Published online Aug 6, 2015. doi: 10.4292/wjgpt.v6.i3.32
Peer-review started: July 9, 2014
First decision: October 28, 2014
Revised: April 21, 2015
Accepted: May 16, 2015
Article in press: May 18, 2015
Published online: August 6, 2015
Processing time: 394 Days and 21.9 Hours
Primary biliary cirrhosis (PBC) is a chronic non-suppurative destructive intrahepatic cholangitis leading to cirrhosis after a protractive non cirrhotic stage. The etiology and pathogenesis are largely unknown and autoimmne mechanisms have been implicated to explain the pathological lesions. Many epitopes and autoantigens have been reported as crucial in the pathophysiology of the disease and T and B cells abnormalities have been described, the exact pathways leading to the destruction of small intrahepatic ductules are mostly speculative. In this review we examined the various epidemiologal and geoepidemiological data as well as the complex pathogenetic aspects of this disease, focusing on recent in vivo and in vitro studies in this field. Initiation and progression of PBC is believed to be a multifactorial process with strong infuences from the patient’s genetic background and by various environmental factors. The role of innate and adaptive immunity, including cytokines, chemokines, macrophages and the involvement of apoptosis and reactive oxygen species are outlined in detailed. The current pathogenetic aspects are presented and a novel pathogenetic theory unifying the accumulated clinical information with in vitro and in vivo data is formulated. A review of clinical manifestations and immunological and pathological diagnosis was presented. Treatment modalities, including the multiple mechanisms of action of ursodeoxycholate were finally discussed.
Core tip: Primary biliary cirrhosis (PBC) is a chronic non-suppurative destructive intrahepatic cholangitis leading to cirrhosis of largely unknown pathophysiology. We examined the epidemiological and geoepidemiological data as well as the pathogenetic aspects of PBC. A novel pathogenetic theory unifying the accumulated clinical information with in vitro and in vivo data is formulated. A review of clinical manifestations and immunological and pathological diagnosis was presented. Treatment modalities, including the multiple mechanisms of action of ursodeoxycholate are discussed.
- Citation: Kouroumalis E, Notas G. Primary biliary cirrhosis: From bench to bedside. World J Gastrointest Pharmacol Ther 2015; 6(3): 32-58
- URL: https://www.wjgnet.com/2150-5349/full/v6/i3/32.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v6.i3.32
Primary biliary cirrhosis (PBC), also known as chronic non-suppurative destructive intrahepatic cholangitis is characterized by a gradual destruction of small intrahepatic bile ducts. Addison et al[1] in 1851 published the first report of a patient with obstructive jaundice without evidence of large bile duct abnormalities. Dauphinee et al[2] in 1949 first used the term PBC but it was Ahrens et al[3] in 1950 that reported on a group of older women with progressive jaundice, pruritus, and hepatosplenomegaly. The basic histological description as chronic intrahepatic non-suppurative destructive cholangitis was reported by Rubin et al[4] in 1965.
Autoimmune mechanisms have been implicated to explain the disease evolution of a condition which is in fact a member of the wider vanishing bile duct syndrome. Many epitopes and autoantigens have been reported as crucial in the pathophysiology of the disease and T and B cells abnormalities have been described, the exact pathways leading to the destruction of small intrahepatic ductules are mostly speculative. However the underlying pathways leading to liver damage are still under investigation. In this review, we examine epidemiological, pathogenetic and clinical characteristics of PBC. Finally we compile the clinical information and the novel data gathered from in vivo and in vitro studies that are presented in this review in order to propose a unifying hypothesis for the pathogenesis of this complex disease.
There is a large variation of the incidence and prevalence of PBC worldwide (Table 1). Moreover there is a tendency to increase over the years. Thus, studies from the region of Victoria in Australia demonstrated that in an earlier population-based survey a prevalence of 19.1 per million was reported[5] while ten years later in the same region a 10-fold higher incidence was reported[6]. In a review of 37 older and more recent published studies the incidence of PBC ranged from 0.7 to 49 per million per year and the prevalence was estimated to range from 6.7 to 402 per million, with a tendency for higher values in the most recent studies[7].
| Ref. | Year of publication | Country/region | Period | Incidence (/106) | Prevalence (/106) |
| Watson et al[5] | 1995 | Australia/Victoria | 1974-1991 | 19.1 | |
| Metcalf et al[11] | 1997 | United Kingdom/Newcastle | 1987-1994 | 14.0-32.0 | 180.0 (1987) 240.0 (1994) |
| James et al[10] | 1999 | United Kingdom | 1987-1994 | 201.9 (1987) 334.6 (1994) | |
| Kim et al[12] | 2000 | United States/Minnesota | 1975-1995 | 27.0 | 402.0 |
| 1Prince et al[7] | 2003 | Multiple | 0.7-49.0 | 6.7-402.0 | |
| Sood et al[6] | 2004 | Australia/Victoria | 1990-2002 | 51.0 | |
| Corpechot et al[8] | 2008 | France | 2006-2007 | 9.0 | 207.0 |
| 1Boonstra et al[9] | 2012 | Multiple | 3.3-58.0 | 19.1-402.0 | |
| Koulentaki et al[13] | 2014 | Greece/Crete | 1990-2010 | 20.9 | 365.0 |
A prospective study performed in 2006-2007 in France shows that the incidence was 9 per million per year, with an estimate of 36 per million in women over 45 years with a prevalence of 207 per million[8], according to a recent systematic review the prevalence of PBC ranges from 1.9 to 40.2 per 100000 inhabitants[9].
Although it is usually stated that the disease is more prevalent in Northern Europe (e.g., England and Scotland)[10,11] or the Northern United States (e.g., Minnesota)[12], we recently reported a prevalence of 365 cases/million population in the island of Crete indicating that in South Europe the disease is highly prevalent with a figure among the higher published in the world[13].
Spatial and geographic differences in prevalence of PBC has been reported in several studies[14-16]. In the North East of England there was a clear spatial distribution of patients with clusters of cases identified in several urban areas (up to 13 cases/km2). No demographic or geographical factors could explain this variation, although the presence of unidentified environmental factor(s) was suggested[17]. Geographic clustering of PBC has also been reported in coastal First Nations of British Columbia, where disease has been recorded to be as high as 1 in 4 within generations of well-characterized multiplex families[18]. In our own study we identified loci of very high prevalence within the homogeneous population of the island of Crete. Prediction map identified areas of high risk (11 patients per 50 km2) in the Eastern part, while low risk areas (122 patients per 50 km2) were located in the Western part of the island[13].
It should be noted that all epidemiological estimations may represent only the tip of the iceberg since it has been reported and confirmed that the incidence of antimitochondrial antibody (AMA) positivity without liver disease is two times higher than the incidence of AMA positivity with liver disease[19,20]. The significance of these findings is unclear. Possibly they do represent a hidden reservoir of future overt PBC or alternatively this is only an immunological abnormality without clinical consequences. If one adds to these observations the very long and often asymptomatic course of the disease, it is clear that epidemiological studies in PBC have many problems as presented in a recent review[21].
As with many other diseases of unclear etiology it is stated that PBC is an autoimmune disease associated with genetic and environmental factors. Both, autoimmunity[22] and environmental factors[23,24] have been emphasized while several genetic associations in particular with recent genome-wide analyses[25-27] have been published. However this is a very general description that may fit to other diseases as distant to PBC as inflammatory bowel disease, rheumatoid arthritis or Hashimoto’s thyroiditis. We will try therefore to delineate these complex association and propose an unifying model to accommodate most aspects of the mechanism leading to the disease.
Epidemiological reports have shown that PBC is a heritable condition with a well proven familial prevalence. Family history of the disease has been reported to vary between 1.33% and 9.00%[9]. In our disease population familial PBC was found to be a high 9.9%. This may reflect the fact that in many parts of the island of Crete societies are relatively close and marriages between distant relatives are common[28]. The relative sibling risk in PBC has been estimated to be 10.5, a figure very close to other classical autoimmune disorders[29]. The existence of a first degree relative with the disease is an independent risk factor disease appearance, with an odds ratio of 6.8-10.7[30,31]. Moreover in monozygotic twins a concordance rate of 0.63 for PBC has been reported, the highest reported among autoimmune diseases[32]. All these data do imply that a strong genetic background participate to the pathophysiology of PBC.
Most gene associations are derived from human leukocyte antigen (HLA) associations. HLA data in European populations has been produced from GWAS and iCHIP studies[33] as for example in a large Italian cohort[34]. Data on HLA alleles were also provided from iCHIP studies by Liu et al[35] and Juran et al[36]. When these studies are combined it seems that in European populations, PBC has been associated with either risk haplotypes (DRB1*08:01-DQA1*04:01-DQB1*04:02 and DRB1*04:04-DQB1*03:02) or protective haplotypes (DRB1*11:01-DQA1*05:01-DQB1*03:01 and DRB1*15:01-DQA1*01:02-DQB1*0602). In the Italian study, the HLA associations were driven by their DRB1 alleles, either the risk alleles DRB1*08 and DRB1*14, or the protective allele DRB1*11.
Apart from HLA associations, genes related to the regulation of components of the immune system appear to be involved in PBC. Candidate genes like SPIB are implicated in the development of plasmacytoid dendritic cells and natural killer (NK) cells, or to the development of dendritic cells, like IRF5 and IRF8[37-39]. Genes that regulate innate immune responses have also been associated with PBC. Thus negative regulators of antigen presenting cells, like RPS6KA4 and DENND1B participating in the signal pathways by pattern recognition receptors and TNFRSF1A respectively are among candidate genes[40-43]. IRF5 in particular is an important mediator since it is also involved in macrophage polarization and Th1-Th17 differentiation and therefore might be a significant link between innate and adaptive immune response[44]. Genes that regulate adaptive immune response have also been associated with PBC like those that drive activated CD4+ve Th0 cells to differentiate into Th1 cells by IL-12 interacting with IL-12R.
Four GWAS and two iCHIP studies of PBC have been recently published[25,27,35,36,45-47].
GWAS studies of PBC from Japan confirmed the genetic heterogeneity of PBC[27]. Risk loci significant in Europeans were not associated with PBC in Japan, including 1p31 (the IL-12RB2 locus, P 1/4 0.054), 3q25 (the IL-12A locus, P 1/4 0.342) and 19q13 (the SPIB locus, P 1/4 0.180). By contrast, other loci like 9p32 (TNFSF15) and 11q23 (POU2AF1) important for the Japanese, were not identified in European populations[25,45,46]. Two excellent reviews on PBC genetics have been recently published[48,49].
Despite extensive studies of specific genes only weak associations were established in PBC. Environmental factors may therefore be equally important. The presence of geographical disease clusters provides evidence for the participation of largely unknown environmental factors in the pathogenesis of PBC. These factors have been recently reviewed[50,51]. A recent geoepidemiological study from Crete with a rather homogeneous and stable population has shown a large heterogeneity in the time-spatial distribution which together with the suggested route of disease spread strongly indicates the importance of environmental factors[13].
Infections, through the mechanism of molecular mimicry, are strong candidate environmental factors in the pathogenesis of PBC. A possible candidate epitope is the mycobacterial hsp65 sharing a common motif with the PDC-E2 antigen[52]. The same motif has been identified in Lactobacillus delbrueckii as the target of IgG3 antibodies in PBC sera[53]. This motif, namely the PDC-E2 221-226 is a candidate common epitope in the model of molecular mimicry. However another pathogen seems to be the most likely candidate in molecular mimicry. Reports have been focused on the intestinal commensal proteobacterium Novosphingobium aromaticivorans (N. aromaticivorans), as an etiological factor in many cases of human PBC[54,55]. N. aromaticivorans was found in fecal samples from 25% of patients with PBC as well as in controls. PDC-E2 from this bacteria has high homology to human PDC-E2, and AMAs in serum samples from patients with PBC have a 1000-fold stronger reaction against N. aromaticivorans PDC-E2 than against Escherichia coli PDC-E2[56,57]. Based on these clinical studies the group of Gershwin has reported on a mouse model of a liver disease closely resembling human PBC, initially triggered by this bacterium[58]. The pathology of liver lesions and the presence of anti-PDC-E2 antibodies were related to the mouse genetic background, the liver persistence of Novosphingobium and the liver infiltration by NKT-cells activated by Novosphingobium glycosphingolipids. The interaction of glycosphingolipids of the Novosphingobium with NKT-cells may be the link between an environmental pathogen and the immune reactions that have been described in PBC. A previously unidentified, infection with Novosphingobium might also explain the reported redistribution of NKT-cells from the blood to the livers of PBC patients and the biliary epithelial expression of CD1d[59,60].
Other infective factors associated with PBC include lipopolysaccharide (LPS), lipoteichoic acid, Helicobacter, β-retrovirus, P. acnes, E. coli and Chlamydia. The evidence however for their implication is not as strong as for the previous infectious agents[61].
Non microbial environmental factors seem to be related to disease progression or disease severity. Thus familiar occurrence of disease, smoking, urinary tract infections or other autoimmune conditions seem to be detrimental while oral contraceptives might have a protective role[31].
There might be a link between genetics and the influence of environmental factors. The mitochondrial autoantigen PDC-E2 contains lipoic acid; PDC-E2 captures electrons to alternate oxidation and reduction of a disulfide bond within lipoic acid. In genetically susceptible individuals, drugs or chemicals that modify this disulfide bond might lead to loss of tolerance to PDC-E2. This protein is well conserved throughout evolution, so loss of tolerance could also result from an immune response against similar epitopes in bacteria (molecular mimicry)[58]. In addition to bacteria, chemical xenobiotic agents might induce PBC[14]. Some halogenated organic compounds can attach to specific epitopes of PDC-E2 and induce production of antibodies that have higher affinities for modified mitochondrial epitopes than for normal PDC-E2. One such agent is 2-octynoic acid, which is a component of many cosmetics like nail polish; in vivo and in vitro data indicate its role in autoimmune biliary disease[62,63].
Non-obese diabetic mice injected with bovine serum albumin-conjugated 2-octynoic acid had high titers of AMAs and developed histologic features of PBC, including liver granulomas and infiltration of portal tracts with CD8+ve T lymphocytes[64]. However in our population of PBC patients no association of the disease with the use of nail polish was found. This might be due to either a different specific genetic background of our patients or to the lack of significant quantities of octynoic acid in the nail polish brands used by our patients[28].
A characteristic of PBC is considered to be the loss of tolerance to a mitochondrial antigen, the PDC-E2, a member of the 2-oxoacid dehydrogenase complexes (2-OADC). The antigenic epitopes are located within the inner lipoyl-binding domain overlapping amino acids 212-226[65-67]. Earlier reports based on the inhibition of 2-OADC enzymatic activity by AMA and the presence in the portal tracts of B cells producing anti-PDC antibodies,suggested a pathogenetic role for AMAs[68]. The best evidence for their pathogenetic role is the presence of secretory IgA anti-PDC in patients saliva, bile and urine of patients which inhibit the enzyme activity[69-71]. It is however possible that AMAs might indirectly implicated in pathogenesis through apoptotic biliary bodies that activate innate responses[72]. Moreover, AMAs is not a specific finding in PBC, since they are occasionally present in other autoimmune and non-autoimmune liver diseases. Therefore loss of tolerance to PDC-E2 alone does not seem to be sufficient to cause autoimmune cholangitis[73,74]. Against the pathogenetic role of AMAs is also the presence of the clinical condition of the so called autoimmune cholangitis or better AMA-negative PBC, a disease clinically and histologically identical to PBC but without detectable AMA. The present evidence does not favour the role of AMA in the pathogenesis of PBC[75].
There have been other antibodies that, although they are not pathogenetically associated, they influence the progress or the severity of the disease like the autoantibodies against the nuclear pore proteins gp210 and p62[76]. The combination of anti-nuclear envelope antibody (ANEA) and anti-gp210 identified a subgroup of patients with increased fibrosis and inflammation and was associated with poor prognosis in our group of PBC patients[77]. There is recent evidence to support deregulated B-cell immune responses in patients with PBC[78].
Adaptive CD4+ve and CD8+ve T-cells and innate responses have been implicated in PBC pathogenesis, following environmental insults in genetically susceptible individuals[72,79,80]. The role of bacterial and viral factors has already been discussed. Biliary epithelial cells express molecules of the toll-like receptor (TLR) family that recognize pathogen-associated molecular patterns (PAMPs) thus triggering the innate immune responses. It is also suggested that cholangiocyte damage might result from a combined dysregulation of TLRs and of peroxisome proliferator-activated receptor-gamma (a negative regulator of intracellular signaling) resulting in Th1-predominant cytokine effects. Moreover, CD4-positive, IL-17 secreting Th17 cells are important for the development of inflammation as tey infiltrate biliary ductules and are directly related to the biliary innate responses to PAMPs[81-85].
In PBC, besides IL-17+ cells infiltrating damaged bile ducts, hepatic NK-cells active against biliary epithelial cells are found, but their role in the loss of immune tolerance reported in PBC has not been elucidated[86,87]. Earlier reports have demonstrated the presence of CD4 and CD8 T-cells reactive against the human PDC in PBC livers during the earliest disease states[88-92].
CD4+ CD28-ve cells are markedly increased as intraepithelial lymphocytes within damaged bile ducts and may therefore participate in the bile duct damage[93]. The residues 159-167 of PDC-E2 seem to be important in the pathogenesis since HLA DR4*0101-restricted T cell epitope, spanning residues 159-167 have been identified[94-98]. while CD8 T cells from PBC livers are cytotoxic against PDC-E2 159-167 pulsed autologous cells[99].
An interesting model implicating T cells in the pathogenesis of PBC is a mouse manipulated for T cell-restricted expression of a dominant-negative (dn) form of transforming growth factor-beta receptor II (TGFbRII). A PBC-like disease, not identical to the human condition develops which further connects the T cell role with the involvement of two cytokines namely TGFb and IL-12[100].
IL-12 consists of the two p35 and p40 subunits, while TGFb signaling is essential in the development of the pro-inflammatory Th17 cells[101]. Further data from this model indicate that it is the p40 subunit which is fundamental for development of bile duct damage[102]. The importance of IL-12 signaling and the PBC-like disease of the dnTGFbRII mice is further supported by genetics relating the IL-12 pathway with human PBC as mentioned in the genetics section[45].
In PBC, as already mentioned, T cells, particularly CD8 cytotoxic cells infiltrating the liver are mandatory for bile duct damage[97]. However in the CD25-negative mouse model, lack of CD8 cells attenuates but does not abolish bile ductular destruction, indicating that an additional mechanism of destruction is also present in PBC[103]. Equally important is the role of T regulatory cells. Animal experiments indicate that deficiency of regulatory T cells (Tregs) results in the development of AMA +ve, autoimmune bile ductular lesions anti[104]. Moreover a reduction of Tregs has been found in the portal tracts of PBC patients[105].
Kupffer cells are the resident liver macrophages and constitute approximately 80% of body macrophages. Kupffer cells respond to all TLR ligands by increasing the production of tumor necrosis factor (TNF)-α, IL-1, IL-6, IL-12, IL-18, and IL-10. Liver DCs express all members of the TLR family, although TLR5 expression is low. Hepatic pDCs activated by TLR7 and TLR9 ligands, produce TNF-α, IL-6, IL-12, and IFN-α[106,107]. Based on the reported connection between the biliary tract and the intestinal lumen, it is possible that intestinal-derived bacterial products could contribute to PBC progression[61,84,108]. In response to ligands for TLR2, TLR3, TLR4, TLR5, and TLR9, PBMCs from PBC patients produce increased amounts of pro-inflammatory cytokines compared to PBMCs from healthy subjects[109]. In PBC patients, high expression of TLR9 leads to increased intracellular immunoglobulin M and AMAs production by B cells[110,111]. Furthermore, a number of TLR9 SNPs may cause B cells to produce higher amount of intracellular immunoglobulin M[112]. High expression of TLR3 and type I interferon in macrophages surrounding portal tract and hepatocytes has been reported in the livers of PBC patients[113]. Liver macrophages produce IFN-α through TLR3 signaling leading to synergistically enhanced LPS-induced NK-cell cytotoxicity to autologous BECs. Furthermore, in PBC patients liver NK-cell cytotoxicity is higher compared to controls, when these cells are incubated with poly I:C and LPS-primed liver macrophages[86].
Mice immunized with 2-octynoic acid have been found to develop a type of autoimmune cholangitis with AMAs, similar to human PBC, but did not develop liver fibrosis. CD8 T cell infiltration, inflammatory cytokine expression and liver fibrosis were induced by Poly I:C treatment in this mouse model of PBC, thus further supporting the impact of TLR3 signaling in PBC[114].
Earlier reports have demonstrated the predominance of a Th1 cytokine profile in PBC with a parallel relative decline of Th2 prevalence[115-117]. Overall, the data suggest that the IL-12 cytokine pathway and downstream JAK-STAT signaling is significant in PBC pathogenesis. The interaction of IL-12 and IL-12 receptor leads to the production of one more candidate gene in PBC, the STAT4 transcription factor that activates of Th1 cytokines production. More experimental data concerning the specific role of IL-12 signaling are needed in PBC and animal models. Interestingly, early onset of biliary cirrhosis has been reported in an IL-12-deficient child but the immediate relevance of this finding to PBC is unclear[118].
Another important cytokine possibly involved in PBC pathogenesis is TGF-β as mentioned before. We have demonstrated that the serum profile of the 3 TGF-β isoforms is different in the liver disease groups studied. An increase in TGF-β3 is a characteristic of PBC irrespective of stage and therefore it may be pathogenetically important. Results from the hepatic vein samples indicate that the liver seems to be the source of the TGF-β abnormalities[119]. Plasma levels of IL-6 are decreased in PBC[120] but monocytes from PBC patients secrete more IL-1 and IL-6 after in vitro challenge with TLR ligands[109] and increased liver expression of IL-6 was described in PBC[117].
As mentioned before, many mechanisms of biliary epithelium death have been proposed, including various cytotoxic T cells subpopulations and NKT-cells[82,83,121-124]. Additional destructive mechanisms involve the activation of TNF, CD 40, and Fas receptors[125]. All these mechanisms however depend on the recruitment of these cells in the area of destruction. Chemokines are a discrete class of cytokines that regulate leukocyte recruitment, positioning and retention in tissues. Epithelial cells produce the chemokine Fractalkine (CX3CL1) and its receptor CX3CR is expressed in CD8+ve and CD4+ve T cells[126]. An overexpression of fractalkine has been reported in injured bile ducts of PBC, while CD4+ve and CD8+ve lymphocytes expressing CX3CR1 predominate in portal tracts and within the biliary epithelium. Other chemokine receptors like CXCR3 and CCR5 are mostly expressed on Th1 cells[124,127]. In early PBC Th1 cells positive for the CXCR3 densely surround the damaged bile ductules. As a consequence the ratio of CXCR3-/CCR4-positive lymphocytes (Th1/Th2) is significantly higher in early compared to late stage PBC[128]. In more recent reports the significance of two other chemokines, namely the IFN-γ-inducible protein 10 (IP-10, CXCL10) and monokine induced by IFN-γ (MIG, CXCL9) has been stressed. These are produced by macrophages, i.e., the Kupffer cells in the liver and are also implicated in the recruitment of Th1 cells via the same receptor CXCR3. Alternatively the sinusoidal endothelial cells may be another site of their production[129,130]. MIG and IP-10 are also secreted by biliary epithelial cells and activated stellate cells[131,132]. Increased plasma IP-10 and MIG levels have been reported in PBC patients accompanied by an increase in their mRNA in the liver tissue. Most importantly increased plasma levels were also found in their first degree relatives[133]. The third CXC chemokine I-TAC was not detected.
We recently reported a significant increase of plasma MIG and IP-10 compared to normal controls. The CXCR3A variant mRNA was found in PBLs from all PBC patients as well as in normal controls. However the CXCR3B variant mRNA was expressed in 4/20 (19%) normal controls and all 20 PBC patients. These data suggest a possible pathogenetic implication of MIG and IP-10 chemokines and their receptor in the pathogenesis of PBC[134]. Recent papers demonstrated that these chemokines and their receptors are also involved in the hepatic recruitment of Tregs and the pro-inflammatory Th17 and Tc17+ lymphocytes[135,136].
There is also evidence that IP-10 is a pro-fibrotic factor participating in the interactions between hepatic stellate cells and NK cells which play a central role in the regulation of hepatic stellate cells activity and fibrosis. IP-10 blockade might be a future target for treatment of liver fibrosis[137].
Experimental and clinical evidence suggests that apoptosis is the most important mechanism of biliary epithelial cell damage. Apoptotic biliary epithelial cells cells identified by the TUNEL reaction are frequently described in PBC liver tissue[138]. Apoptosis markers have been demonstrated within the portal tracts in liver specimens from PBC patients[139,140] including a reduced expression of the anti-apoptotic protein bcl-2[141]. Cells responsible for apoptosis induction are the cytotoxic CD8+ve T-cells. Biliary epithelia, unlike other cell types, have a rather unique characteristic considering the fate of the pyruvate dehydrogenase complex during apoptosis. Glutathione fails to bind to the lysine-lipoyl residue of the dehydrogenase complex and thereby the autoreactive epitope is not cleaved[142].
PDC-E2 is a ubiquitous protein present in mitochondria of nucleated cells; biliary epithelial cells translocate intact PDC-E2 to apoptotic bodies and create an apotope, which is immunoreactive. PDC-E2 remains intact and immunogenic during biliary epithelial cell apoptosis; This allows biliary PDC-E2 to be exposed to dendritic cells and its epitopes to be presented to T cells in draining lymph nodes, leading to production of AMAs[72,80,143]. However in established transgenic mice that constitutively express PDC-E2, aberrant expression of PDC-E2 in the cytoplasm of BECs was not followed by biochemical, serological or histological features of PBC. The results indicated that PDC-E2 expression BECs is not sufficient for the initiation of autoimmunity. Additional factors may be required to establish a model of PBC[144].
There are few data on the significance of reactive oxygen species (ROS) in bile ductular damage in PBC. Biliary epithelial cells express low levels of glutathione-S-transferase with a resultant decrease of intracellular glutathione. On the other hand, lipid peroxidation seem to be increased as reflected by increased perinuclear expression of 4-hydroxynonenal[145].
Other antioxidants including vitamin E, retinol, alpha-tocopherol, total carotenoids, lutein, zeaxanthin, lycopene, alpha and beta-carotene are reduced in PBC[146-148]. Total serum antioxidant capacity (measured with chemiluminescence) is reduced in PBC patients[149]. In contrast, we have reported increased levels of corrected total antioxidant capacity in PBC[150] a fact that may reflect a compensatory but probably not sufficient increase to counteract an increased ROS production.
In vitro studies further support the role of ROS as an effector mechanism in bile duct damage in PBC. Ursodeoxycholic acid (UDCA), the current drug of choice in PBC treatment, is a potent ROS scavenger, inhibiting mitochondrial oxidative stress and lipid peroxidation in a dose-dependent manner[151-153]. Indirect evidence for the role of ROS comes from the rat bile duct-ligated model. Lipid peroxidation is a late event in the bile ligated model and a correlation seems to exist between lipid peroxidation and the activation of inflammatory cells[154,155]. In this model overproduction of Free radicals is due to activation of NF-κB resulting in increased production of the proinflammatory cytokines TNF-α, IL-6 and IL-1b[156]. Moreover, in vitro experiments have shown that retention of several bile acids like taurochenodeoxycholic acid and taurocholic acid lead to hepatocyte damage producing hydroperoxide by mitochondria[157,158]. The same bile acids induce hepatocyte apoptosis in a time and concentration-dependent manner due to ROS generation by mitochondria[159]. These findings are relevant to human PBC because increased bile acid retention is a feature of at least late PBC.
A further connection of increased ROS and apoptosis of biliary epithelia has been provided by Celli et al[160] They demonstrated, that the reduction in the intracellular level of the antioxidant molecule glutathione leads to increased degradation of the anti-apoptotic bcl-2 protein and therefore to an increase in biliary epithelial apoptosis.
Every effort to elucidate the complex pathogenetic mechanisms leading to the development of PBC should account for two facts that are critical in the disease pathophysiology. First, the main PDC auto-antigen is an inner mitochondrial membrane component and therefore any attempt of the immune cells to contact its epitopes should overcome three different membranes. This problem has been partly answered by the unique characteristic of biliary epithelial cell apoptosis and the retention of immunologically active antigens within the apoptotic blebs as mentioned before. Moreover apoptotic blebs does not seem to be an early phenomenon. The initiation event in a genetically predisposed individual still is not known. Second, PBC initially affects only the small intrahepatic bile ductular cells, while the antigen is widely distributed in many tissues including the extrahepatic bile ducts.
Pathogenetical models proposed so far consider as the crucial event the break down of T cell self tolerance to PDC epitopes. The generation of antibodies against PDC by themselves is not enough to damage the biliary ductules,as already mentioned[144]. The molecular mimicry model of self-tolerance breakdown was proposed as a more logical approach compared with the rather obscure concept of autoimmunity[161].
According to this theory,bacterial or retroviral infections, probably trigger an immune attack leading to apoptosis of epithelial cells. This T-cell attack is mediated by TLR interaction with a micro-organism epitope cross-reacting with a self-PDC epitope. The retroviral etiology of PBC has been reviewed in detail[75].
Endotoxins and PAMPs are potent activators of the immune response. These bacterial products are normally cleared by the liver and eliminated in bile[162]. Lipid A is dephosphorylated and inactivated through the action of alkaline phosphatase in bile[163]. Lipid A, the immunogenic constituent of gram-negative bacterial LPS, and other PAMPs accumulate in hepatocytes and biliary epithelium, thus contributing to small bile duct inflammation[82,84,164,165]. PBC patients exhibit a strong immune response to LPS[166,167]. The loss of tolerance to bacterial products could be at least partly secondary to cholestasis and, more specifically, to impaired alkaline choleresis or a defect in transporters involved in the generation of ductal choleresis[168].
Apart from bacterial antigens viruses have been investigated as triggering agents in PBC.Thus viral particles have been described within biliary epithelial cells of PBC patients, and antibodies against retroviruses have been found in their sera[169,170]. In a cohort of patients genetic material of a human beta retrovirus (95% homologous to the mouse mammary tumour virus) has been identified in the lymph nodes of 75% of patients[171].
Epidemiological evidence may be interpreted as supportive of viral implication in the pathogenesis of PBC. Description of PBC clusters among individuals who emigrated from low PBC to high PBC prevalence areas and PBC cases among unrelated individuals living in the same house are some of these. Furthermore, in patients treated with tacrolimus PBC recurrence after liver transplantation occurs earlier and is mere severe, while the contrary is observed with cyclosporine administration[172-174]. However the retroviral etiology of PBC remains debatable[175].
In accordance with the molecular mimicry model, Jones described an alternative pathogenetic model where virals or bacterial epitopes with homology to PDC are the initial trigger[176]. Self-PDC reactive T cells escape negative selection in the thymus, because of the low affinity of their T cell receptor towards the the complex of self-peptide and major histocompatibility complex (MHC). An interesting feature of this model is that the state of activation of APC may determine the efficacy of antigen presentation and promote tolerance breakdown[109].
As mentioned before,several other cells of the innate immune system are implicated in the pathophysiology of PBC. Granulomas common in PBC, increased levels of polyclonal IgM, hyper-responsiveness to CpG oligodeoxynucleotides and increased numbers of NK cells are related to innate responses. Bacterial and viral epitopes induce innate immune responses through binding to TLRs. TLRs were described in cultured human biliary epithelial cells[109-111,177]. These findings make the inclusion of innate immunity mandatory in any pathogenetic model of PBC.
Another finding connects senescence and shortened telomere length as a unique feature of damaged BEC in PBC. Significantly decreased telomere length in the affected biliary epithelial cells compared to histologically normal BEC in both PBC patients and controls has been reported Immunohistochemistry confirmed cellular senescence. These changes are not inconsistent with the retroviral hypothesis, since viruses can directly damage the DNA in susceptible individuals[178].
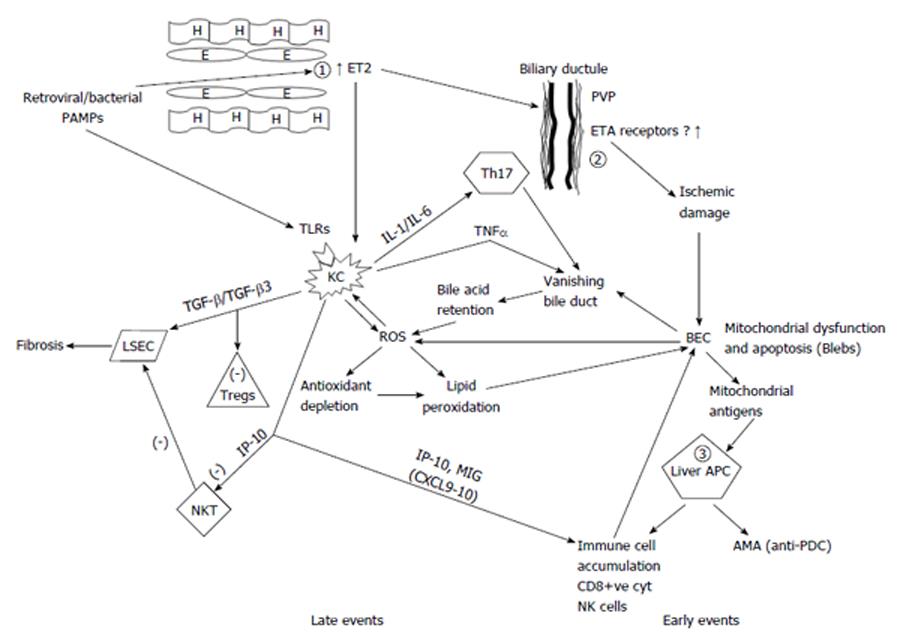
A significant increase of endothelins, particularly ET2 (and to a lesser extent of ET1) both in peripheral blood and in the hepatic vein of PBC patients from the early stages of the disease has been reported in our earlier study[179]. We then proposed a new hypothesis for the pathogenesis of PBC. Here we modify our previous hypothesis to incorporate new data that have emerged since our previous publication[180]. We consider that a primary defect is the overproduction by the liver endothelial cells of ET2 (and to a lesser extent ET1). This defect might be genetically controlled. It is possible that the initiating event in genetically susceptible patients is the effect of either a retrovirus or a pathogen like N. aromaticivorans or PAMPs. The presence of scavenger receptor type B capable of internalizing foreign antigens has been described in endothelial cells. A strongly antigenic component of gram positive bacteria namely lipoteicholic acid, has been described in endothelial cells of PBC patients[181]. ET2 may induce Kupffer cells to produce pro-inflammatory cytokines, such as IL-1 and IL-6. This has been already described for mouse peritoneal macrophages[182]. On the other hand, PAMPs acting on the TLRs of the Kupffer cells may lead to the production of TNF-α and further augmentation of IL-1 and IL-6. ET2 has a similar peptide homology with CXC chemokines and through ETB receptor on macrophages is a strong chemoattractant of these cells[183]. Indeed macrophages comprise 30% of the cells infiltrating portal areas and are mostly found around damaged bile ducts[184]. Electron microscopy verifies the presence of activated macrophages that differentiate into epithelioid cells near bile epithelial cells[185].
Many cells at the periphery of epithelioid granulomas are MCP2 and MCP3 positive and almost 60% co-express CD68, a finding compatible with their macrophage origin[186]. A possible link with the progression to cirrhosis in stage 3 and 4 PBC is the observation that Kupffer cells and myofibroblasts are increased and closely associated in periseptal and periportal areas. This might be an indication of an interaction between Kupffer and stellate cells leading to fibrosis[187].
Endothelins also cause contraction of stellate cells thus helping in the early appearance of portal hypertension in PBC[188]. In agreement with this hypothesis are findings from our group indicating that disease specific antibodies against liver sinusoidal cells circulate in PBC[189]. In addition we reported that polymorphisms related to endothelial cells were associated with PBC. Both eNOS intron4 VNTR and eNOS exon7 G894T SNP were related to increased risk in PBC while endothelin-1 rs2071942 ‘‘A’’ and rs5370 ‘‘T’’ alleles had a tendency for association with disease progression[190].
Bile ducts are supplied with blood only from hepatic arteries; the epithelial cells receives blood from a network of capillaries originating from the terminal branches of the hepatic artery. It is called the peribiliary vascular plexus (PVP) and drains into the sinusoids. This specific vascular supply, is responsible for the extensive involvement of the interlobular bile ducts in ischemic injury[191]. Reduced perfusion of this network can cause extensive bile duct damage[192].
There is evidence that endothelins (ETs) and nitric oxide are the principal regulators of circulation of the PVP[193]. We therefore propose that patients with PBC an initiating damage of the biliary epithelium is caused by ischaemia due to ET2 induced vasoconstriction observed from the ealy stages PBC. This finding was disease specific since it was not observed in other chronic liver disease patient with or without cirrhosis[179]. Ischaemia might then lead to apoptosis of bile ductular cells a mechanism already reported in epithelial destruction of PBC. As mentioned earlier, biliary epithelial cells undergo a unique apoptosis with an antigenically active PDC complex shed from mitochondria into the cytoplasm as early as 6 h after induction of apoptosis possibly through the action of caspase 3[194]. These immunoreactive epitopes, are then taken up by peribiliary antigen presenting cells expressing MHC II (this uptake might be either genetically regulated or alternatively be induced by proinflammatory cytokines[195]. This is a mechanism that could explain the proposed similarities between PBC and graft vs host disease (GVHD)[196-198] which is also attributed to endothelial cell injury[199]. Interestingly in graft vs host disease observed after small bowel transplantation, increased levels of ET1 have been histochemically demonstrated in endothelial and epithelial cells some days before GVHD, strongly suggesting a pathogenetic significance[200]. An additional feature of this model is that increased secretion of chemokines IP-10 and MIG possibly by endothelial or Kupffer cells is an early event that leads to accumulation of immune cells and also inactivation of NKT-cells by IP-10 with resultant reduced apoptosis of liver sinusoid endothelial cells[137].
A possible role for TGF-β abnormalities have also been incorporated in this model. It is TGF-β1 that modulates FoxP3 expression and the regulatory activity of CD4 cells[201]. Could it be that TGF-β3 influences the development of Tregs? Differences in TGF-β isoforms may lead to a deranged balance between Tregs and Th17 cells. We have also proposed that these findings may be the causative link leading to Treg and/or Th17 deregulations reported in PBC. The presence of increased local levels of TGF-β3 (and the concomitant relative lack of TGF-β1) in conjunction with increased levels of IL-6 may shift the balance towards an increased activity of the proinflammatory Th17 cells adding to the CD8 cytotoxic lymphocytes destructive mechanism, while at the same time leading to functionally defective Treg. The increased presence of TGF-β3 instead of TGF-β1 could be a further mechanism that favors Th17 activity since one can postulate that TGF-β3 could either be associated with functionally defective Tregs or could directly favor the increase of Th17. Further evidence for such a hypothesis is provided by a report that mice with mutation of the gene encoding the FoxP3 transcriptional factor developed AMA positivity and features resembling PBC with increased levels of IL-17 and IL-23, cytokines associated with Th17 cells[104].
The proposed model requires that additional aspects have to be verified in future. An important point is that the increased concentration of ET2 we reported was also observed in the systemic circulation.The vasoconstriction and the consequent ischemic injury therefore ought to be present in other organs away from the liver and PVP. This liver selectivity might be due to increased expression or increased affinity of ET receptors for ET2 in the PVP of PBC patients, also genetically determined, but this explanation requires further research.
Our proposed model is diagrammatically presented in Figure 1 and pathophysiological abnormalities are subdivided into early and late events. This hypothetical model has several advantages. An interaction between innate and adaptive immunity in the pathogenesis of PBC is described. The initiating event involves innate immunity while adaptive immunity causes perpetuation of the disease even after disappearance of the initiating event. It also integrates the role of infective agents whether retroviral or bacterial and the similarity of PBC with GVHD. Interaction of endothelial and Kupffer cells with stellate cells allows for the development of fibrosis and cirrhosis.
In this model AMA production is a secondary phenomenon rather than pathogenetically related to bile ductular damage. Therefore the existence of AMA negative PBC is also explained as well as the presence of many other autoantibodies both disease and non-disease specific. Most importantly, it is recurrence with the idea of a complex genetical predisposition as it offers many levels for genetic control as outlined before. Candidates are endothelial cells (and possibly Kupffer cells), the perivascular plexus and ET receptor expression and affinity and peribiliary antigen presenting cells that might be genetically hyper-responsive. Furthermore some of them may be estrogen dependent, thus explaining the extreme female prevalence of the disease, but this requires further research.
The typical PBC is characterized by a gradual long term disappearance of small intrahepatic bile ductules and a parallel long term increase in fibrosis, resulting in biliary cirrhosis over a period of 10-20 years. Ten percent to twenty percent of patients, are presented with fluctuating or persistent biochemical and histological evidence of auto-immune hepatitis. These patients have a more rapid progression of liver fibrosis and liver failure. A small minority of patients, develop a very rapid onset of vanishing bile duct syndrome with serious cholestatic jaundice and quick progress to cirrhosis in less than 5 years[202,203]. At diagnosis, many patients are asymptomatic diagnosed at a routine check by an increase in cholestatic liver enzymes. Approximately 30% of them will remain asymptomatic for many years. The rest will develop symptoms within the next 4 years. The commonest symptom at presentation is fatigue followed by pruritus (21% and 19% respectively)[204-206]. Severe fatigue impairs quality of life and appears in up to 80% of PBC during the course of the disease but it is not correlated with disease severity[207,208].
However symptoms of autonomic dysfunction like orthostatic hypotension, excessive daytime somnolence and sleep disturbance have been reported[209]. The pathophysiological mechanisms of chronic fatigue in PBC are not clear. Treatment is particularly difficult. UDCA is often ineffective while liver transplantation can not ameliorate the symptom. The second commoner symptom is pruritus in 20% to 70% of patients and can be debilitating at times[210]. The severity of cholestasis and the histological stage of the disease do not correlate with the intensity of pruritus. In our population pruritus is extremely rare at any stage of the disease for unknown reasons. The pathophysiology is obscure and the molecules that may be responsible for itching in cholestasis have not been identified. Proposed causative agents include bile salt metabolites, progesterone metabolites, histamine and endogenous opioids but the existing evidence for the implication of these agents is weak[211,212].
Cutaneous manifestations with various degrees of severity are common in PBC patients. We have described fungal infections of the skin even in the earlier histological stages. Plantar mycoses, onychomycoses, and interdigital mycoses were more frequent in PBC patients compared to normal controls. Dermographism and melanosis were also very common. In as many as 38.7% of patients the presenting symptom was a dermatologic lesion[213].
An increased portal pressure defined as the difference between the wedge and free pressure in the hepatic veins is frequently found in PBC. A reliable predictor of survival is the stability or improvement of this pressure gradient[214,215]. Cirrhosis is present only at stage 4, but variceal hemorrhage has been described early in the disease due to early development of nodular regenerative hyperplasia[216].
A relative lack of intestinal bile acids leads to malabsorption of calcium and vitamin D and hence to osteoporosis. This is usually a late finding. Decreased osteoblastic and increased osteoclastic activity have been incriminated in the progress of osteoporosis in PBC patients[217,218].
Regarding breast cancer, both increased risk[219,220] and equal risk[221] compared to a healthy women have been reported in PBC patients. The incidence of hepatocellular carcinoma in advanced PBC is more or less similar to other types of cirrhosis and is more frequent in male patients. The incidence of hepatocellular carcinoma (HCC) amongst patients with a diagnosis of PBC is estimated at 0.36 per 100 person years. An increasing risk for HCC has been observed in the most advanced stages of PBC[222-224]. As with other autoimmune diseases PBC has been reported to be associated with many immune-mediated diseases. Thyroid abnormalities are often reported in PBC, sometimes preceding its diagnosis[225]. Other autoimmune diseases develop in approximately 33% of patients with PBC (for example scleroderma, Hashimoto’s disease, Raynaud syndrome, and Sjögren’s syndrome), as do cholestasis or pruritus during pregnancy[30,31,226-228].
Dyslipidemia was found in 69.4% of our patients, in 60% of their first degree relatives and in 40.9% of the study controls.Multivariate analysis confirmed the association with the presence of at least another autoimmune disease while a cancer history was mere frequently reported in patients than in controls (P = 0.033)[28]. Coeliac disease is far more common in PBC compared to inflammatory bowel diseases and has been reported in up to 6% of PBC patients[229]. However this is not true in all populations and criteria for diagnosis should always include duodenal and small bowel biopsies. In our series of patients a very high proportion of false positive results of celiac disease associated antibodies was found. Twenty-one percent of PBC patients and 35% of patients with AMA negative PBC had anti-gliadin antibodies while the corresponding numbers for anti-transglutaminase were 10% and 18% respectively. By contrast anti-reticulin and anti-endomysial antibodies were not detected. Duodenal and small bowel biopsies performed in two thirds of patients with either AMA positive or AMA negative PBC patients with at least one antibody present, failed to confirm histologicaly even a single case of celiac disease and therefore in our patient population an increased risk for celiac disease could not be demonstrated. In accordance with our findings a high prevalence of false-positive anti-gliadin and anti-tissue transglutaminase antibodies has been reported in chronic liver disease[230].
Presence of serum AMAs, elevations of cholestatic enzymes mainly of alkaline phosphatase and increased levels of IgM immunoglobulin in serum, are the criteria used for diagnosis of PBC. The M2 subclass of AMAs is considered highly specific for PBC. The presence of two criteria indicate a probable diagnosis, but a definitive diagnosis requires all three[231,232].
Histology compatible with PBC, although usually not necessary for diagnosis, is mandatory for identification of the histological stage of the disease.
Various other autoantibodies are frequently found in PBC. Most prominent are the antinuclear antibodies (ANAs) reported in 30% of PBC patients[233-235]. The so-called “multiple nuclear dots” ANA pattern corresponds to the Sp100 and Sp140 antigens, pro-myelocytic leukemia nuclear body proteins and small ubiquitin-like modifiers[236,237] is considered specific for PBC. A second immunofluorescence pattern of AMAs, the “nuclear membrane” (rim) pattern, corresponds to ANEAs, such as gp210 and nucleoporin p62[238,239]. Earlier reports indicated that the anti-gp210 antibodies are specific for PBC and their presence is associated with more severe and active disease[76,240]. An increased expression of gp210 on the nuclear envelope of bile ductules and portal mononuclear cells and peri-portal hepatocytes was reported by Nakamura et al[241] who also confirmed their association with active disease. We also reported that 46.9% of patients with PBC have detectable ANEAs and 21% of them had detectable anti-gp210 antibodies in a subgroup of PBC patients with histologically advanced disease with poor prognosis[77].
The role of serum surrogate markers in PBC diagnosis is doubtful to say the least. We recently reported on simple biochemical markers of liver fibrosis in patients with PBC and other chronic liver diseases. Significantly increased levels of hyaluronan and collagen IV, were found in all chronic liver diseases compared to controls, but laminin was increased only in HBV and HCV cirrhosis. Serum Hyaluronan was significantly increased in late PBC compared to early stage disease (154.5 ng/mL; 95%CI: 55.3-764.4 and 54.5 ng/mL; 95%CI: 27.3-426.9 respectively, P < 0.05). The areas under the curve for late PBC were relatively high for hyaluronan and collagen IV (0.74 and 0.70 respectively) and low for leptin and laminin (0.63 and 0.59 respectively). Moreover hyaluronan had 96% sensitivity and 90% Negative Prognostic Value for late stages of PBC. However no single measurement could differentiate between early and late stage PBC. Serum hyaluronan nonetheless proved to be a useful serum marker for sequential follow up studies in PBC[242]. Other surrogate markers like APRI, Forns, Fibroindex and FIB-4 do not offer any advantage over the single biochemical measurements we used[243].
Other non invasive techniques, namely transient elastography, supersonic shear imaging and acoustic radiation force impulse elastography have been tested in patients with PBC, in an effort to establish advanced fibrosis[244,245]. It is doubtful however that their use can replace the gold standard of liver biopsy particularly in early PBC. The evolution of fibrosis over the years might be a field where these techniques can assist in the follow up of these patients.
PBC is histologically classified into 4 stages according to Ludwig[246] or Scheuer classification[247]. In early stages, lesions mostly progress in small interlobular bile ductules. Swelling and degeneration of their epithelium finally leading to necrosis is a prominent feature of early PBC[248]. There is extensive infiltration of the portal tracts with mononuclear cells, including macrophages and eosinophils mostly around affected bile ductules. In some cases granulomas or aggregates of epithelioid cells are found either in portal tracts or within the lobule. Granulomas can be found in any histological stage. Occasionally mild Kupffer cell hyperplasia or infiltration of sinusoids by lymphocytes can be found. A histological finding associated with disease progression is the presence of periportal hepatitis. In later stages, the injured bile ducts gradually disappear probably through apoptosis and fibrosis and ultimately cirrhosis develop. A useful finding in diagnosis is an excessive accumulation of copper within hepatocytes present from the early stages. As already mentioned a liver biopsy may not be necessary for diagnosis.This is true but staging of the disease still requires a liver biopsy since neither serum surrogate markers nor elastography can replace an adequate liver tissue sample. In particular elastography, although relative simple to perform, can only assess fibrosis and not the other parameters a liver biopsy can provide. Moreover the distinction between stages II and III in the METAVIR scale is extremely difficult[243,244].
Kakuda et al[249] analyzed the usefulness of a new histological classification system in predicting retrospectively the clinical outcome of patients with PBC. The prognostic performance of this system, which was developed a few years ago by this Japanese team[250-252] was compared to those of the Scheuer’s and Ludwig’s classical systems. They concluded that, while the place of liver biopsy in PBC remains clearly limited for diagnostic purposes, an accurate histological examination of the liver based on the evaluation of several individual lesions of prognostic relevance may be useful to predict the long-term prognosis and the outcome under UDCA treatment.
The introduction of the UDCA in the treatment of PBC has altered the natural course of the disease. Before UDCA, an increasing serum bilirubin after a relatively stable phase, was an ominous sign and death was the final event after a few months[253]. Bilirubin above 2 mg/L, was associated with a median survival of 4 years and for levels over 6 mg/L, it was only 2 years. At a multicenter European study of 236 patients, (54% on stage 1-2 and 19% on stage 3), were followed up for a period of 4 years. Almost half of the patients developed histologically-proven cirrhosis[254]. In another large study of 770 patients from the Northeast England liver failure appeared in 15% of them within a 5-year follow up[255]. Very high rates of histological deterioration was reported in three large 4 years follow up studies of untreated patients with a median 2 years for advanced fibrosis to develop and the probability of remaining in the early stage of only 29% (95%CI: 15%-52%)[254,256]. In a additional report of 256 patients prospectively studied for 5.6 years the appearance of esophageal varices was 31%[257].
Medical management in PBC is aimed at two specific goals; first treatment of symptoms (jaundice, fatigue and pruritus) and complications (ascites, metabolic bone disease, hypercholesterolemia, malabsorption, anemia and vitamin deficiencies) resulting from chronic cholestasis. The second aim focuses on suppressing the underlying pathogenetic process that revolves around the destruction of intralobular bile ducts[258].
Treatment PBC patients is not disease specific and virtually has not changed over the last 25 years. Standard treatment is the long term administration of the secondary bile acid UDCA. The first trial looking at UDCA in PBC was conducted by Poupon et al[259] in 1987 and demonstrated dramatic improvement in liver biochemistries in PBC patients receiving 12-15 mg/kg per day of UDCA. Several independent cohort analyses showed that survival in UDCA-treated PBC patients (13-15 mg/kg per day) was significantly longer than expected from natural history models of the disease[260-262]. The effect of UDCA on survival has been studied with either observational studies or Markov models. Two hundred and sixty-two patients who received 13-15 mg/kg UDCA daily were followed up for 1-20 years (mean 8 years). Transplantation free survival rates were 84% and 66% at 10 and 20 years. By contrast, there was a significantly high probability of death or liver transplantation in patients treated in the late stages of the disease with a relative risk of 2.2[263].
We also reported similar results in our cohort of patients with the favourable outcome more prominent when UDCA was commenced in histological stage 1-2 of the disease[264]. Although these comparisons are indirect, they further support a sustained beneficial effect of UDCA on survival in PBC patients. Withdrawal of UDCA even after more than 6 years of treatment was followed by immediate biochemical rebound[265].
In addition to survival, a large number of randomized controlled trials were performed to assess the effect of UDCA on progression of fibrosis. In these trials the dose of UDCA administered was usually 13-15 mg/kg per day[266-271]. In an early report, UDCA treated patients were followed for 6 years and compared with untreated patients. There was a significant reduction of cirrhosis development (13% vs 49%)[272]. Similar results were reported in another study where treated patients had a 5-fold lower rate of advancement of fibrosis or development of cirrhosis. In a 4-year follow up 76% of the treated patients were still in early stages as compared to only 29% of placebo controls[273]. UDCA was equally effective in reducing the rate of esophageal varices appearance in a 4-year study of 180 patients. Only 16% of patients developed varices compared to 58% on the placebo arm[274].
On the other hand, a recent Cochrane systematic review of 16 randomized trials of 1447 patients in total has concluded that UDCA has a significant effect on reducing jaundice, ascites and liver biochemistries but had no significant effect on mortality or liver transplantation[275] in accordance with earlier meta-analyses[276,277]. However, other meta-analyses that excluded short duration trials (< 2 years) and trials using suboptimal UDCA doses,less than 13 mg/kg per day confirmed in fact the beneficial effect on transplant-free survival of UDCA (13-15 mg/kg per day) in early stage PBC patients[278].
Not all patients respond to UDCA treatment. Age is associated with response while male sex is a predictor of non-response to UDCA[279]. Biliary enrichment of UDCA by at least 40% is usually regarded as a surrogate of a therapeutic response; it rarely exceeds 50% with standard doses, but can reach as high as 65%-75% with very high doses (30 mg/kg per day)[280]. Several studies tried to define the best way to assess if a response to UDCA therapy has occurred in patients with PBC. The Barcelona criteria suggests that a decrease of 40% in serum alkaline phosphatase (ALP) level is a predictor of favourable response to UDCA[262]. Corpechot et al[281], looking at the efficiency of several combinations of threshold lab biochemistries in predicting outcomes of 292 patients with PBC established the Paris I criteria (ALP < 3 time the upper limit of normal (ULN), aspartate aminotransferase (AST) < 2 ULN and bilirubin < 1 mg/dL after 1 year of UDCA). A transplant-free survival rate of 90% at ten years was observed in patients fulfilling these criteria and only 51% for those who did not[281]. The same group recently established the better validated Paris II criteria which defined the best biochemical response in early stage (histological stage 1-2) as ALP and AST < 1.5 ULN, with a normal bilirubin[282]. The suboptimal therapeutic response evaluated by different biochemical criteria has been recently reviewed by Corpechot[283]. Normalization of all biochemical abnormalities is achieved in 20% of patients after 2 years treatment with UDCA[284].
The biochemical response to UDCA should be monitored every 3 to 6 mo. Improvement in liver tests may be observed as early as the first month, and 90% of the improvement usually occurs within the first year. In patients with non-advanced disease, the maximum response is observed after 3 years and maintained in the long-term despite slight and marginal increases in bilirubin and aspartate aminotransferase[285].
Today there is a more or less general agreement that a biochemical response to a dose of 13-15 mg/kg per day of UDCA delays progression of disease in the majority of patients. Major changes in the natural history of PBC have been achieved, mainly resulting in decreased rates of liver transplantation and prolonged survival due to earlier diagnosis of the disease and hence timely intervention with UDCA[214,262,271,281,286,287].
An increasing use of UDCA is accompanied by a similar decrease in the number of patients transplanted[288,289]. UDCA has very few adverse effects even after many years of treatment. A slight weight gain not exceeding an average of 2 kg, is reported by many patients particularly those who have stopped smoking. It is possible that an alteration of bile acid pool composition might lead to signaling through the TGR5 pathway and subsequent weight gain[290]. Administration of UDCA in patients with pruritus may in fact cause deterioration of pruritus and it is recommended that a low dose of 200-400 mg/d should initially be given with a gradual increase in 4-8 wk up to the usual daily dosage. UDCA is not approved for use during the first trimester of pregnancy, although embryotoxicity has not been reported in humans. A number of retrospective studies found no serious side-effects in women with PBC who started gestation during UDCA therapy and who continued the treatment throughout their pregnancy[291,292]. Breast feeding with UDCA treatment is also not approved, but was incidentally reported to cause no detrimental effects in the newborn[293].
Despite the favourable response of most patients to UDCA, there is a number of patients with incomplete response to the drug. Various combinations with additional drugs have been tested. Recent studies aimed at combining other agents to UDCA have shown promise. Bezfibrate has proved to have an additional anticholestatic effect in combination with UDCA[294,295]. Two meta-analyses showed different results. A Cochran meta-analysis concludes that the addition of bezafibrate offers no advantage over UDCA alone[296], while favorable results were reported in a very recent meta-analysis[297].
Immunosuppressants such as colchicine or methotrexate demonstrated a sustained clinical response. The addition of methotrexate was reported to improve both liver biochemistry and histology[298,299] although some studies reported no effect from this combination[300-302]. Furthermore, in a 10-year study, the addition to UDCA of either methotrexate or colchicines offered no survival advantage to that predicted by the Mayo model. However pre-cirrhotic patients taking methotrexate showed no progression therefore verifying that the drug is a useful addition to UDCA at least in some patients[303,304].
Non responders could be treated with a combination of UDCA and budesonide but there is not a general consensus for that treatment. In newly diagnosed PBC patients a combination of UDCA and budesonide improves liver biochemistry and histology but whether this is accompanied by a clear survival benefit remains to be established[305,306].
Chrorambucil has also been tried. Despite improvement in some outcome measures such as serum bilirubin levels in PBC patients treated with chlorambucil, a Cochrane systemic review concluded that evidence is inadequate for the usage of chlorambucil in PBC patients. Bone marrow suppression was a serious side-effect with this drug[307].
Tetrathiomolybdate was also investigated and the agent was efficacious for the management of PBC. Only biochemical endpoints were used without a proof of histological improvement. Hence, as the authors suggest longer clinical trials to examine transplant-free survival and histological progression are needed[308].
Novel treatment options have also been suggested for non-responders to UDCA; B cell depletion using rituximab was investigated with promising results that require verification by additional studies[309-311].
The T cell receptor, cytotoxic T lymphocyte antigen 4 (CTLA-4) acts as a co-stimulatory molecule in T-cell activation which in turn leads to biliary damage. Recent evidence supports the utilization of an optimized course of therapy by means of CTLA-4 Ig to potentially aid in the therapy of PBC patients[312]. However the most promising new treatment since the introduction of UDCA is a derivative of chenodeoxycholic acid, obeticholic acid (OCA). The effect of obeticholic acid is mediated through its action in bile acid homeostasis, acting as a potent farnesoid X receptor (FXR) ligand[313,314]. FXR is the key nuclear receptor for homeostasis and entero-hepatic circulation of bile acids. Obeticholic acid is approximately 100 times more potent FXR activator than chenodeoxycholic acid, the strongest natural activator of FXR known so far[315]. Activation of FXR promotes bile acid secretion by activating bile acid transporters in the apical membrane of hepatocytes together with a parallel reduction in bile acid uptake at the basolateral membrane. FXR agonists also reduce bile acid synthesis modulating the transcription of cholesterol 7-alpha-hydroxylase, the rate-limiting enzyme in bile acid synthesis, via induction of the suppressor protein small heterodimer partner and activation of the fibroblast growth factor 19 (FGF-19) enterohepatic signaling, which mediates bile acid feedback regulation[316,317].
Since OCA and UDCA exert their secretagogue effects on bile acids through distinct mechanisms, there is a potential for synergistic therapeutic effects. Experimental evidence indicates that OCA has also antifibrotic properties consistent with FXR agonist effects in vivo[318,319]. Clinical evaluation of OCA is currently ongoing, but phase-II efficacy and safety data in PBC have already been reported. One hundred sixty-five patients with persistently high ALP levels (> 1.5 × ULN) were randomized to receive either a placebo or OCA at 10, 25 or 50 mg once daily for 12 wk while maintained on the usual UDCA treatment Results were initially presented as abstracts and very recently as a full paper[320,321]. Seventy percent of patients demonstrated a 25% reduction in ALP in the three OCA arms compared with a 3% change from baseline in the placebo group was reported. In addition, levels of aminotransferases, gamma-glutamyltransferase (GGT) as well as C4 sterol used as a surrogate marker of bile acid synthesis and the primary bile acids cholic acid and CDCA, were all significantly decreased while FGF-19 concentrations increased[322]. Biochemical improvement was maintained for an additional 12 mo open label extension of the initial trial. However an 85% incidence of pruritus with a dose response relationship was also observed but only 13% were forced to withdraw from the extended trial due to severe pruritus.
In another study, 59 UDCA-naive patients were randomized to either a placebo or OCA at 10 or 50 mg once daily as monotherapy for 12 wk[323]. A 50% reduction in ALP levels from baseline compared with no change in the placebo group was noted. Similar results were reported with aminotransferases, GGT and IgM levels significantly decreased in the OCA groups, but pruritus was seen in > 70% of patients and led to treatment discontinuation in up to 38% of cases.
Endogenous primary bile acids cholic acid and CDCA in both serum and bile are significantly decreased by the oral administration of UDCA, with a parallel marked increase in UDCA concentration, which then accounts for up to 50% of the circulating bile acid pool[324,325]. UDCA interrupts the enterohepatic circulation of endogenous bile acids increasing therefore their fecal elimination[326-328]. It also facilitates the endogenous secretion of bile acids into bile by promoting insertion and possibly activation of two bile carriers (the bile salt export pump bile salt export pump and the conjugate export pump, multidrug resistance-associated protein 2) into the canalicular membrane of the hepatocyte. UDCA also protects hepatocytes from apoptosis induced by bile acid. This is attributed to inhibition of mitochondrial membrane permeability transition, and stimulation of a survival pathway[329]. Another mechanism by which UDCA probably acts is the restoration of ductal expression of the anion exchanger 2, which is thought to compensate for defective biliary alkaline secretion thus contributing to the reduction of cytotoxicity of endogenous bile acids against cholangiocytes (the so called “biliary HCO3-umbrella hypothesis”)[330]. Enhancement of defenses against oxidative stress is also a mechanism by which UDCA protects cholangiocytes and hepatocytes. In chronic cholestasis there is an excessive production of peroxide and hydroxynonenal protein adducts which is probably counteracted by an UDCA-induced enhancement of liver glutathione levels and the upregulation of J-glutamylcysteine synthetase and methionine S-adenosyltransferase[151,331-335]. In experimental animals[336] and patients with PBC[337,338], additional oxidative stress protective mechanisms, namely Nrf2-mediated hepatocellular transport, detoxification and anti-oxidative stress systems, have been induced by UDCA. UDCA may also exert hepatic as well as systemic anti-apoptotic effects. In vitro UDCA and tauroursodeoxycholic acid can prevent apoptosis caused by different agents like hydrophobic bile acids, ethanol, TGFb1, Fas ligand and okadaic acid in both hepatic and non-hepatic cells[339]. In patients with PBC, UDCA also exerts anti-apoptotic properties in cholangiocytes and so may inhibit the availability of mitochondrial antigens in dendritic cells and macrophages[340]. The mechanisms involved in the anti-apoptotic properties of UDCA include targeting of mitochondrial function and integrity, reduction of endoplasmic reticulum stress, and interactions with various survival signals in the cAMP, Akt, NF-κB, MAPK and PI3K signaling pathways[341-344].
We have demonstrated in HepG2 cells that UDCA modulates caspase activation and apoptosis in a concentration-dependent, while activation of the caspase cascade does not always correlates with increased apoptosis. We therefore concluded that serum UDCA concentrations should adjusted through dosage modifications to achieve the required effect[345].
UDCA is an extraordinary molecule since apart from the action mentioned above it seems likely, that it exerts anti-inflammatory[346] and immunological protective effects as well. UDCA strongly reduced MHC class I expression in the liver in PBC[347]. In several experimental models, UDCA exerted immunosuppressive properties by interfering with B-and T-cell functions. UDCA inhibited CpG-induced immunoglobulin production by B cells[348] cytokine (IL-2, IL-4, IFN-J) release by mononuclear cells when challenged with LPS[349] and intrahepatic TNF-α and MIP-2 in the concanavalin A model of liver damage in experimental animals[350]. These findings suggest that UDCA modifies TLR TLR4 and TLR9 signaling pathways, therefore reducing inflammation[351]. The anti-inflammatory properties of UDCA might, in addition, be mediated by activation of the vitamin D receptor in cholangiocytes to promote the secretion of antimicrobial peptides in human bile[352,353].
UDCA administration in PBC blunts the accumulation of endotoxin in bile and suppresses the immune reaction against lipid A[164,166,167]. The mechanisms involved in UDCA-induced tolerance to endotoxin are both a reduced intestinal absorption of gut-derived endotoxin[354] and protection against endotoxemia by enhancing the transport of LPS across hepatocytes from blood to bile[355]. LPS is inactivated in alkaline bile by alkaline phosphatase as it becomes less active in a lower pH milieu[163].
In many autoimmune mediated liver diseases an important pathogenetic role in the initiation and perpetuation of the hepatocyte injury is played by the NKT-cells[58]. UDCA corrects NKT-cell activity by inhibiting prostaglandin E2 production in PBC. Since, as mentioned before, a reduced function of NKT-cells induced by IP-10 inhibits liver stellate cell apoptosis, restoration of NKT activity is evidently favourable in PBC[356].
Two additional mechanisms have been proposed by us to further explain the beneficial effect of UDCA in PBC. We have suggested that an increase in ET2 observed from the early stages of the disease may be the initiative mechanism causing ischemic damage to cholangiocytes. UDCA treatment caused a significant reduction of ET2, possibly interrupting the early detrimental effect of ET2[179]. A second mechanism may involve the CXCL chemokines. As mentioned before, PBC patients have increased levels of the chemokines IP-10 and MIG both in plasma and the liver[133]. These chemokines are important for the attraction of cells cytotoxic for cholangiocytes.
We recently reported a significant serum decrease of these chemokines after UDCA administration. Moreover we demonstrated using flow cytometry a significantly lower CXCR3 expression in normal controls (13.5%) compared to PBC (37.2%), which was decreased (28.1%, P < 0.01) after UDCA treatment, a finding that might indicate a new additional mechanism of action for UDCA[134].
Liver transplantation is the only curative treatment of PBC which is a very frequent indication for liver transplantation in the United States[288]. The indications are the same with any other cirrhosis and include refractory ascites, hepatic encephalopathy, and portal hypertension leading to hepatorenal syndrome, spontaneous bacterial peritonitis. However in PBC there are two additional indications unique in this disease. These are intractable pruritus and debilitating fatigue.
Liver transplantation improves long term survival in PBC patients. An earlier study of 161 PBC patients showed a significant improvement (P < 0.01) of outcomes when expected patient survival through utilization of the Mayo natural history model was compared to actual survival. Moreover the 2 year survival was 74% for transplanted patients as compared to 31% for non-transplanted patients[357]. These survival figures have been considerably improved in recent years[358].
| 1. | Addison T, Gull W. On a certain affection of the skin, vitiligoidea - a. plana, b. tuberosa. Guys Hospital Reports. 1851;7:265-276. |
| 2. | Dauphinee JA, Sinclair JC. Primary biliary cirrhosis. Can Med Assoc J. 1949;61:1-6. [PubMed] |
| 3. | Ahrens EH, Payne MA, Kunkel HG, Eisenmenger WJ, Blondheim SH. Primary biliary cirrhosis. Medicine (Baltimore). 1950;29:299-364. [PubMed] |
| 4. | Rubin E, Schaffner F, Popper H. Primary biliary cirrhosis. chronic non-suppurative destructive cholangitis. Am J Pathol. 1965;46:387-407. [PubMed] |
| 5. | Watson RG, Angus PW, Dewar M, Goss B, Sewell RB, Smallwood RA. Low prevalence of primary biliary cirrhosis in Victoria, Australia. Melbourne Liver Group. Gut. 1995;36:927-930. [PubMed] |
| 6. | Sood S, Gow PJ, Christie JM, Angus PW. Epidemiology of primary biliary cirrhosis in Victoria, Australia: high prevalence in migrant populations. Gastroenterology. 2004;127:470-475. [PubMed] |
| 7. | Prince MI, James OF. The epidemiology of primary biliary cirrhosis. Clin Liver Dis. 2003;7:795-819. [PubMed] |
| 8. | Corpechot C, Gaouar F, Salle A-V, Diemert B, Carrat F, Johanet C, Chazouilleres O, Poupon R. Epidemiology of primary biliary cirrhosis: results of a prospective study performed on the overall metropolitan French population. Hepatology. 2008;48:602A. |
| 9. | Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56:1181-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 455] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 10. | James OF, Bhopal R, Howel D, Gray J, Burt AD, Metcalf JV. Primary biliary cirrhosis once rare, now common in the United Kingdom? Hepatology. 1999;30:390-394. [PubMed] |
| 11. | Metcalf JV, Bhopal RS, Gray J, Howel D, James OF. Incidence and prevalence of primary biliary cirrhosis in the city of Newcastle upon Tyne, England. Int J Epidemiol. 1997;26:830-836. [PubMed] |
| 12. | Kim WR, Lindor KD, Locke GR, Therneau TM, Homburger HA, Batts KP, Yawn BP, Petz JL, Melton LJ, Dickson ER. Epidemiology and natural history of primary biliary cirrhosis in a US community. Gastroenterology. 2000;119:1631-1636. [PubMed] |
| 13. | Koulentaki M, Mantaka A, Sifaki-Pistolla D, Thalassinos E, Tzanakis N, Kouroumalis E. Geoepidemiology and space-time analysis of Primary biliary cirrhosis in Crete, Greece. Liver Int. 2014;34:e200-e207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Ala A, Stanca CM, Bu-Ghanim M, Ahmado I, Branch AD, Schiano TD, Odin JA, Bach N. Increased prevalence of primary biliary cirrhosis near Superfund toxic waste sites. Hepatology. 2006;43:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Abu-Mouch S, Selmi C, Benson GD, Kenny TP, Invernizzi P, Zuin M, Podda M, Rossaro L, Gershwin ME. Geographic clusters of primary biliary cirrhosis. Clin Dev Immunol. 2003;10:127-131. [PubMed] |
| 16. | Arbour L, Field L, Ross P, Erikson A, Yoshida E. The mystery of primary biliary cirrhosis in British Columbia’s First Nations people. Int J Circumpolar Health. 2004;63 Suppl 2:185-188. [PubMed] |
| 17. | Prince MI, Chetwynd A, Diggle P, Jarner M, Metcalf JV, James OF. The geographical distribution of primary biliary cirrhosis in a well-defined cohort. Hepatology. 2001;34:1083-1088. [PubMed] |
| 18. | Arbour L, Rupps R, Field L, Ross P, Erikson A, Henderson H, Hill W, Yoshida E. Characteristics of primary biliary cirrhosis in British Columbia’s First Nations population. Can J Gastroenterol. 2005;19:305-310. [PubMed] |
| 19. | Mattalia A, Quaranta S, Leung PS, Bauducci M, Van de Water J, Calvo PL, Danielle F, Rizzetto M, Ansari A, Coppel RL. Characterization of antimitochondrial antibodies in health adults. Hepatology. 1998;27:656-661. [PubMed] |
| 20. | Ohba K, Omagari K, Kinoshita H, Soda H, Masuda J, Hazama H, Tagawa M, Hata T, Nakamura H, Murata I. Primary biliary cirrhosis among atomic bomb survivors in Nagasaki, Japan. J Clin Epidemiol. 2001;54:845-850. [PubMed] |
| 21. | Podda M, Selmi C, Lleo A, Moroni L, Invernizzi P. The limitations and hidden gems of the epidemiology of primary biliary cirrhosis. J Autoimmun. 2013;46:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Solís Herruzo JA, Solís Muñoz P, Muñoz Yagüe T. The pathogenesis of primary biliary cirrhosis. Rev Esp Enferm Dig. 2009;101:413-423. |
| 23. | McNally RJ, Ducker S, James OF. Are transient environmental agents involved in the cause of primary biliary cirrhosis? Evidence from space-time clustering analysis. Hepatology. 2009;50:1169-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Selmi C, Gershwin ME. The role of environmental factors in primary biliary cirrhosis. Trends Immunol. 2009;30:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Mells GF, Floyd JA, Morley KI, Cordell HJ, Franklin CS, Shin SY, Heneghan MA, Neuberger JM, Donaldson PT, Day DB. Genome-wide association study identifies 12 new susceptibility loci for primary biliary cirrhosis. Nat Genet. 2011;43:329-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 388] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 26. | Tanaka A, Ohira H, Kikuchi K, Nezu S, Shibuya A, Bianchi I, Podda M, Invernizzi P, Takikawa H. Genetic association of Fc receptor-like 3 polymorphisms with susceptibility to primary biliary cirrhosis: ethnic comparative study in Japanese and Italian patients. Tissue Antigens. 2011;77:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Nakamura M, Nishida N, Kawashima M, Aiba Y, Tanaka A, Yasunami M, Nakamura H, Komori A, Nakamuta M, Zeniya M. Genome-wide association study identifies TNFSF15 and POU2AF1 as susceptibility loci for primary biliary cirrhosis in the Japanese population. Am J Hum Genet. 2012;91:721-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 28. | Mantaka A, Koulentaki M, Chlouverakis G, Enele-Melono JM, Darivianaki A, Tzardi M, Kouroumalis EA. Primary biliary cirrhosis in a genetically homogeneous population: disease associations and familial occurrence rates. BMC Gastroenterol. 2012;12:110. [PubMed] |
| 29. | Jones DE, Watt FE, Metcalf JV, Bassendine MF, James OF. Familial primary biliary cirrhosis reassessed: a geographically-based population study. J Hepatol. 1999;30:402-407. [PubMed] |
| 30. | Gershwin ME, Selmi C, Worman HJ, Gold EB, Watnik M, Utts J, Lindor KD, Kaplan MM, Vierling JM. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 443] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 31. | Corpechot C, Chrétien Y, Chazouillères O, Poupon R. Demographic, lifestyle, medical and familial factors associated with primary biliary cirrhosis. J Hepatol. 2010;53:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 32. | Selmi C, Mayo MJ, Bach N, Ishibashi H, Invernizzi P, Gish RG, Gordon SC, Wright HI, Zweiban B, Podda M. Primary biliary cirrhosis in monozygotic and dizygotic twins: genetics, epigenetics, and environment. Gastroenterology. 2004;127:485-492. [PubMed] |
| 33. | Dilthey AT, Moutsianas L, Leslie S, McVean G. HLA*IMP--an integrated framework for imputing classical HLA alleles from SNP genotypes. Bioinformatics. 2011;27:968-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 34. | Invernizzi P, Ransom M, Raychaudhuri S, Kosoy R, Lleo A, Shigeta R, Franke A, Bossa F, Amos CI, Gregersen PK. Classical HLA-DRB1 and DPB1 alleles account for HLA associations with primary biliary cirrhosis. Genes Immun. 2012;13:461-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Liu JZ, Almarri MA, Gaffney DJ, Mells GF, Jostins L, Cordell HJ, Ducker SJ, Day DB, Heneghan MA, Neuberger JM. Dense fine-mapping study identifies new susceptibility loci for primary biliary cirrhosis. Nat Genet. 2012;44:1137-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 36. | Juran BD, Hirschfield GM, Invernizzi P, Atkinson EJ, Li Y, Xie G, Kosoy R, Ransom M, Sun Y, Bianchi I. Immunochip analyses identify a novel risk locus for primary biliary cirrhosis at 13q14, multiple independent associations at four established risk loci and epistasis between 1p31 and 7q32 risk variants. Hum Mol Genet. 2012;21:5209-5221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 37. | Schotte R, Rissoan MC, Bendriss-Vermare N, Bridon JM, Duhen T, Weijer K, Brière F, Spits H. The transcription factor Spi-B is expressed in plasmacytoid DC precursors and inhibits T-, B-, and NK-cell development. Blood. 2003;101:1015-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Becker AM, Michael DG, Satpathy AT, Sciammas R, Singh H, Bhattacharya D. IRF-8 extinguishes neutrophil production and promotes dendritic cell lineage commitment in both myeloid and lymphoid mouse progenitors. Blood. 2012;119:2003-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 39. | Tailor P, Tamura T, Ozato K. IRF family proteins and type I interferon induction in dendritic cells. Cell Res. 2006;16:134-140. [PubMed] |
| 40. | Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644-658. [PubMed] |
| 41. | Kaisho T, Tanaka T. Turning NF-kappaB and IRFs on and off in DC. Trends Immunol. 2008;29:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Arthur JS. MSK activation and physiological roles. Front Biosci. 2008;13:5866-5879. [PubMed] |
| 43. | Cabal-Hierro L, Lazo PS. Signal transduction by tumor necrosis factor receptors. Cell Signal. 2012;24:1297-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 337] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 44. | Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1043] [Cited by in RCA: 1048] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 45. | Hirschfield GM, Liu X, Xu C, Lu Y, Xie G, Lu Y, Gu X, Walker EJ, Jing K, Juran BD. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med. 2009;360:2544-2555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 510] [Cited by in RCA: 476] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 46. | Liu X, Invernizzi P, Lu Y, Kosoy R, Lu Y, Bianchi I, Podda M, Xu C, Xie G, Macciardi F. Genome-wide meta-analyses identify three loci associated with primary biliary cirrhosis. Nat Genet. 2010;42:658-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 315] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 47. | Hirschfield GM, Liu X, Han Y, Gorlov IP, Lu Y, Xu C, Lu Y, Chen W, Juran BD, Coltescu C. Variants at IRF5-TNPO3, 17q12-21 and MMEL1 are associated with primary biliary cirrhosis. Nat Genet. 2010;42:655-657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 48. | Mells GF, Kaser A, Karlsen TH. Novel insights into autoimmune liver diseases provided by genome-wide association studies. J Autoimmun. 2013;46:41-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 49. | Hirschfield GM, Chapman RW, Karlsen TH, Lammert F, Lazaridis KN, Mason AL. The genetics of complex cholestatic disorders. Gastroenterology. 2013;144:1357-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 50. | Kouroumalis E. Environmental agents involved in the cause of primary biliary cirrhosis. Dis Markers. 2010;29:329-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 51. | Selmi C, De Santis M, Cavaciocchi F, Gershwin ME. Infectious agents and xenobiotics in the etiology of primary biliary cirrhosis. Dis Markers. 2010;29:287-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 52. | Bogdanos DP, Pares A, Baum H, Caballeria L, Rigopoulou EI, Ma Y, Burroughs AK, Rodes J, Vergani D. Disease-specific cross-reactivity between mimicking peptides of heat shock protein of Mycobacterium gordonae and dominant epitope of E2 subunit of pyruvate dehydrogenase is common in Spanish but not British patients with primary biliary cirrhosis. J Autoimmun. 2004;22:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Bogdanos DP, Baum H, Okamoto M, Montalto P, Sharma UC, Rigopoulou EI, Vlachogiannakos J, Ma Y, Burroughs AK, Vergani D. Primary biliary cirrhosis is characterized by IgG3 antibodies cross-reactive with the major mitochondrial autoepitope and its Lactobacillus mimic. Hepatology. 2005;42:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Selmi C, Balkwill DL, Invernizzi P, Ansari AA, Coppel RL, Podda M, Leung PS, Kenny TP, Van De Water J, Nantz MH. Patients with primary biliary cirrhosis react against a ubiquitous xenobiotic-metabolizing bacterium. Hepatology. 2003;38:1250-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 55. | Kaplan MM. Novosphingobium aromaticivorans: a potential initiator of primary biliary cirrhosis. Am J Gastroenterol. 2004;99:2147-2149. [PubMed] |
| 56. | Padgett KA, Selmi C, Kenny TP, Leung PS, Balkwill DL, Ansari AA, Coppel RL, Gershwin ME. Phylogenetic and immunological definition of four lipoylated proteins from Novosphingobium aromaticivorans, implications for primary biliary cirrhosis. J Autoimmun. 2005;24:209-219. [PubMed] |
| 57. | Olafsson S, Gudjonsson H, Selmi C, Amano K, Invernizzi P, Podda M, Gershwin ME. Antimitochondrial antibodies and reactivity to N. aromaticivorans proteins in Icelandic patients with primary biliary cirrhosis and their relatives. Am J Gastroenterol. 2004;99:2143-2146. [PubMed] |
| 58. | Mattner J, Savage PB, Leung P, Oertelt SS, Wang V, Trivedi O, Scanlon ST, Pendem K, Teyton L, Hart J. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe. 2008;3:304-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 59. | Kita H, Naidenko OV, Kronenberg M, Ansari AA, Rogers P, He XS, Koning F, Mikayama T, Van De Water J, Coppel RL. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123:1031-1043. [PubMed] |
| 60. | Harada K, Isse K, Tsuneyama K, Ohta H, Nakanuma Y. Accumulating CD57 + CD3 + natural killer T cells are related to intrahepatic bile duct lesions in primary biliary cirrhosis. Liver Int. 2003;23:94-100. [PubMed] |
| 61. | Harada K, Nakanuma Y. Biliary innate immunity: function and modulation. Mediators Inflamm. 2010;2010:pii: 373878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 62. | Amano K, Leung PS, Rieger R, Quan C, Wang X, Marik J, Suen YF, Kurth MJ, Nantz MH, Ansari AA. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. J Immunol. 2005;174:5874-5883. [PubMed] |
| 63. | Rieger R, Leung PS, Jeddeloh MR, Kurth MJ, Nantz MH, Lam KS, Barsky D, Ansari AA, Coppel RL, Mackay IR. Identification of 2-nonynoic acid, a cosmetic component, as a potential trigger of primary biliary cirrhosis. J Autoimmun. 2006;27:7-16. [PubMed] |
| 64. | Wakabayashi K, Yoshida K, Leung PS, Moritoki Y, Yang GX, Tsuneyama K, Lian ZX, Hibi T, Ansari AA, Wicker LS. Induction of autoimmune cholangitis in non-obese diabetic (NOD).1101 mice following a chemical xenobiotic immunization. Clin Exp Immunol. 2009;155:577-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Yeaman SJ, Fussey SP, Danner DJ, James OF, Mutimer DJ, Bassendine MF. Primary biliary cirrhosis: identification of two major M2 mitochondrial autoantigens. Lancet. 1988;1:1067-1070. [PubMed] |
| 66. | Fussey SP, Guest JR, James OF, Bassendine MF, Yeaman SJ. Identification and analysis of the major M2 autoantigens in primary biliary cirrhosis. Proc Natl Acad Sci USA. 1988;85:8654-8658. [PubMed] |
| 67. | Coppel RL, McNeilage LJ, Surh CD, Van de Water J, Spithill TW, Whittingham S, Gershwin ME. Primary structure of the human M2 mitochondrial autoantigen of primary biliary cirrhosis: dihydrolipoamide acetyltransferase. Proc Natl Acad Sci USA. 1988;85:7317-7321. [PubMed] |
| 68. | Björkland A, Lööf L, Mendel-Hartvig I, Tötterman TH. Primary biliary cirrhosis. High proportions of B cells in blood and liver tissue produce anti-mitochondrial antibodies of several Ig classes. J Immunol. 1994;153:2750-2757. [PubMed] |
| 69. | Nishio A, Van de Water J, Leung PS, Joplin R, Neuberger JM, Lake J, Björkland A, Tötterman TH, Peters M, Worman HJ. Comparative studies of antimitochondrial autoantibodies in sera and bile in primary biliary cirrhosis. Hepatology. 1997;25:1085-1089. [PubMed] |
| 70. | Tanaka A, Nalbandian G, Leung PS, Benson GD, Munoz S, Findor JA, Branch AD, Coppel RL, Ansari AA, Gershwin ME. Mucosal immunity and primary biliary cirrhosis: presence of antimitochondrial antibodies in urine. Hepatology. 2000;32:910-915. [PubMed] |
| 71. | Reynoso-Paz S, Leung PS, Van De Water J, Tanaka A, Munoz S, Bass N, Lindor K, Donald PJ, Coppel RL, Ansari AA. Evidence for a locally driven mucosal response and the presence of mitochondrial antigens in saliva in primary biliary cirrhosis. Hepatology. 2000;31:24-29. [PubMed] |
| 72. | Lleo A, Bowlus CL, Yang GX, Invernizzi P, Podda M, Van de Water J, Ansari AA, Coppel RL, Worman HJ, Gores GJ. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010;52:987-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 73. | Leung PS, Rossaro L, Davis PA, Park O, Tanaka A, Kikuchi K, Miyakawa H, Norman GL, Lee W, Gershwin ME. Antimitochondrial antibodies in acute liver failure: implications for primary biliary cirrhosis. Hepatology. 2007;46:1436-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 74. | Bhat M, Guindi M, Heathcote EJ, Hirschfield GM. Transient development of anti-mitochondrial antibodies accompanies autoimmune hepatitis-sclerosing cholangitis overlap. Gut. 2009;58:152-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Sutton I, Neuberger J. Primary biliary cirrhosis: seeking the silent partner of autoimmunity. Gut. 2002;50:743-746. [PubMed] |
| 76. | Invernizzi P, Podda M, Battezzati PM, Crosignani A, Zuin M, Hitchman E, Maggioni M, Meroni PL, Penner E, Wesierska-Gadek J. Autoantibodies against nuclear pore complexes are associated with more active and severe liver disease in primary biliary cirrhosis. J Hepatol. 2001;34:366-372. [PubMed] |
| 77. | Sfakianaki O, Koulentaki M, Tzardi M, Tsangaridou E, Theodoropoulos PA, Castanas E, Kouroumalis EA. Peri-nuclear antibodies correlate with survival in Greek primary biliary cirrhosis patients. World J Gastroenterol. 2010;16:4938-4943. [PubMed] |
| 78. | Takahashi T, Miura T, Nakamura J, Yamada S, Miura T, Yanagi M, Matsuda Y, Usuda H, Emura I, Tsuneyama K. Plasma cells and the chronic nonsuppurative destructive cholangitis of primary biliary cirrhosis. Hepatology. 2012;55:846-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 79. | Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology. 2009;50:291-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 908] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 80. | Lleo A, Selmi C, Invernizzi P, Podda M, Coppel RL, Mackay IR, Gores GJ, Ansari AA, Van de Water J, Gershwin ME. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009;49:871-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 81. | Rong G, Zhou Y, Xiong Y, Zhou L, Geng H, Jiang T, Zhu Y, Lu H, Zhang S, Wang P. Imbalance between T helper type 17 and T regulatory cells in patients with primary biliary cirrhosis: the serum cytokine profile and peripheral cell population. Clin Exp Immunol. 2009;156:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 82. | Harada K, Shimoda S, Sato Y, Isse K, Ikeda H, Nakanuma Y. Periductal interleukin-17 production in association with biliary innate immunity contributes to the pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin Exp Immunol. 2009;157:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 83. | Lan RY, Salunga TL, Tsuneyama K, Lian ZX, Yang GX, Hsu W, Moritoki Y, Ansari AA, Kemper C, Price J. Hepatic IL-17 responses in human and murine primary biliary cirrhosis. J Autoimmun. 2009;32:43-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 84. | Harada K, Nakanuma Y. Biliary innate immunity in the pathogenesis of biliary diseases. Inflamm Allergy Drug Targets. 2010;9:83-90. [PubMed] |
| 85. | Poggi A, Zocchi MR. NK cell autoreactivity and autoimmune diseases. Front Immunol. 2014;5:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 86. | Shimoda S, Harada K, Niiro H, Shirabe K, Taketomi A, Maehara Y, Tsuneyama K, Nakanuma Y, Leung P, Ansari AA. Interaction between Toll-like receptors and natural killer cells in the destruction of bile ducts in primary biliary cirrhosis. Hepatology. 2011;53:1270-1281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 87. | Shimoda S, Tsuneyama K, Kikuchi K, Harada K, Nakanuma Y, Nakamura M, Ishibashi H, Hisamoto S, Niiro H, Leung PS. The role of natural killer (NK) and NK T cells in the loss of tolerance in murine primary biliary cirrhosis. Clin Exp Immunol. 2012;168:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 88. | Van de Water J, Ansari A, Prindiville T, Coppel RL, Ricalton N, Kotzin BL, Liu S, Roche TE, Krams SM, Munoz S. Heterogeneity of autoreactive T cell clones specific for the E2 component of the pyruvate dehydrogenase complex in primary biliary cirrhosis. J Exp Med. 1995;181:723-733. [PubMed] |
| 89. | Jones DE, Palmer JM, James OF, Yeaman SJ, Bassendine MF, Diamond AG. T-cell responses to the components of pyruvate dehydrogenase complex in primary biliary cirrhosis. Hepatology. 1995;21:995-1002. [PubMed] |
| 90. | Shimoda S, Van de Water J, Ansari A, Nakamura M, Ishibashi H, Coppel RL, Lake J, Keeffe EB, Roche TE, Gershwin ME. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest. 1998;102:1831-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 202] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 91. | Jones DE, Palmer JM, Yeaman SJ, Bassendine MF, Diamond AG. T cell responses to natural human proteins in primary biliary cirrhosis. Clin Exp Immunol. 1997;107:562-568. [PubMed] |
| 92. | Akbar SM, Yamamoto K, Miyakawa H, Ninomiya T, Abe M, Hiasa Y, Masumoto T, Horiike N, Onji M. Peripheral blood T-cell responses to pyruvate dehydrogenase complex in primary biliary cirrhosis: role of antigen-presenting dendritic cells. Eur J Clin Invest. 2001;31:639-646. [PubMed] |
| 93. | Kamihira T, Shimoda S, Harada K, Kawano A, Handa M, Baba E, Tsuneyama K, Nakamura M, Ishibashi H, Nakanuma Y. Distinct costimulation dependent and independent autoreactive T-cell clones in primary biliary cirrhosis. Gastroenterology. 2003;125:1379-1387. [PubMed] |
| 94. | Shimoda S, Nakamura M, Ishibashi H, Hayashida K, Niho Y. HLA DRB4 0101-restricted immunodominant T cell autoepitope of pyruvate dehydrogenase complex in primary biliary cirrhosis: evidence of molecular mimicry in human autoimmune diseases. J Exp Med. 1995;181:1835-1845. [PubMed] |
| 95. | Shigematsu H, Shimoda S, Nakamura M, Matsushita S, Nishimura Y, Sakamoto N, Ichiki Y, Niho Y, Gershwin ME, Ishibashi H. Fine specificity of T cells reactive to human PDC-E2 163-176 peptide, the immunodominant autoantigen in primary biliary cirrhosis: implications for molecular mimicry and cross-recognition among mitochondrial autoantigens. Hepatology. 2000;32:901-909. [PubMed] |
| 96. | Shimoda S, Nakamura M, Shigematsu H, Tanimoto H, Gushima T, Gershwin ME, Ishibashi H. Mimicry peptides of human PDC-E2 163-176 peptide, the immunodominant T-cell epitope of primary biliary cirrhosis. Hepatology. 2000;31:1212-1216. [PubMed] |
| 97. | Kita H, Lian ZX, Van de Water J, He XS, Matsumura S, Kaplan M, Luketic V, Coppel RL, Ansari AA, Gershwin ME. Identification of HLA-A2-restricted CD8(+) cytotoxic T cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross-presented by dendritic cells. J Exp Med. 2002;195:113-123. [PubMed] |
| 98. | Matsumura S, Kita H, He XS, Ansari AA, Lian ZX, Van De Water J, Yamamoto K, Tsuji T, Coppel RL, Kaplan M. Comprehensive mapping of HLA-A0201-restricted CD8 T-cell epitopes on PDC-E2 in primary biliary cirrhosis. Hepatology. 2002;36:1125-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Kita H, Matsumura S, He XS, Ansari AA, Lian ZX, Van de Water J, Coppel RL, Kaplan MM, Gershwin ME. Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J Clin Invest. 2002;109:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 100. | Oertelt S, Lian ZX, Cheng CM, Chuang YH, Padgett KA, He XS, Ridgway WM, Ansari AA, Coppel RL, Li MO. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177:1655-1660. [PubMed] |
| 101. | Goriely S, Cavoy R, Goldman M. Interleukin-12 family members and type I interferons in Th17-mediated inflammatory disorders. Allergy. 2009;64:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 102. | Yoshida K, Yang GX, Zhang W, Tsuda M, Tsuneyama K, Moritoki Y, Ansari AA, Okazaki K, Lian ZX, Coppel RL. Deletion of interleukin-12p40 suppresses autoimmune cholangitis in dominant negative transforming growth factor beta receptor type II mice. Hepatology. 2009;50:1494-1500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 103. | Hsu W, Zhang W, Tsuneyama K, Moritoki Y, Ridgway WM, Ansari AA, Coppel RL, Lian ZX, Mackay I, Gershwin ME. Differential mechanisms in the pathogenesis of autoimmune cholangitis versus inflammatory bowel disease in interleukin-2Ralpha(-/-) mice. Hepatology. 2009;49:133-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 104. | Zhang W, Sharma R, Ju ST, He XS, Tao Y, Tsuneyama K, Tian Z, Lian ZX, Fu SM, Gershwin ME. Deficiency in regulatory T cells results in development of antimitochondrial antibodies and autoimmune cholangitis. Hepatology. 2009;49:545-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 105. | Lan RY, Cheng C, Lian ZX, Tsuneyama K, Yang GX, Moritoki Y, Chuang YH, Nakamura T, Saito S, Shimoda S. Liver-targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhosis. Hepatology. 2006;43:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 240] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 106. | Seki E, Tsutsui H, Nakano H, Tsuji N, Hoshino K, Adachi O, Adachi K, Futatsugi S, Kuida K, Takeuchi O. Lipopolysaccharide-induced IL-18 secretion from murine Kupffer cells independently of myeloid differentiation factor 88 that is critically involved in induction of production of IL-12 and IL-1beta. J Immunol. 2001;166:2651-2657. [PubMed] |
| 107. | Wu J, Meng Z, Jiang M, Zhang E, Trippler M, Broering R, Bucchi A, Krux F, Dittmer U, Yang D. Toll-like receptor-induced innate immune responses in non-parenchymal liver cells are cell type-specific. Immunology. 2010;129:363-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 108. | Yang L, Seki E. Toll-like receptors in liver fibrosis: cellular crosstalk and mechanisms. Front Physiol. 2012;3:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 109. | Mao TK, Lian ZX, Selmi C, Ichiki Y, Ashwood P, Ansari AA, Coppel RL, Shimoda S, Ishibashi H, Gershwin ME. Altered monocyte responses to defined TLR ligands in patients with primary biliary cirrhosis. Hepatology. 2005;42:802-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 110. | Kikuchi K, Lian ZX, Yang GX, Ansari AA, Ikehara S, Kaplan M, Miyakawa H, Coppel RL, Gershwin ME. Bacterial CpG induces hyper-IgM production in CD27(+) memory B cells in primary biliary cirrhosis. Gastroenterology. 2005;128:304-312. [PubMed] |
| 111. | Moritoki Y, Lian ZX, Wulff H, Yang GX, Chuang YH, Lan RY, Ueno Y, Ansari AA, Coppel RL, Mackay IR. AMA production in primary biliary cirrhosis is promoted by the TLR9 ligand CpG and suppressed by potassium channel blockers. Hepatology. 2007;45:314-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 112. | Kikuchi K, Lian ZX, Kimura Y, Selmi C, Yang GX, Gordon SC, Invernizzi P, Podda M, Coppel RL, Ansari AA. Genetic polymorphisms of toll-like receptor 9 influence the immune response to CpG and contribute to hyper-IgM in primary biliary cirrhosis. J Autoimmun. 2005;24:347-352. [PubMed] |
| 113. | Takii Y, Nakamura M, Ito M, Yokoyama T, Komori A, Shimizu-Yoshida Y, Nakao R, Kusumoto K, Nagaoka S, Yano K. Enhanced expression of type I interferon and toll-like receptor-3 in primary biliary cirrhosis. Lab Invest. 2005;85:908-920. [PubMed] |
| 114. | Ambrosini YM, Yang GX, Zhang W, Tsuda M, Shu S, Tsuneyama K, Leung PS, Ansari AA, Coppel RL, Gershwin ME. The multi-hit hypothesis of primary biliary cirrhosis: polyinosinic-polycytidylic acid (poly I: C) and murine autoimmune cholangitis. Clin Exp Immunol. 2011;166:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 115. | Harada K, Van de Water J, Leung PS, Coppel RL, Ansari A, Nakanuma Y, Gershwin ME. In situ nucleic acid hybridization of cytokines in primary biliary cirrhosis: predominance of the Th1 subset. Hepatology. 1997;25:791-796. [PubMed] |
| 116. | Sekiya H, Komatsu T, Isono E, Furukawa M, Matsushima S, Yamaguchi N, Yamauchi K, Hayashi N. Decrease in the prevalence of IL-4-producing CD4+ T cells in patients with advanced stage of primary biliary cirrhosis. Am J Gastroenterol. 1999;94:3589-3594. [PubMed] |
| 117. | Nagano T, Yamamoto K, Matsumoto S, Okamoto R, Tagashira M, Ibuki N, Matsumura S, Yabushita K, Okano N, Tsuji T. Cytokine profile in the liver of primary biliary cirrhosis. J Clin Immunol. 1999;19:422-427. [PubMed] |
| 118. | Pulickal AS, Hambleton S, Callaghan MJ, Moore CE, Goulding J, Goodsall A, Baretto R, Lammas DA, Anderson ST, Levin M. Biliary cirrhosis in a child with inherited interleukin-12 deficiency. J Trop Pediatr. 2008;54:269-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 119. | Voumvouraki A, Koulentaki M, Tzardi M, Sfakianaki O, Manousou P, Notas G, Kouroumalis E. Increased ΤGF-β3 in primary biliary cirrhosis: an abnormality related to pathogenesis? World J Gastroenterol. 2010;16:5057-5064. [PubMed] |
| 120. | Chuang YH, Lian ZX, Tsuneyama K, Chiang BL, Ansari AA, Coppel RL, Gershwin ME. Increased killing activity and decreased cytokine production in NK cells in patients with primary biliary cirrhosis. J Autoimmun. 2006;26:232-240. [PubMed] |
| 121. | Isse K, Harada K, Zen Y, Kamihira T, Shimoda S, Harada M, Nakanuma Y. Fractalkine and CX3CR1 are involved in the recruitment of intraepithelial lymphocytes of intrahepatic bile ducts. Hepatology. 2005;41:506-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 122. | Shimoda S, Harada K, Niiro H, Yoshizumi T, Soejima Y, Taketomi A, Maehara Y, Tsuneyama K, Nakamura M, Komori A. Biliary epithelial cells and primary biliary cirrhosis: the role of liver-infiltrating mononuclear cells. Hepatology. 2008;47:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 123. | Borchers AT, Shimoda S, Bowlus C, Keen CL, Gershwin ME. Lymphocyte recruitment and homing to the liver in primary biliary cirrhosis and primary sclerosing cholangitis. Semin Immunopathol. 2009;31:309-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 124. | Shimoda S, Harada K, Niiro H, Taketomi A, Maehara Y, Tsuneyama K, Kikuchi K, Nakanuma Y, Mackay IR, Gershwin ME. CX3CL1 (fractalkine): a signpost for biliary inflammation in primary biliary cirrhosis. Hepatology. 2010;51:567-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 125. | Afford SC, Ahmed-Choudhury J, Randhawa S, Russell C, Youster J, Crosby HA, Eliopoulos A, Hubscher SG, Young LS, Adams DH. CD40 activation-induced, Fas-dependent apoptosis and NF-kappaB/AP-1 signaling in human intrahepatic biliary epithelial cells. FASEB J. 2001;15:2345-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 126. | Muehlhoefer A, Saubermann LJ, Gu X, Luedtke-Heckenkamp K, Xavier R, Blumberg RS, Podolsky DK, MacDermott RP, Reinecker HC. Fractalkine is an epithelial and endothelial cell-derived chemoattractant for intraepithelial lymphocytes in the small intestinal mucosa. J Immunol. 2000;164:3368-3376. [PubMed] |
| 127. | Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1064] [Cited by in RCA: 1089] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 128. | Harada K, Tsuneyama K, Yasoshima M, Kanemori Y, Ohta H, Masuda S, Onai N, Matsushima K, Nakanuma Y. Type1 and type2 memory T cells imbalance shown by expression of intrahepatic chemokine receptors relates to pathogenesis of primary biliary cirrhosis. Hepatol Res. 2002;24:290. [PubMed] |
| 129. | Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163:6236-6243. [PubMed] |
| 130. | Schrage A, Wechsung K, Neumann K, Schumann M, Schulzke JD, Engelhardt B, Zeitz M, Hamann A, Klugewitz K. Enhanced T cell transmigration across the murine liver sinusoidal endothelium is mediated by transcytosis and surface presentation of chemokines. Hepatology. 2008;48:1262-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 131. | Shetty S, Lalor PF, Adams DH. Lymphocyte recruitment to the liver: molecular insights into the pathogenesis of liver injury and hepatitis. Toxicology. 2008;254:136-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 132. | Holt AP, Haughton EL, Lalor PF, Filer A, Buckley CD, Adams DH. Liver myofibroblasts regulate infiltration and positioning of lymphocytes in human liver. Gastroenterology. 2009;136:705-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 133. | Chuang YH, Lian ZX, Cheng CM, Lan RY, Yang GX, Moritoki Y, Chiang BL, Ansari AA, Tsuneyama K, Coppel RL. Increased levels of chemokine receptor CXCR3 and chemokines IP-10 and MIG in patients with primary biliary cirrhosis and their first degree relatives. J Autoimmun. 2005;25:126-132. [PubMed] |
| 134. | Manousou P, Kolios G, Drygiannakis I, Koulentaki M, Pyrovolaki K, Voumvouraki A, Notas G, Bourikas L, Papadaki HA, Kouroumalis E. CXCR3 axis in patients with primary biliary cirrhosis: a possible novel mechanism of the effect of ursodeoxycholic acid. Clin Exp Immunol. 2013;172:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 135. | Santodomingo-Garzon T, Han J, Le T, Yang Y, Swain MG. Natural killer T cells regulate the homing of chemokine CXC receptor 3-positive regulatory T cells to the liver in mice. Hepatology. 2009;49:1267-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 136. | Oo YH, Banz V, Kavanagh D, Liaskou E, Withers DR, Humphreys E, Reynolds GM, Lee-Turner L, Kalia N, Hubscher SG. CXCR3-dependent recruitment and CCR6-mediated positioning of Th-17 cells in the inflamed liver. J Hepatol. 2012;57:1044-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 137. | Hintermann E, Bayer M, Pfeilschifter JM, Luster AD, Christen U. CXCL10 promotes liver fibrosis by prevention of NK cell mediated hepatic stellate cell inactivation. J Autoimmun. 2010;35:424-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 138. | Harada K, Nakanuma Y. Molecular mechanisms of cholangiopathy in primary biliary cirrhosis. Med Mol Morphol. 2006;39:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 139. | Graham AM, Dollinger MM, Howie SE, Harrison DJ. Bile duct cells in primary biliary cirrhosis are ‘primed’ for apoptosis. Eur J Gastroenterol Hepatol. 1998;10:553-557. [PubMed] |
| 140. | Tinmouth J, Lee M, Wanless IR, Tsui FW, Inman R, Heathcote EJ. Apoptosis of biliary epithelial cells in primary biliary cirrhosis and primary sclerosing cholangitis. Liver. 2002;22:228-234. [PubMed] |
| 141. | Iwata M, Harada K, Kono N, Kaneko S, Kobayashi K, Nakanuma Y. Expression of Bcl-2 familial proteins is reduced in small bile duct lesions of primary biliary cirrhosis. Hum Pathol. 2000;31:179-184. [PubMed] |
| 142. | Odin JA, Huebert RC, Casciola-Rosen L, LaRusso NF, Rosen A. Bcl-2-dependent oxidation of pyruvate dehydrogenase-E2, a primary biliary cirrhosis autoantigen, during apoptosis. J Clin Invest. 2001;108:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 143. | Allina J, Hu B, Sullivan DM, Fiel MI, Thung SN, Bronk SF, Huebert RC, van de Water J, LaRusso NF, Gershwin ME. T cell targeting and phagocytosis of apoptotic biliary epithelial cells in primary biliary cirrhosis. J Autoimmun. 2006;27:232-241. [PubMed] |
| 144. | Inamura K, Tsuji H, Nakamoto Y, Suzuki M, Kaneko S. Transgenic mice aberrantly expressing pyruvate dehydrogenase complex E2 component on biliary epithelial cells do not show primary biliary cirrhosis. Clin Exp Immunol. 2006;145:93-100. [PubMed] |
| 145. | Tsuneyama K, Harada K, Kono N, Sasaki M, Saito T, Gershwin ME, Ikemoto M, Arai H, Nakanuma Y. Damaged interlobular bile ducts in primary biliary cirrhosis show reduced expression of glutathione-S-transferase-pi and aberrant expression of 4-hydroxynonenal. J Hepatol. 2002;37:176-183. [PubMed] |
| 146. | Kaplan MM, Elta GH, Furie B, Sadowski JA, Russell RM. Fat-soluble vitamin nutriture in primary biliary cirrhosis. Gastroenterology. 1988;95:787-792. [PubMed] |
| 147. | Sokol RJ, Kim YS, Hoofnagle JH, Heubi JE, Jones EA, Balistreri WF. Intestinal malabsorption of vitamin E in primary biliary cirrhosis. Gastroenterology. 1989;96:479-486. [PubMed] |
| 148. | Muñoz SJ, Heubi JE, Balistreri WF, Maddrey WC. Vitamin E deficiency in primary biliary cirrhosis: gastrointestinal malabsorption, frequency and relationship to other lipid-soluble vitamins. Hepatology. 1989;9:525-531. [PubMed] |
| 149. | Aboutwerat A, Pemberton PW, Smith A, Burrows PC, McMahon RF, Jain SK, Warnes TW. Oxidant stress is a significant feature of primary biliary cirrhosis. Biochim Biophys Acta. 2003;1637:142-150. [PubMed] |
| 150. | Notas G, Miliaraki N, Kampa M, Dimoulios F, Matrella E, Hatzidakis A, Castanas E, Kouroumalis E. Patients with primary biliary cirrhosis have increased serum total antioxidant capacity measured with the crocin bleaching assay. World J Gastroenterol. 2005;11:4194-4198. [PubMed] |
| 151. | Ljubuncic P, Tanne Z, Bomzon A. Ursodeoxycholic acid suppresses extent of lipid peroxidation in diseased liver in experimental cholestatic liver disease. Dig Dis Sci. 2000;45:1921-1928. [PubMed] |
| 152. | Ljubuncic P, Abu-Salach O, Bomzon A. Ursodeoxycholic acid and superoxide anion. World J Gastroenterol. 2005;11:4875-4878. [PubMed] |
| 153. | Serviddio G, Pereda J, Pallardó FV, Carretero J, Borras C, Cutrin J, Vendemiale G, Poli G, Viña J, Sastre J. Ursodeoxycholic acid protects against secondary biliary cirrhosis in rats by preventing mitochondrial oxidative stress. Hepatology. 2004;39:711-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 154. | Parola M, Leonarduzzi G, Robino G, Albano E, Poli G, Dianzani MU. On the role of lipid peroxidation in the pathogenesis of liver damage induced by long-standing cholestasis. Free Radic Biol Med. 1996;20:351-359. [PubMed] |
| 155. | Huang YT, Hsu YC, Chen CJ, Liu CT, Wei YH. Oxidative-stress-related changes in the livers of bile-duct-ligated rats. J Biomed Sci. 2003;10:170-178. [PubMed] |
| 156. | Liu TZ, Lee KT, Chern CL, Cheng JT, Stern A, Tsai LY. Free radical-triggered hepatic injury of experimental obstructive jaundice of rats involves overproduction of proinflammatory cytokines and enhanced activation of nuclear factor kappaB. Ann Clin Lab Sci. 2001;31:383-390. [PubMed] |
| 157. | Sokol RJ, Straka MS, Dahl R, Devereaux MW, Yerushalmi B, Gumpricht E, Elkins N, Everson G. Role of oxidant stress in the permeability transition induced in rat hepatic mitochondria by hydrophobic bile acids. Pediatr Res. 2001;49:519-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 158. | Sokol RJ, Winklhofer-Roob BM, Devereaux MW, McKim JM. Generation of hydroperoxides in isolated rat hepatocytes and hepatic mitochondria exposed to hydrophobic bile acids. Gastroenterology. 1995;109:1249-1256. [PubMed] |
| 159. | Sokol RJ, Dahl R, Devereaux MW, Yerushalmi B, Kobak GE, Gumpricht E. Human hepatic mitochondria generate reactive oxygen species and undergo the permeability transition in response to hydrophobic bile acids. J Pediatr Gastroenterol Nutr. 2005;41:235-243. [PubMed] |
| 160. | Celli A, Que FG, Gores GJ, LaRusso NF. Glutathione depletion is associated with decreased Bcl-2 expression and increased apoptosis in cholangiocytes. Am J Physiol. 1998;275:G749-G757. [PubMed] |
| 161. | Butler P, Hamilton-Miller J, Baum H, Burroughs AK. Detection of M2 antibodies in patients with recurrent urinary tract infection using an ELISA and purified PBC specific antigens. Evidence for a molecular mimicry mechanism in the pathogenesis of primary biliary cirrhosis? Biochem Mol Biol Int. 1995;35:473-485. [PubMed] |
| 162. | Mimura Y, Sakisaka S, Harada M, Sata M, Tanikawa K. Role of hepatocytes in direct clearance of lipopolysaccharide in rats. Gastroenterology. 1995;109:1969-1976. [PubMed] |
| 163. | Tuin A, Huizinga-Van der Vlag A, van Loenen-Weemaes AM, Meijer DK, Poelstra K. On the role and fate of LPS-dephosphorylating activity in the rat liver. Am J Physiol Gastrointest Liver Physiol. 2006;290:G377-G385. [PubMed] |
| 164. | Sasatomi K, Noguchi K, Sakisaka S, Sata M, Tanikawa K. Abnormal accumulation of endotoxin in biliary epithelial cells in primary biliary cirrhosis and primary sclerosing cholangitis. J Hepatol. 1998;29:409-416. [PubMed] |
| 165. | Harada K, Nakanuma Y. Cholangiopathy with respect to biliary innate immunity. Int J Hepatol. 2012;2012:793569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 166. | Ide T, Sata M, Nakano H, Suzuki H, Tanikawa K. Increased serum IgM class anti-lipid A antibody and therapeutic effect of ursodeoxycholic acid in primary biliary cirrhosis. Hepatogastroenterology. 1997;44:1569-1573. [PubMed] |
| 167. | Ballot E, Bandin O, Chazouilleres O, Johanet C, Poupon R. Immune response to lipopolysaccharide in primary biliary cirrhosis and autoimmune diseases. J Autoimmun. 2004;22:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 168. | Fiorotto R, Scirpo R, Trauner M, Fabris L, Hoque R, Spirli C, Strazzabosco M. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-κB-mediated inflammatory response in mice. Gastroenterology. 2011;141:1498-1508, 1508.e1-e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 169. | Mason A, Xu L, Shen Z, Fodera B, Joplin R, Neuberger J, O’Donnell B. Patients with primary biliary cirrhosis make anti-viral and anti-mitochondrial antibodies to mouse mammary tumor virus. Gastroenterology. 2004;127:1863-1864; author reply 1864-1865. [PubMed] |
| 170. | Mason AL, Xu L, Guo L, Munoz S, Jaspan JB, Bryer-Ash M, Cao Y, Sander DM, Shoenfeld Y, Ahmed A. Detection of retroviral antibodies in primary biliary cirrhosis and other idiopathic biliary disorders. Lancet. 1998;351:1620-1624. [PubMed] |
| 171. | Xu L, Shen Z, Guo L, Fodera B, Keogh A, Joplin R, O’Donnell B, Aitken J, Carman W, Neuberger J. Does a betaretrovirus infection trigger primary biliary cirrhosis? Proc Natl Acad Sci USA. 2003;100:8454-8459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 172. | Mason AL, Zhang G. Linking human beta retrovirus infection with primary biliary cirrhosis. Gastroenterol Clin Biol. 2010;34:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 173. | Mason AL. The evidence supports a viral aetiology for primary biliary cirrhosis. J Hepatol. 2011;54:1312-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 174. | Montano-Loza AJ, Wasilenko S, Bintner J, Mason AL. Cyclosporine A inhibits in vitro replication of betaretrovirus associated with primary biliary cirrhosis. Liver Int. 2010;30:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 175. | Selmi C. The evidence does not support a viral etiology for primary biliary cirrhosis. J Hepatol. 2011;54:1315-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 177. | Selmi C, Lleo A, Pasini S, Zuin M, Gershwin ME. Innate immunity and primary biliary cirrhosis. Curr Mol Med. 2009;9:45-51. [PubMed] |
| 178. | Sasaki M, Ikeda H, Yamaguchi J, Nakada S, Nakanuma Y. Telomere shortening in the damaged small bile ducts in primary biliary cirrhosis reflects ongoing cellular senescence. Hepatology. 2008;48:186-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 179. | Dimoulios P, Kolios G, Notas G, Matrella E, Xidakis C, Koulentaki M, Tsagarakis N, Kouroumalis A, Kouroumalis E. Ursodeoxycholic acid reduces increased circulating endothelin 2 in primary biliary cirrhosis. Aliment Pharmacol Ther. 2005;21:227-234. [PubMed] |
| 180. | Kouroumalis E, Notas G. Pathogenesis of primary biliary cirrhosis: a unifying model. World J Gastroenterol. 2006;12:2320-2327. [PubMed] |
| 181. | Tsuneyama K, Harada K, Kono N, Hiramatsu K, Zen Y, Sudo Y, Gershwin ME, Ikemoto M, Arai H, Nakanuma Y. Scavenger cells with gram-positive bacterial lipoteichoic acid infiltrate around the damaged interlobular bile ducts of primary biliary cirrhosis. J Hepatol. 2001;35:156-163. [PubMed] |
| 182. | Speciale L, Roda K, Saresella M, Taramelli D, Ferrante P. Different endothelins stimulate cytokine production by peritoneal macrophages and microglial cell line. Immunology. 1998;93:109-114. [PubMed] |
| 183. | Grimshaw MJ, Wilson JL, Balkwill FR. Endothelin-2 is a macrophage chemoattractant: implications for macrophage distribution in tumors. Eur J Immunol. 2002;32:2393-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 184. | Colucci G, Schaffner F, Paronetto F. In situ characterization of the cell-surface antigens of the mononuclear cell infiltrate and bile duct epithelium in primary biliary cirrhosis. Clin Immunol Immunopathol. 1986;41:35-42. [PubMed] |
| 185. | Tobe K, Tsuchiya T, Itoshima T, Nagashima H, Kobayashi T. Electron microscopy of fat-storing cells in liver diseases with special reference to cilia and cytoplasmic cholesterol crystals. Arch Histol Jpn. 1985;48:435-441. [PubMed] |
| 186. | Tsuneyama K, Harada K, Yasoshima M, Hiramatsu K, Mackay CR, Mackay IR, Gershwin ME, Nakanuma Y. Monocyte chemotactic protein-1, -2, and -3 are distinctively expressed in portal tracts and granulomata in primary biliary cirrhosis: implications for pathogenesis. J Pathol. 2001;193:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 187. | Mathew J, Hines JE, Toole K, Johnson SJ, James OF, Burt AD. Quantitative analysis of macrophages and perisinusoidal cells in primary biliary cirrhosis. Histopathology. 1994;25:65-70. [PubMed] |
| 188. | Rockey D. The cellular pathogenesis of portal hypertension: stellate cell contractility, endothelin, and nitric oxide. Hepatology. 1997;25:2-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 189. | Sfakianaki O, Tzardi M, Voumvouraki A, Afgoustaki A, Koulentaki M, Kouroumalis E. Presence of disease specific autoantibodies against liver sinusoidal cells in primary biliary cirrhosis. World J Hepatol. 2013;5:568-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 190. | Mantaka A, Goulielmos GN, Koulentaki M, Tsagournis O, Voumvouraki A, Kouroumalis EA. Polymorphisms of genes related to endothelial cells are associated with primary biliary cirrhosis patients of Cretan origin. Hum Immunol. 2012;73:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 191. | Strazzabosco M, Fabris L. Functional anatomy of normal bile ducts. Anat Rec (Hoboken). 2008;291:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 192. | Ludwig J, Batts KP, MacCarty RL. Ischemic cholangitis in hepatic allografts. Mayo Clin Proc. 1992;67:519-526. [PubMed] |
| 193. | Koda W, Harada K, Tsuneyama K, Kono N, Sasaki M, Matsui O, Nakanuma Y. Evidence of the participation of peribiliary mast cells in regulation of the peribiliary vascular plexus along the intrahepatic biliary tree. Lab Invest. 2000;80:1007-1017. [PubMed] |
| 194. | Matsumura S, Van De Water J, Kita H, Coppel RL, Tsuji T, Yamamoto K, Ansari AA, Gershwin ME. Contribution to antimitochondrial antibody production: cleavage of pyruvate dehydrogenase complex-E2 by apoptosis-related proteases. Hepatology. 2002;35:14-22. [PubMed] |
| 195. | Ayres RC, Neuberger JM, Shaw J, Joplin R, Adams DH. Intercellular adhesion molecule-1 and MHC antigens on human intrahepatic bile duct cells: effect of pro-inflammatory cytokines. Gut. 1993;34:1245-1249. [PubMed] |
| 196. | Howell CD, Li J, Chen W. Role of intercellular adhesion molecule-1 and lymphocyte function-associated antigen-1 during nonsuppurative destructive cholangitis in a mouse graft-versus-host disease model. Hepatology. 1999;29:766-776. [PubMed] |
| 197. | Kimura T, Suzuki K, Inada S, Hayashi A, Isobe M, Matsuzaki Y, Tanaka N, Osuga T, Fujiwara M. Monoclonal antibody against lymphocyte function-associated antigen 1 inhibits the formation of primary biliary cirrhosis-like lesions induced by murine graft-versus-host reaction. Hepatology. 1996;24:888-894. [PubMed] |
| 198. | Wakae T, Takatsuka H, Seto Y, Iwata N, Mori A, Okada M, Fujimori Y, Okamoto T, Kakishita E, Hara H. Similarity between hepatic graft-versus-host disease and primary biliary cirrhosis. Hematology. 2002;7:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 199. | Beschorner WE, Shinn CA, Hess AD, Suresch DL, Santos GW. Immune-related injury to endothelium associated with acute graft-versus-host disease in the rat. Transplant Proc. 1989;21:3025-3027. [PubMed] |
| 200. | Hiroyasu S, Shiraishi M, Kusano T, Muto Y. Involvement of endothelin in graft-versus-host disease after rat small bowel transplantation. Transpl Int. 1997;10:121-124. [PubMed] |
| 201. | Pyzik M, Piccirillo CA. TGF-beta1 modulates Foxp3 expression and regulatory activity in distinct CD4+ T cell subsets. J Leukoc Biol. 2007;82:335-346. [PubMed] |
| 202. | Poupon R. Autoimmune overlapping syndromes. Clin Liver Dis. 2003;7:865-878. [PubMed] |
| 203. | Vleggaar FP, van Buuren HR, Zondervan PE, ten Kate FJ, Hop WC. Jaundice in non-cirrhotic primary biliary cirrhosis: the premature ductopenic variant. Gut. 2001;49:276-281. [PubMed] |
| 204. | Parés A, Rodés J. Natural history of primary biliary cirrhosis. Clin Liver Dis. 2003;7:779-794. [PubMed] |
| 205. | Prince MI, Chetwynd A, Craig WL, Metcalf JV, James OF. Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut. 2004;53:865-870. [PubMed] |
| 207. | Cauch-Dudek K, Abbey S, Stewart DE, Heathcote EJ. Fatigue in primary biliary cirrhosis. Gut. 1998;43:705-710. [PubMed] |
| 208. | Huet PM, Deslauriers J, Tran A, Faucher C, Charbonneau J. Impact of fatigue on the quality of life of patients with primary biliary cirrhosis. Am J Gastroenterol. 2000;95:760-767. [PubMed] |
| 209. | Newton JL, Pairman J, Sutcliffe K, Wilton K, Jones DE. A predictive model for fatigue and its etiologic associations in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2008;6:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 210. | Talwalkar JA, Souto E, Jorgensen RA, Lindor KD. Natural history of pruritus in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2003;1:297-302. [PubMed] |
| 211. | Jones DE. Fatigue in cholestatic liver disease: is it all in the mind? J Hepatol. 2007;46:992-994. [PubMed] |
| 212. | Kremer AE, Beuers U, Oude-Elferink RP, Pusl T. Pathogenesis and treatment of pruritus in cholestasis. Drugs. 2008;68:2163-2182. [PubMed] |
| 213. | Koulentaki M, Ioannidou D, Stefanidou M, Maraki S, Drigiannakis I, Dimoulios P, Melono JM, Tosca A, Kouroumalis EA. Dermatological manifestations in primary biliary cirrhosis patients: a case control study. Am J Gastroenterol. 2006;101:541-546. [PubMed] |
| 214. | Huet PM, Vincent C, Deslaurier J, Coté J, Matsutami S, Boileau R, Huet-van Kerckvoorde J. Portal hypertension and primary biliary cirrhosis: effect of long-term ursodeoxycholic acid treatment. Gastroenterology. 2008;135:1552-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 215. | Mayo MJ. Portal hypertension in primary biliary cirrhosis: a potentially reversible harbinger of demise. Gastroenterology. 2008;135:1450-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 216. | Colina F, Pinedo F, Solís JA, Moreno D, Nevado M. Nodular regenerative hyperplasia of the liver in early histological stages of primary biliary cirrhosis. Gastroenterology. 1992;102:1319-1324. [PubMed] |
| 217. | Hodgson SF, Dickson ER, Wahner HW, Johnson KA, Mann KG, Riggs BL. Bone loss and reduced osteoblast function in primary biliary cirrhosis. Ann Intern Med. 1985;103:855-860. [PubMed] |
| 218. | Springer JE, Cole DE, Rubin LA, Cauch-Dudek K, Harewood L, Evrovski J, Peltekova VD, Heathcote EJ. Vitamin D-receptor genotypes as independent genetic predictors of decreased bone mineral density in primary biliary cirrhosis. Gastroenterology. 2000;118:145-151. [PubMed] |
| 219. | Wolke AM, Schaffner F, Kapelman B, Sacks HS. Malignancy in primary biliary cirrhosis. High incidence of breast cancer in affected women. Am J Med. 1984;76:1075-1078. [PubMed] |
| 220. | Goudie BM, Burt AD, Boyle P, Macfarlane G, Birnie GG, Mills PR, Gillis CR, MacSween RN, Watkinson G. Breast cancer in women with primary biliary cirrhosis. Br Med J (Clin Res Ed). 1985;291:1597-1598. [PubMed] |
| 221. | Lööf L, Adami HO, Sparén P, Danielsson A, Eriksson LS, Hultcrantz R, Lindgren S, Olsson R, Prytz H, Ryden BO. Cancer risk in primary biliary cirrhosis: a population-based study from Sweden. Hepatology. 1994;20:101-104. [PubMed] |
| 222. | Jones DE, Metcalf JV, Collier JD, Bassendine MF, James OF. Hepatocellular carcinoma in primary biliary cirrhosis and its impact on outcomes. Hepatology. 1997;26:1138-1142. [PubMed] |
| 223. | Nijhawan PK, Therneau TM, Dickson ER, Boynton J, Lindor KD. Incidence of cancer in primary biliary cirrhosis: the Mayo experience. Hepatology. 1999;29:1396-1398. [PubMed] |
| 224. | Shibuya A, Tanaka K, Miyakawa H, Shibata M, Takatori M, Sekiyama K, Hashimoto N, Amaki S, Komatsu T, Morizane T. Hepatocellular carcinoma and survival in patients with primary biliary cirrhosis. Hepatology. 2002;35:1172-1178. [PubMed] |
| 225. | Crowe JP, Christensen E, Butler J, Wheeler P, Doniach D, Keenan J, Williams R. Primary biliary cirrhosis: the prevalence of hypothyroidism and its relationship to thyroid autoantibodies and sicca syndrome. Gastroenterology. 1980;78:1437-1441. [PubMed] |
| 226. | Mang FW, Michieletti P, O’Rourke K, Cauch-Dudek K, Diamant N, Bookman A, Heathcote J. Primary biliary cirrhosis, sicca complex, and dysphagia. Dysphagia. 1997;12:167-170. [PubMed] |
| 227. | Watt FE, James OF, Jones DE. Patterns of autoimmunity in primary biliary cirrhosis patients and their families: a population-based cohort study. QJM. 2004;97:397-406. [PubMed] |
| 228. | Prince MI, Ducker SJ, James OF. Case-control studies of risk factors for primary biliary cirrhosis in two United Kingdom populations. Gut. 2010;59:508-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 229. | Bush A, Mitchison H, Walt R, Baron JH, Boylston AW, Summerfield JA. Primary biliary cirrhosis and ulcerative colitis. Gastroenterology. 1987;92:2009-2013. [PubMed] |
| 230. | Chatzicostas C, Roussomoustakaki M, Drygiannakis D, Niniraki M, Tzardi M, Koulentaki M, Dimoulios P, Mouzas I, Kouroumalis E. Primary biliary cirrhosis and autoimmune cholangitis are not associated with coeliac disease in Crete. BMC Gastroenterol. 2002;2:5. [PubMed] |
| 231. | Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261-1273. [PubMed] |
| 232. | Drebber U, Mueller JJ, Klein E, Kasper HU, Schulze F, Schardt K, Quasdorff M, Schulte S, Odenthal M, Dienes HP. Liver biopsy in primary biliary cirrhosis: clinicopathological data and stage. Pathol Int. 2009;59:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 233. | Szostecki C, Guldner HH, Will H. Autoantibodies against “nuclear dots” in primary biliary cirrhosis. Semin Liver Dis. 1997;17:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 234. | Invernizzi P, Selmi C, Ranftler C, Podda M, Wesierska-Gadek J. Antinuclear antibodies in primary biliary cirrhosis. Semin Liver Dis. 2005;25:298-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 131] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 235. | Bogdanos DP, Komorowski L. Disease-specific autoantibodies in primary biliary cirrhosis. Clin Chim Acta. 2011;412:502-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 236. | Sternsdorf T, Guldner HH, Szostecki C, Grötzinger T, Will H. Two nuclear dot-associated proteins, PML and Sp100, are often co-autoimmunogenic in patients with primary biliary cirrhosis. Scand J Immunol. 1995;42:257-268. [PubMed] |
| 237. | Granito A, Yang WH, Muratori L, Lim MJ, Nakajima A, Ferri S, Pappas G, Quarneti C, Bianchi FB, Bloch DB. PML nuclear body component Sp140 is a novel autoantigen in primary biliary cirrhosis. Am J Gastroenterol. 2010;105:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 238. | Courvalin JC, Lassoued K, Bartnik E, Blobel G, Wozniak RW. The 210-kD nuclear envelope polypeptide recognized by human autoantibodies in primary biliary cirrhosis is the major glycoprotein of the nuclear pore. J Clin Invest. 1990;86:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 239. | Wesierska-Gadek J, Hohenuer H, Hitchman E, Penner E. Autoantibodies against nucleoporin p62 constitute a novel marker of primary biliary cirrhosis. Gastroenterology. 1996;110:840-847. [PubMed] |
| 240. | Muratori P, Muratori L, Ferrari R, Cassani F, Bianchi G, Lenzi M, Rodrigo L, Linares A, Fuentes D, Bianchi FB. Characterization and clinical impact of antinuclear antibodies in primary biliary cirrhosis. Am J Gastroenterol. 2003;98:431-437. [PubMed] |
| 241. | Nakamura M, Takii Y, Ito M, Komori A, Yokoyama T, Shimizu-Yoshida Y, Koyabu M, Matsuyama M, Mori T, Kamihira T. Increased expression of nuclear envelope gp210 antigen in small bile ducts in primary biliary cirrhosis. J Autoimmun. 2006;26:138-145. [PubMed] |
| 242. | Voumvouraki A, Koulentaki M, Notas G, Sfakianaki O, Kouroumalis E. Serum surrogate markers of liver fibrosis in primary biliary cirrhosis. Eur J Intern Med. 2011;22:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 243. | Floreani A, Cazzagon N, Martines D, Cavalletto L, Baldo V, Chemello L. Performance and utility of transient elastography and noninvasive markers of liver fibrosis in primary biliary cirrhosis. Dig Liver Dis. 2011;43:887-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 244. | Corpechot C, Carrat F, Poujol-Robert A, Gaouar F, Wendum D, Chazouillères O, Poupon R. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology. 2012;56:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 245. | Cassinotto C, Lapuyade B, Mouries A, Hiriart JB, Vergniol J, Gaye D, Castain C, Le Bail B, Chermak F, Foucher J. Non-invasive assessment of liver fibrosis with impulse elastography: comparison of Supersonic Shear Imaging with ARFI and FibroScan®. J Hepatol. 2014;61:550-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (1)] |
| 246. | Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Arch A Pathol Anat Histol. 1978;379:103-112. [PubMed] |
| 247. | Scheuer P. Primary biliary cirrhosis. Proc R Soc Med. 1967;60:1257-1260. [PubMed] |
| 248. | Nakanuma Y, Harada K. Florid duct lesion in primary biliary cirrhosis shows highly proliferative activities. J Hepatol. 1993;19:216-221. [PubMed] |
| 249. | Kakuda Y, Harada K, Sawada-Kitamura S, Ikeda H, Sato Y, Sasaki M, Okafuji H, Mizukoshi E, Terasaki S, Ohta H. Evaluation of a new histologic staging and grading system for primary biliary cirrhosis in comparison with classical systems. Hum Pathol. 2013;44:1107-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 250. | Nakanuma Y, Harada K. The role of the pathologist in diagnosing and grading biliary diseases. Clin Res Hepatol Gastroenterol. 2011;35:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 251. | Nakanuma Y, Zen Y, Harada K, Sasaki M, Nonomura A, Uehara T, Sano K, Kondo F, Fukusato T, Tsuneyama K. Application of a new histological staging and grading system for primary biliary cirrhosis to liver biopsy specimens: Interobserver agreement. Pathol Int. 2010;60:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 252. | Bioulac-Sage P. Primary biliary cirrhosis: a new histological staging and grading system proposed by Japanese authors. Clin Res Hepatol Gastroenterol. 2011;35:333-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 253. | Shapiro JM, Smith H, Schaffner F. Serum bilirubin: a prognostic factor in primary biliary cirrhosis. Gut. 1979;20:137-140. [PubMed] |
| 254. | Christensen E, Crowe J, Doniach D, Popper H, Ranek L, Rodés J, Tygstrup N, Williams R. Clinical pattern and course of disease in primary biliary cirrhosis based on an analysis of 236 patients. Gastroenterology. 1980;78:236-246. [PubMed] |
| 255. | Prince M, Chetwynd A, Newman W, Metcalf JV, James OF. Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: follow-up for up to 28 years. Gastroenterology. 2002;123:1044-1051. [PubMed] |
| 256. | Locke GR, Therneau TM, Ludwig J, Dickson ER, Lindor KD. Time course of histological progression in primary biliary cirrhosis. Hepatology. 1996;23:52-56. [PubMed] |
| 257. | Gores GJ, Wiesner RH, Dickson ER, Zinsmeister AR, Jorgensen RA, Langworthy A. Prospective evaluation of esophageal varices in primary biliary cirrhosis: development, natural history, and influence on survival. Gastroenterology. 1989;96:1552-1559. [PubMed] |
| 258. | Imam MH, Talwalkar JA, Lindor KD. Clinical management of autoimmune biliary diseases. J Autoimmun. 2013;46:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 259. | Poupon R, Chrétien Y, Poupon RE, Ballet F, Calmus Y, Darnis F. Is ursodeoxycholic acid an effective treatment for primary biliary cirrhosis? Lancet. 1987;1:834-836. [PubMed] |
| 260. | Poupon RE, Bonnand AM, Chrétien Y, Poupon R. Ten-year survival in ursodeoxycholic acid-treated patients with primary biliary cirrhosis. The UDCA-PBC Study Group. Hepatology. 1999;29:1668-1671. [PubMed] |
| 261. | ter Borg PC, Schalm SW, Hansen BE, van Buuren HR. Prognosis of ursodeoxycholic Acid-treated patients with primary biliary cirrhosis. Results of a 10-yr cohort study involving 297 patients. Am J Gastroenterol. 2006;101:2044-2050. [PubMed] |
| 262. | Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology. 2006;130:715-720. [PubMed] |
| 263. | Corpechot C, Carrat F, Bahr A, Chrétien Y, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on the natural course of primary biliary cirrhosis. Gastroenterology. 2005;128:297-303. [PubMed] |
| 264. | Koulentaki M, Moscandrea J, Dimoulios P, Chatzicostas C, Kouroumalis EA. Survival of anti-mitochondrial antibody-positive and -negative primary biliary cirrhosis patients on ursodeoxycholic acid treatment. Dig Dis Sci. 2004;49:1190-1195. [PubMed] |
| 265. | Leuschner M. UDCA, PBC, and biochemistry, what does normal mean? Gut. 2000;47:595-596. [PubMed] |
| 266. | Poupon RE, Balkau B, Eschwège E, Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med. 1991;324:1548-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 560] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 267. | Poupon RE, Poupon R, Balkau B. Ursodiol for the long-term treatment of primary biliary cirrhosis. The UDCA-PBC Study Group. N Engl J Med. 1994;330:1342-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 348] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 268. | Poupon RE, Lindor KD, Cauch-Dudek K, Dickson ER, Poupon R, Heathcote EJ. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997;113:884-890. [PubMed] |
| 269. | Lindor KD, Therneau TM, Jorgensen RA, Malinchoc M, Dickson ER. Effects of ursodeoxycholic acid on survival in patients with primary biliary cirrhosis. Gastroenterology. 1996;110:1515-1518. [PubMed] |
| 270. | Heathcote EJ, Cauch-Dudek K, Walker V, Bailey RJ, Blendis LM, Ghent CN, Michieletti P, Minuk GY, Pappas SC, Scully LJ. The Canadian Multicenter Double-blind Randomized Controlled Trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1994;19:1149-1156. [PubMed] |
| 271. | Poupon RE, Lindor KD, Parés A, Chazouillères O, Poupon R, Heathcote EJ. Combined analysis of the effect of treatment with ursodeoxycholic acid on histologic progression in primary biliary cirrhosis. J Hepatol. 2003;39:12-16. [PubMed] |
| 272. | Angulo P, Batts KP, Therneau TM, Jorgensen RA, Dickson ER, Lindor KD. Long-term ursodeoxycholic acid delays histological progression in primary biliary cirrhosis. Hepatology. 1999;29:644-647. [PubMed] |
| 273. | Corpechot C, Carrat F, Bonnand AM, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on liver fibrosis progression in primary biliary cirrhosis. Hepatology. 2000;32:1196-1199. [PubMed] |
| 274. | Lindor KD, Jorgensen RA, Therneau TM, Malinchoc M, Dickson ER. Ursodeoxycholic acid delays the onset of esophageal varices in primary biliary cirrhosis. Mayo Clin Proc. 1997;72:1137-1140. [PubMed] |
| 275. | Rudic JS, Poropat G, Krstic MN, Bjelakovic G, Gluud C. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database Syst Rev. 2012;12:CD000551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 276. | Goulis J, Leandro G, Burroughs AK. Randomised controlled trials of ursodeoxycholic-acid therapy for primary biliary cirrhosis: a meta-analysis. Lancet. 1999;354:1053-1060. [PubMed] |
| 277. | Gong Y, Huang Z, Christensen E, Gluud C. Ursodeoxycholic acid for patients with primary biliary cirrhosis: an updated systematic review and meta-analysis of randomized clinical trials using Bayesian approach as sensitivity analyses. Am J Gastroenterol. 2007;102:1799-1807. [PubMed] |
| 278. | Shi J, Wu C, Lin Y, Chen YX, Zhu L, Xie WF. Long-term effects of mid-dose ursodeoxycholic acid in primary biliary cirrhosis: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2006;101:1529-1538. [PubMed] |
| 279. | Carbone M, Mells GF, Pells G, Dawwas MF, Newton JL, Heneghan MA, Neuberger JM, Day DB, Ducker SJ, Sandford RN. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology. 2013;144:560-569.e7; quiz e13-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 304] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 280. | Roda E, Azzaroli F, Nigro G, Piazza F, Jaboli F, Ferrara F, Liva S, Giovanelli S, Miracolo A, Colecchia A. Improved liver tests and greater biliary enrichment with high dose ursodeoxycholic acid in early stage primary biliary cirrhosis. Dig Liver Dis. 2002;34:523-527. [PubMed] |
| 281. | Corpechot C, Abenavoli L, Rabahi N, Chrétien Y, Andréani T, Johanet C, Chazouillères O, Poupon R. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 498] [Article Influence: 27.7] [Reference Citation Analysis (1)] |
| 282. | Corpechot C, Chazouillères O, Poupon R. Early primary biliary cirrhosis: biochemical response to treatment and prediction of long-term outcome. J Hepatol. 2011;55:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 349] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 283. | Corpechot C. Primary biliary cirrhosis and bile acids. Clin Res Hepatol Gastroenterol. 2012;36 Suppl 1:S13-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 284. | Jorgensen RA, Dickson ER, Hofmann AF, Rossi SS, Lindor KD. Characterisation of patients with a complete biochemical response to ursodeoxycholic acid. Gut. 1995;36:935-938. [PubMed] |
| 285. | Kuiper EM, Hansen BE, Lesterhuis W, Robijn RJ, Thijs JC, Engels LG, Koek GH, Aparicio MN, Kerbert-Dreteler MJ, van Buuren HR. The long-term effect of ursodeoxycholic acid on laboratory liver parameters in biochemically non-advanced primary biliary cirrhosis. Clin Res Hepatol Gastroenterol. 2011;35:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 286. | Kuiper EM, Hansen BE, de Vries RA, den Ouden-Muller JW, van Ditzhuijsen TJ, Haagsma EB, Houben MH, Witteman BJ, van Erpecum KJ, van Buuren HR. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009;136:1281-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 367] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 287. | Kumagi T, Guindi M, Fischer SE, Arenovich T, Abdalian R, Coltescu C, Heathcote EJ, Hirschfield GM. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol. 2010;105:2186-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 288. | Lee J, Belanger A, Doucette JT, Stanca C, Friedman S, Bach N. Transplantation trends in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2007;5:1313-1315. [PubMed] |
| 289. | Corpechot C, Poupon R. Geotherapeutics of primary biliary cirrhosis: bright and sunny around the Mediterranean but still cloudy and foggy in the United Kingdom. Hepatology. 2007;46:963-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 290. | Thomas C, Auwerx J, Schoonjans K. Bile acids and the membrane bile acid receptor TGR5--connecting nutrition and metabolism. Thyroid. 2008;18:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 291. | Poupon R, Chrétien Y, Chazouillères O, Poupon RE. Pregnancy in women with ursodeoxycholic acid-treated primary biliary cirrhosis. J Hepatol. 2005;42:418-419. [PubMed] |
| 292. | Westbrook R, Hughes S, O’Grady J, Devlin J, Harrison P, Heneghan M. Pregnancy in cholestatic disease. Hepatology. 2011;54:926A. |
| 293. | Kumagi T, Al - Harthy N, Coltescu C, GM H. Pregnancy and primary biliary cirrhosis: cross- sectional and retrospective analyses demonstrate a high prevalence of symptoms and a risk of disease progression. Hepatology. 2009;50:1008A. |
| 294. | Iwasaki S, Ohira H, Nishiguchi S, Zeniya M, Kaneko S, Onji M, Ishibashi H, Sakaida I, Kuriyama S, Ichida T. The efficacy of ursodeoxycholic acid and bezafibrate combination therapy for primary biliary cirrhosis: A prospective, multicenter study. Hepatol Res. 2008;38:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 295. | Honda A, Ikegami T, Nakamuta M, Miyazaki T, Iwamoto J, Hirayama T, Saito Y, Takikawa H, Imawari M, Matsuzaki Y. Anticholestatic effects of bezafibrate in patients with primary biliary cirrhosis treated with ursodeoxycholic acid. Hepatology. 2013;57:1931-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 296. | Rudic JS, Poropat G, Krstic MN, Bjelakovic G, Gluud C. Bezafibrate for primary biliary cirrhosis. Cochrane Database Syst Rev. 2012;1:CD009145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 297. | Zhang Y, Chen K, Dai W, Xia Y, Wang F, Shen M, Cheng P, Wang C, Yang J, Zhu R. Combination therapy of bezafibrate and ursodeoxycholic acid for primary biliary cirrhosis: A meta-analysis. Hepatol Res. 2015;45:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 298. | Kaplan MM, Schmid C, Provenzale D, Sharma A, Dickstein G, McKusick A. A prospective trial of colchicine and methotrexate in the treatment of primary biliary cirrhosis. Gastroenterology. 1999;117:1173-1180. [PubMed] |
| 299. | Babatin MA, Sanai FM, Swain MG. Methotrexate therapy for the symptomatic treatment of primary biliary cirrhosis patients, who are biochemical incomplete responders to ursodeoxycholic acid therapy. Aliment Pharmacol Ther. 2006;24:813-820. [PubMed] |
| 300. | Lindor KD, Dickson ER, Jorgensen RA, Anderson ML, Wiesner RH, Gores GJ, Lange SM, Rossi SS, Hofmann AF, Baldus WP. The combination of ursodeoxycholic acid and methotrexate for patients with primary biliary cirrhosis: the results of a pilot study. Hepatology. 1995;22:1158-1162. [PubMed] |
| 301. | González-Koch A, Brahm J, Antezana C, Smok G, Cumsille MA. The combination of ursodeoxycholic acid and methotrexate for primary biliary cirrhosis is not better than ursodeoxycholic acid alone. J Hepatol. 1997;27:143-149. [PubMed] |
| 302. | Combes B, Emerson SS, Flye NL, Munoz SJ, Luketic VA, Mayo MJ, McCashland TM, Zetterman RK, Peters MG, Di Bisceglie AM. Methotrexate (MTX) plus ursodeoxycholic acid (UDCA) in the treatment of primary biliary cirrhosis. Hepatology. 2005;42:1184-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 303. | Kaplan MM, Cheng S, Price LL, Bonis PA. A randomized controlled trial of colchicine plus ursodiol versus methotrexate plus ursodiol in primary biliary cirrhosis: ten-year results. Hepatology. 2004;39:915-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 304. | Leung J, Bonis PA, Kaplan MM. Colchicine or methotrexate, with ursodiol, are effective after 20 years in a subset of patients with primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2011;9:776-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 305. | Leuschner M, Maier KP, Schlichting J, Strahl S, Herrmann G, Dahm HH, Ackermann H, Happ J, Leuschner U. Oral budesonide and ursodeoxycholic acid for treatment of primary biliary cirrhosis: results of a prospective double-blind trial. Gastroenterology. 1999;117:918-925. [PubMed] |
| 306. | Rautiainen H, Kärkkäinen P, Karvonen AL, Nurmi H, Pikkarainen P, Nuutinen H, Färkkilä M. Budesonide combined with UDCA to improve liver histology in primary biliary cirrhosis: a three-year randomized trial. Hepatology. 2005;41:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 161] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 307. | Li WX, Yan X, Shi CR, Zhang AP. Chlorambucil for patients with primary biliary cirrhosis. Cochrane Database Syst Rev. 2012;9:CD008714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 308. | Askari F, Innis D, Dick RB, Hou G, Marrero J, Greenson J, Brewer GJ. Treatment of primary biliary cirrhosis with tetrathiomolybdate: results of a double-blind trial. Transl Res. 2010;155:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 309. | Myers RP, Swain MG, Lee SS, Shaheen AA, Burak KW. B-cell depletion with rituximab in patients with primary biliary cirrhosis refractory to ursodeoxycholic acid. Am J Gastroenterol. 2013;108:933-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 310. | Moritoki Y, Lian ZX, Lindor K, Tuscano J, Tsuneyama K, Zhang W, Ueno Y, Dunn R, Kehry M, Coppel RL. B-cell depletion with anti-CD20 ameliorates autoimmune cholangitis but exacerbates colitis in transforming growth factor-beta receptor II dominant negative mice. Hepatology. 2009;50:1893-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 311. | Tsuda M, Moritoki Y, Lian ZX, Zhang W, Yoshida K, Wakabayashi K, Yang GX, Nakatani T, Vierling J, Lindor K. Biochemical and immunologic effects of rituximab in patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Hepatology. 2012;55:512-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 312. | Dhirapong A, Yang GX, Nadler S, Zhang W, Tsuneyama K, Leung P, Knechtle S, Ansari AA, Coppel RL, Liu FT. Therapeutic effect of cytotoxic T lymphocyte antigen 4/immunoglobulin on a murine model of primary biliary cirrhosis. Hepatology. 2013;57:708-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (4)] |
| 313. | Chignard N, Poupon R. Targeting farnesoid x receptor in hepatic and biliary inflammatory diseases. Gastroenterology. 2009;137:734-735; discussion 736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 314. | Kowdley K, Luketic V, Jones D, Chapman R, Burroughs A, Hirschfield G, Poupon R, Schramm C, Vincent C, Rust C. The first new monotherapy therapeutic PBC study in a decade? An international study evaluating the farnesoid X receptor agonist obeticholic acid in PBC. Hepatology. 2011;54:416A. |
| 315. | Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, Morelli A, Parks DJ, Willson TM. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569-3572. [PubMed] |
| 316. | Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009;49:297-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 349] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 317. | Hofmann AF, Mangelsdorf DJ, Kliewer SA. Chronic diarrhea due to excessive bile acid synthesis and not defective ileal transport: a new syndrome of defective fibroblast growth factor 19 release. Clin Gastroenterol Hepatol. 2009;7:1151-1154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 318. | Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pellicciari R, Morelli A. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497-1512. [PubMed] |
| 319. | Fiorucci S, Rizzo G, Antonelli E, Renga B, Mencarelli A, Riccardi L, Morelli A, Pruzanski M, Pellicciari R. Cross-talk between farnesoid-X-receptor (FXR) and peroxisome proliferator-activated receptor gamma contributes to the antifibrotic activity of FXR ligands in rodent models of liver cirrhosis. J Pharmacol Exp Ther. 2005;315:58-68. [PubMed] |
| 320. | Mason A, Luketic V, Lindor K, Hirschfield G, Gordon S, Mayo M, Kowdley K, Pares A, Trauner M, Sciacca C. Farnesoid - X Receptor Agonists: a New Class of Drugs for the Treatment of PBC? An International Study Evaluating the Addition of Obeticholic Acid ( INT - 747 ) to Ursodeoxycholic Acid. Hepatology. 2010;52:357A. |
| 321. | Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, Kowdley KV, Vincent C, Bodhenheimer HC, Parés A. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751-761.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 448] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 322. | Marschall HU, Luketic VA, Mason AL, Lindor KD, Hirschfield GM, Gordon SC, Mayo MJ, Kowdley KV, Pares A, Trauner M. The Farnecoid X receptor (FXR) agonist obeticholic acid (INT-747, 6 alpha-ethyl chenodeoxycholic acid) in combination with urodeoxycholic acid (UDCA) increases plasma FGF-19 concentration or profile in Primary Biliary Cirrhosis (PBC). Hepatology. 2010;52:355a-356a. |
| 323. | Kowdley KV, Jones D, Luketic V, Chapman R, Burroughs A, Hirschfield G, Poupon R, Schramm C, Vincent C, Rust C. An International Study Evaluating the Farnesoid X Receptor Agonist Obeticholic Acid as Monotherapy in Pbc. Hepatology. 2011;54:S13-S13. |
| 324. | Poupon RE, Chrétien Y, Poupon R, Paumgartner G. Serum bile acids in primary biliary cirrhosis: effect of ursodeoxycholic acid therapy. Hepatology. 1993;17:599-604. [PubMed] |
| 325. | Lindor KD, Lacerda MA, Jorgensen RA, DeSotel CK, Batta AK, Salen G, Dickson ER, Rossi SS, Hofmann AF. Relationship between biliary and serum bile acids and response to ursodeoxycholic acid in patients with primary biliary cirrhosis. Am J Gastroenterol. 1998;93:1498-1504. [PubMed] |
| 326. | Marteau P, Chazouilléres O, Myara A, Jian R, Rambaud JC, Poupon R. Effect of chronic administration of ursodeoxycholic acid on the ileal absorption of endogenous bile acids in man. Hepatology. 1990;12:1206-1208. [PubMed] |
| 327. | Eusufzai S, Ericsson S, Cederlund T, Einarsson K, Angelin B. Effect of ursodeoxycholic acid treatment on ileal absorption of bile acids in man as determined by the SeHCAT test. Gut. 1991;32:1044-1048. [PubMed] |
| 328. | Marschall HU, Wagner M, Zollner G, Fickert P, Diczfalusy U, Gumhold J, Silbert D, Fuchsbichler A, Benthin L, Grundström R. Complementary stimulation of hepatobiliary transport and detoxification systems by rifampicin and ursodeoxycholic acid in humans. Gastroenterology. 2005;129:476-485. [PubMed] |
| 329. | Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525-531. [PubMed] |
| 330. | Prieto J, García N, Martí-Climent JM, Peñuelas I, Richter JA, Medina JF. Assessment of biliary bicarbonate secretion in humans by positron emission tomography. Gastroenterology. 1999;117:167-172. [PubMed] |
| 331. | Mitsuyoshi H, Nakashima T, Sumida Y, Yoh T, Nakajima Y, Ishikawa H, Inaba K, Sakamoto Y, Okanoue T, Kashima K. Ursodeoxycholic acid protects hepatocytes against oxidative injury via induction of antioxidants. Biochem Biophys Res Commun. 1999;263:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 332. | Rodríguez-Ortigosa CM, Cincu RN, Sanz S, Ruiz F, Quiroga J, Prieto J. Effect of ursodeoxycholic acid on methionine adenosyltransferase activity and hepatic glutathione metabolism in rats. Gut. 2002;50:701-706. [PubMed] |
| 333. | Bernstein C, Payne CM, Bernstein H, Garewal H. Activation of the metallothionein IIA promoter and other key stress response elements by ursodeoxycholate in HepG2 cells: relevance to the cytoprotective function of ursodeoxycholate. Pharmacology. 2002;65:2-9. [PubMed] |
| 334. | Lapenna D, Ciofani G, Festi D, Neri M, Pierdomenico SD, Giamberardino MA, Cuccurullo F. Antioxidant properties of ursodeoxycholic acid. Biochem Pharmacol. 2002;64:1661-1667. [PubMed] |
| 335. | Arisawa S, Ishida K, Kameyama N, Ueyama J, Hattori A, Tatsumi Y, Hayashi H, Yano M, Hayashi K, Katano Y. Ursodeoxycholic acid induces glutathione synthesis through activation of PI3K/Akt pathway in HepG2 cells. Biochem Pharmacol. 2009;77:858-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 336. | Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, Ohtsuki M, Goto N, Takeda K, Utsunomiya H. Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G735-G747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 337. | Yang H, Ramani K, Xia M, Ko KS, Li TW, Oh P, Li J, Lu SC. Dysregulation of glutathione synthesis during cholestasis in mice: molecular mechanisms and therapeutic implications. Hepatology. 2009;49:1982-1991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 338. | Kawata K, Kobayashi Y, Souda K, Kawamura K, Sumiyoshi S, Takahashi Y, Noritake H, Watanabe S, Suehiro T, Nakamura H. Enhanced hepatic Nrf2 activation after ursodeoxycholic acid treatment in patients with primary biliary cirrhosis. Antioxid Redox Signal. 2010;13:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 339. | Rodrigues CM, Fan G, Ma X, Kren BT, Steer CJ. A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J Clin Invest. 1998;101:2790-2799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 385] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 340. | Koga H, Sakisaka S, Ohishi M, Sata M, Tanikawa K. Nuclear DNA fragmentation and expression of Bcl-2 in primary biliary cirrhosis. Hepatology. 1997;25:1077-1084. [PubMed] |
| 341. | Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, Yoffe B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology. 2002;36:592-601. [PubMed] |
| 342. | Qiao L, Yacoub A, Studer E, Gupta S, Pei XY, Grant S, Hylemon PB, Dent P. Inhibition of the MAPK and PI3K pathways enhances UDCA-induced apoptosis in primary rodent hepatocytes. Hepatology. 2002;35:779-789. [PubMed] |
| 343. | Schoemaker MH, Conde de la Rosa L, Buist-Homan M, Vrenken TE, Havinga R, Poelstra K, Haisma HJ, Jansen PL, Moshage H. Tauroursodeoxycholic acid protects rat hepatocytes from bile acid-induced apoptosis via activation of survival pathways. Hepatology. 2004;39:1563-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 182] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 344. | Solá S, Castro RE, Kren BT, Steer CJ, Rodrigues CM. Modulation of nuclear steroid receptors by ursodeoxycholic acid inhibits TGF-beta1-induced E2F-1/p53-mediated apoptosis of rat hepatocytes. Biochemistry. 2004;43:8429-8438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 345. | Tsagarakis NJ, Drygiannakis I, Batistakis AG, Kolios G, Kouroumalis EA. A concentration-dependent effect of ursodeoxycholate on apoptosis and caspases activities of HepG2 hepatocellular carcinoma cells. Eur J Pharmacol. 2010;640:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 346. | Ikegami T, Matsuzaki Y, Fukushima S, Shoda J, Olivier JL, Bouscarel B, Tanaka N. Suppressive effect of ursodeoxycholic acid on type IIA phospholipase A2 expression in HepG2 cells. Hepatology. 2005;41:896-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 347. | Calmus Y, Gane P, Rouger P, Poupon R. Hepatic expression of class I and class II major histocompatibility complex molecules in primary biliary cirrhosis: effect of ursodeoxycholic acid. Hepatology. 1990;11:12-15. [PubMed] |
| 348. | Kikuchi K, Hsu W, Hosoya N, Moritoki Y, Kajiyama Y, Kawai T, Takai A, Hayami E, Selmi C, Gershwin ME. Ursodeoxycholic acid reduces CpG-induced IgM production in patients with primary biliary cirrhosis. Hepatol Res. 2009;39:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 349. | Yoshikawa M, Tsujii T, Matsumura K, Yamao J, Matsumura Y, Kubo R, Fukui H, Ishizaka S. Immunomodulatory effects of ursodeoxycholic acid on immune responses. Hepatology. 1992;16:358-364. [PubMed] |
| 350. | Ishizaki K, Iwaki T, Kinoshita S, Koyama M, Fukunari A, Tanaka H, Tsurufuji M, Sakata K, Maeda Y, Imada T. Ursodeoxycholic acid protects concanavalin A-induced mouse liver injury through inhibition of intrahepatic tumor necrosis factor-alpha and macrophage inflammatory protein-2 production. Eur J Pharmacol. 2008;578:57-64. [PubMed] |
| 351. | Tu CT, Han B, Yao QY, Zhang YA, Liu HC, Zhang SC. Curcumin attenuates Concanavalin A-induced liver injury in mice by inhibition of Toll-like receptor (TLR) 2, TLR4 and TLR9 expression. Int Immunopharmacol. 2012;12:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 352. | Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, Zügel U, Steinmeyer A, Pollak A, Roth E. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 378] [Article Influence: 18.9] [Reference Citation Analysis (9)] |
| 353. | D’Aldebert E, Biyeyeme Bi Mve MJ, Mergey M, Wendum D, Firrincieli D, Coilly A, Fouassier L, Corpechot C, Poupon R, Housset C. Bile salts control the antimicrobial peptide cathelicidin through nuclear receptors in the human biliary epithelium. Gastroenterology. 2009;136:1435-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 354. | Schwarzenberg SJ, Bundy M. Ursodeoxycholic acid modifies gut-derived endotoxemia in neonatal rats. Pediatr Res. 1994;35:214-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 355. | Hori Y, Ohyanagi H. Protective effect of the intravenous administration of ursodeoxycholic acid against endotoxemia in rats with obstructive jaundice. Surg Today. 1997;27:140-144. [PubMed] |
| 356. | Nishigaki Y, Ohnishi H, Moriwaki H, Muto Y. Ursodeoxycholic acid corrects defective natural killer activity by inhibiting prostaglandin E2 production in primary biliary cirrhosis. Dig Dis Sci. 1996;41:1487-1493. [PubMed] |
| 357. | Markus BH, Dickson ER, Grambsch PM, Fleming TR, Mazzaferro V, Klintmalm GB, Wiesner RH, Van Thiel DH, Starzl TE. Efficiency of liver transplantation in patients with primary biliary cirrhosis. N Engl J Med. 1989;320:1709-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 136] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 358. | Karlsen TH, Vesterhus M, Boberg KM. Review article: controversies in the management of primary biliary cirrhosis and primary sclerosing cholangitis. Aliment Pharmacol Ther. 2014;39:282-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
P- Reviewer: Lisotti A, Lin CW S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/