Published online Feb 6, 2016. doi: 10.4292/wjgpt.v7.i1.145
Peer-review started: July 30, 2015
First decision: September 14, 2015
Revised: October 9, 2015
Accepted: December 3, 2015
Article in press: December 4, 2015
Published online: February 6, 2016
Processing time: 192 Days and 0.9 Hours
AIM: To evaluate the efficacy of lower esophageal sphincter (LES)-electrical stimulation therapy (EST) in a subgroup of patients that reported only partial response to proton pump inhibitors (PPIs) therapy, compared to a group of patient with complete response.
METHODS: Bipolar stitch electrodes were laparoscopically placed in the LES and connected to an implantable pulse generator (EndoStim BV, the Hague, the Netherlands), placed subcutaneously in the anterior abdominal wall. Stimulation at 20 Hz, 215 μsec, 3-8 mAmp in 30 min sessions was delivered starting on day 1 post-implant. Patients were evaluated using gastroesophageal reflux disease (GERD)-HRQL, symptom diaries; esophageal pH and esophageal manometry before and up to 24 mo after therapy and results were compared between partial and complete responders.
RESULTS: Twenty-three patients with GERD on LES-EST were enrolled and received continuous per-protocol stimulation through 12 mo and 21 patients completed 24 mo of therapy. Of the 23 patients, 16 (8 male, mean age 52.1 ± 12 years) had incomplete response to PPIs prior to LES-EST, while 7 patients (5 male, mean age 52.7 ± 4.7) had complete response to PPIs. In the sub-group with incomplete response to PPIs, median (IQR) composite GERD-HRQL score improved significantly from 9.5 (9.0-10.0) at baseline on-PPI and 24.0 (20.8-26.3) at baseline off-PPI to 2.5 (0.0-4.0) at 12-mo and 0.0 (0.0-2.5) at 24-mo follow-up (P < 0.05 compared to on-and off-PPI at baseline). Median (IQR) % 24-h esophageal pH < 4.0 at baseline in this sub-group improved significantly from 9.8% (7.8-11.5) at baseline to 3.0% (1.9-6.3) at 12 mo (P < 0.001) and 4.6% (2.0-5.8) at 24 mo follow-up (P < 0.01). At their 24-mo follow-up, 9/11 patients in this sub-group were completely free of PPI use. These results were comparable to the sub-group that reported complete response to PPI therapy at baseline. No unanticipated implantation or stimulation-related adverse events, or any untoward sensation due to stimulation were reported in either group and LES-EST was safely tolerated by both groups.
CONCLUSION: LES-EST is safe and effective in controlling symptoms and esophageal acid exposure in GERD patients with incomplete response to PPIs. These results were comparable to those observed PPI responders.
Core tip: Proton pump Inhibitors (PPI) are the main medical therapy for gastroesophageal reflux disease (GERD). However, 30%-40% of patients are unsatisfied with PPI therapy. Traditional antireflux surgery is effective but is associated with adverse effects and its numbers are declining, resulting in an unmet need for alternative therapies. Electrical stimulation therapy (EST) of the LES has been shown to significantly improve GERD symptoms and esophageal acid exposure in patients with GERD. The current study shows that patients who respond to PPI but are concerned about the drugs, as well as those with incomplete response to PPI respond equally to EST of the LES.
- Citation: Soffer E, Rodríguez L, Rodriguez P, Gómez B, Neto MG, Crowell MD. Effect of electrical stimulation of the lower esophageal sphincter in gastroesophageal reflux disease patients refractory to proton pump inhibitors. World J Gastrointest Pharmacol Ther 2016; 7(1): 145-155
- URL: https://www.wjgnet.com/2150-5349/full/v7/i1/145.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i1.145
Gastroesophageal reflux disease (GERD) is a common and widespread condition[1-3], impacting patients’ wellbeing and driving health care cost[4]. Acid suppression agents and surgical therapy with laparoscopic Nissen fundoplication have been shown to significantly improve the care of many patients with GERD[5]. However, acid suppression therapy targets acid secretion rather than a dysfunctional lower esophageal sphincter and reflux, with inadequate control of symptoms resulting in poor quality of life in approximately 30%-40% of patients with GERD[6]. While fundoplication is effective in expert hands, results are inferior in community centers as compared to high volume ones[7], and the intervention can be associated with significant long-term adverse effects including dysphagia and gas bloat[8]. These limitations have led to a decline of traditional anti-reflux procedures and to a search for alternative treatment modalities of GERD[9].
Lower esophageal sphincter (LES)-electrical stimulation therapy (EST) has been shown to increase resting LES pressure in both acute and chronic animal models[10-12]. Short term LES-EST, using temporary leads implanted in the LES of subjects with GERD has confirmed these results and demonstrated a significant enhancement in LES tone without impairing swallow-induced LES relaxation and without inducing any adverse sensation[13,14]. These results suggested that LES-EST might be an effective method of restoring the anti-reflux function of the LES in GERD patients and led to the development of the EndoStim® LES Stimulation System for treatment of GERD.
We have previously reported the safety and efficacy of 1 and 2-year LES-EST therapy in patients with GERD enrolled in an open-label, single -enter trial, using a permanently implanted stimulation system[15,16]. The objectives of this post-hoc analysis of this open-label human trial were to compare the effects of LES-EST on GERD symptoms and medication use between GERD patients with partial response to daily PPI medications and those reporting a complete response at their baseline evaluation. Additionally, esophageal acid exposure, esophageal motor function and healing of erosive esophagitis, evaluated at 12 and 24 mo of LES-EST, were compared between the groups.
This is a post-hoc analysis of a prospective, single-center, open-label, and treatment only trial that evaluated the safety and efficacy of LES stimulation for the treatment of GERD. The objective of this post-hoc analysis was to assess the response to LES-EST in a sub-group of patients with incomplete response to PPIs, defined as bothersome symptoms of heartburn at least 1 d a week while ON-PPIs[17] and compared to the sub-group of complete responders. The incidence of serious device- and/or procedure-related adverse effects (AEs) was the primary safety endpoint of the trial, while the incidence of non-serious device- and procedure-related AEs was the secondary safety endpoint. The primary efficacy endpoint was a reduction in the GERD-HRQL composite score on LES-EST compared with baseline score while on and OFF-PPIs. Additional efficacy endpoints included improvement in esophageal acid exposure, GERD symptoms reported in daily symptom diaries and medication use on LES-EST compared with baseline, and improvement in esophagitis grade and mean respiratory and end-expiratory LES pressures.
The open-label trial was approved by the Servicio de Salud Metropolitano Oriente, Santiago, Chile ethics committee and all subjects signed informed consent.
All patients enrolled in the trial suffered from chronic GERD symptoms, were not satisfied with their medical therapy or were concerned about long-term acid suppression therapy, and contemplated surgical intervention for GERD. Key inclusion criteria included subjects between 21 and 65 years of age with a history of heartburn, regurgitation or both for > 6 mo prompting physician recommendation of chronic daily use of PPI before study entry, and a baseline GERD-HRQL heartburn score of ≥ 20 OFF-PPI with a symptomatic response to a course of GERD therapy (≥ 2 wk) and a GERD-HRQL heartburn score improvement of ≥ 10 on therapy. Subjects had to exhibit excessive esophageal acid exposure during 24-h pH-measurement off antisecretory therapy defined as pH < 4 for ≥ 5% of total or ≥ 3% of supine time. Exclusion criteria were: A resting LES end expiratory pressure ≤ 5mm Hg on a high resolution manometry; esophageal body contraction amplitude ≤ 30 mmHg for ≥ 70% of swallows; ≤ 50% peristaltic contractions on high resolution manometry; esophagitis > Grade C (LA classification) on upper endoscopy performed within 6 mo prior to enrollment; Barrett’s epithelium (> M2; > C1) with any grade of dysplasia; hiatus hernia greater than 3 cm; BMI > than 35 kg/m2; uncontrolled Type 2 diabetes mellitus (T2DM) defined as HbA1c > 9.5 in the previous 6 mo; a history of T2DM for > 10 years or Type 1 diabetes mellitus. Detailed inclusion, exclusion criteria and study details have been previously reported[15,16].
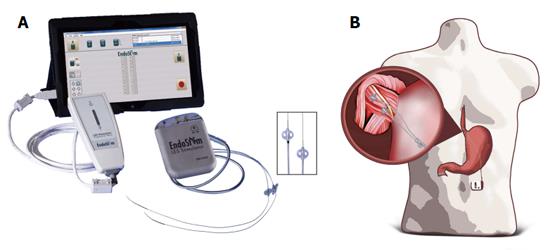
The LES stimulation system is similar to traditional neurostimulators with three components: a bipolar stimulation lead with two stitch electrodes, an implantable pulse generator (IPG) and an external programmer (Figure 1A), and the details were described previously[16]. In brief, the IPG and stimulation lead were all implanted by laparoscopy, using 4-5 ports. The anterior right aspect of the abdominal esophagus was exposed through dissection of the paraesophageal fat and pars flaccida of the hepatogastric ligament. A rectangular longitudinal area of approximately 3 cm × 1 cm is needed in which the electrodes are implanted. This approach minimized dissection of the phreno-esophageal attachment and damage to the anterior vagal nerve. The 2 stitch electrodes were implanted via a superficial bite into the LES muscle along the main esophageal axis with approximately 10 mm between the electrodes. Each electrode was then secured by a clip on the proximal edge of the electrode on to the nylon suture wire and also by suturing the distal anchoring ‘‘butterfly’’ present on the back end of the electrode. Upper gastrointestinal endoscopy was performed to verify electrode position in the LES and to confirm that no perforation of the esophageal lumen had occurred with the needle or electrode. The lead delivered through the abdominal wall and secured to the IPG located in a subcutaneous pocket in the left upper quadrant (Figure 1B). Interrogation and programming of the IPG were provided via a wireless external programmer and computer software.
The LES stimulation system delivers therapy personalized to individual patient needs. The stimulation pulse is monophasic followed by a charge-balancing phase. The pulse is 215 μsec wide and nominally 5 mA in amplitude (range 3-8 mA). The stimulation pulse is delivered at a rate of 20 Hz and continues for a period of 30 min. Up to twelve 30-min sessions per day were delivered. Electrical stimulation could be optimized using the external programmer to tailor therapy to individual patients’ needs. Therapy could be adjusted at follow-up to address residual symptoms or acid events seen on pH testing by altering stimulation parameters such as number or timing of stimulation sessions, electrode polarity and stimulation amplitude.
After signing an informed consent, symptoms were assessed while on-PPIs and after a period of 2 wk off-PPIs. High resolution esophageal manometry and ambulatory esophageal pH test were performed 2 wk after being OFF-PPIs. Data on GERD medication usage was recorded. Patients fulfilling entry criteria underwent a laparoscopic LES stimulation system implant procedure, EST was started immediately post-procedure and PPI therapy was discontinued. Patients were allowed to use antacid or antisecretory medications for control of breakthrough symptoms on LES-EST. Patients were evaluated at regular intervals after implantation per-protocol.
GERD symptoms were assessed by the validated GERD-Health Related Quality of Life (GERD-HRQL) questionnaire[17], which provides a composite score based on the frequency and severity of symptoms. Patients’ GERD-HRQL scores were assessed at baseline while ON-PPI therapy and at 10-14 d off-PPI, before initiation of LES-EST and at follow-up periods of 1, 3, 6, 12, 15, 18 and 24 mo. Symptoms of heartburn and regurgitation and medication use were assessed by 14-d daily diary, and overall quality of life was assessed by the SF-12 Physical and Mental Health Surveys at each follow-up time point. High resolution esophageal manometry at baseline was performed using the Medical Measurement System (MMS, Dover, NH) in 6 patients and the Sierra Scientific Instruments system (Given Imaging, Los Angeles, California) in 18 patients. Due to equipment availability, all 12-mo manometries were performed with the MMS system.
Esophageal acid exposure was assessed with 24-h esophageal pH-metry at baseline and at 3, 6, and 12 and 24 mo of follow-up with patients off-PPI for at least 5 d (AL1 Sistema de pH-Metria, Ver 1.26, Alacer Biomedica, Brazil).
The degree of esophagitis was assessed by upper endoscopy performed within 6 mo prior to enrollment, and at 12-mo follow-up.
Safety evaluation was descriptive and included the incidence, severity, and type of AEs, and clinically significant changes or abnormalities in each patient’s physical examination, vital signs, clinical tests and EKG results, for patients in both sub-groups. All reported adverse events were adjudicated by an independent Data Monitoring Committee for relatedness to the procedure, device and/or therapy.
The effect of LES-EST on patient symptoms was assessed by comparing patients’ GERD-HRQL at 12 and 24-mo follow-up with baseline scores (both on and 2-wk off-PPI). Frequency and severity of symptoms and medication use were assessed by 14 d daily diary and compared between 12 and 24-mo follow-up and baseline. The impact of GERD symptoms on global quality of life measured by SF-12 was also compared at 12 and 24-mo follow-up vs baseline.
Esophageal acid exposure was expressed as the proportion of time during 24-h pH-metry with distal esophageal pH < 4.0. Esophageal acid exposure was compared between baseline and 12 and 24 mo follow-up. A reviewer (MDC), blinded to all identifying patient and visit data, independently analyzed all pH data. Manometry was performed as described above. Due to the change in equipment only descriptive manometry findings are presented. Esophagitis, if present by endoscopy, was classified using the Los Angeles (LA) Classification scheme.
Data were analyzed and presented as mean ± SD, or median and quartiles. All comparisons between the two groups and changes from baseline were made at the P <0.05 level using related-samples Wilcoxon Sign Rank test (for continuous or scale measures) or McNemar’s test (for categorical measures), SAS version 9.4 (SAS Institute, Cary, NC, United States).
Twenty-five patients were enrolled in the LES stimulation study. One patient withdrew consent 2 wk post-implant due to the demanding nature of the protocol and underwent an uneventful explant of the stimulator 6 wk after implantation. One patient quit the study for an elective surgical procedure for control of diabetes. Twenty three and 21 patients respectively completed the 1 and 2 years follow up.
Of the 23 patients that were enrolled in the study, a subgroup of 16 patients (8 male, mean age 52.1 ± 12 years) reported incomplete response to PPI therapy and 7 patients (5 male, mean age 52.7 ± 4.7) reported complete response to PPI therapy prior to LES-EST. All patients completed 12 mo follow up, while 14/16 incomplete responders and all 7 complete responders completed the 24 mo follow up.
Patients with incomplete response had a mean duration of GERD of 12.9 ± 9.0 years and all were on chronic PPI therapy prior to implantation for a mean duration of 6.3 ± 3.4 years (QD = 12, BID = 4). Patients with complete response had a mean duration of GERD of 8.6 ± 4.3 years and all were on chronic PPI therapy prior to implantation for a mean duration of 4.7 ± 3.4 years (QD = 6, BID = 1). Baseline characteristics and signal optimization were comparable among the two groups.
During the 24 mo following implant 11 out of the 16 patients who were incomplete responders to PPI reported 28 AEs. One serious not related adverse event was reported; a diagnosis of nodular thyroid disease requiring hospitalization and surgery. The remaining 27 AEs were non-serious. Two events in 1 patient were reported as probably or possibly related to the device or the laparoscopic implant procedure (implant site pain = 1, abdominal pain and excess salivation = 1). Additionally, 1 event (post-operative nausea/vomiting) in 1 patient was reported as possibly procedure related, which was resolved with medication.
During the 24 mo following implant, 6 out of the 7 patients who were complete responders to PPI reported 25 AEs. One not related serious adverse event was reported; an episode of non-cardiac chest pain not related to LES stimulation. Similar episodes were experienced by the patient prior to starting LES-EST. The patient was hospitalized and cardiac evaluation, including cardiac catheterization, was normal. Chest X-ray revealed stable lead position without any evidence of migration. The episode resolved spontaneously and stimulation was restarted. One event in one patient was reported as probably device related (implant site pain = 1) and 6 events were procedure related (implant site pain = 1, post-op nausea = 2, hypertensive crisis = 1, acute shoulder pain = 1, localized infection = 1).
None of the patients in either group reported any GI side effects of bloating, inability to belch or new dysphagia associated with LES-EST. Rates of AE/SAE were comparable among the two groups.
GERD symptoms improved significantly in both groups following treatmet and improvement persisted over the next 2 years. There was a significant reduction of GERD-HRQL composite and individual symptom scores compared to baseline (Table 1).
| Incomplete responders | Complete responders | |||||||
| Visit interval(subject number) | Median (IQR) | P value | Visit interval(subject number) | Median (IQR) | P value | |||
| Baseline ON-PPI | Baseline OFF-PPI | Baseline ON-PPI | Baseline OFF-PPI | |||||
| GERD-HRQL scores | Baseline on PPI (16) | 9.5 (9-10) | 0.012 | Baseline ON PPI (7) | 4 (2.5-6) | 0.13 | 0.02 | |
| Baseline off PPI (16) | 24 (20.8-26.3) | 0.0013 | < 0.001 | Baseline OFF PPI (7) (16) | 21 (21-23) | 0.40 | 0.02 | |
| 12 mo (16) | 2.5 (0-4) | 0.0011 | 12 mo (7) | 1 (0-2.5) | ||||
| 24 mo (14) | 0 (0-2.8) | 24 mo (7) | 0 (0-3) | |||||
| Percent of 24-h esophageal pH < 4.0 | Baseline off PPI (16) | 9.8 (7.8-11.5) | < 0.001 | Baseline OFF PPI (7) | 16.7 (7.5-17.8) | 0.09 | ||
| Total | 12 mo (16) | 3.0 (1.9-6.3) | 0.003 | 12 mo (6) | 3.5 (2.1-6.1) | 0.12 | ||
| 24 mo (13) | 4.6 (1.6-5.1) | 24 mo (5) | 7.5 (5.1-12.6) | |||||
| Percent 24-h esophageal pH < 4.0 | Baseline off PPI (16) | 9.4 (7.3-13.6) | < 0.001 | Baseline OFF PPI (7) | 10.4 (10.1-15.2) | 0.03 | ||
| Upright | 12 mo (16) | 3.9 (3.1-5.2) | < 0.001 | 12 mo (6) | 5.0 (2.3-7.8) | 0.06 | ||
| 24 mo (13) | 5.5 (1.8-7.2) | 24 mo (5) | 5.0 (4.3-6.8) | |||||
| % 24-h esophageal pH < 4.0 | Baseline off PPI (16) | 5.8 (1.7-10.3) | 0.10 | Baseline OFF PPI (7) | 8.3 (3.2-22) | 0.30 | ||
| Supine | 12 mo (16) | 0.4 (0.0-5.9) | 0.56 | 12 mo (6) | 1.0 (0.0-3) | 1.00 | ||
| 24 mo (13) | 0.5 (0.2-4.1) | 24 mo (5) | 11.1 (0.6-18.9) | |||||
| DeMeester score | Baseline off PPI (16) | 34.8 (29.6-43.8) | < 0.001 | Baseline OFF PPI (7) | 67 (31.9-70.2) | 0.09 | ||
| 12 mo (16) | 10.8 (5.9-28.3) | 0.02 | 12 mo (6) | 17.6 (10.2-24.5) | 0.13 | |||
| 24 mo (13) | 15.4 (8-20) | 24 mo (5) | 30.0 (18.1-54.5) | |||||
| Percent patients with abnormal distal esophageal pH (pH < 4.0 for > 4%) | Baseline off PPI (16) | 94% | 0.008 | Baseline OFF PPI (7) | 100% | 0.13 | ||
| 12 mo (16) | 38% | 0.021 | 12 mo (6) | 33% | 0.33 | |||
| 24 mo (13) | 54% | 24 mo (5) | 80% | |||||
| Percent 24-h proximal esophageal pH < 4.0 | Baseline off PPI (16) | 0.2 (0.1-1.2) | 0.006 | Baseline OFF PPI (7) | 0.6 (0.4-2) | 0.06 | ||
| 12 mo (16) | 0.0 (0-0) | 0.006 | 12 mo (6) | 0.0 (0-0.1) | 0.13 | |||
| 24 mo (13) | 0.0 (0-0) | 24 mo (5) | 0.0 (0-0.1) | |||||
| Percent 24-h proximal esophageal pH < 4.0 | Baseline off PPI (16) | 0.4 (0.2-1.9) | 0.006 | Baseline OFF PPI (7) | 1.1 (0.6-2.6) | |||
| 12 mo (16) | 0.0 (0-0) | 0.008 | 12 mo (6) | 0.0 (0-0.1) | 0.06 | |||
| 24 mo (13) | 0.0 (0-0.1) | 24 mo (5) | 0.0 (0-0.1) | 0.13 | ||||
| Percent 24-h proximal esophageal pH < 4.0 | Baseline off PPI (16) | 0.0 (0-0.1) | 0.20 | Baseline OFF PPI (7) | 0.1 (0.0-1.3) | 0.37 | ||
| Supine | 12 mo (16) | 0.0 (0-0) | 0.37 | 12 mo (6) | 0.0 (0-0) | 0.37 | ||
| 24 mo (13) | 0.0 (0-0) | 24 mo (5) | 0.0 (0-0) | |||||
| Percent patients with abnormal proximal esophageal pH (pH < 4.0 for > 1.1%) | Baseline OFF PPI (16) | 29% | 0.13 | Baseline OFF PPI (7) | 33% | 0.50 | ||
| 12 mo (16) | 0% | 0.18 | 12 mo (6) | 0% | 0.14 | |||
| 24 mo (13) | 0% | 24 mo (5) | 0% | |||||
| LES end expiratory pressure (mmHg) | Baseline OFF PPI (16) | 8.2 (6.8-9.8) | Baseline OFF PPI (7) | 8.8 (5.7-10.5) | ||||
| 12 mo (16) | 12 (7.8-18) | 12 mo (6) | 9.5 (4.5-14.5) | |||||
| 24 mo (13) | 6 (4-10) | 24 mo (5) | 6 (4-9) | |||||
| LES respiratory mean pressure (mmHg) | Baseline OFF PPI (16) | 17.2 (16.1-20.9) | Baseline OFF PPI (7) | 2 (1.9-2.5) | ||||
| 12 mo (16) | 12 (17.5-29) | 12 mo (6) | 2.7 (2.2-3.0) | |||||
| 24 mo (13) | 19 (16-22) | 24 mo (5) | 2.5 (2.4-2.6) | |||||
| LES length (cm) | Baseline off PPI (16) | 2.1 (1.8-2.6) | Baseline OFF PPI (7) | 2.0 (1.9-2.5) | ||||
| 12 mo (16) | 2.6 (2.3-2.9) | 12 mo (6) | 2.7 (2.2-3) | |||||
| 24 mo (11) | 2.3 (2.0-2.5) | 24 mo (5) | 2.5 (2.4-2.6) | |||||
| Percent peristaltic swallows | Baseline OFF PPI (16) | 100 (95-100) | Baseline OFF PPI (7) | 100 (98-100) | ||||
| 12 mo (16) | 100 (91-100) | 12 mo (6) | 98 (70-100) | |||||
| 24 mo (11) | 100 (85-100) | 24 mo (5) | 86 (85-100) | |||||
| Distal esophageal contraction amplitude (mmHg) | Baseline OFF PPI (16) | 78.3 (67-95) | Baseline OFF PPI (7) | 46.1 (39.9-54.5) | ||||
| 12 mo (16) | 71.5 (48.5-80.5) | 12 mo (6) | 40.5 (34.5-46.5) | |||||
| 24 mo (11) | 53.0 (42.5-69.5) | 24 mo (5) | 45.0 (41.0-53.0) | |||||
| Number of esophageal contractions > 30 mmHg | Baseline OFF PPI (16) | 18 (14-20) | Baseline OFF PPI (7) | 19 (11.5-19.5) | ||||
| 12 mo (16) | 19.5 (18.5-20.0) | 12 mo (6) | 16 (9.3-19.8) | |||||
| 24 mo (11) | 18.0 (8.0-20.0) | 24 mo (5) | 11.0 (6.0-15.0) | |||||
| Residual LES pressure (mmHg) | Baseline OFF PPI (16) | 6.5 (2.9-10.9) | Baseline OFF PPI (7) | 8.9 (7.4-10.9) | ||||
| 12 mo (16) | 8.0 (4.0-11.0) | 12 mo (6) | 7.0 (1.5-8.0) | |||||
| 24 mo (11) | 7.0 (4.5-11.5) | 24 mo (5) | 5.0 (4.0-10.0) | |||||
| SF-12 PCS | Baseline ON PPI (16) | 45 (41.5-49.5) | 0.38 | 0.28 | Baseline ON PPI (7) | 50 (46-52.5) | 0.34 | 0.03 |
| Baseline OFF PPI (16) | 46.5 (41.2-53) | 0.004 | 0.002 | Baseline OFF PPI (7) (16) | 45 (40.5-47) | 0.09 | 0.02 | |
| 12 mo (16) | 51.0 (42.8-55.2) | 12 mo (7) | 55 (47-55) | |||||
| 24 mo (14) | 54.5 (52.2-56.5) | 24 mo (7) | 55 (53-58.5) | |||||
| SF-12 MCS | Baseline ON PPI (16) | 49 (42.5-59) | 0.68 | 0.18 | Baseline ON PPI (16) | 43.0 (40.5-47.5) | 0.38 | 0.83 |
| Baseline OFF PPI (16) | 45 (36.5-54.2) | 0.01 | 0.002 | Baseline OFF PPI (16) | 54 (51-55.5) | 0.61 | 0.16 | |
| 12 mo (16) | 52.5 (43.8-58.0) | 12 mo (16) | 50 (47.5-53.5) | |||||
| 24 mo (14) | 60.5 (54.5-62) | 24 mo (14) | 44 (38.5-50) | |||||
Composite GERD-HRQL scores are presented in Table 1. Median (IQR) scores of the incomplete responders patients at baseline on-and-OFF-PPI were 9.5 (9-10) and 24 (20.8-26.3) respectively. Scores significantly improved at both 12- and 24-mo of treatment to 2.5 (0-4) and 0 (0-2.5) respectively (P < 0.01). Median (IQR) composite GERD-HRQL scores for the sub-group of patients responding completely to PPI improved from 4 (2.5-6) at baseline on-PPI and 21 (21-23) at baseline off-PPI to 1 (0.0-2.5) and 0.0 (0.0-3.0) at 12- and 24-mo of treatment (P = 0.02). The percentage improvement in GERD-HRQL scores from baseline on-PPI and off- PPI scores was greater in the incomplete responder subgroup than the complete responder subgroup at both 12- and 24-mo of LES-EST.
Dysphagia In the incomplete responder group was reported in 14/16 and 6/16 patients at baseline off-PPI and on-PPI repectively, while only 2 patients reported dysphagia at 24-mo (P = 0.01 vs baseline). In the complete responder group, 7/7 patients reported dysphagia at baseline off-PPI and 2/7 reported dysphagia at baseline on-PPI, while only one patient reported dysphagia at 24-mo follow-up (P = 0.18 vs baseline on-PPI; P = 0.002 vs baseline off-PPI).
In the incomplete responder group, reflux affecting sleep was reported by 15/16 patients at baseline off-PPI and 13/16 patients on-PPI, while two patients reported reflux affecting sleep at 24-mo follow-up (P < 0.01 vs baseline). In the complete responder group, nocturnal reflux was reported by 7/7 patients at baseline off-PPI and 3/7 patients at baseline on-PPI, while one patient reported reflux affecting sleep at 24-mo follow-up (P = 0.05 vs baseline).
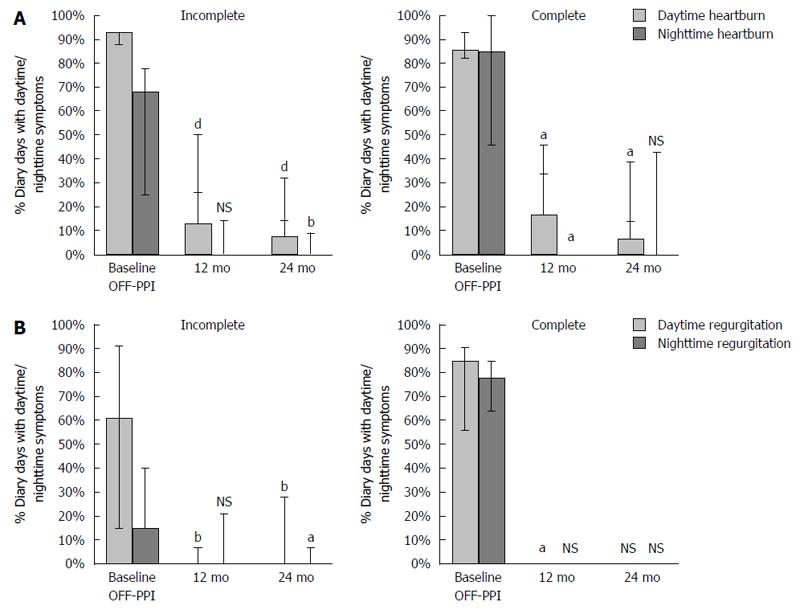
Fourteen-day symptom diaries evaluating heartburn and regurgitation symptoms were completed in 14 of the 16 patients in the incomplete responder subgroup and in 7 of 7 patients in the complete responder subgroup (Figure 2 and Table 2). Frequency and severity of heartburn and regurgitation, both during the day and night, improved significantly over time with LES-EST.
| Incomplete responders | Complete responders | |||||||
| Visit interval(subject number) | Median (IQR) | P value | Visit interval(subject number) | Median (IQR) | P value | |||
| Baseline ON-PPI | Baseline OFF-PPI | Baseline ON-PPI | Baseline OFF-PPI | |||||
| Percent diary days with heartburn severity | Baseline OFF PPI (14) | Baseline OFF PPI (6) | ||||||
| 12 mo (15) | < 0.001 | 12 mo (6) | 0.03 | |||||
| 24 mo (12) | < 0.001 | 24 mo (7) | 0.03 | |||||
| None | Baseline OFF PPI (14) | 0 (0-10) | Baseline OFF PPI (6) | 0 (0-14) | ||||
| 12 mo (15) | 88 (42-100) | 12 mo (6) | 83 (54-100) | |||||
| 24 mo (12) | 86 (73-100) | 24 mo (7) | 79 (32-93) | |||||
| Mild | Baseline OFF PPI (14) | 19 (14-29) | Baseline OFF PPI (6) | 14 (0-14) | ||||
| 12 mo (15) | 0 (0-32) | 12 mo (6) | 0 (0-25) | |||||
| 24 mo (12) | 7 (0-14) | 24 mo (7) | 7 (0-18) | |||||
| Moderate | Baseline OFF PPI (14) | 46 (31-66) | Baseline OFF PPI (6) | 62 (46-68) | ||||
| 12 mo (15) | 0 (0-7) | 12 mo (6) | 0 (0-11) | |||||
| 24 mo (12) | 0 (0-7) | 24 mo (7) | 7 (0-29) | |||||
| Baseline OFF PPI (14) | 11 (0-29) | Baseline OFF PPI (6) | 8 (4-21) | |||||
| Severe | 12 mo (15) | 0 (0-0) | 12 mo (6) | 0 (0-1) | ||||
| 24 mo (12) | 0 (0-0) | 24 mo (7) | 0 (0-4) | |||||
| % Diary days with regurgitation severity | Baseline OFF PPI (14) | Baseline OFF PPI (6) | 0.03 | 0.03 | ||||
| 12 mo (15) | < 0.003 | 12 mo (6) | ||||||
| 24 mo (12) | < 0.03 | 24 mo (7) | ||||||
| None | Baseline OFF PPI (14) | 35 (7-70) | Baseline OFF PPI (6) | 4 (0-8) | ||||
| 12 mo (15) | 100 (100-100) | 12 mo (6) | 100 (100-100) | |||||
| 24 mo (12) | 100 (95-100) | 24 mo (7) | 100 (86-100) | |||||
| Mild | Baseline OFF PPI (14) | 8 (7-20) | Baseline OFF PPI (6) | 19 (9-54) | ||||
| 12 mo (15) | 0 (0-0) | 12 mo (6) | 0 (0-0) | |||||
| 24 mo (12) | 0 (0-0) | 24 mo (7) | 0 (0-4) | |||||
| Moderate | Baseline OFF PPI (14) | 30 (4-57) | Baseline OFF PPI (6) | 29 (11-46) | ||||
| 12 mo (15) | 0 (0-0) | 12 mo (6) | 0 (0-0) | |||||
| 24 mo (12) | 0 (0-0) | 24 mo (7) | 0 (0-4) | |||||
| Baseline OFF PPI (14) | 0 (0-21) | Baseline OFF PPI (6) | 0 (0-16) | |||||
| Severe | 12 mo (15) | 0 (0-0) | 12 mo (6) | 0 (0-0) | ||||
| 24 mo (12) | 24 mo (7) | 0 (0-0) | ||||||
At 24 mo, 14/16 (88%) incomplete responders were completely OFF-PPI and only 2 patients (13%) were still using PPIs. At 24 mo, 4/7 (57%) patients in the responder sub-group were completely OFF-PPI and one (14%) patient used the medication occasionally (< 50% of diary days). Two out of seven (29%) used PPI regularly (100% of diary days).
Patients in both groups reported improvement of both SF-12 Physical Component Score and the SF-12 Mental Component Score over time, compared to baseline, and most scores were significantly improved (Table 1).
At baseline none of the patients in the incomplete responder group were satisfied with their condition, while at 12 mo 69% (11/16) were (P < 0.01). In the complete responder group all patients were satisfied at one year (7/7), as compared to only 1 patients satisfied at baseline (P = 0.12).
Variables of esophageal acid exposure improved in both groups, and significantly so in the subgroup of patients with incomplete response to PPI (Table 1). In the incomplete responder group, 24-h esophageal pH-metry was completed in all 16 patients at baseline and 12 mo, and 10 of the 16 patients at 24-mo who received continuous stimulation through 24-mo follow-up. Median (IQR) percent time with pH < 4.0 improved from 9.8% (7.8-11.5) at baseline to 3.0% (1.9-6.3) at 12 mo (P < 0.001) and 4.6% (2.0-5.8) at 24 mo (P < 0.01). Esophageal acid exposure was normalized in 7/10 (70%) of patients at their 24 mo follow-up. In the complete responder group, 24-h esophageal pH-metry was performed in all 7 patients at baseline, 6 out of 7 at 12 mo, and 4 out of 7 at 24 mo. Esophageal acid exposure was normalized in 67% (4/ 6) patients and > 50% improvement was observed in an additional 16% (1/6) patients at 12 mo. At 24 mo, 50% (2/4) of patients were normalized or improved by at least 50%. Improvement in esophageal pH was comparable between the groups.
High-resolution manometry revealed no substantial change in end expiratory LES pressure following stimulation therapy. Importantly, LES-EST had no effect on Swallow-induced LES residual pressure with 6.5 mmHg (2.9-10.9), 8.0 mmHg (4.0-11.0) and 7.0 mmHg (4.5-11.5) at baseline, 12- and 24-mo respectively in the incomplete responder group and 8.9 mmHg (7.4-10.9), 7.0 mmHg (1.5-8.0) and 5.0 mmHg (4.0-10.0) in the responder group.
At baseline endoscopy, all patients had esophagitis, for the most part mild. In the incomplete responder group, 11 patients had LA grade A esophagitis, 4 LA grade B and 1 LA grade C. At 12 mo, esophagitis resolved in 6 patients, 7 had LA grade A, 2 LA grade B and 1 LA grade C. Esophagitis improved by at least 1 grade in 63% of patients at 12-mo (P = 0.02). In the complete responder group, 4 patients had LA grade A esophagitis, 2 LA grade B and 1 LA grade C. At 12 mo, esophagitis resolved in 1 patient, 5 had LA grade A, 1 LA grade B and none with LA grade C. Esophagitis improved by at least 1 grade in 43 % of patients at 12-mo (P = 0.15).
Our post-hoc analysis shows that LES electrical stimulation was as effective in incomplete responders as it was in complete responders. LES stimulation was not associated with any adverse effects or sensations that are typically observed with traditional anti-reflux surgery (as dysphagia, gas-bloat, and diarrhea), in either group. In both groups, LES electrical stimulation safely improved symptoms, significantly reduced esophageal acid exposure, and almost completely eliminated the need for daily acid suppression medications (PPIs). Swallow-induced LES relaxation or peristaltic activity was not affected by electrical stimulation in either group.
The definition of GERD by the Montreal consensus emphasizes both subjective complaints as well as complications of GERD, defining it as “a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications”[18]. GERD symptoms are common and affect 10%-20% of adults in Western countries[19] and up to 40% in the United States[20]. Proton pump inhibitors are potent suppressors of gastric acid secretion and have revolutionized the treatment of GERD, however, a substantial number of patients remain symptomatic in spite of maximal medical therapy[6].
There has been increasing awareness of partial responders to medical therapy. A meta-analysis of randomized, controlled studies conducted in the secondary care practices showed that 10%-40% of GERD patients reported partial- or nonresponse of their reflux symptoms to PPI therapy[21]. Comparable rates were recently reported in a systematic review of persistent reflux symptoms in patients ON-PPI therapy evaluated in primary care and community studies[22]. In this study, the persistence of GERD symptoms was associated with decreased psychological and physical well-being. A recent observational study, conducted in primary care as well as specialized settings, supported these findings, reporting that incomplete response to PPI therapy was associated with considerable direct and indirect costs, and substantial impairment in HRQL and productivity of such patients[23]. Failure of PPI treatment to resolve GERD-related symptoms has become the most common presentation of GERD in gastrointestinal practice, and presents a significant therapeutic challenge to the practicing physician.
Both the “AGA position statement on the management of GERD” and “SAGES guidelines for surgical treatment of GERD” recommend anti-reflux surgery for GERD patients with incomplete response to PPI, with persistent and troublesome GERD symptoms despite medical therapy, or in those intolerant to PPIs[24,25]. Other recommended reasons for surgery include the lifelong need for medication intake, expense of medications, and side-effects[24,25]. Indeed, almost 50% of patients chose to undergo surgery because inadequate control of GERD symptoms in spite of acid suppression therapy[7]. The patients enrolled in our study met these two sets of indications, with 2/3 selecting a surgical intervention because of continuing bothersome symptoms despite daily single or double-dose PPI therapy, while a 1/3 chose surgical therapy because of quality of life concerns with continuous use of daily medications.
A number of mechanisms are thought to contribute to incomplete response, an important one being the persistent reflux of weakly acidic or non-acidic gastric content while ON-PPIs since PPIs target acid secretion rather than reflux of gastric contents into the esophagus[26]. In a group of 145 patients with refractory symptoms on twice daily PPI; only 13% of persistent heartburn events and 8% of persistent regurgitation events were associated with acid reflux; meanwhile, 27% of heartburn events and 58% of regurgitation events were associated with weakly acid reflux[6]. In fact, PPIs are effective in control of the symptom of heartburn but not regurgitation, as highlighted by a recent study showing a higher therapeutic gain with PPI therapy for heartburn over regurgitation[27], highlighting the important role of regurgitation in PPI failure[28], and its being one of the indications for antireflux surgery. Accordingly, in our study, regurgitation reported by 21/23 patients at baseline. Regurgitation was reported by 3/15 patients with incomplete response to PPI at 12 mo and by 3/14 at 12 mo when OFF-PPI (data not reported in 1 and 2 patients respectively). In the complete responder group, none of the 7 patients had regurgitation at 12 mo and only 1/7 reported regurgitation at 24 mo, thus supporting the notion that LES-EST is truly an antireflux therapy.
Anti-reflux surgery has been the main alternative to medical therapy. However, complications and the need for continuous GERD medications despite surgery remain a concern, resulting in a declining number of anti-reflux surgeries in the United States[29]. Moreover, data suggest that laparoscopic fundoplication is less effective at reducing symptoms in partial responders to medical therapy than in complete responders[30]. Consequently, surgical therapy is generally not recommended in patients who are complete non-responders to PPI therapy. This highlights the need for an alternative therapy in this challenging group of patients.
Our results demonstrate that a comparable, significant and sustained improvement in esophageal acid exposure and symptoms, and almost completely eliminated the need for daily PPIs in patients with incomplete response to PPI, who may have been less than optimal candidates for anti-reflux surgery. The improvement in symptoms was sustained over 24 mo. Symptoms continued to improve over time which was likely the result of the ability to non-invasively optimize therapy to individual needs, a feature not available with any of the endoscopic or surgical interventions.
Our results also demonstrate the safety of this intervention and its excellent adverse effects profile, without the symptoms that are commonly observed after surgery[29], reflecting the “non-disruptive” nature of this intervention on the GE junction anatomy. No device or procedure-related SAEs were reported with LES-EST during the study, only minor anticipated AEs typically observed in the postoperative state. All events resolved without a need for any significant intervention, in line with previous experience with gastric electrical stimulation[31]. Importantly, EST had no effect on LES residual pressure in response to swallows, and none of the patients in this trial reported dysphagia or any other gastrointestinal symptoms associated with stimulation. The technical simplicity of this intervention is likely to result in less procedural variability and more uniform long-term outcomes, unlike laparoscopic fundoplication where outcomes from low-volume centers are less favorable compared to those reported from high-volume centers[7].
There are limitations to this study. The open label design cannot control for placebo effect and a “regression to mean”, affecting subjective variables such as GERD-HRQL and other patient reported outcomes. However, objective variables, such as esophageal acid exposure are much less likely to be influenced by placebo. Also, because of the selective enrollment criteria, the results may not be generalizable to these patients. The efficacy of LES-EST in patients with large hernia, combined with hernia repair, remains to be established. Finally, these are small numbers and some of the comparisons are susceptible to a type-II error.
In conclusion, the results of the study support the safety and efficacy of electrical stimulation of the LES in the treatment of GERD patients with incomplete response to PPI therapy. These results were comparable to those seen in patients with complete response to PPI at baseline.
A substantial number of gastroesophageal reflux disease (GERD) patients are not satisfied with medical therapy, and are reluctant to consider fundoplication, as evident by the declining number of such intervention.
Surgical therapy for GERD.
A novel therapy for GERD, using application of electrical stimulation to the lower esophageal sphincter.
Patients with GERD who are not controlled or are not unsatisfied with medical therapy and are seeking an alternative to established surgical interventions.
This paper is concerning an interesting topic, i.e., alternative treatment to proton pump inhibitors (PPI) for patients poorly responding to PPI. Obviously, as pointed by the authors, the paper has two limits: the very low number of patients in both arms (one arm include 7 patients), which strongly reduces its power, and the “open label” design. Likely more data should be elaborated in order to support the final conclusions.
| 1. | Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1062] [Cited by in RCA: 1043] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 2. | Everhart JE. Digestive diseases in the United States: Epidemiology and Impact. NIDDK: NIH Publication 1994; 94-1447. |
| 3. | El-Serag HB. Time trends of gastroesophageal reflux disease: a systematic review. Clin Gastroenterol Hepatol. 2007;5:17-26. |
| 4. | Wahlqvist P, Reilly MC, Barkun A. Systematic review: the impact of gastro-oesophageal reflux disease on work productivity. Aliment Pharmacol Ther. 2006;24:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | McQuaid KR, Laine L. Early heartburn relief with proton pump inhibitors: a systematic review and meta-analysis of clinical trials. Clin Gastroenterol Hepatol. 2005;3:553-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Fass R, Shapiro M, Dekel R, Sewell J. Systematic review: proton-pump inhibitor failure in gastro-oesophageal reflux disease--where next? Aliment Pharmacol Ther. 2005;22:79-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 298] [Article Influence: 14.2] [Reference Citation Analysis (1)] |
| 7. | Vakil N, Shaw M, Kirby R. Clinical effectiveness of laparoscopic fundoplication in a U.S. community. Am J Med. 2003;114:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Richter JE. Gastroesophageal reflux disease treatment: side effects and complications of fundoplication. Clin Gastroenterol Hepatol. 2013;11:465-471; quiz e39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 9. | Vassiliou MC, von Renteln D, Rothstein RI. Recent advances in endoscopic antireflux techniques. Gastrointest Endosc Clin N Am. 2010;20:89-101, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Ellis F, Berne TV, Settevig K. The prevention of experimentally induced reflux by electrical stimulation of the distal esophagus. Am J Surg. 1968;115:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Clarke JO, Jagannath SB, Kalloo AN, Long VR, Beitler DM, Kantsevoy SV. An endoscopically implantable device stimulates the lower esophageal sphincter on demand by remote control: a study using a canine model. Endoscopy. 2007;39:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Sanmiguel CP, Hagiike M, Mintchev MP, Cruz RD, Phillips EH, Cunneen SA, Conklin JL, Soffer EE. Effect of electrical stimulation of the LES on LES pressure in a canine model. Am J Physiol Gastrointest Liver Physiol. 2008;295:G389-G394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Banerjee R, Pratap N, Kalpala R, Reddy DN. Effect of electrical stimulation of the lower esophageal sphincter using endoscopically implanted temporary stimulation leads in patients with reflux disease. Surg Endosc. 2014;28:1003-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Rodríguez L, Rodríguez P, Neto MG, Ayala JC, Saba J, Berel D, Conklin J, Soffer E. Short-term electrical stimulation of the lower esophageal sphincter increases sphincter pressure in patients with gastroesophageal reflux disease. Neurogastroenterol Motil. 2012;24:446-450, e213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Rodríguez L, Rodriguez P, Gómez B, Ayala JC, Oksenberg D, Perez-Castilla A, Netto MG, Soffer E, Crowell MD. Long-term results of electrical stimulation of the lower esophageal sphincter for the treatment of gastroesophageal reflux disease. Endoscopy. 2013;45:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Rodríguez L, Rodriguez P, Gómez B, Ayala JC, Oxenberg D, Perez-Castilla A, Netto MG, Soffer E, Boscardin WJ, Crowell MD. Two-year results of intermittent electrical stimulation of the lower esophageal sphincter treatment of gastroesophageal reflux disease. Surgery. 2015;157:556-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Velanovich V. The development of the GERD-HRQL symptom severity instrument. Dis Esophagus. 2007;20:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 409] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 18. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920; quiz 1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2368] [Cited by in RCA: 2520] [Article Influence: 126.0] [Reference Citation Analysis (2)] |
| 19. | El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1322] [Article Influence: 110.2] [Reference Citation Analysis (2)] |
| 20. | Locke GR, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1384] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 21. | Crawley JA, Schmitt CM. How satisfied are chronic heartburn sufferers with their prescription medications? Results of the patient unmet needs study. J Clin Outcomes Manag. 2000;7:29. |
| 22. | El-Serag H, Becher A, Jones R. Systematic review: persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther. 2010;32:720-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 212] [Article Influence: 13.3] [Reference Citation Analysis (40)] |
| 23. | Stålhammar N, Spiegel BM, Löfman HG, Karlsson M, Wahlqvist P, Næsdal J, Nelson MT, Despiége N. Partial response to proton pump inhibitor therapy for GERD: observational study of patient characteristics, burden of disease, and costs in the USA. Pragm Obser Res. 2012;3: 57-67. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Kahrilas PJ, Shaheen NJ, Vaezi MF, Hiltz SW, Black E, Modlin IM, Johnson SP, Allen J, Brill JV. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391, 1391.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 399] [Article Influence: 22.2] [Reference Citation Analysis (35)] |
| 25. | Stefanidis D, Hope WW, Kohn GP, Reardon PR, Richardson WS, Fanelli RD. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc. 2010;24:2647-2669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 255] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 26. | Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut. 2012;61:1340-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 27. | Kahrilas PJ, Howden CW, Hughes N. Response of regurgitation to proton pump inhibitor therapy in clinical trials of gastroesophageal reflux disease. Am J Gastroenterol. 2011;106:1419-1425; quiz 1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Kahrilas PJ, Jonsson A, Denison H, Wernersson B, Hughes N, Howden CW. Regurgitation is less responsive to acid suppression than heartburn in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2012;10:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Wang YR, Dempsey DT, Richter JE. Trends and perioperative outcomes of inpatient antireflux surgery in the United States, 1993-2006. Dis Esophagus. 2011;24:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Lundell L. Borderline indications and selection of gastroesophageal reflux disease patients: ‘Is surgery better than medical therapy’? Dig Dis. 2014;32:152-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | McCallum RW, Lin Z, Forster J, Roeser K, Hou Q, Sarosiek I. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Contini S, Luo HS, Mann O S- Editor: Qi Y L- Editor: A E- Editor: Wang CH