Published online Nov 6, 2015. doi: 10.4292/wjgpt.v6.i4.120
Peer-review started: April 18, 2015
First decision: June 18, 2015
Revised: July 11, 2015
Accepted: October 12, 2015
Article in press: October 13, 2015
Published online: November 6, 2015
Processing time: 211 Days and 2.4 Hours
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors belonging to the nuclear hormone receptor superfamily. Three subtypes, PPARα, PPARβ/δ, and PPARγ, have been identified so far. PPARα is expressed in the liver, kidney, small intestine, heart, and muscle, where it activates the fatty acid catabolism and control lipoprotein assembly in response to long-chain unsaturated fatty acids, eicosanoids, and hypolipidemic drugs (e.g., fenofibrate). PPARβ/δ is more broadly expressed and is implicated in fatty acid oxidation, keratinocyte differentiation, wound healing, and macrophage response to very low density lipoprotein metabolism. This isoform has been implicated in transcriptional-repression functions and has been shown to repress the activity of PPARα or PPARγ target genes. PPARγ1 and γ2 are generated from a single-gene peroxisome proliferator-activated receptors gamma by differential promoter usage and alternative splicing. PPARγ1 is expressed in colon, immune system (e.g., monocytes and macrophages), and other tissues where it participates in the modulation of inflammation, cell proliferation, and differentiation. PPARs regulate gene expression through distinct mechanisms: Ligand-dependent transactivation, ligand-independent repression, and ligand-dependent transrepression. Studies in animals have demonstrated the gastric antisecretory activity of PPARα agonists like ciprofibrate, bezafibrate and clofibrate. Study by Pathak et al also demonstrated the effect of PPARα agonist, bezafibrate, on gastric secretion and gastric cytoprotection in various gastric ulcer models in rats. The majority of the experimental studies is on pioglitazone and rosiglitazone, which are PPARγ activators. In all the studies, both the PPARγ activators showed protection against the gastric ulcer and also accelerate the ulcer healing in gastric ulcer model in rats. Therefore, PPARα and PPARγ may be a target for gastric ulcer therapy. Finally, more studies are also needed to confirm the involvement of PPARs α and γ in gastric ulcer.
Core tip: Peroxisome proliferator-activated receptors (PPARs) are a nuclear hormone receptor family and act as transcription factors. PPARs are of three subtypes, i.e., PPARα, PPARβ/δ, and PPARγ. The common sites where PPARα is expressed are muscle, heart, liver, small intestine and kidney. PPARγ is involved in modulation of various functions like inflammation, cell proliferation, and differentiation and it is commonly expressed in white blood cells (e.g., macrophages and monocytes) which are involved in immune activity and in the colon. Studies in animals have demonstrated the gastric antisecretory activity of PPARα agonists like ciprofibrate, bezafibrate and clofibrate. The majority of the experimental studies regarding the role of PPARγ activators is on pioglitazone and rosiglitazone. In all the studies, both the PPARγ activators showed protection against the gastric ulcer and also accelerate the ulcer healing in gastric ulcer model in rats. Therefore, PPARα and PPARγ can be explored as a target of gastric ulcer treatment. The aim of the present paper is to discuss the experimental evidences of the role of PPARs in gastric ulcer.
- Citation: Saha L. Role of peroxisome proliferator-activated receptors alpha and gamma in gastric ulcer: An overview of experimental evidences. World J Gastrointest Pharmacol Ther 2015; 6(4): 120-126
- URL: https://www.wjgnet.com/2150-5349/full/v6/i4/120.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v6.i4.120
Peroxisome proliferator-activated receptors (PPARs) are a nuclear hormone receptor family and act as transcription factors. PPARs are of three subtypes, i.e., PPARα, PPARβ/δ, and PPARγ. PPARα is involved in fatty acid catabolism and also in controlling of lipoprotein assembly. Factors which activate PPARα are hypolipidemic drugs (e.g., Fenofibrate), long-chain unsaturated fatty acids and eicosanoids. PPARα is distributed in many tissues like heart, kidney, muscle, liver, and small intestine[1,2]. PPARβ/δ isoform of PPARs has been shown to involve in transcriptional-repression functions and inhibits PPARα or PPARγ target genes[2-7]. This isoform is abundantly distributed in the body and take part in various activities like keratinocyte differentiation, fatty acid oxidation, healing and very low density lipoprotein metabolism. PPARγ1 and γ2 are two subtype of PPARγ and derived from a single gene peroxisome proliferator-activated receptors gamma[8-12]. PPARγ1 is involved in modulation of various physiological functions like inflammation, cell proliferation, and differentiation and is found in tissues like white blood cells (e.g., monocytes and macrophages) of the immune system, colon, and other tissues. Whereas PPARγ2 found in adipose tissue and plays an important role in the differentiation of adipocyte, storage of lipid, and energy dissipation[12]. Not only that, PPARγ is also taking part in the metabolism of glucose and improving the insulin sensitivity. Thiazolidinediones which is a PPARγ agonist, are commonly used for the treatment of type 2 diabetes as insulin-sensitizing drugs[2,4,5].
PPARs are consist of following function domains: (A/B) N-terminal region, (C) DBD (DNA-binding domain), (D) flexible hinge region, (E) LBD (ligand binding domain) and (F) C-terminal region[13,14]. The DBD contains two zinc finger motifs, which bind to specific sequences of DNA known as hormone response elements when the receptor is activated. The LBD has an extensive secondary structure consisting of 13 alpha helices and a beta sheet. Natural and synthetic ligands bind to the LBD, either activating or repressing the receptor. The transcriptional activating function (AF-1) motif is present in the N-terminus and it is not activated by ligands. On the other hand, E/F domain or LBD also contains a transcriptional activating function (AF-2) motif at the C-terminus helix 12, which is activated by ligands[13]. A large numbers of synthetic and natural ligands like eicosanoids, fatty acids, linoleic acid derivatives, oxidized and nitrated fatty acids, bind to the large binding pocket present on the E/F (LBD) domain. The dimerization of PPARs with the 9-cis retinoic acid receptor (RXR) requires both the D and E/F domains. Then this dimerized PPARs and RXR bound to their respective peroxisome proliferator-activated receptor response elements (PPREs) present on the DNA molecule.
Genes which take part in various body functions like the metabolism of lipid, homeostasis of energy, proliferation, differentiation, and survival of cells has their functional PPREs in their regulatory regions[1,2,13,15].
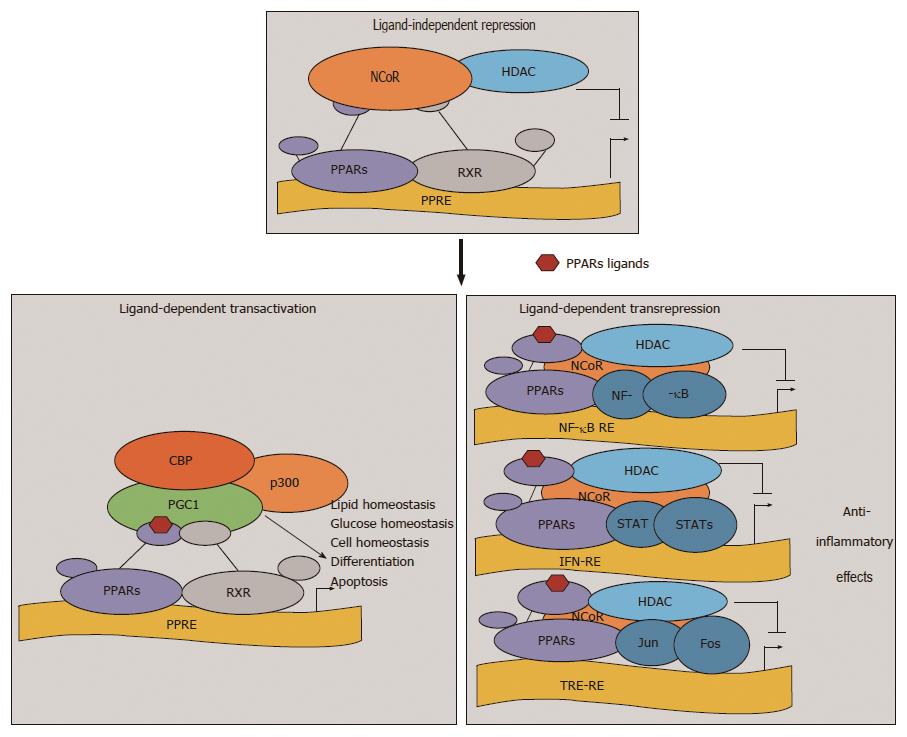
The expression of various genes which involved in various physiological functions are regulated by PPARs through three mechanisms: Transactivation (ligand-dependent), repression (ligand-independent) and transrepression (ligand-dependent)[16,17] (Figure 1).
The classical mode of action of PPARs is the ligand-dependent transactivation. In this mechanism when a ligand binds to the PPARs, there is folding back of the helix 12 of the LBD which leads to the exposure of the AF-2 motif. The AF-2 motif is crucial for the recruitment of transcriptional coactivators. All these changes help in fitting together of all the transcriptional machinery at PPRE-containing promoters[16,17]. When there is no ligand, transcription of target genes is inhibited by PPARs by recruitment of co repressor complexes like nuclear receptor corepressor and silencing mediator for retinoid and thyroid receptors (Figure 1). “Transrepression” has recently been discovered as an additional nongenomic, ligand-dependent gene repression mechanism of PPARs which involves protein-protein interactions of NFκB, AP1, Smads, signal transducers and activators of transcription, and nuclear factor of activated T cells[17-19]. In both the genomic mechanisms of regulation of various genes by PPARs, i.e., repression and transactivation required the binding of PPARs to PPREs, but transrepression, which is a nongenomic mechanism, does not require the binding of PPARs to PPREs. The anti-inflammatory properties of PPARs might be explained by the transrepression mechanism, where there is recruitment and stabilization of the corepressor complexes on the promoters of pro-inflammatory genes[17-20].
PPARα is widely distributed in the small and large intestinal mucosa where dietary fatty acids delivered[21,22]. The genes which involved in functions like inflammation, cell cycle progression, angiogenesis and lipid metabolism are regulated by PPARα[23-27]. The role of PPARα in the processes like angiogenesis and cell cycle progression has been suggested its contribution toward the formation and progression tumor. To the best of my knowledge till date, no data have been available which indicates its role in gastric and esophageal cancer. However, its role in colorectal cancer has been investigated both in vivo and in vitro studies[24-30].
PPARα agonist enhanced the release of gastrin following the stimulation of PPARα[31] . The PPARα agonist induced hypergastrinemia is associated with less number of granules per cell as well as a relative increase in the number of electron-dense granules. All these changes with PPARα agonist are similar to those effects induced by proton pump inhibitor, pantoprazole, which indicates the signs of activation of the gastric cells in general[32]. Gastrin is a peptide hormone. It has two principal biological effects: Stimulation of acid secretion from gastric parietal cells and stimulation of mucosal growth in the acid-secreting part of the stomach (gastric cytoprotective effect)[33]. Nitric oxide is one of the well know gastro-cytoprotective agent and there is increased in the release of nitric oxide following PPARα stimulation[34]. Reports in the published literature have indirectly shown the antigastric ulcer effect of PPARα. The gastric antisecretory activity of PPARα agonists like ciprofibrate, bezafibrate and clofibrate have been demonstrated in animal studies[35,36]. A short study by Eason et al[36] in the mid-1970s first time demonstrated the antisecretory activity of clofibrate in rats. In the same study other phenoxyisobutyrate derivatives like ciprofibrate and bezafibrate have also demonstrated a significant reduction in gastric acid secretion in rats like clofibrate, but the duration of action of ciprofibrate was longer as compared to ciprofibrate and bezafibrate[36].
Another study by Pathak et al[35] also studied the effect of bezafibrate, a PPARα agonist, on acid secretion and gastric cytoprotection in various rat models of gastric ulcer. Various gastric ulcer models were used in this study like acetic acid-induced chronic gastric ulcers, pylorus ligation, ethanol-induced, indomethacin-induced and ischemia-reperfusion-induced gastric ulcers. Bezafibrate (10 mg/kg and 100 mg/kg body weight) were used intraperitonealy. Significant antiulcer effects were seen with both the doses of bezafibrate in all the gastric ulcer models except acetic acid-induced chronic gastric ulcers and ischemia-reperfusion-induced gastric ulcer models. The healing of gastric ulcer was improved with bezafibrate in acetic acid-induced chronic gastric ulcer model. Bezafibrate (10 and 100 mg/kg) was also able to inhibit gastric ulcer formation induced by ischemia-reperfusion. So, this study not only demonstrated the antigastric ulcer property of the PPARα agonists, but also demonstrated its ulcer healing property in gastric ulcer models in rats[35].
We have also demonstrated the antigastric ulcer activity of bezafibrate in our laboratory (unpublished data). The present study was undertaken to validate antiulcer activity and the mechanism of action of bezafibrate in gastric ulcer. The aspirin induced gastric ulcer model was used. Bezafibrate was administered in graded doses (10-200 mg/kg) to detect the best effective anti-ulcer dose of bezafibrate. Keeping in view the diversity of defensive mechanisms, the present study was limited to exploring the involvement of the mucosal oxidant system, apoptotic pathway and nitric oxide pathway in the mechanism of the antigastric ulcer effect of bezafibrate. To explore the nitric oxide mechanism, a nitric oxide synthase inhibitor, Nω-nitro-l-arginine was used. The following parameters were measured: Ulcer Index, Histopathological scoring of gastric ulcer, gastric juice analysis, Gastric mucosal lipid peroxidation parameters, Estimation of nitric oxide metabolite in blood, mRNA expression of inducible nitric oxide synthase (iNOS) and constitutive nitric oxide synthase (cNOS) enzyme in gastric mucosa, Gastric mucosal DNA fragmentation study. Bezafibrate demonstrated dose-dependent antiulcer activity, showed antisecretory and gastro protective action, reduced lipid peroxidation, inhibit iNOS expression, preserve cNOS expression and qualitatively inhibited DNA fragmentation and improved upon the Histopathological score of gastric mucosa.
The histopathological findings of the gastric mucosa: Aspirin administration showed superficial erosion and ulceration on the mucosa and infiltration of inflammatory cells. Co administration of bezafibrate with aspirin showed gastric mucosa with reepithilization, formation of pits and decreased infiltration of inflammatory cells. From this finding we can say that bezafibrate (a PPARα agonist) might have an anti inflammatory activity in gastric ulcer. This is the new finding seen with Bezafibrate and if proven clinically can be used in combination with aspirin (unpublished data).
PPARγ is a subtype type of PPARs which is a nuclear hormone receptor super family. The transcription of numerous cellular processes and various cytokines is controlled by activation of PPARγ[37]. The function and differentiation of immune cells and synthesis of many inflammatory cytokines like tumor necrosis factor alpha (TNF-α) might be controlled by stimulation of PPARγ[38]. Cytoprotective and antioxidant activities of PPARγ activation have been demonstrated in several experimental studies[39]. The role of PPARγ has been implicated in various disease conditions like atherosclerosis, inflammation, cancer and infertility. In adipose tissue, PPARγ is highly expressed and it plays an important role in the differentiation of adipocyte and maintenance of insulin responses.
PPARγ is widely distributed in the colon and the major sources might be the macrophages and epithelial cells. The other tissues which expressed PPARγ are small intestine, liver, pancreas and stomach. Low concentrations of PPARγ are seen in tissues like kidneys, glial cells, cartilage, airway epithelial cells and skin. Whereas gene of PPARγ is almost undetectable in muscles[40]. Inspite the fact that colonic epithelial cells highly expressed PPARγ, investigators have also demonstrated the effect of the PPARγ/RXR in gastric disorders. Most gastric cancer cell lines expressed PPARγ and RXRα both at mRNA and protein level[41]. Studies have not been fully explored the distribution of PPARγ in human stomach tissue. In the published literatures, reports are there about the constitutive and the ubiquitous expression of PPARγ mainly by epithelial cells in normal human gastric mucosa[42]. During gastritis, there noticeably increased in the expression of PPARγ in gastric mucosa[41]. This finding may substantiate the preventive regulatory role of PPARγ in inflammatory cascade and could be a target for the prevention of gastric inflammation. This hypothesis has been supported by many studies in which PPARγ agonists demonstrated the protective role in gastric inflammation in chemical-induced gastritis models in rats[43,44]. A study by Hamaguchi et al[43] demonstrated the ulcer healing property of 15d-prostaglandin J2, a natural PPARγ agonist, in gastric ulcer model without the effect on gastric-acid secretion. A study by Takagi et al[44] also supported this finding of Hamaguchi and colleagues by demonstrating the beneficial effects of pioglitazone, a specific ligand of PPARγ on aspirin-induced gastric mucosal injury, which could be attributable to the inhibition of production of gastric TNF-α. Another study by Naito et al[45] further substantiated the anti-inflammatory properties of pioglitazone in experimentally induced gastric mucosal injury in rat which could be attributable to its. Naito et al[45] also explores the antioxidative property of pioglitazone in their study. The ulcer healing property of pioglitazone was also demonstrated by Konturek et al[46] in rat. They determined the effect of pioglitazone on the healing of gastric ulcers in rats. Konturek et al[46] explored the following parameters in their study to demonstrate the ulcer healing property of pioglitazone: Measuring the gastric mucosal blood flow, measuring the expression of various cytokines like interleukin-1α, TNF-α and measuring the expression of various protective proteins like heat shock protein 70 (HSP70), cNOS, iNOS, cyclooxygenase-1 and cyclooxygenase-2 in gastric mucosa. There was significant increased in gastric ulcer healing by pioglitazone pre-treatment as ther was significant increased in blood flow at the ulcer margin and there was reduction in the area of gastric ulcers. There was significant increased in the expression of PPARγ mRNA in the gastric mucosa (ulcerated). Therefore the study by Konturek et al[46] demonstrated the ulcer healing property of pioglitazone and also supports the anti-inflammatory action of pioglitazone.
The gastroprotective effect of another PPARγ agonist, rosiglitazone, has been documented by Villegas et al[47] against ischemia - reperfusion induced injury in the rat stomach. The potent gastro protective and ulcer healing properties of pioglitazone was also determined in another study by Brzozowski et al[37]. In this study, the authors were also able to demonstrate the role of endogenous PG and NO in gastroprotective and hyperaemic actions of pioglitazone. There were significantly decreased in the release and expression of various proinflammatory cytokines like TNF-α and IL-1β by pioglitazone. The acceleration of ulcer healing by the PPARγ ligand may be attributed to the increase in angiogenesis at the ulcer margin by a significant increase in the expression of PECAM-1 protein, a marker of angiogenesis[37].
The latest study by El-Moselhy et al[48] further documented the involvement of PPARγ activation in nitric oxide mediated gastric ulcer healing in rats. Stress - induced ulcer model was used in this study. The nitric oxide pathway was explored. To demonstrate the gastroprotective effect of rosiglitazone, the authors used the following groups of animals: Rosiglitazone alone treated group, rosiglitazone + PPARγ antagonist (BADGE) group, rosiglitazone + nitric oxide synthase inhibitor (L-NAME) group. The authors concluded that the gastroprotective effect of rosiglitazone might be because of its antisecretory, antioxidant and anti-inflammatory properties[48].
The findings of the experimental studies suggest the involvement of PPARα and γ in gastric ulcer. Regarding the involvement of PPARα, there are two studies published in the literature which showed gastric antisecretory effects of phenoxyisobutyrate (ciprofibrate, bezafibrate and clofibrate) and gastric cytoprotective effects of bezafibrate in the rat gastric ulcer model. The majority of the experimental studies is on pioglitazone and rosiglitazone, which are PPARγ activators. In all the studies, both the PPARγ activators showed protection against the gastric ulcer and also accelerate the ulcer healing in gastric ulcer model in rats. The mechanisms involved in the protection of gastric ulcer explored in these studies are antioxidant activity, anti-inflammatory activity, angiogenic activity and over expression of HSP70. Therefore, PPARα and PPARγ may be a target for gastric ulcer therapy. Finally, more studies are also needed to confirm the involvement of PPARα and γ in gastric ulcer.
| 1. | Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 828] [Article Influence: 30.7] [Reference Citation Analysis (2)] |
| 2. | Kersten S, Wahli W. Peroxisome proliferator activated receptor agonists. EXS. 2000;89:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer. 2004;4:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 455] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 4. | Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1230] [Article Influence: 55.9] [Reference Citation Analysis (1)] |
| 5. | Nahlé Z. PPAR trilogy from metabolism to cancer. Curr Opin Clin Nutr Metab Care. 2004;7:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T, Lazar MA, O’Rahilly S. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 2006;58:726-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 772] [Cited by in RCA: 751] [Article Influence: 37.6] [Reference Citation Analysis (6)] |
| 7. | Michalik L, Wahli W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. J Clin Invest. 2006;116:598-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 182] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Fajas L, Fruchart JC, Auwerx J. PPARgamma3 mRNA: a distinct PPARgamma mRNA subtype transcribed from an independent promoter. FEBS Lett. 1998;438:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 233] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Al-Shali K, Cao H, Knoers N, Hermus AR, Tack CJ, Hegele RA. A single-base mutation in the peroxisome proliferator-activated receptor gamma4 promoter associated with altered in vitro expression and partial lipodystrophy. J Clin Endocrinol Metab. 2004;89:5655-5660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Zhu Y, Alvares K, Huang Q, Rao MS, Reddy JK. Cloning of a new member of the peroxisome proliferator-activated receptor gene family from mouse liver. J Biol Chem. 1993;268:26817-26820. [PubMed] |
| 11. | Meirhaeghe A, Amouyel P. Impact of genetic variation of PPARgamma in humans. Mol Genet Metab. 2004;83:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1711] [Cited by in RCA: 1738] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 13. | Diradourian C, Girard J, Pégorier JP. Phosphorylation of PPARs: from molecular characterization to physiological relevance. Biochimie. 2005;87:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Renaud JP, Moras D. Structural studies on nuclear receptors. Cell Mol Life Sci. 2000;57:1748-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 173] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2414] [Cited by in RCA: 2384] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 16. | Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1865] [Cited by in RCA: 1991] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 17. | Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771:926-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 429] [Cited by in RCA: 428] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 18. | Yessoufou A, Wahli W. Multifaceted roles of peroxisome proliferator-activated receptors (PPARs) at the cellular and whole organism levels. Swiss Med Wkly. 2010;140:w13071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Torchia J, Glass C, Rosenfeld MG. Co-activators and co-repressors in the integration of transcriptional responses. Curr Opin Cell Biol. 1998;10:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 439] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 20. | Li M, Pascual G, Glass CK. Peroxisome proliferator-activated receptor gamma-dependent repression of the inducible nitric oxide synthase gene. Mol Cell Biol. 2000;20:4699-4707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 297] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 21. | Huin C, Corriveau L, Bianchi A, Keller JM, Collet P, Krémarik-Bouillaud P, Domenjoud L, Bécuwe P, Schohn H, Ménard D. Differential expression of peroxisome proliferator-activated receptors (PPARs) in the developing human fetal digestive tract. J Histochem Cytochem. 2000;48:603-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Braissant O, Wahli W. Differential expression of peroxisome proliferator-activated receptor-alpha, -beta, and -gamma during rat embryonic development. Endocrinology. 1998;139:2748-2754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 102] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Cuzzocrea S, Di Paola R, Mazzon E, Genovese T, Muià C, Centorrino T, Caputi AP. Role of endogenous and exogenous ligands for the peroxisome proliferators activated receptors alpha (PPAR-alpha) in the development of inflammatory bowel disease in mice. Lab Invest. 2004;84:1643-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Griffioen M, Steegenga WT, Ouwerkerk IJ, Peltenburg LT, Jochemsen AG, Schrier PI. Repression of the minimal HLA-B promoter by c-myc and p53 occurs through independent mechanisms. Mol Immunol. 1998;35:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Watson JD, Oster SK, Shago M, Khosravi F, Penn LZ. Identifying genes regulated in a Myc-dependent manner. J Biol Chem. 2002;277:36921-36930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Varet J, Vincent L, Mirshahi P, Pille JV, Legrand E, Opolon P, Mishal Z, Soria J, Li H, Soria C. Fenofibrate inhibits angiogenesis in vitro and in vivo. Cell Mol Life Sci. 2003;60:810-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Kasai T, Miyauchi K, Yokoyama T, Aihara K, Daida H. Efficacy of peroxisome proliferative activated receptor (PPAR)-alpha ligands, fenofibrate, on intimal hyperplasia and constrictive remodeling after coronary angioplasty in porcine models. Atherosclerosis. 2006;188:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Pozzi A, Capdevila JH. PPARalpha Ligands as Antitumorigenic and Antiangiogenic Agents. PPAR Res. 2008;2008:906542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Martinasso G, Oraldi M, Trombetta A, Maggiora M, Bertetto O, Canuto RA, Muzio G. Involvement of PPARs in Cell Proliferation and Apoptosis in Human Colon Cancer Specimens and in Normal and Cancer Cell Lines. PPAR Res. 2007;2007:93416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Gizard F, Amant C, Barbier O, Bellosta S, Robillard R, Percevault F, Sevestre H, Krimpenfort P, Corsini A, Rochette J. PPAR alpha inhibits vascular smooth muscle cell proliferation underlying intimal hyperplasia by inducing the tumor suppressor p16INK4a. J Clin Invest. 2005;115:3228-3238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | Lachal S, Ford J, Shulkes A, Baldwin GS. PPARalpha agonists stimulate progastrin production in human colorectal carcinoma cells. Regul Pept. 2004;120:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Martinsen TC, Skogaker NE, Bendheim MØ, Waldum HL. Antral G cells in rats during dosing with a PPAR alpha agonist: a morphometric and immunocytochemical study. Med Electron Microsc. 2003;36:18-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Allen A, Flemström G, Garner A, Kivilaakso E. Gastroduodenal mucosal protection. Physiol Rev. 1993;73:823-857. [PubMed] |
| 34. | Newaz M, Blanton A, Fidelis P, Oyekan A. NAD(P)H oxidase/nitric oxide interactions in peroxisome proliferator activated receptor (PPAR)alpha-mediated cardiovascular effects. Mutat Res. 2005;579:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 35. | Pathak R, Asad M, Hrishikeshavan HJ, Prasad S. Effect of peroxisome proliferator-activated receptor-alpha agonist (bezafibrate) on gastric secretion and gastric cytoprotection in rats. Fundam Clin Pharmacol. 2007;21:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Eason CT, Pattison A, Howells DD, Spencer AJ, Bonner FW. Assessment of gastric antisecretory effects of phenoxyisobutyrate derivatives in the rat and the mouse. Scand J Gastroenterol. 1988;23:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Brzozowski T, Konturek PC, Pajdo R, Kwiecień SN, Konturek S, Targosz A, Burnat G, Cieszkowski J, Pawlik WW, Hahn EG. Agonist of peroxisome proliferator-activated receptor gamma (PPAR-gamma): a new compound with potent gastroprotective and ulcer healing properties. Inflammopharmacology. 2005;13:317-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Wada K, Nakajima A, Takahashi H, Yoneda M, Fujisawa N, Ohsawa E, Kadowaki T, Kubota N, Terauchi Y, Matsuhashi N. Protective effect of endogenous PPARgamma against acute gastric mucosal lesions associated with ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2004;287:G452-G458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (2)] |
| 39. | Gumieniczek A. Modification of cardiac oxidative stress in alloxan-induced diabetic rabbits with repaglinide treatment. Life Sci. 2005;78:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Dubuquoy L, Dharancy S, Nutten S, Pettersson S, Auwerx J, Desreumaux P. Role of peroxisome proliferator-activated receptor gamma and retinoid X receptor heterodimer in hepatogastroenterological diseases. Lancet. 2002;360:1410-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 154] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 41. | Wu K, Yao L, Wu H, Liu Z, Li L, Qiao T, Fan D. Expression of PPAR gamma and RXR alpha in tissue and cell lines of gastric carcinoma. Gastroenterology. 2001;120:A64. [DOI] [Full Text] |
| 42. | Nomura S, Nakajima A, Osawa E, Sekihara H, Matsuhashi N, Kaminishi M. Gastric expression of peroxisome proliferator-activated receptor γ (PPARγ) in gastritis and gastric tumors. Gastroenterology. 2001;120:A519. [DOI] [Full Text] |
| 43. | Hamaguchi M, Watanabe T, Higuchi K, Tominaga K, Fujiwara Y, Arakawa T. Marked enhancement of gastric ulcer healing by peroxisome proliferator-activated receptor γ ligands in rats. Gastroenterology. 2001;120:A162. [DOI] [Full Text] |
| 44. | Takagi T, Naito Y, Matsuyama K, Yagi N, Yoshida N, Yoshikawa T. Pioglitazone, a specific PPARγ ligand, inhibits aspirin-induced gastric injury in rats. Gastroenterology. 2001;120:A609. |
| 45. | Naito Y, Takagi T, Matsuyama K, Yoshida N, Yoshikawa T. Pioglitazone, a specific PPAR-gamma ligand, inhibits aspirin-induced gastric mucosal injury in rats. Aliment Pharmacol Ther. 2001;15:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Konturek PC, Brzozowski T, Kania J, Konturek SJ, Kwiecien S, Pajdo R, Hahn EG. Pioglitazone, a specific ligand of peroxisome proliferator-activated receptor-gamma, accelerates gastric ulcer healing in rat. Eur J Pharmacol. 2003;472:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Villegas I, Martín AR, Toma W, de la Lastra CA. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, protects against gastric ischemia-reperfusion damage in rats: role of oxygen free radicals generation. Eur J Pharmacol. 2004;505:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | El-Moselhy MA, Nazmy WH. Involvement of PPAR γ activation in NO mediated gastric ulcer healing in rats. Asian J Pharm Clin Res. 2013;6:200-205. |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Actis GC, Bashashati M S- Editor: Tian YL
L- Editor: A E- Editor: Li D