Published online May 6, 2016. doi: 10.4292/wjgpt.v7.i2.242
Peer-review started: November 28, 2015
First decision: December 24, 2015
Revised: January 20, 2016
Accepted: February 16, 2016
Article in press: February 17, 2016
Published online: May 6, 2016
Processing time: 145 Days and 8.5 Hours
AIM: To investigate whether oral tolerance is inducible during the active phase of dextran sulfate sodium (DSS)-induced colitis.
METHODS: Colitis was induced in 6- to 8-wk-old female BALB/c mice by the administration of 2% DSS. To induce oral tolerance, mice that received water with DSS [DSS (+)] and mice that received autoclaved water [DSS (-)] were intragastrically (i.g.) administered ovalbumin (OVA) as a tolerogen before systemic challenge with OVA. Following this, serum levels of OVA-specific IgE antibodies were measured. In mice with active colitis, CD4+CD25+Foxp3+ cell and B10 cell frequencies were evaluated using flow cytometry. Cytokine mRNA expression profiles were evaluated by reverse transcription real-time polymerase chain reaction.
RESULTS: Regardless of the presence of DSS colitis, OVA-specific immunoglobulin E concentrations were significantly reduced in mice that were i.g. administered OVA compared to mice that were i.g. administered PBS [DSS (+): 4.4 (4.2-9.5) ng/mL vs 83.9 (66.1-123.2) ng/mL, P < 0.01; DSS (-): 27.7 (0.1-54.5) ng/mL vs 116.5 (80.6-213.6) ng/mL, P < 0.01]. These results demonstrated that oral tolerance was induced in both the presence and absence of colitis. In the spleen and mesenteric lymph nodes (MLN), the frequencies of CD4+CD25+Foxp3+ cells and B10 cells, both of which are associated with oral tolerance, did not significantly change. In the spleen, interferon-γ mRNA expression significantly decreased in mice with colitis [DSS (+): 0.42 (0.31-0.53) vs DSS (-): 1.00 (0.84-1.39), P < 0.01]. The expression levels of other cytokines did not significantly change.
CONCLUSION: Oral tolerance is inducible during active DSS colitis. The stability of regulatory cell populations in the spleen and MLN in colitis might correlate with these results.
Core tip: Our study is the first to demonstrate that oral tolerance is inducible during the active phase of dextran sulfate sodium (DSS)-induced colitis. Lymphocytic infiltration into the large intestine mucosa associated with epithelial defects did not influence oral tolerance. In DSS colitis, the frequencies of CD4+CD25+Foxp3+ T cells and B10 cells in the spleen and mesenteric lymph nodes remained stable. This stability might have led to the induction of oral tolerance in DSS colitis. Accordingly, if an appropriate antigen is chosen, then oral immunotherapy may be applicable for the treatment of ulcerative colitis.
- Citation: Ino S, Kohda C, Takeshima K, Ishikawa H, Norose T, Yamochi T, Takimoto M, Takahashi H, Tanaka K. Oral tolerance is inducible during active dextran sulfate sodium-induced colitis. World J Gastrointest Pharmacol Ther 2016; 7(2): 242-253
- URL: https://www.wjgnet.com/2150-5349/full/v7/i2/242.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i2.242
Inflammatory bowel disease (IBD) includes Crohn’s disease (CD) and ulcerative colitis (UC). The precise cause of IBD remains unknown. IBD is a multifactorial disease resulting from excessive immune responses to various environmental factors and is associated with genetic background. As a result of these excessive immune responses, T helper cell type (Th)0 cells differentiate into Th1, Th17 or Th2 cells in response to interleukin (IL)-12/IL-18, IL-6/tissue growth factor (TGF)-β or IL-4, respectively[1].
Regulatory T cells (Tregs) have been shown to suppress conventional T cells through multiple mechanisms, including the generation of immunosuppressive cytokines, such as TGF-β and IL-10, and via direct contact with effector T cells or antigen-presenting cells[2]. Decreases in the anti-inflammatory activity of Tregs may therefore be equal in importance to the enhancement of effector mechanisms in contributing to IBD pathogenesis[1].
Regulatory B cells (Bregs) are functionally characterized by their capacity to produce IL-10, a potent inhibitory cytokine. These cells have been designated as B10 cells because their ability to downregulate immune responses and inflammatory disease is attributable to IL-10. The absence of B10 cells exacerbates disease symptoms in mouse models[3]. Breg dysfunction has been reported to influence the pathogeneses of various autoimmune and allergic diseases. In addition to autoimmune and allergic diseases, intestinal inflammation is also regulated by Breg functions, a relationship that has been confirmed by several studies using mouse models of colitis[4]. Additionally, in humans, the depletion of B cells using anti-CD20 (rituximab) for various disorders has been reported to either exacerbate colitis or result in spontaneous colitis[5,6].
Oral tolerance is a phenomenon in which systemic immunity is suppressed relative to orally administered antigens. Treg involvement has been demonstrated as a mechanism for the induction of oral tolerance. Tregs are naturally produced in the thymus (nTreg) and are also induced in peripheral tissues (pTreg). Induced, antigen-specific Tregs can then circulate and establish systemic tolerance to their corresponding antigens. This phenomenon largely contributes to the induction of oral tolerance[7]. In recent years, Bregs have also been indicated to be involved in oral tolerance[8]. Consistent with the regulatory role of B cells, B cell-deficient mice are defective in developing oral tolerance[9].
The administration of dextran sulfate sodium (DSS) can induce colitis in animal models. This induced colitis is similar in appearance to human UC both clinically and histologically[10,11]. Several reports have evaluated Treg dynamics during active DSS colitis tolerance[12,13]; however, few reports have assessed Breg dynamics during active DSS colitis.
Although oral immunotherapy has been applied for various immune disorders, this treatment modality is considered ineffective for IBD because IBD patients have dysfunctional oral tolerance[14,15]. Although the effectiveness of oral immunotherapy for CD patients was recently reported[16,17], there are currently few reports regarding oral immunotherapy for UC patients. In this study, we utilized a DSS colitis model to explore the potential use of oral immunotherapy during the active phase of UC. The oral administration of colon extracted protein (CEP) prior to the onset of DSS colitis has been shown to induce immune tolerance, downregulate the inflammatory immune response and alleviate DSS-induced colitis[18,19]. However, to the best of our knowledge, no report thus far has evaluated the effectiveness of the oral administration of CEP after the onset of DSS colitis, during the active phase of the disease.
The purpose of this study was to investigate whether oral tolerance is inducible during the active phase of DSS colitis. Additionally, we determined how cytokine levels and regulatory cell populations change in colitis in the mesenteric lymph nodes (MLN) and the spleen and how these changes influence the induction of oral tolerance. Furthermore, we explored the potential use of oral immunotherapy during the active phase of UC.
Specific pathogen-free (SPF) BALB/c mice were purchased from Charles River Laboratories Japan (Yokohama, Kanagawa, Japan). All experiments were performed using 6- to 8-wk-old female mice. The protocol used in the current study was designed to minimize pain and discomfort to the animals and was approved by the Institutional Animal Care and Use Committee of Showa University. The mice had access to water and food ad libitum and were housed in SPF conditions with alternating light-dark cycles for one week prior to experimentation. Intragastric gavage was performed using straight gavage needles appropriate for each animal’s size. All animals were euthanized using CO2 prior to tissue collection.
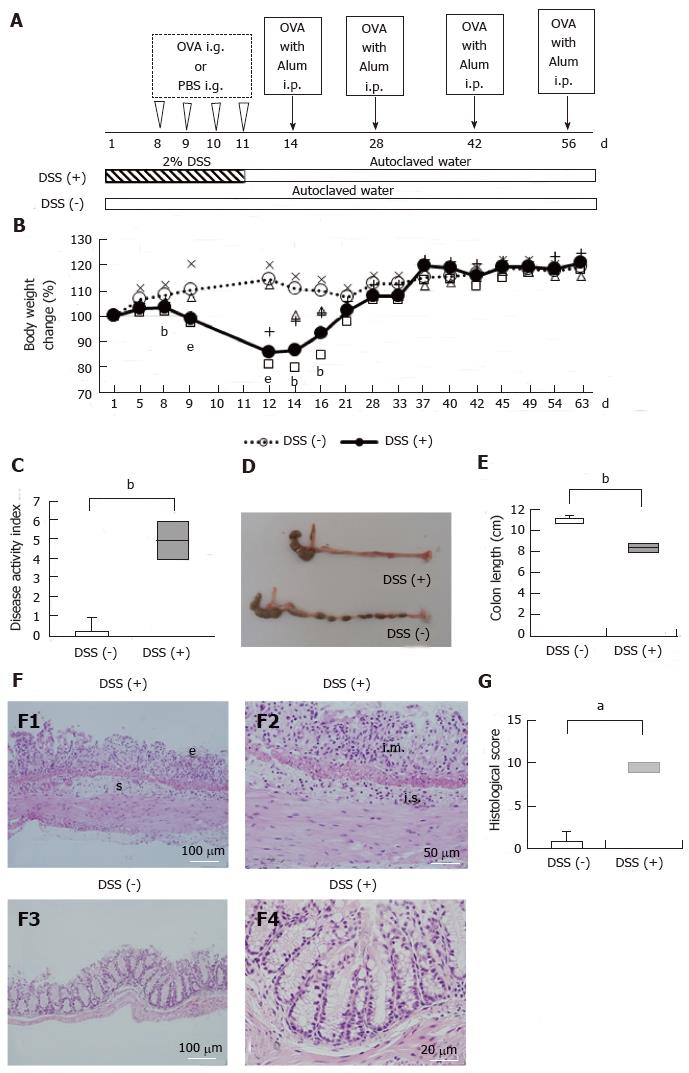
An overview of the experimental setup is provided in Figure 1A and B. DSS administration was performed as previously described with slight modification[11,20]. DSS colitis was induced by the administration of 2% DSS with a molecular weight ranging between 36 and 50 kDa (MP Biomedicals, Solon, OH, United States) ad libitum from day 1 through day 11. As a control, a subset of mice was provided with autoclaved water for the entire study period. All mice were clinically evaluated based on body weight and a scoring system comprising evaluations of stool consistency and fecal blood, as described previously[20].
On day 8, the colons of the mice were removed and fixed in 10% buffered formalin and then embedded in paraffin, sliced into sections, and stained with hematoxylin and eosin. The stained sections were examined by two pathologists for evidence of colitis using a previously described histological scoring method[21].
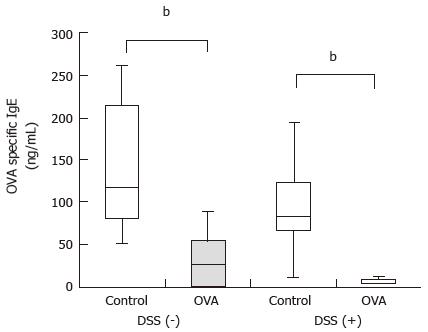
To induce oral tolerance, the mice that received 2% DSS from day 1 through day 11 and the mice that received autoclaved water were intragastrically (i.g.) administered either 5 mg/d ovalbumin (OVA) or PBS as a control for 4 consecutive days from day 8 through day 11. To induce systemic antibody (Ab) production in response to OVA antigen, the mice were intraperitoneally (i.p.) injected with 1 µg of OVA antigen plus 0.1 mg of aluminum hydroxide (alum) (Thermo Scientific, Rockford, IL, United States) on days 14, 28, 42, and 56. Following this, blood samples were collected on day 63 to measure serum anti-OVA-specific IgE Ab concentrations, as described previously[22].
Serum OVA-specific IgE concentrations were measured by ELISA. Briefly, 50 μg/mL of OVA was dissolved in 0.1 mol/L sodium carbonate buffer (pH 9.5) and incubated with serum samples in a 96-well immunoplate at 4 °C overnight. The samples were treated with protein-free blocking buffer T20 (PBS) (Thermo Scientific) to inhibit nonspecific binding. After washing, the serum samples or OVA-specific IgE antibody (Ab) standards (Acris Antibodies, San Diego, CA, United States) and biotin-conjugated anti-mouse IgE Abs (Southern Biotech, Birmingham, AL, United States) were then plated in the wells and incubated for 1 h. All wells were sequentially incubated with HRP-conjugated streptavidin (eBioscience, San Diego, CA, United States). OVA-specific IgE Ab was detected using a TMB Microwell Peroxidase Substrate System (KPL, Gaithersburg, MD, United States) and measured at an absorbance of 450 nm after the addition of H2SO4.
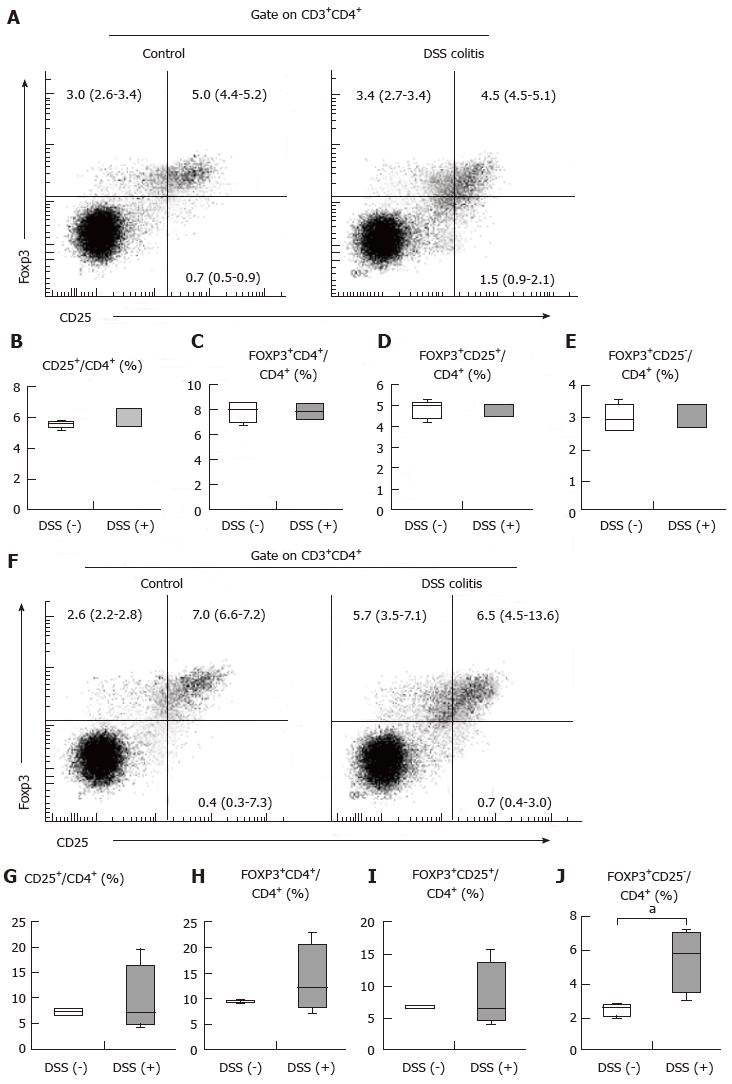
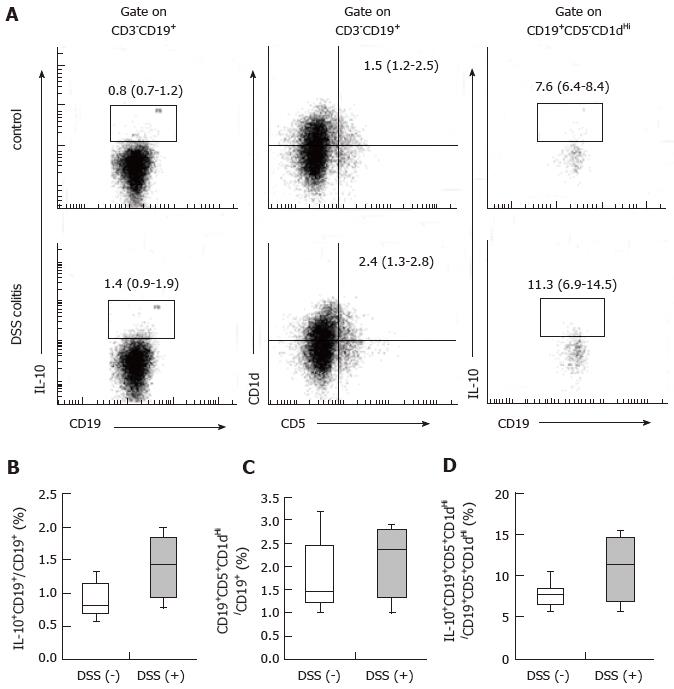
Single-cell suspensions were prepared from the MLN and spleen on day 14. The following antibodies were used in this study: Anti-CD16/CD32 Ab as an Fc-blocker; FITC-conjugated anti-CD4 Ab; BV421-conjugated anti-CD25 Ab; Alexa Fluor-conjugated anti-Foxp3 Ab (BD Biosciences, San Diego, CA, United States); BV650-conjugated anti-CD3 Ab; FITC-conjugated anti-CD19 Ab; BV510-conjugated anti-CD5 Ab; PE-conjugated anti-CD1d Ab; and PE/Cy7-conjugated anti-IL-10 Ab (BioLegend, San Diego, CA, United States). Dead cells were detected using a Zombie Red Fixable Viability Kit (BioLegend) according to the manufacturer’s recommended protocol. Following the staining of surface antigens, intracellular Foxp3 or IL-10 staining was performed using a Transcription Factor Buffer set (BD Biosciences) according to the manufacturer’s recommended protocol. All cells were analyzed using a LSRFortessa flow cytometer (BD Biosciences).
Analysis of intracellular IL-10 was performed using flow cytometry as previously described[23]. Briefly, isolated spleen cells were resuspended (2 × 106/mL) in complete medium (RPMI 1640) (Wako Pure Chemical Industries, Osaka, Japan) containing 10% FBS with 5 × 10-5 mol/L 2-mercaptoethanol, 10 μg/mL lipopolysaccharide (LPS), 50 ng/mL phorbol 12-myristate 13-acetate (PMA), 500 ng/mL ionomycin (Sigma-Aldrich, St. Louis, MO, United States) and 1 μg/mL Brefeldin A (BioLegend) for 5 h in 24-well flat-bottom plates.
Total RNA was extracted from whole MLN and spleen cells on day 14 using an RNeasy Mini Kit (Qiagen, Tokyo, Japan). Reverse transcription was performed using a QuantiTect Reverse Transcription Kit (Qiagen). Real-time polymerase chain reaction (PCR) was performed using a LightCycler 480II system (Roche Diagnostics, Mannheim, Germany) with LightCycler 480 Probes Master (Roche Diagnostics). The following PCR primers were used: TaqMan® Gene Expression Assays for mouse IL-4 (Assay ID: Mm00445259_m1), IL-6 (Assay ID: Mm00446190_m1), IL-10 (Assay ID: Mm 00439614_m1), interferon-γ (IFN-γ) (Assay ID: Mm01168134_m1), tumor necrosis factor (TNF)-α (Assay ID: Mm00443258_m1), and GAPDH (Assay ID: Mm99999915_g1) (Applied Biosystems, Foster City, CA, United States). All values were normalized against the expression of the housekeeping gene GAPDH.
Statistical analyses were performed using the Mann-Whitney U test, and a P value < 0.05 was considered to indicate a statistically significant difference.
DSS is widely used to induce intestinal inflammation. From day 8 to day 16, the mice that received water containing 2% DSS [DSS (+) mice] had significantly lower body weights than the mice that received autoclaved water [DSS (-) mice] (Figure 1B, P < 0.05 to P < 0.001). With the progression of colitis, the DSS (+) mice exhibited diarrhea and visible fecal blood. On day 12, the disease activity index (DAI) of the DSS (+) mice was significantly higher than that of the DSS (-) mice (Figure 1C, P < 0.01). On day 8, the average colon length of the DSS (+) mice was shorter than that of the DSS (-) mice (Figure 1D and E, P < 0.01). Histologically, on day 8, DSS colitis was characterized by epithelial defects, submucosal edema (F1) and inflammatory cell infiltration (F2) (Figure 1F). The histological scores for the DSS (+) mice were significantly higher than those of the DSS (-) mice (Figure 1G, P < 0.05).
DSS (+) mice and DSS (-) mice were i.g. administered 5 mg/d OVA or PBS, respectively, for 4 consecutive days before undergoing an i.p. administered challenge with 1 μg OVA plus 0.1 mg of alum every two weeks for a total of four times. Serum samples were collected from the mice 1 wk after each challenge, and OVA-specific IgE concentrations were measured. Regardless of the presence of DSS colitis, the mice that were i.g. administered OVA had significantly lower OVA-specific IgE concentrations than the mice i.g. administered PBS [DSS (+): 4.4 (4.2-9.5) ng/mL vs 83.9 (66.1-123.2) ng/mL, P < 0.01; DSS (-): 27.7 (0.1-54.5) ng/mL vs 116.5 (80.6-213.6) ng/mL, P < 0.01] (Figure 2). These results demonstrated that oral tolerance was inducible with or without colitis.
As indicated above, oral tolerance was inducible with or without colitis. We hypothesized that MLN and spleen populations of CD4+CD25+Foxp3+ cells, which are involved in oral tolerance, remained stable regardless of the presence of colitis, although the colitis mice did exhibit epithelial defects and inflammatory cell infiltration into the colonic mucosa. In the spleen, the frequency of CD4+CD25+ cells among CD4+ T cells and the frequency of CD4+Foxp3+ cells among CD4+ T cells were 5.6% (5.35%-5.75%) for the control mice and 6.6% (5.4%-6.6%) for the DSS (+) mice (Figure 3B) and 8.0% (7.0%-8.6%) for the control mice and 7.9% (7.2%-8.5%) for the DSS (+) mice (Figure 3C), respectively. The CD4+Foxp3+ cells included both CD4+CD25+Foxp3+ T cell and CD4+CD25-Foxp3+ T cell populations. In the spleen, the frequencies of CD4+CD25+Foxp3+ cells and CD4+CD25-Foxp3+ cells among CD4+ T cells were 5.0% (4.4%-5.2%) for the control mice and 4.5% (4.5%-5.1%) for the DSS (+) mice (Figure 3D) and 3.0% (2.6%-3.4%) for the control mice and 3.4% (2.7%-3.4%) for the DSS (+) mice (Figure 3E), respectively. In the MLN, the frequencies of CD4+ CD25+ T cells among CD4+ T cells and CD4+Foxp3+ T cells among CD4+ T cells were 7.4% (6.8%-7.9%) for the control mice and 7.1% (4.9%-16.6%) for the DSS (+) mice (Figure 3G) and 9.4% (9.1%-9.8%) for the control mice and 12.2% (8.3%-20.4%) for the DSS (+) mice (Figure 3H), respectively. In the MLN, the frequencies of CD4+CD25+Foxp3+ cells and CD4+CD25-Foxp3+ cells among CD4+ T cells were 7.0% (6.6%-7.2%) for the control mice and 6.5% (4.5%-13.6%) for the DSS (+) mice (Figure 3I) and 2.6% (2.2%-2.8%) for the control mice and 5.7% (3.5%-7.1%) for the DSS (+) mice (Figure 3J), respectively. These findings demonstrate that CD4+CD25+ cell frequency among CD4+ T cells in the spleen tended to increase in colitis, while the frequencies of CD4+CD25+Foxp3+ cells and CD4+CD25-Foxp3+ cells among CD4+ T cells did not change. Our findings additionally revealed that CD4+CD25+Foxp3+ cell frequency among CD4+ T cells in the MLN did not change during colitis, while CD4+CD25-Foxp3+ Treg frequency increased significantly (Figure 3J, P < 0.05). Inflammatory cell infiltration into the colonic mucosa did not influence the stability of CD4+CD25+Foxp3+ T cell populations in the spleen, although CD4+ T cells in the spleen were activated during colitis. Moreover, inflammatory cell infiltration did not influence the stability of CD4+CD25+Foxp3+ T cell populations in the MLN; however, inflammatory cell infiltration increased the population of CD4+CD25-Foxp3+ cells in the MLN. These results suggest that the stability of CD4+CD25+Foxp3+ T cell populations in the spleen and MLN may play a role in oral tolerance induction in DSS colitis, and elevated numbers of CD4+CD25-Foxp3+ Tregs in the MLN may help sustain homeostasis during colitis.
B10 cells are associated with the induction of oral tolerance[8]. Consistent with the regulatory role of B cells, B cell-deficient mice are defective in the ability to develop oral tolerance[9]. We hypothesized that B10 cell populations, similarly to CD4+CD25+Foxp3+ cell populations, remain stable during colitis. Therefore, we examined whether any differences existed in CD19+IL-10+ cell populations in the spleens of mice with and without DSS colitis. The frequencies of CD19+IL-10+ cells among CD19+ cells and CD19+CD5+CD1dHi cells among CD19+ cells, as well as CD19+CD5+CD1dHi IL-10+ cells among CD19+CD5+CD1dHi cells were 0.8% (0.7%-1.2%) for the control mice and 1.4% (0.9%-1.9%) for the DSS (+) mice (Figure 4B), 1.5% (1.2%-2.5%) for the control mice and 2.4% (1.3%-2.8%) for the DSS (+) mice (Figure 4C), and 7.6% (6.4%-8.4%) for the control mice and 11.3% (6.9%-14.5%) for the DSS (+) mice (Figure 4D), respectively. The frequencies of CD19+IL-10+ cells in the spleens of the DSS colitis mice were comparable to those in the spleens of the control mice. CD19+CD5+CD1dHi cell frequency among CD19+ cells did not change during DSS colitis. However, IL-10+ cell frequency among CD19+CD5+CD1dHi cells tended to increase in the spleens of the DSS colitis mice relative to the control mice. These results suggest that DSS colitis may act either directly or indirectly to promote IL-10 production within CD19+CD5+CD1dHi cells from the spleen.
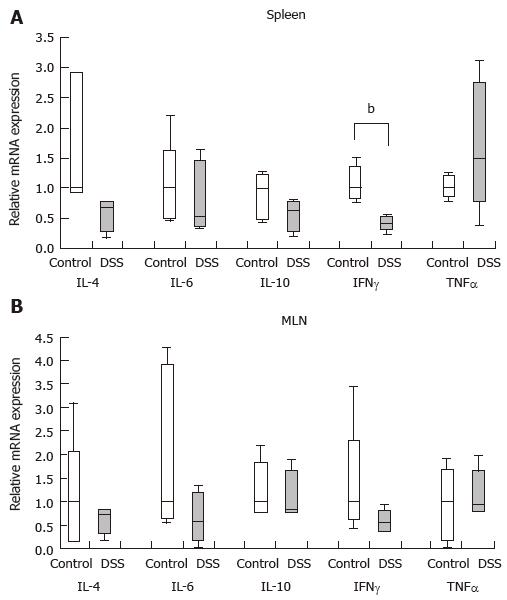
As indicated above, CD4+CD25+Foxp3+ Treg and B10 cell populations did not decrease during DSS colitis. We next investigated how the levels of cytokines, which influence the function and differentiation of Tregs and B10 cells, change during colitis. In the spleens of the mice with colitis, IFN-γ mRNA expression was significantly lower than that in the mice without colitis (P < 0.01) (Figure 5A). However, IFN-γ expression in the MLN were comparable between mice with and without colitis (Figure 5B). Additionally, there were no significant differences in IL-4, IL-6, IL-10 or TNF-α mRNA expression in the spleens or MLNs of mice with and without colitis (Figure 5).
In the current study, we revealed that oral tolerance is inducible during the active phase of DSS colitis. We hypothesized that the MLN and spleen, both of which are involved in oral tolerance induction, maintained stability during colitis. We also investigated the manners in which cytokine levels and regulatory cell populations, such as those for Foxp3+ T cells and B10 cells, change during colitis.
Histologically, DSS colitis was characterized by epithelial defects and inflammatory cell infiltration. In previous studies, DSS colitis has been shown to exhibit a Th1-predominant profile[24] or a Th1-Th17-predominant profile within the colonic mucosa[25]. During the acute phase of DSS colitis, no differences were found in Foxp3 mRNA expression in colonic tissues from DSS colitis mice and normal mice; however, during the chronic phase, Foxp3 mRNA expression increased[12]. In the current study, we revealed that CD4+CD25+ cell frequency among CD4+ T cells in the spleen tended to increase during colitis, while CD4+Foxp3+ cell frequency among CD4+ T cells did not change. CD25 is known as an activation marker. These results suggest that DSS colitis activated CD4+ cells in the spleen while sustaining the stability of CD4+Foxp3+ cell populations in the spleen. We further revealed that CD4+CD25+ cell frequency among CD4+ T cells in the MLN did not change during colitis, while CD4+Foxp3+ cell frequency among CD4+ T cells tended to increase. In previous studies, CD4+Foxp3+ Treg frequencies in the MLNs and spleens of mice with colitis were lower than those in mice without colitis[13]. The target organs and mouse species assessed, as well as the concentrations of DSS, evaluation timing and evaluation methodology used may explain the differences in these results.
CD4+Foxp3+ T cells include CD4+CD25+Foxp3+ cell and CD4+CD25-Foxp3+ cell populations. The function of CD4+CD25-Foxp3+ Tregs remains unclear. One previous study indicated that CD4+CD25-FoxP3+ Tregs act similarly to conventional Tregs to a certain extent[26]; however, another study demonstrated that CD4+CD25-Foxp3+ Tregs differ from CD4+CD25+ Tregs both phenotypically and functionally[27,28]. We revealed that mice with and without colitis had comparable frequencies of CD4+CD25+Foxp3+ Tregs within the MLN, while CD4+CD25-Foxp3+ Treg frequency significantly increased during colitis. CD4+CD25-FoxP3+ Tregs may retain a suppressive function in an inflammatory environment[28]. Taken together, the above data indicate that CD4+CD25-Foxp3+ Tregs may play roles in maintaining homeostasis in the MLN and in inducing oral tolerance during DSS colitis.
Recently, Bregs have been shown to play an important role in oral tolerance in addition to Tregs. Allergen-specific, IL-10-producing B cells are involved in the development of tolerance to food allergens[8]. The proportion of IL-10-producing B cells following antigen stimulation was shown to decrease in an allergy group, whereas it increased in a tolerant group[29]. We hypothesized that, similarly to CD4+CD25+Foxp3+ cell populations, B10 cell populations remain stable during colitis. The frequencies of CD19+IL-10+ cells in the spleens of DSS colitis mice were comparable to those in the spleens of control mice. CD19+CD5+CD1dHi is the predominant source of IL-10 production[30]. CD19+CD5+CD1dHi cell frequency among CD19+ cells did not change during DSS colitis; however, CD19+CD5+CD1dHiIL-10+ cell frequency within the CD19+CD5+CD1dHi cell population tended to increase. These results suggest that DSS colitis may act either directly or indirectly to promote IL-10 production from CD19+CD5+CD1dHi cells in the spleen. B10 cells have also been shown to inhibit intestinal injury in DSS colitis mice[31]. A previous study showed that B10 cell populations did not decrease in the spleen during DSS colitis, similar to the present results[32]. Both the current study and the referenced study suggest that DSS colitis does not decrease B10 cell frequency, inhibit B cell IL-10 production, or inhibit B10 cell functions associated with oral tolerance. However, there were limitations associated with our analysis of B10 cells. Whole cells from the spleen were stimulated with LPS, PMA and ionomycin. Thus, it is not possible to exclude the effects of cells other than B cells on Bregs.
Cytokines can influence Treg function[33] and Breg differentiation[34,35]. As indicated above, the frequencies of CD4+CD25+Foxp3+ Tregs and B10 cells did not decrease during DSS colitis. We therefore investigated how cytokines, which influence the functions of Tregs and B10 cells, change during colitis.
IFN-γ appears to play an important role in food allergen tolerance induction. Specific oral immunotherapy using IFN-γ may induce tolerance induction in both IgE-mediated[36,37] and non-IgE-mediated food allergies[37]. IFN-γ can both promote and subvert Treg suppressive activity in various settings, and the balance between these opposing functions likely depends on contextual factors, such as the timing and extent of the expression[33]. IFN-γ induces murine CD5+ B1 cells to adopt a macrophage-like morphology. Macrophage-like B1 cells express high levels of CD5[38]. Moreover, IFN-γ induces allergen-specific B10 responses and promotes tolerogenic function[29,34]. In previous studies, during the acute phase of DSS colitis, colonic Th cells have been shown to exhibit a Th1 profile, rather than a Th2 or Th17 profile[24]. Moreover, DSS colitis leads to a Th1-Th17 response during its active phase[25]. In the current study, we revealed that IFN-γ mRNA expression was reduced in the spleens of mice with DSS, while its expression did not change in the MLN. This change in IFN-γ mRNA expression in the spleen did not influence oral tolerance, whereas the stability of IFN-γ expression in the MLN may have influenced the induction of oral tolerance.
IL-4 inhibits Treg function[33]. IL-4 receptor signaling has been shown to impair the capacity of Tregs to suppress mast cell activation and expansion, which in turn drives Th2-cell reprogramming of Tregs[39]. Moreover, IL-4 inhibits mouse CD5+ B1 cells from adopting a macrophage-like morphology[38]. In previous studies, DSS colitis mice have not exhibited increased IL-4 production from colonic T cells[24]. Similarly, in the current study, we revealed that DSS colitis mice did not exhibit increased IL-4 mRNA expression in either the spleen or MLN. This stability of IL-4 mRNA expression in the spleen and MLN may influence oral tolerance induction.
IL-6 subverts Treg cell function[33] and is essential for the differentiation of IL-10-producing B cells. Bregs are induced by the gut microbiota; this induction is driven by IL-6 production[35]. During both the acute and chronic phases of DSS colitis, serum IL-6 concentrations increase[25]. In the current study, we revealed that IL-6 mRNA expression was stable in the spleens and MLNs of mice with DSS colitis. This stability may influence oral tolerance induction.
IL-10 signaling is required to maintain Treg and Breg functions. IL-10 exhibits anti-inflammatory effects in part through its regulation of Treg stability and function both under steady-state conditions and during inflammation[33]. Autocrine stimulation of IL-10 is critical toward enriching IL-10 production in CD40HiCD5+ Bregs both in vitro and in vivo[40]. In DSS colitis, serum IL-10 levels have been shown to remain stable during the acute phase, whereas these levels increase during the chronic phase[25]. In the current study, we revealed that IL-10 mRNA expression was stable in the spleens and MLNs of mice with DSS colitis. This stability may influence oral tolerance induction.
TNF-α can both promote and subvert Treg cell function[34]. In DSS colitis, serum TNF-α concentrations increase during the acute phase but remain stable during the chronic phase[25]. We revealed that TNF-α mRNA expression was stable in the spleens and MLNs of mice with DSS colitis. This stability may influence oral tolerance induction.
In our cytokine analysis, IFN-γ expression decreased in the spleens of mice with DSS colitis, whereas IFN-γ expression in the MLN and IL-4, IL-6, IL-10 and TNF-α expression in both the spleen and the MLN remained stable. The cytokine profiles associated with DSS colitis may help to maintain the function and differentiation of Tregs and Bregs, which in turn are associated with oral tolerance. However, it should be noted that we only evaluated cytokine mRNA expression and not cytokine production.
Oral immunotherapy has been utilized for various immune disorders. However, oral immunotherapy is considered only poorly effective for IBD because IBD patients have dysfunctional oral tolerance[14,15]. One mechanism underlying this dysfunction is small intestinal permeability. In an IL-10 knock out model, increasing small intestinal permeability was shown to prevent the development of oral tolerance[41]. Several studies have also shown that a defect in intestinal epithelial permeability may be involved in the pathogenesis of IBD. Supporting this concept, other studies have shown that increased intestinal permeability precedes the onset of colitis in experimental animal models of IBD[41-43]. Conversely, IBD family members with no clinical symptoms exhibit dysfunctional oral tolerance, although small intestinal permeability is within the normal range. Thus, other genetic backgrounds are likely involved in dysfunctional oral tolerance[44]. In previous reports, the absence of functional inducible nitric oxide synthase (iNOS) enhanced the efficacy of oral tolerance[45]. Conversely, nitric oxide (NO) and iNOS production were increased both in colonic tissues collected from IBD patients and in a DSS-induced colitis model[46,47]. These data suggest that dysfunctional oral tolerance in IBD patients might be due to NO induction in addition to inflammation.
Although the effectiveness of oral immunotherapy for CD patients has recently been reported[16,17], there are few reports regarding the use of oral immunotherapy for UC patients. DSS-induced colitis serves as an experimental animal model of UC[10,11]. In the present study, we used this DSS colitis model to explore the potential use of oral immunotherapy as a treatment during the active phase of UC. Oral administration of CEP prior to the onset DSS colitis has been shown to induce immune tolerance, downregulate the inflammatory immune response and alleviate DSS-induced colitis[18,19]. However, no reports have evaluated oral tolerance following the oral administration of CEP after DSS colitis has developed.
To the best of our knowledge, the current study is the first to demonstrate that oral tolerance is inducible during the active phase of DSS colitis. Lymphocytic infiltration into the large intestine mucosa associated with epithelial defects did not influence oral tolerance. In addition to that used here, there are many other mouse models of IBD available for use. Further research evaluating oral tolerance in these models is warranted prior to clinical translation. Our study suggests that the choice of an appropriate antigen will enhance the effectiveness of oral immunotherapy for the treatment of UC.
Oral immunotherapy is considered only poorly effective for inflammatory bowel disease (IBD) because IBD patients have dysfunctional oral tolerance. Although the effectiveness of oral immunotherapy for Crohn’s disease patients has recently been reported, there are few reports regarding the use of oral immunotherapy for ulcerative colitis (UC) patients. Dextran sulfate sodium (DSS) colitis serves as an animal model of UC. Oral administration of colon extract protein (CEP) prior to the onset of DSS colitis has been shown to alleviate colitis; however, the effectiveness of oral administration of CEP after the onset of DSS colitis has not been evaluated.
The purpose of this study was to investigate whether oral tolerance is inducible during the active phase of DSS colitis. Additionally, The authors determined how cytokine levels and regulatory cell populations, such as those of Foxp3+ T cells and B10 cells, which are associated with oral tolerance, change in colitis in the mesenteric lymph nodes and the spleen.
This study is the first to demonstrate that oral tolerance is inducible during the active phase of DSS colitis. Lymphocytic infiltration into the large intestine mucosa associated with epithelial defects did not influence oral tolerance. The frequency of CD4+CD25+Foxp3+ cells and B10 cells, which are associated with oral tolerance, did not change significantly. In the spleen, IFN-γ mRNA expression decreased in mice with colitis, but the expression levels of other cytokines did not significantly change. This stability in regulatory cell populations and the observed cytokine profiles might influence oral tolerance induction during DSS colitis.
This study suggests that if an appropriate antigen is chosen, then oral immunotherapy may be applicable for the treatment of UC.
Oral tolerance is a phenomenon in which systemic immunity is suppressed following the oral administration of antigens. Oral immunotherapy has been applied for various immune disorders.
The manuscript by Ino et al is an interesting study and first to demonstrate that oral tolerance is inducible in the active phase of DSS colitis. In addition the authors tried to make a link between oral tolerance and the numbers of Treg and B10 cells.
| 1. | Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 745] [Article Influence: 57.3] [Reference Citation Analysis (2)] |
| 2. | Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2593] [Cited by in RCA: 2577] [Article Influence: 143.2] [Reference Citation Analysis (0)] |
| 3. | Tedder TF. B10 cells: a functionally defined regulatory B cell subset. J Immunol. 2015;194:1395-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 242] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 4. | Oka A, Ishihara S, Mishima Y, Tada Y, Kusunoki R, Fukuba N, Yuki T, Kawashima K, Matsumoto S, Kinoshita Y. Role of regulatory B cells in chronic intestinal inflammation: association with pathogenesis of Crohn’s disease. Inflamm Bowel Dis. 2014;20:315-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | El Fassi D, Nielsen CH, Kjeldsen J, Clemmensen O, Hegedüs L. Ulcerative colitis following B lymphocyte depletion with rituximab in a patient with Graves’ disease. Gut. 2008;57:714-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Ardelean DS, Gonska T, Wires S, Cutz E, Griffiths A, Harvey E, Tse SM, Benseler SM. Severe ulcerative colitis after rituximab therapy. Pediatrics. 2010;126:e243-e246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Adel-Patient K, Wavrin S, Bernard H, Meziti N, Ah-Leung S, Wal JM. Oral tolerance and Treg cells are induced in BALB/c mice after gavage with bovine β-lactoglobulin. Allergy. 2011;66:1312-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Noh J, Lee JH, Noh G, Bang SY, Kim HS, Choi WS, Cho S, Lee SS. Characterisation of allergen-specific responses of IL-10-producing regulatory B cells (Br1) in Cow Milk Allergy. Cell Immunol. 2010;264:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Gonnella PA, Waldner HP, Weiner HL. B cell-deficient (mu MT) mice have alterations in the cytokine microenvironment of the gut-associated lymphoid tissue (GALT) and a defect in the low dose mechanism of oral tolerance. J Immunol. 2001;166:4456-4464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-249. [PubMed] |
| 11. | Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694-702. [PubMed] |
| 12. | Bento AF, Leite DF, Marcon R, Claudino RF, Dutra RC, Cola M, Martini AC, Calixto JB. Evaluation of chemical mediators and cellular response during acute and chronic gut inflammatory response induced by dextran sodium sulfate in mice. Biochem Pharmacol. 2012;84:1459-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Chen J, Xie L, Toyama S, Hünig T, Takahara S, Li XK, Zhong L. The effects of Foxp3-expressing regulatory T cells expanded with CD28 superagonist antibody in DSS-induced mice colitis. Int Immunopharmacol. 2011;11:610-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Kraus TA, Toy L, Chan L, Childs J, Mayer L. Failure to induce oral tolerance to a soluble protein in patients with inflammatory bowel disease. Gastroenterology. 2004;126:1771-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Kraus TA, Cheifetz A, Toy L, Meddings JB, Mayer L. Evidence for a genetic defect in oral tolerance induction in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:82-88; discussion 81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Margalit M, Israeli E, Shibolet O, Zigmond E, Klein A, Hemed N, Donegan JJ, Rabbani E, Goldin E, Ilan Y. A double-blind clinical trial for treatment of Crohn’s disease by oral administration of Alequel, a mixture of autologous colon-extracted proteins: a patient-tailored approach. Am J Gastroenterol. 2006;101:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Israeli E, Zigmond E, Lalazar G, Klein A, Hemed N, Goldin E, Ilan Y. Oral mixture of autologous colon-extracted proteins for the Crohn’s disease: A double-blind trial. World J Gastroenterol. 2015;21:5685-5694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Ye Y, Yue M, Jin X, Chen S, Li Y. The effect of oral tolerance on the roles of small intestinal intraepithelial lymphocytes in murine colitis induced by dextran sodium sulfate. Int J Colorectal Dis. 2012;27:583-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Yue M, Shen Z, Yu CH, Ye H, Ye YF, Li YM. Effects of appendectomy and oral tolerance on dextran sulfate sodium colitis. World J Gastroenterol. 2011;17:2437-2445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1294] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 21. | Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643-1652. [PubMed] |
| 22. | Ishikawa H, Tanaka K, Maeda Y, Aiba Y, Hata A, Tsuji NM, Koga Y, Matsumoto T. Effect of intestinal microbiota on the induction of regulatory CD25+ CD4+ T cells. Clin Exp Immunol. 2008;153:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Hong C, Gao XM. Purification and immunophenotypic characterization of murine B10 B cells. Methods Mol Biol. 2014;1190:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Kim YS, Lee MH, Ju AS, Rhee KJ. Th17 responses are not induced in dextran sodium sulfate model of acute colitis. Immune Netw. 2011;11:416-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 616] [Cited by in RCA: 637] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 26. | Bonelli M, Savitskaya A, Steiner CW, Rath E, Smolen JS, Scheinecker C. Phenotypic and functional analysis of CD4+ CD25- Foxp3+ T cells in patients with systemic lupus erythematosus. J Immunol. 2009;182:1689-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 27. | Iikuni N, Lourenço EV, Hahn BH, La Cava A. Cutting edge: Regulatory T cells directly suppress B cells in systemic lupus erythematosus. J Immunol. 2009;183:1518-1522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 28. | Tang Y, Peng LP, Qin GX, Sun JT, Xu LJ, Jiang YF. CD4(+)CD25(-)Foxp3(+) T cells play a role in tuberculous hydrothorax rather than malignant hydrothorax. J Transl Med. 2015;13:268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Lee SJ, Noh G, Lee JH. In Vitro Induction of Allergen-Specific Interleukin-10-Producing Regulatory B Cell Responses by Interferon-γ in Non-Immunoglobulin E-Mediated Milk Allergy. Allergy Asthma Immunol Res. 2013;5:48-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 264] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 31. | Yanaba K, Yoshizaki A, Asano Y, Kadono T, Tedder TF, Sato S. IL-10-producing regulatory B10 cells inhibit intestinal injury in a mouse model. Am J Pathol. 2011;178:735-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 32. | Wang L, Ray A, Jiang X, Wang JY, Basu S, Liu X, Qian T, He R, Dittel BN, Chu Y. T regulatory cells and B cells cooperate to form a regulatory loop that maintains gut homeostasis and suppresses dextran sulfate sodium-induced colitis. Mucosal Immunol. 2015;8:1297-1312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Smigiel KS, Srivastava S, Stolley JM, Campbell DJ. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunol Rev. 2014;259:40-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 34. | Noh J, Noh G, Lee SJ, Lee JH, Kim A, Kim HS, Choi WS. Tolerogenic effects of interferon-gamma with induction of allergen-specific interleukin-10-producing regulatory B cell (Br1) changes in non-IgE-mediated food allergy. Cell Immunol. 2012;273:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, Harris KA, Jones SA, Klein N, Mauri C. Regulatory B cells are induced by gut microbiota-driven interleukin-1β and interleukin-6 production. Nat Med. 2014;20:1334-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 381] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 36. | Noh G, Lee SS. A pilot study of interferon-gamma-induced specific oral tolerance induction (ISOTI) for immunoglobulin E-mediated anaphylactic food allergy. J Interferon Cytokine Res. 2009;29:667-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Lee JH, Noh G, Noh J, Lee S, Choi WS, Kim HS, Lee K, Choi S, Jin H, Cho S. Clinical characteristics of oral tolerance induction of IgE-mediated and non-IgE-mediated food allergy using interferon gamma. Allergy Asthma Proc. 2010;31:e39-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Koide N, Sugiyama T, Mori I, Mu MM, Hamano T, Yoshida T, Yokochi T. Change of mouse CD5(+) B1 cells to a macrophage-like morphology induced by gamma interferon and inhibited by interleukin-4. Clin Diagn Lab Immunol. 2002;9:1169-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, Rachid R, Chatila TA. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity. 2015;42:512-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 300] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 40. | Kim HS, Lee JH, Han HD, Kim AR, Nam ST, Kim HW, Park YH, Lee D, Lee MB, Park YM. Autocrine stimulation of IL-10 is critical to the enrichment of IL-10-producing CD40(hi)CD5(+) regulatory B cells in vitro and in vivo. BMB Rep. 2015;48:54-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Arrieta MC, Madsen KL, Field CJ, Meddings JB. Increasing small intestinal permeability worsens colitis in the IL-10-/- mouse and prevents the induction of oral tolerance to ovalbumin. Inflamm Bowel Dis. 2015;21:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Resta-Lenert S, Smitham J, Barrett KE. Epithelial dysfunction associated with the development of colitis in conventionally housed mdr1a-/- mice. Am J Physiol Gastrointest Liver Physiol. 2005;289:G153-G162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | Kraus TA, Toy L, Chan L, Childs J, Cheifetz A, Mayer L. Failure to induce oral tolerance in Crohn’s and ulcerative colitis patients: possible genetic risk. Ann N Y Acad Sci. 2004;1029:225-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Kahn DA, Archer DC, Kelly CJ. Absence of functional inducible NO synthase enhances the efficacy of tolerance induced by high dose antigen feeding. J Immunol. 2000;165:6116-6122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Martín MC, Martinez A, Mendoza JL, Taxonera C, Díaz-Rubio M, Fernández-Arquero M, de la Concha EG, Urcelay E. Influence of the inducible nitric oxide synthase gene (NOS2A) on inflammatory bowel disease susceptibility. Immunogenetics. 2007;59:833-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Beck PL, Xavier R, Wong J, Ezedi I, Mashimo H, Mizoguchi A, Mizoguchi E, Bhan AK, Podolsky DK. Paradoxical roles of different nitric oxide synthase isoforms in colonic injury. Am J Physiol Gastrointest Liver Physiol. 2004;286:G137-G147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Hokama A, Trifan A, Velin D S- Editor: Gong ZM L- Editor: A E- Editor: Wu HL