Published online Aug 6, 2015. doi: 10.4292/wjgpt.v6.i3.73
Peer-review started: November 18, 2014
First decision: December 12, 2014
Revised: April 26, 2015
Accepted: May 16, 2015
Article in press: May 18, 2015
Published online: August 6, 2015
Processing time: 263 Days and 9.2 Hours
AIM: To study the effect of the opioid-receptor like-1 (ORL1) agonist nociceptin on gastrointestinal (GI) myenteric neurotransmission and motility.
METHODS: Reverse transcriptase - polymerase chain reaction and immunohistochemistry were used to localize nociceptin and ORL1 in mouse tissues. Intracellular electrophysiological recordings of excitatory and inhibitory junction potentials (EJP, IJP) were made in a chambered organ bath. Intestinal motility was measured in vivo.
RESULTS: Nociceptin accelerated whole and upper GI transit, but slowed colonic expulsion in vivo in an ORL1-dependent manner, as shown using [Nphe1]NOC and AS ODN pretreatment. ORL1 and nociceptin immunoreactivity were found on enteric neurons. Nociceptin reduced the EJP and the nitric oxide-sensitive slow IJP in an ORL1-dependent manner, whereas the fast IJP was unchanged. Nociceptin further reduced the spatial spreading of the EJP up to 2 cm.
CONCLUSION: Compounds acting at ORL1 are good candidates for the future treatment of disorders associated with increased colonic transit, such as diarrhea or diarrhea-predominant irritable bowel syndrome.
Core tip: Here we aimed to study the effect of the endogenous opioid-receptor like-1 (ORL1) agonist nociceptin on gastrointestinal (GI) myenteric neurotransmission and motility. We observed that nociceptin reduces excitatory and inhibitory neurotransmission in motorneurons and interneurons and the overall effect on the GI tract may depend on co-localization of ORL1 receptors with nitric oxide synthase. Since ORL1 activation, unlike other opioid receptor-active drugs, is not associated with central side effects, compounds acting at the ORL1 may be good candidates for the future treatment of disorders associated with increased colonic transit, such as diarrhea or diarrhea-predominant irritable bowel syndrome.
- Citation: Sibaev A, Fichna J, Saur D, Yuece B, Timmermans JP, Storr M. Nociceptin effect on intestinal motility depends on opioid-receptor like-1 receptors and nitric oxide synthase co-localization. World J Gastrointest Pharmacol Ther 2015; 6(3): 73-83
- URL: https://www.wjgnet.com/2150-5349/full/v6/i3/73.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v6.i3.73
The heptadecapeptide nociceptin, also known as orphanin FQ, is the only known endogenous agonist at the opioid-receptor like-1 (ORL1) receptor[1,2]. From a structural perspective nociceptin is closely related to the dynorphin family, but differs from other endogenous opioids[1-5]. Although the ORL1 receptor, alternatively named nociceptin or opioid-4, is a G protein-coupled receptor that shares significant (> 60%) sequence homology to the classical opioid binding sites (μ, δ, κ)[4,6-8], it remains the only target for nociceptin. Furthermore, the effects of nociceptin are not blocked by naloxone, which would further argue against grouping the ORL1 with classical opioid receptors.
ORL1 mRNA is widely distributed in the central and peripheral nervous systems, and in peripheral organs. In the gastrointestinal (GI) tract ORL1 was localized by molecular and immunohistochemical methods. In the guinea pig colon nociceptin is co-localized with calretinin, substance P and enkephalin and, to a small extent, also with nitric oxide synthase (NOS) and VIP immunoreactivity, suggesting that it may be localized in excitatory motorneurons, as well as in descending interneurons[9].
The GI tract is one site where opioids show actions and opioids are known to slow motility[10-13], by reducing peripheral neurotransmitter release[14,15] or by blocking enteric neurotransmission at multiple sites within the peristaltic reflex[10,16-21].
Over the last decade, organ bath experiments revealed that nociceptin induces contractions by reducing inhibitory neurotransmission and causes relaxation by reducing cholinergic neurotransmission[22-24]. More complex preparations disclosed that nociceptin reduces the excitatory components of the peristaltic reflex and thus slows GI motility[25]. Depending on the model used colonic transit was increased or reduced and it seems that timing and route of administration of nociceptin injections may also influence the direction of the results[26,27]. Many of the previously published results are conflicting and it is intriguing that the majority of these studies characterized nociceptin, but failed to shed some light on the involvement of ORL1. Some of the confusion may be caused since previous in vivo studies used different ORL1 agonists; however, ORL1 antagonists were never employed in vivo, thus probing an ORL1 involvement of the observed effects.
The aim of our study was to investigate the endogenous nociceptin system in attempt to elucidate the role and function of ORL1 receptors in the GI tract. Based on the effects of nociceptin on the GI motility observed in vivo, localization and co-localization of the peptide and its receptor were examined in the mouse ileum and colon. Electrophysiology was employed to investigate whether cholinergic excitatory junction potentials (EJPs), purinergic fast excitatory and inhibitory junction potentials (fIJPs) and nitrergic slow IJPs (sIJPs) are changed by nociceptin, if nociceptin has effects on long-distance neurotransmission and motorneurons and interneurons are involved. Our results shed new light on the endogenous nociceptin system physiology, what may have important clinical implications in future.
Animal use for these studies was approved by the Animal Care Committee of the Government of Bavaria/Germany and the University of Calgary Animal Care Committee and all experiments were performed in accordance with institutional Animal Ethics Committee guidelines.
Whole gut transit and colonic expulsion test was performed as described previously in non-fasted male mice[28,29].
To test the involvement of ORL1 receptors, co-administration studies with selective antagonist [NPhe1]NOC [100 pmol, intravenous (iv)] were performed. To interfere with the expression of the ORL1 protein, a phosphorothioate antisense oligodeoxynucleotide AS ODN, synthesized by the UCDNA Synthesis Lab (Calgary, AB, Canada) was used. The AS ODN sequence (5’-C*A*G*G*C*A*C*T*C*G*A*T-3’, where an asterisk marks the phosphorothioate linkage) was chosen to selectively target exon 4 of ORL1, but not the ORL1 alternative variant. The control consisted of a mismatched sequence (MM ODN), in which four bases were switched (5’-C*G*G*G*T*A*C*G*C*G*C*T-3’, where bold indicates the mismatch). ODN solutions were prepared in sterile water immediately prior to use. Mice received either AS or MM ODN at a dose of 5 μg per animal (i.p.), injected every 12 h over 2 d (in total 4 injections). Twelve hours after last injection the animals were used to test colonic bead expulsion. Treatment with AS or MM ODN did not alter the normal behaviour of mice.
Total RNA from mouse brain, longitudinal muscle-myenteric plexus layer (LMMP) and mucosa of ileum and LMMP and mucosa of colon was reversely transcribed in complementary DNA as described before[30,31]. mRNA expression of mouse ORL1 was tested using specific primers [mORL1 (S): 5’-CTGCCTCGTCATGTATGTCATC-3’; mORL1 (AS): 5’-GGAAGATGCAGATGGCAAATACA-3’]. As control, we used GAPDH [mGAPDH (S): 5’-GCTGAACGGGAAGCTCACTG-3’; mGAPDH (AS): 5’- GCTGTTGAAGTCGCAGGAGAC-3’].
Cryosections and whole mounts of mouse colon and ileum were used for immunohistochemistry. Immunohistochemical incubations were carried out as detailed previously[25]. Antisera and streptavidin complexes are listed in Table 1. Negative controls and specificity controls were performed as described previously[25].
| Antisera (host) | Source | Dilution |
| Primary antisera | ||
| ORL1 (KOR-3) (H-85) (rabbit) | Santa Cruz Biotechnology (sc-15309) | 1:50 |
| ORL1 (KOR-3) (N-19) (goat) | Santa Cruz Biotechnology (sc-9759) | 1:50 |
| Nociceptin (guinea-pig) | Abcam (ab10276) | 1:100-200 |
| NOS (rabbit) | Euro Diagnostica (B 220-1) | 0.389 |
| NOS (guinea pig) | Euro Diagnostica (B-GP 225-1) | 0.736 |
| PGP 9.5 (guinea pig) | Chemicon (AB5898) | 0.389 |
| Secondary antisera and streptavidin complexes | ||
| FITC-conjugated donkey anti rabbit | Jackson ImmunoResearch (711-095-152) | 0.111 |
| Cy3-conjugated donkey anti rabbit | Jackson ImmunoResearch (711-165-152) | 0.215 |
| Biotinylated donkey anti rabbit | Jackson ImmunoResearch (711-065-152) | 0.111 |
| Biotinylated donkey anti goat | Jackson ImmunoResearch (705-065-147) | 0.111 |
| Biotinylated donkey anti goat | Jackson ImmunoResearch (706-065-148) | 0.111 |
| FITC-conjugated donkey anti goat | Jackson ImmunoResearch (705-095-147) | 1:50 |
| FITC-conjugated donkey anti guinea pig | Jackson ImmunoResearch (706-095-148) | 1:50 |
| Cy3-conjugated donkey anti guinea pig | Jackson ImmunoResearch (706-165-148) | 0.215 |
| Cy3-conjugated Streptavidin | Jackson ImmunoResearch (016-160-084) | 0.215 |
| FITC-conjugated Streptavidin | Jackson ImmunoResearch (016-010-084) | 1:100 Streptavidin FITC: Jackson ImmunoResearch 016-010-084; 1:100 |
Tissue preparation was performed as described previously[25].
Intracellular recordings of circular colonic smooth muscle cells were performed as detailed previously[32,33]. Neurons were stimulated with single electrical pulses (15 V; 0.3 ms duration) using single or multiple platinum electrodes as described previously[32,33].
Membrane potentials were recorded as described previously[25].
Hexamethonium, atropine, tetrodotoxin (TTX) methylene blue were obtained from Sigma (Irvine, United Kingdom); [NPhe1]NOCNH2 ([NPhe1]NOC) and nociceptin were from Biotrend (Köln, Germany). Drugs were added to the bath in microliter volumes and appropriate control experiments were performed with vehicles to exclude vehicle-induced effects. For the in vivo experiments drugs were first dissolved in DMSO and then further diluted in saline.
Data analysis and statistics was performed as described previously[25].
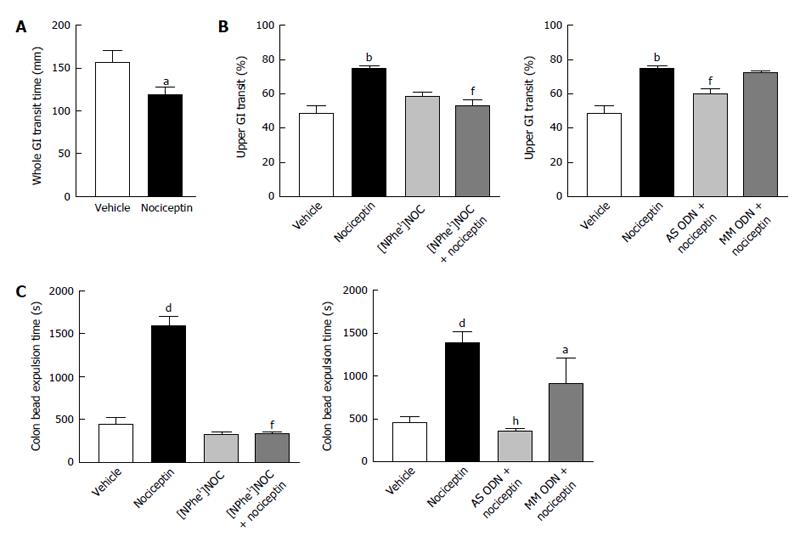
As shown in Figure 1A and B, nociceptin (100 pmol, iv) significantly increased the passage through whole and upper GI tract. The effect of nociceptin on upper GI transit time was blocked by the selective ORL1 antagonist, [NPhe1]NOC (100 pmol, iv) and the pretreatment with AS ODN for two days. On the contrary, a significant delay in bead expulsion time was observed in mice that received nociceptin iv (100 pmol) (Figure 1C). [NPhe1]NOC alone (100 pmol, iv) did not change colonic bead expulsion, but significantly reversed the effects of nociceptin, confirming the involvement of ORL1. Blocking ORL1 by i.p. treatment of mice with AS ODN over 48 h abolished the inhibitory effect of nociceptin (nociceptin 100 pmol, iv: 1383 ± 146.8 s vs AS ODN + nociceptin 100 pmol, iv: 373 ± 33.6 s, P < 0.001) (Figure 1C). The repeated administration of MM ODN produced a minor decrease of the nociceptin action in the mouse colon.
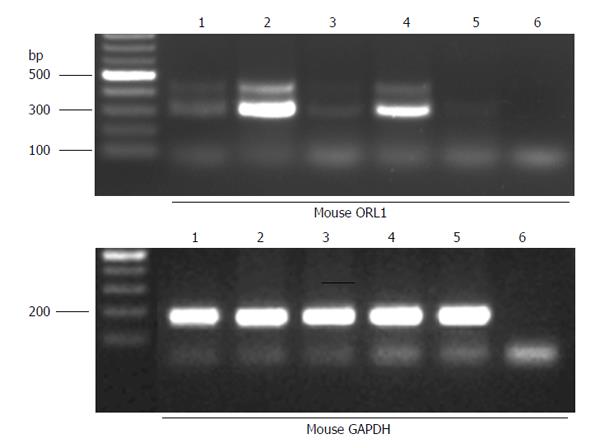
To verify the mechanisms underlying the differential effect of nociceptin based on the site of action, the presence of ORL1 mRNA in mouse GI tract was determined by RT-PCR (Figure 2). Low abundant expression of an alternative ORL1 variant with an 81 bp insertion of the unspliced intron 3 was observed in mouse brain, in LMMP and mucosa of mouse ileum and in LMMP of mouse colon (Figure 1; PCR product of approximately 400 bp. This alternative splicing event generates an in-frame stop codon and therefore creates a C-terminally truncated ORL1 protein with unknown function. All investigated mouse tissues displayed expression of the short ORL1 mRNA variant lacking intron 3 with a PCR product size of approximately 320 bp (Figure 2).
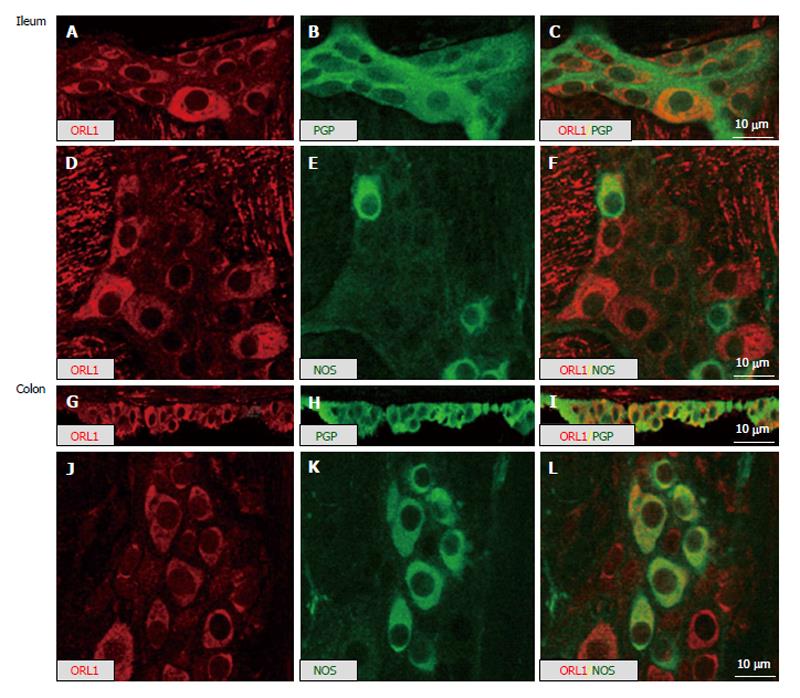
In line with the single labeling, the RT-PCR data and the double labeling with the general neuronal marker PGP9.5 showed that a majority of myenteric neurons in mouse ileum and colon expressed IR for ORL1 (Figure 3). This ORL1 labeling was mainly restricted to the neuronal somata. Double staining with a nitrergic marker revealed high level of co-expression of ORL1 with NOS in mouse large intestine, but not in the small intestine. Corresponding single labeling studies, showed comparable staining, thus non-specific findings were ruled out (Figure 3).
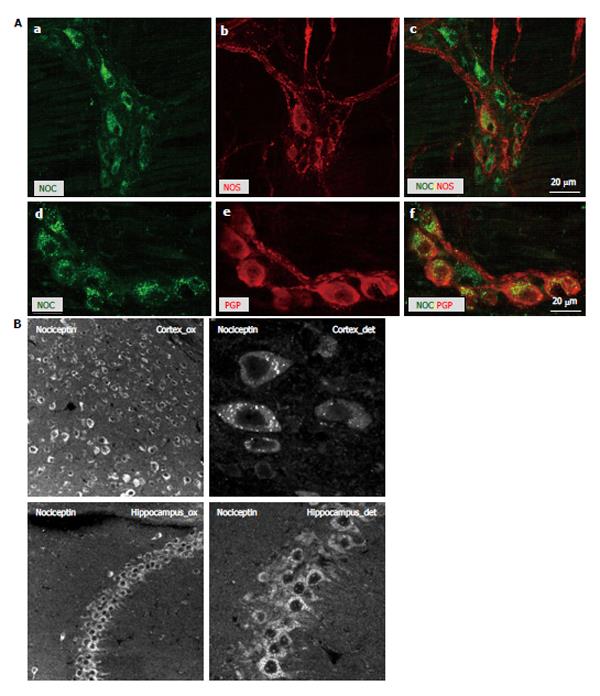
Moreover, the presence of the endogenous peptide nociceptin was confirmed intracellularly in the majority of the myenteric neurons of the mouse colon and both NOS and PGP9.5 double-labeled with nociceptin (Figure 4A). A punctuate intracellular staining pattern suggested a localization in vesicles. Since granular staining was observed in 100% of the neurons, we performed additional control staining in cortex and hippocampus, i.e., regions where distinct subpopulations of neurons are known to stain positive for nociceptin (Figure 4B).
Under basal conditions, circular smooth muscle cells of mouse large bowel displayed stable resting membrane potentials (-53.9 ± 3.7 mV, n = 17). Stimulation of myenteric neurons with EFS gave rise to junction potentials sensitive to TTX.
In the colon (proximal), an EJP was followed by a biphasic IJP, with an initial fIJP followed by a sIJP. In the middle colon, EFS with the same stimulation parameter elicited a biphasic IJP without an EJP and in the distal colon only a monophasic IJP was seen.
The amplitudes of junction potentials were measured and compared to membrane potential prior to electrical stimulus application. The EJP represents excitatory cholinergic neurotransmission, the sIJP inhibitory nitrergic neurotransmission and the fIJP purinergic inhibitory neurotransmission as detailed earlier[33].
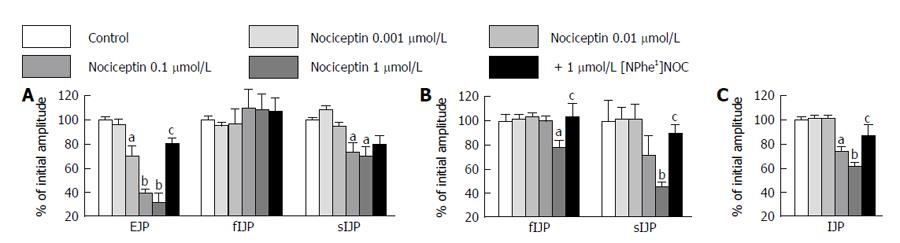
Increasing the nociceptin concentrations (1 nmol/L-1 μmol/L), led to a significant reduction in EJP in the mouse proximal colon and this effect was reversed by addition of [NPhe1]NOC, (n = 6; Figure 5A). The fIJP was not affected by nociceptin, whereas the sIJP was significantly reduced. The effect of nociceptin on the sIJP was reversed by [NPhe1]NOC (n = 6; Figure 5A). In the middle colon, nociceptin (1 nmol/L-1 μmol/L) reduced the sIJP, an effect that was reversed by [NPhe1]NOC (n = 6; Figure 5B). The fIJP was not changed. Finally, in the distal colon nociceptin (10 nmol/L-1 μmol/L) significantly reduced the monophasic IJP, and this effect that was also reversed by [NPhe1]NOC (n = 6; Figure 5C).
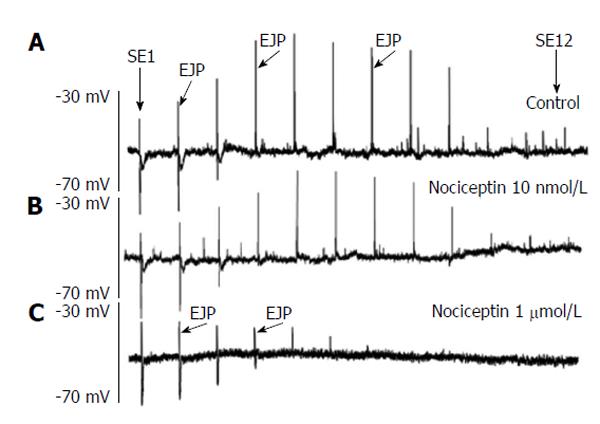
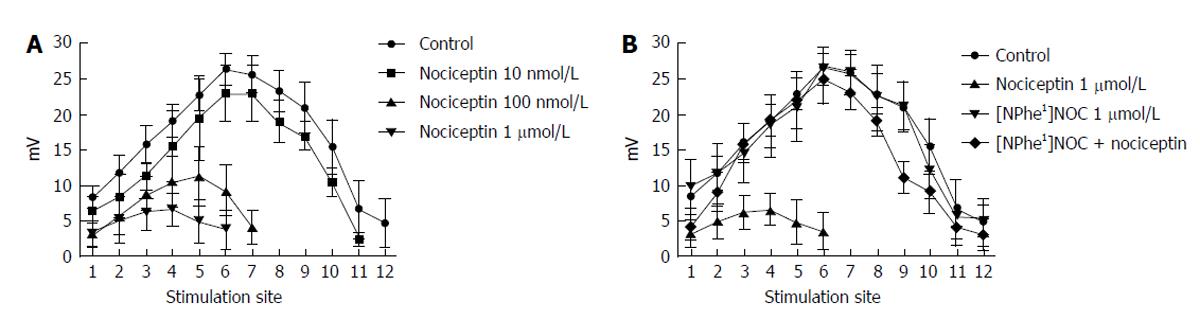
The ORL1 agonist nociceptin was added in increasing doses (100 nmol/L-10 μmol/L) to the organ bath and the spatial distribution of the ascending EJP was reported. EJPs could be recorded up to a distance of 18-20 mm. Incubation of the tissues with nociceptin caused a reduction in EJP responses to electrical stimulation (Figures 6 and 7). For SE 1-6 this decrease represented an ORL1 activity on the motor neurons, whereas for SE > 7 the effects represented influences on interneurones, as characterized previously[34]. Furthermore, the distance over which the EJP was recordable, was concentration dependently shortened. This suggests that ORL1 agonism reduces the excitatory responses not only on amplitude, but also on the spatial fast propagation in the myenteric reflex (ascending part) (Figure 7A). The ORL1 antagonist [NPhe1]NOC (1 μmol/L), when given alone, did not change the spatial distribution of EJPs (Figure 7B).
In additional experiments with two separately perfused electrophysiological chambers, TTX (3 mmol/L), when applied to the stimulatory chamber, reduced the EJP in the chamber where recording was performed (data not shown). Atropine (1 μmol/L) when added to the stimulation chamber did not alter the EJP in the recording chamber (data not shown). Hexamethonium (100 mmol/L) when given to the stimulatory chamber reduced the EJP significantly in the chamber where recording was performed [control 29.6 ± 4.3 mV, hexamethonium (100 mmol/L) 24.5 ± 4.7 mV; n = 5, P < 0.05]. In separate experiments nociceptin was given to the stimulatory chamber and significantly reduced the EJP in the chamber where the anal recording was performed [control 27.9 ± 5.3 mV, nociceptin (1 μmol/L) 21.9 ± 4.5 mV; n = 7, P < 0.05].
Due to powerful actions on GI motility opioids are clinically used to slow increased GI transit. However, opioids are associated with side effects like addiction, respiratory depression and sedation, limiting their use. The ORL1 receptor and its endogenous ligand, nociceptin, have been known for approximately 15 years, but their effects on GI motility are still not fully understood. Since ORL1 activation, unlike the classical opioids, appears to be devoid of central side effects, drugs targeting the ORL1 receptor may be of future use in humans. The present study showed that nociceptin and ORL1 are present in the mouse colon and both are involved in the regulation of motility and neurotransmission. Most importantly, this study for the first time proves that the co-expression of ORL1 with NOS is crucial for nociceptin effects on GI motility in vivo. It also demonstrates that not only motor neurons, but also interneurons are modulated by nociceptin in an ORL1-dependent manner.
The effect of nociceptin on the GI motility has already been investigated by several groups and the results are often contradictory, depending on the site of administration, dose used and the animal model employed in the study[23,26,35,36]. Our observations in vivo clearly showed a differential, ORL1-dependent action of nociceptin in the mouse ileum and colon, where it accelerates and decreases the intestinal motility, respectively. In an attempt to investigate the possible influence of the ORL1 expression patterns on the effect of nociceptin, RT-PCR and immunohistochemistry studies were then executed. We found that ORL1 mRNA and immunoreactivity are present in the GI tract and there is practically no difference in expression levels between small and large intestine, excluding this factor from further investigations. Interestingly, we found the expression of an alternatively spliced ORL1 mRNA variant[37] in mice that has not been reported in other rodents like rats[25]. It remains unresolved whether the presence of ORL1 splice variants is associated with different receptor functionality; however, in view of our latter findings this seems rather unlikely. Immunohistochemistry localized ORL1 staining in the majority of myenteric neurons of the colon and co-localisation with NOS suggests that ORL1 is expressed on inhibitory neurons, what was previously suggested for rats[25]. Of note, there was no co-expression of ORL1 with NOS in the mouse ileum, what may indicate the lack of involvement of nitrergic pathways in the action of nociceptin in the small intestine and may explain in part the effect that we observed in mice.
Using specific antibodies, nociceptin has previously been localized mainly on fibers of myenteric neurons of rat colon and neuronal cell bodies of guinea pig colon[9,36]. Our study extends these observations to mouse colon and for the first time reports that nociceptin appears to be present in vesicles in the cell body of the majority of myenteric neurons, where it double labels with NOS and PGP9.5. In contrast to the guinea pig, where nociceptin can be found only on a limited number of neurons, in the mouse nociceptin is present in the majority of neurons.
We proceeded with our investigation by performing electrophysiological recordings and found that nociceptin significantly reduced electrically-induced contractions in vitro; moreover, these effects showed regional differences. The notion that the ORL1 receptor is involved modulation of intestinal motility was indicated by the finding that [Nphe1]NOC antagonized the effects of nociceptin. Since the nociceptin effects were unchanged in naloxone presence no other opioid receptors are involved and this is in agreement with observations published by others[27,38,39]. Furthermore, we found in agreement with others no effect of nociceptin or [Nphe1]NOC on pharmacologically stimulated smooth muscle or basal tone[22,24,25,40] and are strongly supported by our immunohistochemical studies, which localize ORL1 receptors on neuronal, but not on smooth muscle cells.
The most important findings in our study were illustrated by further intracellular recordings in the mouse colon. Nociceptin produced a significant inhibitory effect on EJPs, which, in addition, was the lowest in the middle colon compared to effects in proximal and distal colonic tissues. Furthermore, sIJP were reduced by nociceptin, suggesting ORL1 involvement in excitatory cholinergic and inhibitory neurotransmission. The sIJP is known to resemble the nitrergic inhibitory neurotransmission, which is further stressed by our immunohistochemical findings, showing that the ORL1 was co-localized with NOS, as well as our observations in vivo.
The present study shows am involvement of nociceptin and the ORL1 in ascending myenteric neuronal network circuits in the colon and increases our current understanding about ORL1 neurotransmission. EJP, which represents excitatory cholinergic neurotransmission was reduced by nociceptin[33], in a [Nphe1]NOC-reversible manner at all involved sites of stimulation, but also shortened the transmission distance. This observation leads us to postulate that not only motor neurons, but also interneurons are modulated by nociceptin. The nociceptin-induced reduction of the EJP amplitude at the shorter distances (SE1-6) points to an action at ORL1 on motor-neurons. However, the shortened distance of transmission of the EJP was caused by ORL1 actions on inter-neurons. Here ORL1 activation reduced spreading of the electrical signal and excitatory neurotransmission. The shortened transmission distances reported here may be explained by different neuronal mechanisms, e.g., reduced pre- or postsynaptic neurotransmitter release or neuronal excitability as underlying mechanisms[41,42].
The experiments with hexamethonium show that transmission involving ganglions is involved in EJP transmission, since EJPs were abolished by hexamethonium beyond 10 mm. In our study EJPs at the closer stimulation sites were reduced and this reduction effect was found to be higher than the remainder amplitude in presence of hexametonium, which suggests that the ORL1 influenced neurotransmission on motor neurons.
[Nphe1]NOC alone had no influence on the electrophysiological parameter observed, but did antagonize the effect of nociceptin, lending clear evidence to the hypothesis that the receptors on motor neurons and interneurons are ORL1 receptors. Our experiments in the separatable electrophysiological chamber clearly indicated that ORL1 activation minimizes interneuron neurotransmission. This happens to an extent comparable to that seen with the ganglionic blocker hexamethonium. Therefore, our study shows for the first time that ORL1 receptors are located on myenteric plexus interneurons and additionally on neuromuscular junctions of motor neurons.
In summary, our study shows for the first time that nociceptin activates ORL1 on motorneurons and interneurons, which leads to a decrease in cholinergic excitatory and inhibitory nitrergic neurotransmission. In the cholinergic excitatory pathways nociceptin reduces not only the local transmission of EJP, but also the spatial spreading of ascending excitatory responses at distances up to 20 mm. Since ORL1 activation, unlike other opioid receptor-active drugs, is not associated with central side effects, compounds acting at the ORL1 may be good candidates for the future treatment of disorders associated with increased colonic transit, such as diarrhea or diarrhea-predominant irritable bowel syndrome.
The heptadecapeptide nociceptin, also known as orphanin FQ, is the only known endogenous agonist at the opioid-receptor like-1 (ORL1) receptor. From a structural perspective nociceptin is closely related to the dynorphin family, but differs from other endogenous opioids in that it does not possess the N-terminal tyrosine residue. Over the last decade, organ bath experiments revealed that nociceptin induces contractions by reducing inhibitory neurotransmission and causes relaxation by reducing cholinergic neurotransmission. More complex preparations disclosed that nociceptin reduces the excitatory components of the peristaltic reflex and thus slows gastrointestinal (GI) motility.
The aim of the study was to investigate the endogenous nociceptin system in attempt to elucidate the role and function of ORL1 receptors in the GI tract. Based on the effects of nociceptin on the GI motility observed in vivo, localization and co-localization of the peptide and its receptor were examined in the mouse ileum and colon. Electrophysiology was employed to investigate whether cholinergic excitatory junction potentials, purinergic fast inhibitory junction potentials (IJPs) and nitrergic slow IJPs are changed by nociceptin, if nociceptin has effects on long-distance neurotransmission and motorneurons and interneurons are involved. The authors’ results shed new light on the endogenous nociceptin system physiology, what may have important clinical implications in future.
The present study shows that nociceptin and ORL1 are present in the mouse colon and both are involved in the regulation of motility and neurotransmission. Most importantly, this study for the first time proves that the co-expression of ORL1 with nitric oxide synthase is crucial for nociceptin effects on GI motility in vivo. It also demonstrates that not only motor neurons, but also interneurons are modulated by nociceptin in an ORL1-dependent manner.
Since ORL1 activation, unlike other opioid receptor-active drugs, is not associated with central side effects, compounds acting at the ORL1 may be good candidates for the future treatment of disorders associated with increased colonic transit, such as diarrhea or diarrhea-predominant irritable bowel syndrome.
The authors’ observations in vivo clearly showed a differential, ORL1-dependent action of nociceptin in the mouse ileum and colon, where it accelerates and decreases the intestinal motility, respectively. The authors proceeded with our investigation by performing electrophysiological recordings and found that nociceptin significantly reduces electrically-induced contractions in vitro; moreover, these effects showed regional differences.
Overall this manuscript is well conducted and written. The hypothesis is also interest and successful.
| 1. | Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1538] [Cited by in RCA: 1527] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 2. | Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1534] [Cited by in RCA: 1486] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 3. | Reinscheid RK, Ardati A, Monsma FJ, Civelli O. Structure-activity relationship studies on the novel neuropeptide orphanin FQ. J Biol Chem. 1996;271:14163-14168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 155] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 855] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 5. | Guerrini R, Calo’ G, Rizzi A, Bigoni R, Rizzi D, Regoli D, Salvadori S. Structure-activity relationships of nociceptin and related peptides: comparison with dynorphin A. Peptides. 2000;21:923-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Chen Y, Fan Y, Liu J, Mestek A, Tian M, Kozak CA, Yu L. Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett. 1994;347:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 268] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Fukuda K, Kato S, Mori K, Nishi M, Takeshima H, Iwabe N, Miyata T, Houtani T, Sugimoto T. cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett. 1994;343:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 335] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Hawes BE, Fried S, Yao X, Weig B, Graziano MP. Nociceptin (ORL-1) and mu-opioid receptors mediate mitogen-activated protein kinase activation in CHO cells through a Gi-coupled signaling pathway: evidence for distinct mechanisms of agonist-mediated desensitization. J Neurochem. 1998;71:1024-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | O’Donnell AM, Ellis LM, Riedl MS, Elde RP, Mawe GM. Distribution and chemical coding of orphanin FQ/nociceptin-immunoreactive neurons in the myenteric plexus of guinea pig intestines and sphincter of Oddi. J Comp Neurol. 2001;430:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Allescher HD, Storr M, Brechmann C, Hahn A, Schusdziarra V. Modulatory effect of endogenous and exogenous opioids on the excitatory reflex pathway of the rat ileum. Neuropeptides. 2000;34:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Grider JR, Makhlouf GM. Role of opioid neurons in the regulation of intestinal peristalsis. Am J Physiol. 1987;253:G226-G231. [PubMed] |
| 12. | Kromer W. The current status of opioid research on gastrointestinal motility. Life Sci. 1989;44:579-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Kaufman PN, Krevsky B, Malmud LS, Maurer AH, Somers MB, Siegel JA, Fisher RS. Role of opiate receptors in the regulation of colonic transit. Gastroenterology. 1988;94:1351-1356. [PubMed] |
| 14. | Manaka H, Manaka Y, Kostolanska F, Fox JE, Daniel EE. Release of VIP and substance P from isolated perfused canine ileum. Am J Physiol. 1989;257:G182-G190. [PubMed] |
| 15. | Donnerer J, Holzer P, Lembeck F. Release of dynorphin, somatostatin and substance P from the vascularly perfused small intestine of the guinea-pig during peristalsis. Br J Pharmacol. 1984;83:919-925. [PubMed] |
| 16. | Allescher HD, Storr M, Piller C, Brantl V, Schusdziarra V. Effect of opioid active therapeutics on the ascending reflex pathway in the rat ileum. Neuropeptides. 2000;34:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Storr M, Hahn A, Gaffal E, Saur D, Allescher HD. Effects of endomorphin-1 and -2 on mu-opioid receptors in myenteric neurons and in the peristaltic reflex in rat small intestine. Clin Exp Pharmacol Physiol. 2002;29:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Coupar IM. The peristaltic reflex in the rat ileum: evidence for functional mu- and delta-opiate receptors. J Pharm Pharmacol. 1995;47:643-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Clark SJ, Smith TW. The release of Met-enkephalin from the guinea-pig ileum at rest and during peristaltic activity. Life Sci. 1983;33 Suppl 1:465-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Clark SJ, Smith TW. Peristalsis abolishes the release of methionine-enkephalin from guinea-pig ileum in vitro. Eur J Pharmacol. 1981;70:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Kromer W, Pretzlaff W, Woinoff R. Opioids modulate periodicity rather than efficacy of peristaltic waves in the guinea pig ileum in vitro. Life Sci. 1980;26:1857-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Zhang G, Murray TF, Grandy DK. Orphanin FQ has an inhibitory effect on the guinea pig ileum and the mouse vas deferens. Brain Res. 1997;772:102-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Yazdani A, Takahashi T, Bagnol D, Akil H, Watson SJ. A newly discovered neuropeptide orphanin FQ: its distribution and action in rat gastrointestinal tract. Gastroenterology. 1997;112:A1201. |
| 24. | Osinski MA, Pampusch MS, Murtaugh MP, Brown DR. Cloning, expression and functional role of a nociceptin/orphanin FQ receptor in the porcine gastrointestinal tract. Eur J Pharmacol. 1999;365:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Yüce B, Sibaev A, Haaken A, Saur D, Allescher HD, Göke B, Timmermans JP, Storr M. ORL-1 receptor mediates the action of nociceptin on ascending myenteric reflex pathways in rats. Gastroenterology. 2007;133:574-586. [PubMed] |
| 26. | Broccardo M, Agostini S, Petrella C, Guerrini R, Improta G. Central and peripheral role of the nociceptin/orphaninFQ system on normal and disturbed colonic motor function and faecal pellet output in the rat. Neurogastroenterol Motil. 2008;20:939-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Osinski MA, Brown DR. Orphanin FQ/nociceptin: a novel neuromodulator of gastrointestinal function? Peptides. 2000;21:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Fichna J, Schicho R, Andrews CN, Bashashati M, Klompus M, McKay DM, Sharkey KA, Zjawiony JK, Janecka A, Storr MA. Salvinorin A inhibits colonic transit and neurogenic ion transport in mice by activating kappa-opioid and cannabinoid receptors. Neurogastroenterol Motil. 2009;21:1326-e128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Kamysz E, Sałaga M, Sobczak M, Kamysz W, Fichna J. Characterization of the effects of opiorphin and sialorphin and their analogs substituted in position 1 with pyroglutamic acid on motility in the mouse ileum. J Pept Sci. 2013;19:166-172. [PubMed] |
| 30. | Saur D, Paehge H, Schusdziarra V, Allescher HD. Distinct expression of splice variants of neuronal nitric oxide synthase in the human gastrointestinal tract. Gastroenterology. 2000;118:849-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Saur D, Seidler B, Paehge H, Schusdziarra V, Allescher HD. Complex regulation of human neuronal nitric-oxide synthase exon 1c gene transcription. Essential role of Sp and ZNF family members of transcription factors. J Biol Chem. 2002;277:25798-25814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Storr M, Sibaev A, Marsicano G, Lutz B, Schusdziarra V, Timmermans JP, Allescher HD. Cannabinoid receptor type 1 modulates excitatory and inhibitory neurotransmission in mouse colon. Am J Physiol Gastrointest Liver Physiol. 2004;286:G110-G117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Sibaev A, Franck H, Vanderwinden JM, Allescher HD, Storr M. Structural differences in the enteric neural network in murine colon: impact on electrophysiology. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1325-G1334. [PubMed] |
| 34. | Sibaev A, Yüce B, Allescher HD, Göke B, Storr M. A new electrophysiological tool to investigate the spatial neuronal projections within the myenteric ascending reflex of the mouse colon. Clin Exp Pharmacol Physiol. 2008;35:744-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Osinski MA, Bass P, Gaumnitz EA. Peripheral and central actions of orphanin FQ (nociceptin) on murine colon. Am J Physiol. 1999;276:G125-G131. [PubMed] |
| 36. | Yazdani A, Takahashi T, Bagnol D, Watson SJ, Owyang C. Functional significance of a newly discovered neuropeptide, orphanin FQ, in rat gastrointestinal motility. Gastroenterology. 1999;116:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Pan YX, Xu J, Wan BL, Zuckerman A, Pasternak GW. Identification and differential regional expression of KOR-3/ORL-1 gene splice variants in mouse brain. FEBS Lett. 1998;435:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Menzies JR, Glen T, Davies MR, Paterson SJ, Corbett AD. In vitro agonist effects of nociceptin and [Phe(1)psi(CH(2)-NH)Gly(2)]nociceptin(1-13)NH(2) in the mouse and rat colon and the mouse vas deferens. Eur J Pharmacol. 1999;385:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Rizzi A, Bigoni R, Caló G, Guerrini R, Salvadori S, Regoli D. [Nphe(1)]nociceptin-(1-13)-NH(2) antagonizes nociceptin effects in the mouse colon. Eur J Pharmacol. 1999;385:R3-R5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Calò G, Rizzi A, Bodin M, Neugebauer W, Salvadori S, Guerrini R, Bianchi C, Regoli D. Pharmacological characterization of nociceptin receptor: an in vitro study. Can J Physiol Pharmacol. 1997;75:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Vaughan CW, Ingram SL, Christie MJ. Actions of the ORL1 receptor ligand nociceptin on membrane properties of rat periaqueductal gray neurons in vitro. J Neurosci. 1997;17:996-1003. [PubMed] |
| 42. | Yu TP, Xie CW. Orphanin FQ/nociceptin inhibits synaptic transmission and long-term potentiation in rat dentate gyrus through postsynaptic mechanisms. J Neurophysiol. 1998;80:1277-1284. [PubMed] |
P- Reviewer: Lambrecht NW, Senates ES S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/