestinal motility and secretion as well as decreasing the absorption of water and electrolytes in the intestine. One study done as a double-blind, placebo-controlled trial used participants diagnosed with slow transit constipation. The trial used low dose colchicine (1 mg daily) in an effort to improve symptoms and increase the number of spontaneous bowel movements. In this trial, patients using colchicine showed improvement in bloating and abdominal pain. They reported increased number of bowel movements. Eventually all participants were placed on open label colchicine for one month after the duration of the study was completed due to the considerable beneficial effects[34].
As the population in the Unites States continues to age, constipation will increase in prevalence, having an impact on the functional status and quality of life for many patients[6]. Traditionally, the first line pharmacological agents used to be lactulose, sorbitol and senna[8]; however, in studies that involved geriatric populations, senna combinations have shown greater efficacy and a more favorable side effect profile. Sorbitol had roughly the same efficacy as lactulose but had a better side effect and cost profile, which makes it an attractive alternative to using lactulose. PEG, another osmotic laxative, was shown to be effective in geriatric populations in comparison to general adult populations. Interestingly, geriatrics patients did not have more side effects than the general adult participants. Trials showed that lubiprostone has good outcomes in geriatric population in terms of both efficacy and safety. Similarly, prucalopride shows a great potential. Negative cardiovascular interactions were the primary concern with prucalopride. However, prucalopride did not produce adverse cardiovascular effects throughout the trial. Both lubiprostone and prucalopride need further studies to determine their efficacy in geriatrics compared to common treatments such as senna and PEG but they remain a potential option in the treatment of constipation. Though studies of linaclotide have shown significant improvement in constipation, clinical trials for linaclotide with a larger geriatric population are needed to determine real life applicability in the elderly. Emerging treatments using CDCA, A3309 and colchicine for constipation show promise for the future. However, studies comparing these medications to older treatments are necessary to evaluate efficacy and whether the benefits can be reproduced for long-term use in the geriatric population.
Constipation in the elderly is a growing health care concern in the United States. It has a remarkable impact on their functional status and quality of life. As physicians treat elderly patients with this condition, it is important to know the efficacy and safety of the drugs they choose.
The current literature lacks a review of the efficacy and safety of different classes of laxatives in the treatment of chronic constipation among elderly populations.
This review suggests that, in geriatric populations, senna combinations and polyethylene glycol are more efficacious than other traditionally-used laxatives including but not limited to lactulose and sorbitol. Lubiprostone, prucalopride, and linaclotide have been showing promising results but further geriatric studies are warranted.
The authors suggest prescribing senna combinations and/or polyethelene glycol as the first line for the treatment of chronic constipation in geriatrics. Routine use of the new, potentially effective medications (i.e., Lubiprostone, Prucalopride, and Linaclotide) is pending further studies in this patient population.
The review is a good starting point.
| 1. | Gandell D, Straus SE, Bundookwala M, Tsui V, Alibhai SM. Treatment of constipation in older people. CMAJ. 2013;185:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Department of Health & Human Services: Adminstration on Aging (5/8/2013). [accessed 2013 Sept 20]. Available from: http://www.aoa.gov/Aging_Statistics/future_growth/future_growth.aspx. |
| 3. | Werth BL, Williams KA, Pont LG. A longitudinal study of constipation and laxative use in a community-dwelling elderly population. Arch Gerontol Geriatr. 2015;60:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 5. | Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377-1390. [PubMed] |
| 6. | Rao SS, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171. [PubMed] |
| 7. | Crane SJ, Talley NJ. Chronic gastrointestinal symptoms in the elderly. Clin Geriatr Med. 2007;23:721-734, v. [PubMed] |
| 8. | Bouras EP, Tangalos EG. Chronic constipation in the elderly. Gastroenterol Clin North Am. 2009;38:463-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Mascolo N, Meli R, Autore G, Capasso F. Senna still causes laxation in rats maintained on a diet deficient in essential fatty acids. J Pharm Pharmacol. 1988;40:882-884. [PubMed] |
| 10. | Dufour P, Gendre P. Ultrastructure of mouse intestinal mucosa and changes observed after long term anthraquinone administration. Gut. 1984;25:1358-1363. [PubMed] |
| 11. | Frexinos J, Staumont G, Fioramonti J, Bueno L. Effects of sennosides on colonic myoelectrical activity in man. Dig Dis Sci. 1989;34:214-219. [PubMed] |
| 12. | Kinnunen O, Winblad I, Koistinen P, Salokannel J. Safety and efficacy of a bulk laxative containing senna versus lactulose in the treatment of chronic constipation in geriatric patients. Pharmacology. 1993;47 Suppl 1:253-255. [PubMed] |
| 13. | Passmore AP, Wilson-Davies K, Stoker C, Scott ME. Chronic constipation in long stay elderly patients: a comparison of lactulose and a senna-fibre combination. BMJ. 1993;307:769-771. [PubMed] |
| 14. | Corazziari E, Badiali D, Bazzocchi G, Bassotti G, Roselli P, Mastropaolo G, Lucà MG, Galeazzi R, Peruzzi E. Long term efficacy, safety, and tolerabilitity of low daily doses of isosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in the treatment of functional chronic constipation. Gut. 2000;46:522-526. [PubMed] |
| 15. | Lashner BA, Winans CS, Blackstone MO. Randomized clinical trial of two colonoscopy preparation methods for elderly patients. J Clin Gastroenterol. 1990;12:405-408. [PubMed] |
| 16. | Lederle FA, Busch DL, Mattox KM, West MJ, Aske DM. Cost-effective treatment of constipation in the elderly: a randomized double-blind comparison of sorbitol and lactulose. Am J Med. 1990;89:597-601. [PubMed] |
| 17. | Dipalma JA, Cleveland MV, McGowan J, Herrera JL. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am J Gastroenterol. 2007;102:1436-1441. [PubMed] |
| 18. | Seinelä L, Sairanen U, Laine T, Kurl S, Pettersson T, Happonen P. Comparison of polyethylene glycol with and without electrolytes in the treatment of constipation in elderly institutionalized patients: a randomized, double-blind, parallel-group study. Drugs Aging. 2009;26:703-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Lacy BE, Levy LC. Lubiprostone: a novel treatment for chronic constipation. Clin Interv Aging. 2008;3:357-364. [PubMed] |
| 20. | Ueno R, Joswick TR, Wahle A, Zhu Y, Holland PC. Efficacy and safety of lubiprostone for the treatment of chronic constipation in elderly vs. non-elderly subjects. Gastroenterology. 2006;130:A189. Abstract S1262. |
| 21. | Ueno R, Panas R, Wahle A, Zhu Y, Holland PC. Long-term safety and efficacy of lubiprostone for the treatment of chronic constipation in elderly subjects. Gastroenterology. 2006;130:A-188. Abstract S1260. |
| 22. | Ueno R, Wahle A, Rivera E. Pooled analysis of the most frequent adverse events associated with the use of lubiprostone. Am J Gastroenterol. 2006;101:S489. Abstract 1264. |
| 23. | Schulz S, Lopez MJ, Kuhn M, Garbers DL. Disruption of the guanylyl cyclase-C gene leads to a paradoxical phenotype of viable but heat-stable enterotoxin-resistant mice. J Clin Invest. 1997;100:1590-1595. [PubMed] |
| 24. | Lembo AJ, Kurtz CB, Macdougall JE, Lavins BJ, Currie MG, Fitch DA, Jeglinski BI, Johnston JM. Efficacy of linaclotide for patients with chronic constipation. Gastroenterology. 2010;138:886-895.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Müller-Lissner S, Rykx A, Kerstens R, Vandeplassche L. A double-blind, placebo-controlled study of prucalopride in elderly patients with chronic constipation. Neurogastroenterol Motil. 2010;22:991-98, e255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Camilleri M, Beyens G, Kerstens R, Robinson P, Vandeplassche L. Safety assessment of prucalopride in elderly patients with constipation: a double-blind, placebo-controlled study. Neurogastroenterol Motil. 2009;21:1256-e117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Mekjian HS, Phillips SF, Hofmann AF. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J Clin Invest. 1971;50:1569-1577. [PubMed] |
| 28. | Bajor A, Gillberg PG, Abrahamsson H. Bile acids: short and long term effects in the intestine. Scand J Gastroenterol. 2010;45:645-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Iser JH, Sali A. Chenodeoxycholic acid: a review of its pharmacological properties and therapeutic use. Drugs. 1981;21:90-119. [PubMed] |
| 30. | Mok HY, Bell GD, Dowling RH. Effect of different doses of chenodeoxycholic acid on bile-lipid composition and on frequency of side-effects in patients with gallstones. Lancet. 1974;2:253-257. [PubMed] |
| 31. | Rao AS, Wong BS, Camilleri M, Odunsi-Shiyanbade ST, McKinzie S, Ryks M, Burton D, Carlson P, Lamsam J, Singh R. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010;139:1549-158, 1558.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 32. | Wong BS, Camilleri M. Elobixibat for the treatment of constipation. Expert Opin Investig Drugs. 2013;22:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Wong BS, Camilleri M, McKinzie S, Burton D, Graffner H, Zinsmeister AR. Effects of A3309, an ileal bile acid transporter inhibitor, on colonic transit and symptoms in females with functional constipation. Am J Gastroenterol. 2011;106:2154-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Taghavi SA, Shabani S, Mehramiri A, Eshraghian A, Kazemi SM, Moeini M, Hosseini-Asl SM, Saberifiroozi M, Alizade-Naeeni M, Mostaghni AA. Colchicine is effective for short-term treatment of slow transit constipation: a double-blind placebo-controlled clinical trial. Int J Colorectal Dis. 2010;25:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Carroccio A, Shailubhai K S- Editor: Ji FF L- Editor: A E- Editor: Wu HL