Published online May 6, 2014. doi: 10.4292/wjgpt.v5.i2.63
Revised: December 27, 2013
Accepted: February 18, 2014
Published online: May 6, 2014
Processing time: 210 Days and 6.9 Hours
Thiopurines are widely used for maintenance treatment of inflammatory bowel disease. Inter-individual variability in clinical response to thiopurines may be attributed to several factors including genetic polymorphisms, severity and chronicity of disease, comorbidities, duration of administration, compliance issues and use of concomitant medication, environmental factors and clinician and patient preferences. The purpose of this review is to summarise the current evidence on thiopurine safety and toxicity, to describe adverse drug events and emphasise the significance of drug interactions, and to discuss the relative safety of thiopurine use in adults, elderly patients, children and pregnant women. Thiopurines are safe to use and well tolerated, however dose adjustment or discontinuation of treatment must be considered in cases of non-response, poor compliance or toxicity. Drug safety, clinical response to treatment and short to long term risks and benefits must be balanced throughout treatment duration for different categories of patients. Treatment should be individualised and stratified according to patient requirements. Enzymatic testing prior to treatment commencement is advised. Surveillance with regular clinic follow-up and monitoring of laboratory markers is important. Data on long term efficacy, safety of thiopurine use and interaction with other disease modifying drugs are lacking, especially in paediatric inflammatory bowel disease. High quality, collaborative clinical research is required so as to inform clinical practice in the future.
Core tip: This review summarises the safety issues around thiopurine use in adult and paediatric inflammatory bowel disease. Adverse drug effects, toxicity and malignancy risks, interactions with concomitant medications, clinical and laboratory drug surveillance and value of pharmacogenetics in therapeutic drug monitoring are discussed.
- Citation: Konidari A, Matary WE. Use of thiopurines in inflammatory bowel disease: Safety issues. World J Gastrointest Pharmacol Ther 2014; 5(2): 63-76
- URL: https://www.wjgnet.com/2150-5349/full/v5/i2/63.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v5.i2.63
Thiopurines are commonly used for maintenance of clinical remission in inflammatory bowel disease (IBD)[1,2]. They include azathioprine, mercaptopurine (MP) and thioguanine (TG). The onset of their action may vary from 4 to 16 wk[3-5]. Thiopurine induce apoptosis of antigen specific T cells following repetitive encounters with the antigen over a prolonged period of time[6]. Continued medium to long term use is recommended[7], however there is lack of evidence about optimal treatment duration so as to sustain the therapeutic efficacy in both Crohn’s disease (CD) and ulcerative colitis (UC)[8-11].
Safety issues and risk/benefit analysis must be considered prior to and during treatment with thiopurines, therefore regular follow up for detection of poor response, dose adjustment or treatment modification is necessary. Surveillance is essential for prompt identification of adverse drug events, thiopurine toxicity, interactions with concomitant medication and loss of response over time. Factors including genetic differences, age, disease duration and severity, comorbidities and the environment may influence treatment efficacy and safety[12-14].
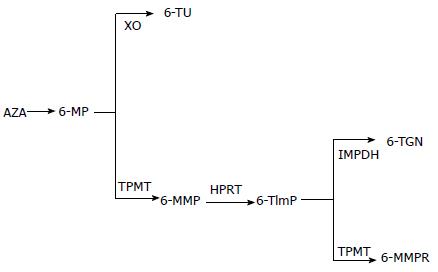
Thiopurines are structural purine analogues which, when orally administered, are absorbed across the gastrointestinal tract epithelium and carried by the portal venous system to the liver before entering systemic circulation. Trials of intravenous thiopurine infusions have been reported[15,16]. Azathioprine is a pro-drug with good oral bioavailability and long duration of action. In the liver, azathioprine undergoes enzymatic[17,18] and non-enzymatic reduction in the presence of glutathione and releases MP and 6-TG. There is a paucity of evidence on the use of thioguanine[19], but its use has been associated with severe irreversible hepatotoxicity and therefore is not currently recommended outside a clinical trial setting[20]. Small scale retrospective cohort studies by van Asseldonk et al[21] and Herrlinger et al[22] advocate short term efficacy of thioguanine for maintenance of remission; however the risk of hepatotoxicity outweighs potential clinical benefits. MP is converted to thioinosine monophosphate (tIMP) by hypoxanthine guanine phosphoribosyltransferase (HGPRT); tIMP is either converted to inactive inosine-triphosphate (ITP by ITPase) or 6TImP (6 thioinosino-5’ monophosphate), by thiopurine methyltransferase (TPMT), which inhibits nucleic acid synthesis. Through this cytotoxic effect on dividing cells, thiopurines inhibit clonal proliferation during the induction phase of the adaptive immune response; as a consequence, antibody and cell mediated immune responses of the effector phase are also suppressed[23]. 6-TG is ribosylated and phosphorylated by HGPRT and irreversibly inhibits 6-thioguanine nucleotides (6-TGN) production. Various metabolites are enzymatically produced during this process: mainly active metabolites are 6-TGN, which mediate the pharmacologic effect of thiopurines, and 6-methylmercaptopurine ribonucleotides (6-MMPR) which are formed by TPMT and principally mediate thiopurine induced hepatotoxicity (Figure 1).
The immunosuppressive effect of thiopurines has been reported to be exerted by alternative mechanisms; for instance by gene expression suppression of inflammatory genes such as a4-integrin, tumour necrosis factor (TNF) ligand superfamily member 10 and TNF receptor superfamily member 7[24]. Other proposed in vitro mechanism by Tiede et al[25] is through inhibition of GTPase Rac1 activation by azathioprine metabolite 6-thioguanine triphosphate (6-Thio-GTP) in CD4+ human T lymphocytes[26].
Recent Cochrane reviews have highlighted that azathioprine is beneficial for maintenance but not induction of remission in active CD and UC[10,27]. Thiopurines exert indirect steroid sparing effect due to their effectiveness in sustaining prolonged clinical remission[10,28,29], especially when used in moderate to severe IBD[30]. Reduced rates of first laparotomy in adults with CD and positive effect in the avoidance of advanced colorectal cancer have been reported[31,32]. No clear evidence on surgery sparing benefit has been demonstrated in a population based study which investigated the effect of immunosuppressive drugs and surgery rates over time[33]. Thiopurine use has been shown to improve quality of life in adult patients with IBD[34,35]. Children also tolerate thiopurines and can achieve prolonged remission[36,37].
A meta-analysis by French et al investigated relapse rates following withdrawal of azathioprine in CD and reported lack of strong evidence in support of continuous thiopurine treatment beyond 18 mo for maintenance of remission in CD[38]. Fraser et al in a 30 years review reported sustained efficacy for five years in adult patients with CD and UC[8]. A randomised controlled trial by Hawthorne et al[39] reported that early withdrawal of azathioprine in UC patients in clinical remission for at least six months, resulted in significantly higher relapse rate, when compared to patients on minimum of two years continuous treatment. Thiopurines are therefore safe, effective medicines with a pivotal role in the treatment of IBD[40].
Adverse drug reactions have historically been divided in idiosyncratic and intrinsic (dose dependent). The first type is probably immune mediated, unpredictable and can occur within few weeks of treatment commencement. Reactions include intolerance and hypersensitivity manifestations, such as malaise, dizziness, vomiting, diarrhoea, fever, myalgia, arthralgia, rash and hypotension[41]. Rare idiosyncratic reactions include renal impairment[42], pneumonitis[43-45], and pancreatitis[46].
Thiopurine-induced liver dysfunction secondary to methylated intermediate metabolites can manifest as elevated liver enzymes, hepatitis, cholestatic jaundice or hepatic veno-occlusive disease. Liver impairment following thiopurine administration has been divided into three categories: hypersensitivity, idiosyncratic cholestatic reactions, and nodular regenerative hyperplasia (NRH). Characteristic nodules of hypertrophied hepatocytes with adjacent areas of atrophied hepatocytes arise following thiopurine induced sinusoidal endothelial injury or obliterative portal venopathy[47,48]. Dose-dependent toxicity is possible in cases of NRH[20,49]. The cumulative incidence of NRH in IBD patients treated with thiopurines is approximately 0.6% and 1.28% at 5 and 10 years respectively[50]; patients on higher dose of thiopurines have increased risk of NRH[51]. NRH however can occur in thiopurine-naïve patients with IBD (reported incidence 6%) and IBD may be an independent predisposing risk factor[51]. Pathogenesis of NRH is obscure. Reported risk factors for NRH, other than thiopurine dose in patients with IBD, are male gender, older age, stricturing disease and small bowel resection[50,52,53]. Mild liver impairment presenting with raised liver function tests, which represents the majority of cases, may resolve with or without dose reduction[20].
Bone marrow toxicity manifesting as myelosuppression and/or aplasia is the most severe haematological adverse drug reaction and leads to discontinuation of treatment[54]. Haematological toxicity presenting as leucopenia is presumed to be avoided with reduced doses[55], however there are studies which do not support that this type of toxicity is dose related[56,57]. Patients with UC treated with immunomodulators have demonstrated a higher incidence of infections than patients not on thiopurines[58], due to impaired immune response. In particular, patients with inflammatory bowel disease are at increased risk of acquiring opportunistic infections (OR = 3.1; 95%CI: 1.7-5.5)[59], for instance cytomegalovirus (CMV) infection (especially pneumonitis or enteritis), which may cause aggravation of underlying disease and failure of immunosuppressive treatment[60]. Pneumocystis jiroveci pneumonitis is an opportunistic infection with severe morbidity. Antibiotic prophylaxis should be considered on a case by case basis, especially in patients with advanced age, increased disease severity and extensive disease[61]. Parasitic or other fungal infections are extremely rare in IBD[62].
Due to thiopurine induced immunosuppression, 3-5 yearly pneumococcal and annual prophylactic influenza vaccinations with trivalent inactivated vaccine are recommended[62]. International consensus guidance recommends varicella, tuberculosis, Hepatitis B, Hepatitis C, HIV screening and prophylactic vaccination (not BCG) prior to treatment with immunomodulator[62,63].
The incidence of adverse drug events are summarised in Table 1.
In the elderly population, there is good evidence of functional alterations in cells from the innate and adaptive immune systems resulting in a state of dysregulated immune function and increased susceptibility to infection[64-66]. There are data to demonstrate that mainly bacterial infections (urinary tract infections and community acquired pneumonia), and few viral infections, such as influenza, are more prevalent and severe in the elderly patients with IBD than in younger adults[66].
Elderly patients (> 65 years of age) on immunosuppressants are at increased risk of developing malignancies[67], when compared to younger adults and children. Past or co-existing comorbidities, such as previous cancer, predispose them to additional malignancy risk, for example lymphoma[68].
In elderly patients therefore, the benefit of long term (over 5 years) thiopurine use may not outweigh the risks[69]; disease chronicity and severity may of course exert a confounding effect in observed outcomes in this population. Adverse drug events such as NRH have been more frequently reported in older age[51].
Variation in disease management is common in paediatric IBD due to lack of high quality randomised controlled trials in children. The ongoing development of service networks will accelerate collaborative standardised research in children with IBD for generation of high quality evidence and improvement of care[70].
The thiopurines have been rarely implicated in lymphoproliferative disorders in childhood IBD. The relative risk is 3-4 folds increased, however the absolute risk is very low[71].
In paediatric IBD, early life onset of disease translates into longstanding disease activity requiring life-long medication[72]. Paediatric-onset UC has a different phenotype than adult-onset disease with more extensive (pan colitis) and more aggressive disease course. Special consideration in the decision making process about treatment in children must be given to growth, puberty and bone density accrual. A large number of children are at risk for steroid-dependency, therefore steroid sparing strategies with early use of immunomodulators such as thiopurines are recommended in high-risk patients[73]. On the other hand, the safety profile of immunosuppressive therapy in children stipulates a more conservative approach, with early treatment intensification applied in patients with severe or refractory disease[12]. Punati et al[30] published a prospective multicentre observational study where early thiopurine use was associated with reduced corticosteroid exposure and possibly fewer hospitalizations per patient. Similarly, Riello et al[37], in a retrospective study of 105 children treated with thiopurines, reported that the majority of patients who were in steroid-free remission by 12 mo, remained in prolonged remission.
Use of thiopurines is not an absolute contraindication throughout pregnancy; however data on their safety profile during pregnancy is insufficient. The human placenta is believed to act as a barrier; a human placental perfusion model has been used to demonstrate the binding of the drug to placental tissue. Maternal pharmacokinetic parameters could restrict the fetal exposure to drug metabolite[74]. Fetal 6-TGN levels correlated with maternal 6-TGN in a prospective study of 28 pregnant women on thiopurines; maternal thiopurine metabolism was affected during pregnancy. 60% of the neonates were noted to be anaemic at birth[75]. Casanova et al[76] conducted a retrospective multicentre study with 571 pregnant women and found no increase in adverse outcomes for pregnant women and newborns following exposure to thiopurine. There is no evidence whether IBD or medical therapy [5 aminosalicylates (5-ASA), thiopurines, corticosteroids] during pregnancy increase the risk of major congenital anomalies in the off-springs; this was recently shown in a retrospective case control study of women with IBD (n = 1703) and women without the disease (n = 384811)[77]. Akbari et al[78] identified the risk of preterm delivery in a recent meta-analysis which reviewed the effects of thiopurines on birth outcomes of female and male patients with IBD; despite this, exposure to thiopurine at the time of conception was not associated with increased risk of congenital abnormalities. The development and immune function of children exposed to thiopurines in utero has not been affected until the age of six years[79].
Thiopurines can increase the incidence of malignancies by different plausible mechanisms, such as by incorporating ‘rogue’ thiopurine nucleotides in the DNA or by rendering DNA highly sensitive to ultraviolet radiation, thereby promoting mutagenesis[80-82].
Four to six fold increased risk of hematologic malignancies[83] has been observed in patients treated with azathioprine; however no causality has been established to date. Risk increases gradually over successive years of therapy and discontinuation of thiopurine therapy reduces the risk[84]. The absolute risk of lymphoma however is low; balancing the potential risk of lymphoma against the risk of undertreatment IBD should inform decision making in the medical management of IBD[85].
Latent or primary opportunistic EBV infection during immunosuppressive therapy may result in post-transplant like lymphoproliferative disease or haemophagocytic lymphohistiocytosis[86,87]; the latter has also been reported following CMV infection[88]. 5% of EBV negative peripheral T cell lymphomas reported in IBD patients are non-Hodgkin’s hepatosplenic T cell lymphomas (HSTCL) with poor prognosis[89,90]. A systematic review on medication, therapy duration and patient age in reported cases of HSTCL concluded that most patients were male, younger than 35 years old, and had received at least 2 years of combined treatment with anti-TNF agent and thiopurine[91] or anti-TNF monotherapy[92].
Relative increase in non-melanoma skin cancer was shown in a large retrospective cohort study of over 50000 adult patients with IBD, conducted by Long et al[93]; thiopurine treatment of a minimum of three months has been associated with increased risk of non-melanoma skin cancers compared to controls. Melanoma skin cancer may increase by 37% according to a meta-analysis investigating the risk of melanoma in a cohort of 172837 patients with IBD; however no specific increase in risk has been associated with thiopurine treatment per se[94].
Since the beginning of this century, there is a controversy in the medical literature with regards to increased risk of gastrointestinal neoplasia in patients with IBD.
A meta-analysis by Eaden et al in 2001 investigated the colorectal cancer risk in UC, and reported a risk of 3% (95%CI: 2.2-3.8) at 10 years, 5.9% (95%CI: 4.3-7.4) at 20 years, and 8.7% (95%CI: 6.4-10.9) at 30 years after diagnosis. A non-significant increase in the risk of colorectal cancer (CRC) by decade of disease was also reported. In 2005, a more recent meta-analysis of population based studies by Jess et al however reported a 2.4 (95%CI: 2.1-2.7) fold increase in CRC risk in patients with UC[95].
Canavan et al[96] performed a meta-analysis to ascertain the combined relative risk of gastrointestinal malignancies and reported increased relative risk in CD. The most recent meta-analysis by Lutgens et al[97] has updated CRC risk in both ulcerative and Crohn’s colitis, by investigating time trends, and identifying high-risk modifiers; it has been concluded that the risk of CRC is increased in patients with IBD but not as high as previously reported and that the risk of CRC is significantly higher in patients with longer disease duration, extensive disease, and IBD diagnosis at young age[97].
Thiopurine treatment did not decrease the risk of colorectal neoplasia in UC (n = 315 patients)[98]. Beaugerie et al[99] nevertheless conducted a large prospective cohort study with 20000 patients and found that thiopurine treatment significantly lowered the multivariate adjusted hazard ratio for colorectal neoplasia (HR = 0.28, 95%CI: 0.1-0.9; P = 0.03). A recent meta-analysis by Gong et al[100] which addressed the same issue concluded that thiopurines exerted a chemo prophylactic effect and a tendency of reducing advanced colorectal neoplasms in IBD, however due to the heterogeneity of included studies, the authors suggested that the results should be interpreted with caution.
Young female smokers with concomitant 5-aminosalicylic acid and thiopurine exposure[101] are at increased risk of cervical cancer; it is still unclear whether the disease itself or whether the treatment may predispose to cervical dysplasia[102]. Evidence to date is inconclusive; no clear increase in relative risk secondary to the use of thiopurines has been shown in the majority of relevant studies, with the exception of a population based case control study by Singh et al[103], which reported increased risk for cervical dysplasia or cancer in patients on both steroids and immunomodulators. Lees et al[104] conducted a large case control study where women with IBD were not shown to have increased rates of abnormal cervical smears unless they smoked; this finding was not affected by immunosuppressant therapy or disease phenotype. Cervical cancer surveillance and HPV vaccination is currently recommended[102].
Treatment with immunosuppressive drugs had no major impact on the increased risk of developing new or recurrent cancer in a prospective cohort of 405 IBD patients with pre-existing history of cancer[105]. Pasternak et al[106] conducted a retrospective cohort study and reported that azathioprine treatment in IBD was associated with marginally increased risk for lymphoma and urinary tract cancer, therefore reported an overall increased risk of malignancy with azathioprine treatment (RR = 1.41; 95%CI: 1.15-1.74) interestingly though, previous use of azathioprine or increasing cumulative received doses did not increase the cancer risk. Finally, a meta-analysis of Masunaga et al[107] investigated whether long-term administration of immunosuppressants in patients with IBD increased the risk of malignancy and concluded that treatment did not increase the overall cancer risk in patients with IBD; drugs other than thiopurines, such as cyclosporine, methotrexate, tacrolimus were included in the analysis.
Clinical surveillance and patient follow up are required for identification of adverse drug events throughout the duration of thiopurine treatment; this can be enhanced by regular monitoring of laboratory indices, such as peripheral blood counts, pancreatic and liver function tests[108]. There is a lack of consensus on the usefulness of active thiopurine metabolite monitoring as surrogate markers of thiopurine efficacy and toxicity. Their use is not widely recommended or adopted[109] and largely depends on local practices and individual clinician preferences.
Higgs et al[110] published a meta-analysis about the increased risk of myelosuppression in patients with intermediate TPMT activity and concluded that in spite of controversial findings between studies, “higher 6-TGN levels were generally associated with clinical remission”. Gonzalez-Lama conducted a prospective multicentre cohort study which did not support determination of TPMT activity or 6-TGN concentrations for prediction of treatment outcome; no clinically useful serum metabolites threshold value was identified for dose adjustment purpose[111]. Poor correlation between 6-TGN levels and thiopurine dose has also been reported by other studies[4,56,112,113], however metabolites levels may contribute to earlier and safer dose tailoring, especially in patients with poor clinical response or non-compliance[114-117].
Measurement of active thiopurine metabolite levels in addition to regular full blood count monitoring, especially over the first 1-2 mo after treatment commencement, and regularly thereafter has been proposed[118-120]. 6-MMPR levels and liver enzymes may be used in combination as surrogate markers of hepatotoxicity; a 6-MMPR cut-off value > 5700 pmol/8 × 108 RBC has been recommended as indication of liver dysfunction[117,121-123]. There is currently no clear consensus on optimal frequency of blood monitoring[124].
Allopurinol is known to reduce thiopurine-induced hepatotoxicity[125] and may have a synergistic therapeutic effect to patients who preferentially produce 6-MMPR, rather than 6-TGN[126-128]. The underlying mechanism remains enigmatic. Different pathways have been proposed: (1) allopurinol inhibition of both xanthine dehydrogenase and TPMT and promotion of 6-TGN production at the expense of 6-MMPR[129]; (2) TPMT inhibition by allopurinol’s active metabolite oxypurinol[130]; and (3) increased HGPRT activity and subsequent 6-TGN increase[131]. Co-administration of allopurinol has been reported to allow thiopurine dose reduction by up to 75%[23]; this may enhance efficacy in azathioprine refractory patients and avoid high doses with potential dose dependent adverse events; conversely combined therapy could potentially induce toxicity in azathioprine-naïve patients due to the synergistic effect of these two medicines. Combination therapy with allopurinol is therefore currently not widely used, due to the lack of adequate high grade evidence from double blinded randomised controlled trials assessing the risk-benefit ratios of dual therapy, especially in paediatrics.
5-ASA have been reported to increase 6-TGN and decrease the production of 6-MMPR in two prospective adult studies[132,133]. de Boer et al[134] conducted a prospective multicentre pharmacokinetic study and reported a significant dose-dependent increase in 6-TGN levels in cases of 5-ASA co-administration. It was concluded that patients refractory to standard thiopurine therapy may benefit from the co-administration of 5-ASA. A systematic review by Andrews et al addressed the clinical outcomes following concomitant 5ASA and thiopurines administration; it was unclear whether combination therapy improved outcomes of disease control, drug toxicity or compliance, but concurrent therapy could decrease colorectal risk at “acceptable cost”[135]. An increased risk of myelotoxicity has been noted in children with IBD treated with combination therapy[136]. Nguyen et al[137] in a study of 71 children with IBD, reported more frequently observed lymphopenia and elevated 6-TGN concentrations, without increase in remission rate in patients on combined treatment. A favourable clinical outcome has been described by Tajiri et al[56] in paediatric UC, however a high rate (40%) of myelosuppression was noted. The clinical benefit of combination treatment therefore needs to be further researched and careful weighted against toxicity risk[132].
New disease modifying drugs such as infliximab, adalimumab have been increasingly used in adult and paediatric gastroenterology. Infliximab is the first anti-TNF alpha agent ever introduced in the treatment of IBD and therefore has been more widely researched to date. It is a partially humanised monoclonal antibody against tumour necrosis factor alpha (TNF-α), a cytokine mediator of inflammation. Historically anti-TNF alpha drugs were principally introduced as rescue medical therapy in patients with treatment refractory, severe or extensive disease[138].
A top-down versus a “bottom-up” approach to treatment is the epicentre of attention within the international community of gastroenterologists; early introduction versus rescue use of anti-TNF agents, with or without concomitant thiopurine for prompt treatment intensification and avoidance of disease complications is the dilemma in current clinical practice[139,140].
Combination therapy with infliximab and azathioprine has been reported more favourable than monotherapy for induction and maintenance of steroid-free remission, and for avoidance of postoperative recurrence[140,141]. Higher serum trough concentrations of infliximab, lower anti-infliximab antibodies and better clinical outcomes may occur more frequently in patients receiving combination therapy with azathioprine[139,142]. Colombel et al[139] specifically reported that combination may be superior to azathioprine or infliximab alone, not only for induction and maintenance of steroid free remission, but also for increased mucosal healing rates in adult patients with moderate to severe CD. The described effect was sustained at one-year follow up. The beneficial effects of combination therapy on mucosal healing, steroid-free remission and sustained increase in quality of life have been reported in both UC[143] and CD[144]. Sokol et al[145] conducted a prospective cohort study and reported favourable six month outcomes with regards to disease activity, infliximab dose and need of other anti-TNF alpha agents such as adalimumab.
On the contrary, Lichtenstein et al[146] pooled data from multicentre prospective randomised controlled trials in adult patients with IBD and concluded that concomitant use of immunomodulators-principally thiopurines but also methotrexate in isolated cases with CD did not significantly increase efficacy or alter thiopurine safety; infusion reactions were about 50% less in combined therapy than in infliximab monotherapy.
Van Assche et al[147] conducted an open label randomised controlled trial to evaluate the influence of anti-TNF alpha discontinuation after patients had been in remission for at least six months with combined therapy; no clear benefit of continuing combined therapy beyond six months from clinical remission was demonstrated.
Combination of anti-TNF agents with thiopurines may therefore enhance immunosuppression, however the risk of related adverse drug reactions and toxicity is also enhanced[148]. Combination therapy may also be associated with higher relative risk of opportunistic infections in UC, but no significantly increased absolute risk of serious infections has been observed[58,149].
Limited evidence exists on drug interactions between azathioprine and adalimumab. The latter has recently obtained approval by the European Medicines Agency and the United States Food and Drug Administration for use in adult patients with moderate-to-severe, active, refractory UC, who are intolerant to corticosteroids and thiopurines[150]. Adalimumab is effective in inducing and maintaining remission in patients with active, moderate-to-severe, luminal or perianal CD, or patients with previous loss of response or intolerance to infliximab[151-153]. A recent prospective case series of twelve adult patients with CD reported that 6-TGN and 6-MMPR were not influenced by concomitant administration of adalimumab during a 12 wk follow up period[154]. The same study reported no change in TPMT, ITPase or HGPRT enzyme activity after 4 wk of combined treatment. More research is required into the efficacy and safety of combination treatment.
Chaparro et al has recently reported the findings of a large prospective nationwide cohort study from Spain, where 67% of 1026 patients had to discontinue thiopurine treatment due to adverse drug events such as nausea, arthralgia, alopecia, abdominal pain, liver and pancreatic toxicity, infection, leucopenia, myelotoxicity. 37% percent of them were restarted on the same thiopurine. 40% had recurrent side effects; 4% following treatment with the same thiopurine and 36% after introduction of an alternative thiopurine[155]. Interestingly even in patients with severe complications such as hepatotoxicity, thiopurine re-introduction was tolerated in 74% of cases; abdominal pain recurred in 18% of cases, nausea and arthralgia recurrence close to 50% was noted. 85% of patients demonstrated recurrence of pancreatic toxicity, recurrence rates of infection and bone marrow failure were 80% and 65% respectively. Overall over half of the patients tolerated re-introduction of thiopurine treatment following drug induced side effects.
Ledder et al[46] published a small case series of four adult patients with successful introduction of mercaptopurine following azathioprine induced pancreatitis.
A recent meta-analysis on alternative use of mercaptopurine in adult patients with IBD who suffered azathioprine-induced toxicity (n = 455 patients), concluded that favourable outcomes had been observed in two-thirds of patients where trial of mercaptopurine was implemented[57]. In detail, 62% with gastrointestinal toxicity, 81% with hepatotoxicity and 36% with flu-like illness had been able to tolerate mercaptopurine. Trial of mercaptopurine is therefore advised in cases of azathioprine intolerance, except in patients with severe pancreatitis or bone marrow aplasia. Among patients who discontinued mercaptopurine for further adverse effects, 59% experienced the same adverse effect as they had with azathioprine.
Further studies are required so as to quantify the hepatotoxicity risk associated with thioguanine as an alternative therapy for IBD treatment; metabolism of this non-conventional thiopurine does not generate 6-methyl mercaptopurine[156].
Observed genetic polymorphisms have been reported to play pivotal role in the occurrence of adverse drug reactions for various drugs, including thiopurine[157,158], warfarin[159], antiepileptic[160] and anti-retroviral drugs[161]. Inter-individual variability in drug response reflects differences in genetic polymorphisms which, to some extent, may be responsible for variation in drug metabolism[116,162].
TPMT is an extensively researched example of the clinical applicability of pharmacogenetics; pre-treatment testing is currently implemented; common variant alleles such as TPMT* 2, TPMT*3A, TPMT*3C, TPMT*8 may give rise to decreased enzyme production; heterozygous or homozygous TPMT deficient patients require decreased dose upon treatment commencement, or thiopurine avoidance respectively. Weinshilboum and Sladek first reported that approximately 0.3% Caucasians have complete deficiency, approximately 10% have low or intermediate activity and about 90% have high activity[163]. Numerous studies have since verified the differences in TPMT levels and activity between different ethnic group[164-166].
Pre-treatment determination of TPMT genotype and phenotype may be useful for prediction of thiopurine toxicity; TPMT testing is however not universally implemented by gastroenterologist[167-169], because evidence on its predictive value for thiopurine toxicity in IBD is still unclear; Colombel et al[170] has reported that the majority of adult patients with myelotoxicity had normal TPMT genotype. The controversy in the medical literature may exist because of the non-absolute concordance between genotype and phenotype (TPMT activity status)[167,171], and the diversity in the laboratory methods (enzymatic, radiochemical, high performance liquid chromatography assays) used for quantification of TPMT activity in red blood cells[172]. A systematic review by Booth et al[173] reported that in patients with intermediate and low enzymatic activity, genotyping sensitivity to identify patients with low enzymatic activity ranged from 70.33% to 86.15% (lower-bound 95%CI: 54.52%-70.88%; upper-bound 95%CI: 78.50%-96.33%) and was therefore imprecise. Despite this finding, variant genotype and low TPMT activity were reported to be strongly associated with haematological toxicity.
Additional polymorphic genes implicated in thiopurine metabolism are under investigation for possible effect on effectiveness and safety. These are the ITPase[174], the guanine monophosphate synthetase (GMPS mediates tIMP conversion to tGMP) and the glutathione S transferases (GST which catalyses MP production from azathioprine)[175,176].
Smith et al[177] reported that single nucleotide polymorphism (SNP) of aldehyde oxidase (AOX1) c.3404A > G may predict lack of response (P = 0.035, OR = 2.54, 95%CI: 1.06-6.13); when combined with TPMT activity, this information allowed stratification of a patient’s chance of response to azathioprine, ranging from 86% in patients where both markers were favourable to 33% where both were unfavourable (P < 0.0001)[177].
A common SNP, associated with a dramatically reduced ABCC4 function, has been identified in approximately 14%-18% of the Japanese population. In these patients, the OR of carrying the ABCC4 variant and having leucopenia following thiopurine introduction has been reported to be 3.30 (95%CI: 1.03-10.57; P = 0.036)[178].
In the future, the pharmacogenetic approach may enhance pre-treatment safety and prompt dose adjustment[19].
Safety of thiopurine treatment in IBD stipulates fine tuning between therapeutic efficacy, intolerance and toxicity. This balance must be achieved on a long term basis. Combination therapy with new disease modifying drugs has further modified the safety profile of thiopurines; the importance of such interactions has yet to be confirmed in large studies across all age groups. There is a need for a standardised approach in therapeutic drug monitoring. Further research into disease pathogenesis and pharmacokinetic/pharmacodynamic pathways may identify potentially useful biomarkers for thiopurine safety monitoring. High quality, prospective and collaborative clinical research will establish a robust evidence foundation which will safely inform future clinical practice.
| 1. | Candy S, Wright J, Gerber M, Adams G, Gerig M, Goodman R. A controlled double blind study of azathioprine in the management of Crohn’s disease. Gut. 1995;37:674-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 410] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 2. | Markowitz J, Grancher K, Kohn N, Lesser M, Daum F. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn’s disease. Gastroenterology. 2000;119:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 525] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 3. | Derijks LJ, Gilissen LP, Engels LG, Bos LP, Bus PJ, Lohman JJ, van Deventer SJ, Hommes DW, Hooymans PM. Pharmacokinetics of 6-thioguanine in patients with inflammatory bowel disease. Ther Drug Monit. 2006;28:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Pozler O, Chládek J, Malý J, Hroch M, Dědek P, Beránek M, Krásničanová P. Steady-state of azathioprine during initiation treatment of pediatric inflammatory bowel disease. J Crohns Colitis. 2010;4:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Hindorf U, Lindqvist M, Peterson C, Söderkvist P, Ström M, Hjortswang H, Pousette A, Almer S. Pharmacogenetics during standardised initiation of thiopurine treatment in inflammatory bowel disease. Gut. 2006;55:1423-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Ben-Horin S, Goldstein I, Fudim E, Picard O, Yerushalmi Z, Barshack I, Bank I, Goldschmid Y, Meir SB, Mayer L. Early preservation of effector functions followed by eventual T cell memory depletion: a model for the delayed onset of the effect of thiopurines. Gut. 2009;58:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Ha C, Dassopoulos T. Thiopurine therapy in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2010;4:575-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002;50:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 464] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 9. | Lémann M, Mary JY, Colombel JF, Duclos B, Soule JC, Lerebours E, Modigliani R, Bouhnik Y. A randomized, double-blind, controlled withdrawal trial in Crohn’s disease patients in long-term remission on azathioprine. Gastroenterology. 2005;128:1812-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 238] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Timmer A, McDonald JW, Tsoulis DJ, Macdonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;9:CD000478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Nielsen CB, Turbiez M, McCulloch I. Recent advances in the development of semiconducting DPP-containing polymers for transistor applications. Adv Mater. 2013;25:1859-1880. [PubMed] |
| 12. | Turner D, Levine A, Escher JC, Griffiths AM, Russell RK, Dignass A, Dias JA, Bronsky J, Braegger CP, Cucchiara S. Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidence-based consensus guidelines. J Pediatr Gastroenterol Nutr. 2012;55:340-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 310] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 13. | LeLeiko NS, Lobato D, Hagin S, McQuaid E, Seifer R, Kopel SJ, Boergers J, Nassau J, Suorsa K, Shapiro J. Rates and predictors of oral medication adherence in pediatric patients with IBD. Inflamm Bowel Dis. 2013;19:832-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Ng SW, Mahadevan U. Management of inflammatory bowel disease in pregnancy. Expert Rev Clin Immunol. 2013;9:161-173; quiz 174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 15. | Kitchen BJ, Moser A, Lowe E, Balis FM, Widemann B, Anderson L, Strong J, Blaney SM, Berg SL, O’Brien M. Thioguanine administered as a continuous intravenous infusion to pediatric patients is metabolized to the novel metabolite 8-hydroxy-thioguanine. J Pharmacol Exp Ther. 1999;291:870-874. [PubMed] |
| 16. | Smith DC, Vick NA, Trump DL, Friedman HS, Friedman AH, Purvis J, Gauspari A, Schold SC. Phase I study of BCNU and intravenous 6-mercaptopurine in patients with anaplastic gliomas. Cancer Chemother Pharmacol. 1992;30:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Watanabe A, Hobara N, Nagashima H. Demonstration of enzymatic activity converting azathioprine to 6-mercaptopurine. Acta Med Okayama. 1978;32:173-179. [PubMed] |
| 18. | Kaplowitz N. Enzymatic thiolysis of azathioprine in vitro. Biochem Pharmacol. 1976;25:2421-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Chouchana L, Narjoz C, Beaune P, Loriot MA, Roblin X. Review article: the benefits of pharmacogenetics for improving thiopurine therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:15-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Gisbert JP, González-Lama Y, Maté J. Thiopurine-induced liver injury in patients with inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2007;102:1518-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | van Asseldonk DP, Seinen ML, de Boer NK, van Bodegraven AA, Mulder CJ. Hepatotoxicity associated with 6-methyl mercaptopurine formation during azathioprine and 6-mercaptopurine therapy does not occur on the short-term during 6-thioguanine therapy in IBD treatment. J Crohns Colitis. 2012;6:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Herrlinger KR, Kreisel W, Schwab M, Schoelmerich J, Fleig WE, Ruhl A, Reinshagen M, Deibert P, Fellermann K, Greinwald R. 6-thioguanine--efficacy and safety in chronic active Crohn’s disease. Aliment Pharmacol Ther. 2003;17:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Barbie DA, Frank DA. Pharmacology of cancer: genome synthesis, stability and maintenance. In Principles of Pharmacology: the pathophysiologic basis of drug therapy. 3rd edition. Golan DE TA, Armstrong EJ, Armstrong AW, editor. Philadelphia: Lippincott Williams and Wilkins 2012; 674-693. |
| 24. | Thomas CW, Myhre GM, Tschumper R, Sreekumar R, Jelinek D, McKean DJ, Lipsky JJ, Sandborn WJ, Egan LJ. Selective inhibition of inflammatory gene expression in activated T lymphocytes: a mechanism of immune suppression by thiopurines. J Pharmacol Exp Ther. 2005;312:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, Lehr HA, Wirtz S, Becker C, Atreya R. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111:1133-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 587] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 26. | Atreya R, Atreya I, Neurath MF. Novel signal transduction pathways: analysis of STAT-3 and Rac-1 signaling in inflammatory bowel disease. Ann N Y Acad Sci. 2006;1072:98-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Chande N, Tsoulis DJ, MacDonald JK. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2013;4:CD000545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Prefontaine E, Macdonald JK, Sutherland LR. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2010;CD000545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 29. | Khan KJ, Dubinsky MC, Ford AC, Ullman TA, Talley NJ, Moayyedi P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106:630-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | Punati J, Markowitz J, Lerer T, Hyams J, Kugathasan S, Griffiths A, Otley A, Rosh J, Pfefferkorn M, Mack D. Effect of early immunomodulator use in moderate to severe pediatric Crohn disease. Inflamm Bowel Dis. 2008;14:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | van Schaik FD, van Oijen MG, Smeets HM, van der Heijden GJ, Siersema PD, Oldenburg B. Thiopurines prevent advanced colorectal neoplasia in patients with inflammatory bowel disease. Gut. 2012;61:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 32. | Szamosi T, Banai J, Lakatos L, Czegledi Z, David G, Zsigmond F, Pandur T, Erdelyi Z, Gemela O, Papp M. Early azathioprine/biological therapy is associated with decreased risk for first surgery and delays time to surgery but not reoperation in both smokers and nonsmokers with Crohn’s disease, while smoking decreases the risk of colectomy in ulcerative colitis. Eur J Gastroenterol Hepatol. 2010;22:872-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Rungoe C, Langholz E, Andersson M, Basit S, Nielsen NM, Wohlfahrt J, Jess T. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979-2011. Gut. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 268] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 34. | Bastida G, Nos P, Aguas M, Beltrán B, Iborra M, Ortiz V, Garrigues V, Estevan R, Ponce J. The effects of thiopurine therapy on health-related quality of life in Inflammatory Bowel Disease patients. BMC Gastroenterol. 2010;10:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Calvet X, Gallardo O, Coronas R, Casellas F, Montserrat A, Torrejón A, Vergara M, Campo R, Brullet E. Remission on thiopurinic immunomodulators normalizes quality of life and psychological status in patients with Crohn’s disease. Inflamm Bowel Dis. 2006;12:692-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Goodhand JR, Tshuma N, Rao A, Kotta S, Wahed M, Croft NM, Sanderson IR, Epstein J, Rampton DS. Do children with IBD really respond better than adults to thiopurines? J Pediatr Gastroenterol Nutr. 2011;52:702-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Riello L, Talbotec C, Garnier-Lengliné H, Pigneur B, Svahn J, Canioni D, Goulet O, Schmitz J, Ruemmele FM. Tolerance and efficacy of azathioprine in pediatric Crohn’s disease. Inflamm Bowel Dis. 2011;17:2138-2143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | French H, Mark Dalzell A, Srinivasan R, El-Matary W. Relapse rate following azathioprine withdrawal in maintaining remission for Crohn’s disease: a meta-analysis. Dig Dis Sci. 2011;56:1929-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Hawthorne AB, Logan RF, Hawkey CJ, Foster PN, Axon AT, Swarbrick ET, Scott BB, Lennard-Jones JE. Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ. 1992;305:20-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 319] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 40. | van Asseldonk DP, Sanderson J, de Boer NK, Sparrow MP, Lémann M, Ansari A, Almer SH, Florin TH, Gearry RB, Mulder CJ. Difficulties and possibilities with thiopurine therapy in inflammatory bowel disease--proceedings of the first Thiopurine Task Force meeting. Dig Liver Dis. 2011;43:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Andrade RJ, Agúndez JA, Lucena MI, Martínez C, Cueto R, García-Martín E. Pharmacogenomics in drug induced liver injury. Curr Drug Metab. 2009;10:956-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Meys E, Devogelaer JP, Geubel A, Rahier J, Nagant de Deuxchaisnes C. Fever, hepatitis and acute interstitial nephritis in a patient with rheumatoid arthritis. Concurrent manifestations of azathioprine hypersensitivity. J Rheumatol. 1992;19:807-809. [PubMed] |
| 43. | Bodelier AG, Masclee AA, Bakker JA, Hameeteman WH, Pierik MJ. Azathioprine induced pneumonitis in a patient with ulcerative colitis. J Crohns Colitis. 2009;3:309-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Ananthakrishnan AN, Attila T, Otterson MF, Lipchik RJ, Massey BT, Komorowski RA, Binion DG. Severe pulmonary toxicity after azathioprine/6-mercaptopurine initiation for the treatment of inflammatory bowel disease. J Clin Gastroenterol. 2007;41:682-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Nagy F, Molnar T, Makula E, Kiss I, Milassin P, Zollei E, Tiszlavicz L, Lonovics J. A case of interstitial pneumonitis in a patient with ulcerative colitis treated with azathioprine. World J Gastroenterol. 2007;13:316-319. [PubMed] |
| 46. | Ledder OD, Lemberg DA, Ooi CY, Day AS. Are thiopurines always contraindicated after thiopurine-induced pancreatitis in inflammatory bowel disease? J Pediatr Gastroenterol Nutr. 2013;57:583-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Wanless IR, Godwin TA, Allen F, Feder A. Nodular regenerative hyperplasia of the liver in hematologic disorders: a possible response to obliterative portal venopathy. A morphometric study of nine cases with an hypothesis on the pathogenesis. Medicine (Baltimore). 1980;59:367-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 184] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 48. | Haboubi NY, Ali HH, Whitwell HL, Ackrill P. Role of endothelial cell injury in the spectrum of azathioprine-induced liver disease after renal transplant: light microscopy and ultrastructural observations. Am J Gastroenterol. 1988;83:256-261. [PubMed] |
| 49. | de Jong DJ, Goullet M, Naber TH. Side effects of azathioprine in patients with Crohn’s disease. Eur J Gastroenterol Hepatol. 2004;16:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Seksik P, Mary JY, Beaugerie L, Lémann M, Colombel JF, Vernier-Massouille G, Cosnes J. Incidence of nodular regenerative hyperplasia in inflammatory bowel disease patients treated with azathioprine. Inflamm Bowel Dis. 2011;17:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Musumba CO. Review article: the association between nodular regenerative hyperplasia, inflammatory bowel disease and thiopurine therapy. Aliment Pharmacol Ther. 2013;38:1025-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Teml A, Schwab M, Hommes DW, Almer S, Lukas M, Feichtenschlager T, Florin T, Seiderer J, Petritsch W, Bokemeyer B. A systematic survey evaluating 6-thioguanine-related hepatotoxicity in patients with inflammatory bowel disease. Wien Klin Wochenschr. 2007;119:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Dubinsky MC, Vasiliauskas EA, Singh H, Abreu MT, Papadakis KA, Tran T, Martin P, Vierling JM, Geller SA, Targan SR. 6-thioguanine can cause serious liver injury in inflammatory bowel disease patients. Gastroenterology. 2003;125:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 191] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 54. | Shih DQ, Nguyen M, Zheng L, Ibanez P, Mei L, Kwan LY, Bradford K, Ting C, Targan SR, Vasiliauskas EA. Split-dose administration of thiopurine drugs: a novel and effective strategy for managing preferential 6-MMP metabolism. Aliment Pharmacol Ther. 2012;36:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Kim DU, Kim YH, Kim BJ, Chang DK, Son HJ, Rhee PL, Kim JJ, Rhee JC. The efficacy of low dose azathioprine/6-mercaptopurine in patients with inflammatory bowel disease. Hepatogastroenterology. 2009;56:1395-1402. [PubMed] |
| 56. | Tajiri H, Tomomasa T, Yoden A, Konno M, Sasaki M, Maisawa S, Sumazaki R, Shimizu T, Toyoda S, Etani Y. Efficacy and safety of azathioprine and 6-mercaptopurine in Japanese pediatric patients with ulcerative colitis: a survey of the Japanese Society for Pediatric Inflammatory Bowel Disease. Digestion. 2008;77:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Kennedy NA, Rhatigan E, Arnott ID, Noble CL, Shand AG, Satsangi J, Lees CW. A trial of mercaptopurine is a safe strategy in patients with inflammatory bowel disease intolerant to azathioprine: an observational study, systematic review and meta-analysis. Aliment Pharmacol Ther. 2013;38:1255-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Lichtenstein GR, Rutgeerts P, Sandborn WJ, Sands BE, Diamond RH, Blank M, Montello J, Tang L, Cornillie F, Colombel JF. A pooled analysis of infections, malignancy, and mortality in infliximab- and immunomodulator-treated adult patients with inflammatory bowel disease. Am J Gastroenterol. 2012;107:1051-1063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 59. | Toruner M, Loftus EV, Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, Colombel JF, Egan LJ. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 763] [Article Influence: 42.4] [Reference Citation Analysis (1)] |
| 60. | Papadakis KA, Tung JK, Binder SW, Kam LY, Abreu MT, Targan SR, Vasiliauskas EA. Outcome of cytomegalovirus infections in patients with inflammatory bowel disease. Am J Gastroenterol. 2001;96:2137-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 202] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 61. | Poppers DM, Scherl EJ. Prophylaxis against Pneumocystis pneumonia in patients with inflammatory bowel disease: toward a standard of care. Inflamm Bowel Dis. 2008;14:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Rahier JF, Ben-Horin S, Chowers Y, Conlon C, De Munter P, D’Haens G, Domènech E, Eliakim R, Eser A, Frater J. European evidence-based Consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2009;3:47-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 378] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 63. | Walsh AJ, Weltman M, Burger D, Vivekanandarajah S, Connor S, Howlett M, Radford-Smith G, Selby W, Veillard AS, Grimm MC. Implementing guidelines on the prevention of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2013;7:e449-e456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 342] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 65. | Solana R, Pawelec G, Tarazona R. Aging and innate immunity. Immunity. 2006;24:491-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 66. | Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis. 2002;2:659-666. [PubMed] |
| 67. | Smith MA, Irving PM, Marinaki AM, Sanderson JD. Review article: malignancy on thiopurine treatment with special reference to inflammatory bowel disease. Aliment Pharmacol Ther. 2010;32:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 68. | Beaugerie L. Lymphoma: the bête noire of the long-term use of thiopurines in adult and elderly patients with inflammatory bowel disease. Gastroenterology. 2013;145:927-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 69. | Lewis JD, Schwartz JS, Lichtenstein GR. Azathioprine for maintenance of remission in Crohn’s disease: benefits outweigh the risk of lymphoma. Gastroenterology. 2000;118:1018-1024. [PubMed] |
| 70. | Crandall WV, Margolis PA, Kappelman MD, King EC, Pratt JM, Boyle BM, Duffy LF, Grunow JE, Kim SC, Leibowitz I. Improved outcomes in a quality improvement collaborative for pediatric inflammatory bowel disease. Pediatrics. 2012;129:e1030-e1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 71. | Boyle B, Mackner L, Ross C, Moses J, Kumar S, Crandall W. A single-center experience with methotrexate after thiopurine therapy in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2010;51:714-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 72. | Hyams JS. Risk/benefit strategies must be employed in the management of pediatric Crohn’s disease. Dig Dis. 2009;27:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Ruemmele FM, Turner D. Differences in the management of pediatric and adult onset ulcerative colitis--lessons from the joint ECCO and ESPGHAN consensus guidelines for the management of pediatric ulcerative colitis. J Crohns Colitis. 2014;8:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 74. | Hutson JR, Garcia-Bournissen F, Davis A, Koren G. The human placental perfusion model: a systematic review and development of a model to predict in vivo transfer of therapeutic drugs. Clin Pharmacol Ther. 2011;90:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 75. | Jharap B, de Boer NK, Stokkers P, Hommes DW, Oldenburg B, Dijkstra G, van der Woude CJ, de Jong DJ, Mulder CJ, van Elburg RM. Intrauterine exposure and pharmacology of conventional thiopurine therapy in pregnant patients with inflammatory bowel disease. Gut. 2014;63:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 76. | Casanova MJ, Chaparro M, Domènech E, Barreiro-de Acosta M, Bermejo F, Iglesias E, Gomollón F, Rodrigo L, Calvet X, Esteve M. Safety of thiopurines and anti-TNF-α drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol. 2013;108:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 77. | Ban L, Tata LJ, Fiaschi L, Card T. Limited risks of major congenital anomalies in children of mothers with IBD and effects of medications. Gastroenterology. 2014;146:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 78. | Akbari M, Shah S, Velayos FS, Mahadevan U, Cheifetz AS. Systematic review and meta-analysis on the effects of thiopurines on birth outcomes from female and male patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 79. | de Meij TG, Jharap B, Kneepkens CM, van Bodegraven AA, de Boer NK. Long-term follow-up of children exposed intrauterine to maternal thiopurine therapy during pregnancy in females with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 80. | Somerville L, Krynetski EY, Krynetskaia NF, Beger RD, Zhang W, Marhefka CA, Evans WE, Kriwacki RW. Structure and dynamics of thioguanine-modified duplex DNA. J Biol Chem. 2003;278:1005-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 81. | O’Donovan P, Perrett CM, Zhang X, Montaner B, Xu YZ, Harwood CA, McGregor JM, Walker SL, Hanaoka F, Karran P. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science. 2005;309:1871-1874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 507] [Cited by in RCA: 442] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 82. | KAPLAN HS, ZAVARINE R, EARLE J. Interaction of the oxygen effect and radiosensitization produced by base analogues incorporated into deoxyribonuclease acid. Nature. 1962;194:662-664. [PubMed] |
| 83. | Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, Hébuterne X, Cortot A, Bouhnik Y, Gendre JP. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 825] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 84. | Khan N, Abbas AM, Lichtenstein GR, Loftus EV, Bazzano LA. Risk of lymphoma in patients with ulcerative colitis treated with thiopurines: a nationwide retrospective cohort study. Gastroenterology. 2013;145:1007-1015.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 85. | Jones JL, Loftus EV. Lymphoma risk in inflammatory bowel disease: is it the disease or its treatment? Inflamm Bowel Dis. 2007;13:1299-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 86. | Subramaniam K, D’Rozario J, Pavli P. Lymphoma and other lymphoproliferative disorders in inflammatory bowel disease: a review. J Gastroenterol Hepatol. 2013;28:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 87. | Fries W, Cottone M, Cascio A. Systematic review: macrophage activation syndrome in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:1033-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 88. | van Langenberg DR, Morrison G, Foley A, Buttigieg RJ, Gibson PR. Cytomegalovirus disease, haemophagocytic syndrome, immunosuppression in patients with IBD: ‘a cocktail best avoided, not stirred’. J Crohns Colitis. 2011;5:469-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 89. | Herrinton LJ, Liu L, Abramson O, Jaffe ES. The incidence of hepatosplenic T-cell lymphoma in a large managed care organization, with reference to anti-tumor necrosis factor therapy, Northern California, 2000-2006. Pharmacoepidemiol Drug Saf. 2012;21:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 90. | Schmidt LA, Lim MS. T cell lymphoproliferative disorders associated with anti-tumor necrosis factor alpha antibody therapy for ulcerative colitis: literature summary. J Hematop. 2009;2:121-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 91. | Kotlyar DS, Osterman MT, Diamond RH, Porter D, Blonski WC, Wasik M, Sampat S, Mendizabal M, Lin MV, Lichtenstein GR. A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:36-41.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 329] [Article Influence: 21.9] [Reference Citation Analysis (2)] |
| 92. | Parakkal D, Sifuentes H, Semer R, Ehrenpreis ED. Hepatosplenic T-cell lymphoma in patients receiving TNF-α inhibitor therapy: expanding the groups at risk. Eur J Gastroenterol Hepatol. 2011;23:1150-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 93. | Long MD, Herfarth HH, Pipkin CA, Porter CQ, Sandler RS, Kappelman MD. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2010;8:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 94. | Singh S, Nagpal SJ, Murad MH, Yadav S, Kane SV, Pardi DS, Talwalkar JA, Loftus EV. Inflammatory bowel disease is associated with an increased risk of melanoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 95. | Jess T, Gamborg M, Matzen P, Munkholm P, Sørensen TI. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 413] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 96. | Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;23:1097-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 436] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 97. | Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013;19:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 398] [Article Influence: 30.6] [Reference Citation Analysis (1)] |
| 98. | Matula S, Croog V, Itzkowitz S, Harpaz N, Bodian C, Hossain S, Ullman T. Chemoprevention of colorectal neoplasia in ulcerative colitis: the effect of 6-mercaptopurine. Clin Gastroenterol Hepatol. 2005;3:1015-1021. [PubMed] |
| 99. | Beaugerie L, Svrcek M, Seksik P, Bouvier AM, Simon T, Allez M, Brixi H, Gornet JM, Altwegg R, Beau P. Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroenterology. 2013;145:166-175.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 100. | Gong J, Zhu L, Guo Z, Li Y, Zhu W, Li N, Li J. Use of thiopurines and risk of colorectal neoplasia in patients with inflammatory bowel diseases: a meta-analysis. PLoS One. 2013;8:e81487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 101. | Jess T, Horváth-Puhó E, Fallingborg J, Rasmussen HH, Jacobsen BA. Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: a Danish population-based cohort study. Am J Gastroenterol. 2013;108:1869-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 102. | Magro F, Peyrin-Biroulet L, Sokol H, Aldeger X, Costa A, Higgins PD, Joyce JC, Katsanos KH, Lopez A, de Xaxars TM. Extra-intestinal malignancies in inflammatory bowel disease: results of the 3rd ECCO Pathogenesis Scientific Workshop (III). J Crohns Colitis. 2014;8:31-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 103. | Singh H, Demers AA, Nugent Z, Mahmud SM, Kliewer EV, Bernstein CN. Risk of cervical abnormalities in women with inflammatory bowel disease: a population-based nested case-control study. Gastroenterology. 2009;136:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 104. | Lees CW, Critchley J, Chee N, Beez T, Gailer RE, Williams AR, Shand AG, Arnott ID, Satsangi J. Lack of association between cervical dysplasia and IBD: a large case-control study. Inflamm Bowel Dis. 2009;15:1621-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 105. | Beaugerie L, Carrat F, Colombel JF, Bouvier AM, Sokol H, Babouri A, Carbonnel F, Laharie D, Faucheron JL, Simon T. Risk of new or recurrent cancer under immunosuppressive therapy in patients with IBD and previous cancer. Gut. 2013;Oct 25; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 106. | Pasternak B, Svanström H, Schmiegelow K, Jess T, Hviid A. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol. 2013;177:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 107. | Masunaga Y, Ohno K, Ogawa R, Hashiguchi M, Echizen H, Ogata H. Meta-analysis of risk of malignancy with immunosuppressive drugs in inflammatory bowel disease. Ann Pharmacother. 2007;41:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 108. | Dewit O, Starkel P, Roblin X. Thiopurine metabolism monitoring: implications in inflammatory bowel diseases. Eur J Clin Invest. 2010;40:1037-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 109. | Travis SP, Stange EF, Lémann M, Oresland T, Bemelman WA, Chowers Y, Colombel JF, D’Haens G, Ghosh S, Marteau P. European evidence-based Consensus on the management of ulcerative colitis: Current management. J Crohns Colitis. 2008;2:24-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 371] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 110. | Higgs JE, Payne K, Roberts C, Newman WG. Are patients with intermediate TPMT activity at increased risk of myelosuppression when taking thiopurine medications? Pharmacogenomics. 2010;11:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 111. | González-Lama Y, Bermejo F, López-Sanromán A, García-Sánchez V, Esteve M, Cabriada JL, McNicholl AG, Pajares R, Casellas F, Merino O. Thiopurine methyl-transferase activity and azathioprine metabolite concentrations do not predict clinical outcome in thiopurine-treated inflammatory bowel disease patients. Aliment Pharmacol Ther. 2011;34:544-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 112. | Ooi CY, Bohane TD, Lee D, Naidoo D, Day AS. Thiopurine metabolite monitoring in paediatric inflammatory bowel disease. Aliment Pharmacol Ther. 2007;25:941-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 113. | Ohtsuka Y, Arai K, Aoyagi Y, Fujii T, Yamakawa Y, Ohtani K, Ikuse T, Baba Y, Inage E, Kudo T. Monitoring 6-thioguanine nucleotide concentrations in Japanese children and adolescents with inflammatory bowel disease. J Gastroenterol Hepatol. 2010;25:1626-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 114. | Smith M, Blaker P, Patel C, Marinaki A, Arenas M, Escuredo E, Anderson S, Irving P, Sanderson J. The impact of introducing thioguanine nucleotide monitoring into an inflammatory bowel disease clinic. Int J Clin Pract. 2013;67:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 115. | Gilissen LP, Wong DR, Engels LG, Bierau J, Bakker JA, Paulussen AD, Romberg-Camps MJ, Stronkhorst A, Bus P, Bos LP. Therapeutic drug monitoring of thiopurine metabolites in adult thiopurine tolerant IBD patients on maintenance therapy. J Crohns Colitis. 2012;6:698-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 116. | Van Asseldonk DP, de Boer NK, Peters GJ, Veldkamp AI, Mulder CJ, Van Bodegraven AA. On therapeutic drug monitoring of thiopurines in inflammatory bowel disease; pharmacology, pharmacogenomics, drug intolerance and clinical relevance. Curr Drug Metab. 2009;10:981-997. [PubMed] |
| 117. | Haines ML, Ajlouni Y, Irving PM, Sparrow MP, Rose R, Gearry RB, Gibson PR. Clinical usefulness of therapeutic drug monitoring of thiopurines in patients with inadequately controlled inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1301-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 118. | Hyams JS, Lerer T, Mack D, Bousvaros A, Griffiths A, Rosh J, Otley A, Evans J, Stephens M, Kay M. Outcome following thiopurine use in children with ulcerative colitis: a prospective multicenter registry study. Am J Gastroenterol. 2011;106:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 119. | Soman S, Ashok D, Connolly SA, Cordell SJ, Taylor CJ, Campbell DI. Change in hematologic indices over time in pediatric inflammatory bowel disease treated with azathioprine. Drugs R D. 2010;10:213-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 120. | Lewis JD, Abramson O, Pascua M, Liu L, Asakura LM, Velayos FS, Hutfless SM, Alison JE, Herrinton LJ. Timing of myelosuppression during thiopurine therapy for inflammatory bowel disease: implications for monitoring recommendations. Clin Gastroenterol Hepatol. 2009;7:1195-201; quiz 1141-2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 121. | Cangemi G, Barabino A, Barco S, Parodi A, Arrigo S, Melioli G. A validated HPLC method for the monitoring of thiopurine metabolites in whole blood in paediatric patients with inflammatory bowel disease. Int J Immunopathol Pharmacol. 2012;25:435-444. [PubMed] |
| 122. | Gupta P, Gokhale R, Kirschner BS. 6-mercaptopurine metabolite levels in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;33:450-454. [PubMed] |
| 123. | Banerjee S, Bishop WP. Evolution of thiopurine use in pediatric inflammatory bowel disease in an academic center. J Pediatr Gastroenterol Nutr. 2006;43:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 124. | El-Matary W. Letter: thiopurine blood monitoring for patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:742; author reply 743-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 125. | Ansari A, Patel N, Sanderson J, O’Donohue J, Duley JA, Florin TH. Low-dose azathioprine or mercaptopurine in combination with allopurinol can bypass many adverse drug reactions in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2010;31:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 126. | Gardiner SJ, Gearry RB, Burt MJ, Chalmers-Watson T, Chapman BA, Ross AG, Stedman CA, Huelsen A, Barclay ML. Allopurinol might improve response to azathioprine and 6-mercaptopurine by correcting an unfavorable metabolite ratio. J Gastroenterol Hepatol. 2011;26:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 127. | Sparrow MP, Hande SA, Friedman S, Lim WC, Reddy SI, Cao D, Hanauer SB. Allopurinol safely and effectively optimizes tioguanine metabolites in inflammatory bowel disease patients not responding to azathioprine and mercaptopurine. Aliment Pharmacol Ther. 2005;22:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 157] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 128. | Smith MA, Blaker P, Marinaki AM, Anderson SH, Irving PM, Sanderson JD. Optimising outcome on thiopurines in inflammatory bowel disease by co-prescription of allopurinol. J Crohns Colitis. 2012;6:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 129. | Roberts RL, Gearry RB, Barclay ML. Allopurinol-thiopurine combination therapy in inflammatory bowel disease: are there genetic clues to this puzzle? Pharmacogenomics. 2010;11:1505-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 130. | Duley JA, Chocair PR, Florin TH. Observations on the use of allopurinol in combination with azathioprine or mercaptopurine. Aliment Pharmacol Ther. 2005;22:1161-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 131. | Seinen ML, de Boer NK, Smid K, van Asseldonk DP, Bouma G, van Bodegraven AA, Peters GJ. Allopurinol enhances the activity of hypoxanthine-guanine phosphoribosyltransferase in inflammatory bowel disease patients during low-dose thiopurine therapy: preliminary data of an ongoing series. Nucleosides Nucleotides Nucleic Acids. 2011;30:1085-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 132. | de Graaf P, de Boer NK, Wong DR, Karner S, Jharap B, Hooymans PM, Veldkamp AI, Mulder CJ, van Bodegraven AA, Schwab M. Influence of 5-aminosalicylic acid on 6-thioguanosine phosphate metabolite levels: a prospective study in patients under steady thiopurine therapy. Br J Pharmacol. 2010;160:1083-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 133. | Dewit O, Vanheuverzwyn R, Desager JP, Horsmans Y. Interaction between azathioprine and aminosalicylates: an in vivo study in patients with Crohn’s disease. Aliment Pharmacol Ther. 2002;16:79-85. [PubMed] |
| 134. | de Boer NK, Wong DR, Jharap B, de Graaf P, Hooymans PM, Mulder CJ, Rijmen F, Engels LG, van Bodegraven AA. Dose-dependent influence of 5-aminosalicylates on thiopurine metabolism. Am J Gastroenterol. 2007;102:2747-2753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 135. | Andrews JM, Travis SP, Gibson PR, Gasche C. Systematic review: does concurrent therapy with 5-ASA and immunomodulators in inflammatory bowel disease improve outcomes? Aliment Pharmacol Ther. 2009;29:459-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 136. | Hande S, Wilson-Rich N, Bousvaros A, Zholudev A, Maurer R, Banks P, Makrauer F, Reddy S, Burakoff R, Friedman S. 5-aminosalicylate therapy is associated with higher 6-thioguanine levels in adults and children with inflammatory bowel disease in remission on 6-mercaptopurine or azathioprine. Inflamm Bowel Dis. 2006;12:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 137. | Nguyen TM, Le Gall C, Lachaux A, Boulieu R. High thiopurine metabolite concentrations associated with lymphopenia in inflammatory bowel disease (IBD) pediatric patients receiving aminosalicylates combined with azathioprine. Int J Clin Pharmacol Ther. 2010;48:275-281. [PubMed] |
| 138. | Sherlock ME, Griffiths AM. Medical therapy for pediatric inflammatory bowel disease. Curr Gastroenterol Rep. 2012;14:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 139. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2452] [Article Influence: 153.3] [Reference Citation Analysis (1)] |
| 140. | Absah I, Stephens M. Adjunctive treatment to antitumor necrosis factor in pediatric patient with refractory Crohn’s disease. Curr Opin Pediatr. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 141. | Vuitton L, Koch S, Peyrin-Biroulet L. Preventing postoperative recurrence in Crohn’s disease: what does the future hold? Drugs. 2013;73:1749-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 142. | Sandborn WJ. State-of-the-art: Immunosuppression and biologic therapy. Dig Dis. 2010;28:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 143. | Garcia-Planella E, Mañosa M, Van Domselaar M, Gordillo J, Zabana Y, Cabré E, López San Román A, Domènech E. Long-term outcome of ulcerative colitis in patients who achieve clinical remission with a first course of corticosteroids. Dig Liver Dis. 2012;44:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 144. | Casellas F, Rodrigo L, Niño P, Pantiga C, Riestra S, Malagelada JR. Sustained improvement of health-related quality of life in Crohn’s disease patients treated with infliximab and azathioprine for 4 years. Inflamm Bowel Dis. 2007;13:1395-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 145. | Sokol H, Seksik P, Carrat F, Nion-Larmurier I, Vienne A, Beaugerie L, Cosnes J. Usefulness of co-treatment with immunomodulators in patients with inflammatory bowel disease treated with scheduled infliximab maintenance therapy. Gut. 2010;59:1363-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 146. | Lichtenstein GR, Diamond RH, Wagner CL, Fasanmade AA, Olson AD, Marano CW, Johanns J, Lang Y, Sandborn WJ. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther. 2009;30:210-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 147. | Van Assche G, Magdelaine-Beuzelin C, D’Haens G, Baert F, Noman M, Vermeire S, Ternant D, Watier H, Paintaud G, Rutgeerts P. Withdrawal of immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology. 2008;134:1861-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 375] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 148. | Herrinton LJ, Liu L, Weng X, Lewis JD, Hutfless S, Allison JE. Role of thiopurine and anti-TNF therapy in lymphoma in inflammatory bowel disease. Am J Gastroenterol. 2011;106:2146-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 149. | Colombel JF. Understanding combination therapy with biologics and immunosuppressives for the treatment of Crohn’s disease. Gastroenterol Hepatol (N Y). 2010;6:486-490. [PubMed] |
| 150. | Armuzzi A, Pugliese D, Nardone OM, Guidi L. Management of difficult-to-treat patients with ulcerative colitis: focus on adalimumab. Drug Des Devel Ther. 2013;7:289-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 151. | Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-33; quiz 591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1212] [Article Influence: 60.6] [Reference Citation Analysis (1)] |
| 152. | Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, Panaccione R, Wolf D, Kent JD, Bittle B. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 783] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 153. | Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1598] [Cited by in RCA: 1654] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 154. | Wong DR, Pierik M, Seinen ML, van Bodegraven AA, Gilissen LP, Bus P, Bakker JA, Masclee AA, Neef C, Engels LG. The pharmacokinetic effect of adalimumab on thiopurine metabolism in Crohn’s disease patients. J Crohns Colitis. 2014;8:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 155. | Chaparro M, Ordás I, Cabré E, Garcia-Sanchez V, Bastida G, Peñalva M, Gomollón F, García-Planella E, Merino O, Gutiérrez A. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis. 2013;19:1404-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 238] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 156. | Bär F, Sina C, Fellermann K. Thiopurines in inflammatory bowel disease revisited. World J Gastroenterol. 2013;19:1699-1706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 157. | Wroblova K, Kolorz M, Batovsky M, Zboril V, Suchankova J, Bartos M, Ulicny B, Pav I, Bartosova L. Gene polymorphisms involved in manifestation of leucopenia, digestive intolerance, and pancreatitis in azathioprine-treated patients. Dig Dis Sci. 2012;57:2394-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 158. | Hawwa AF, Millership JS, Collier PS, Vandenbroeck K, McCarthy A, Dempsey S, Cairns C, Collins J, Rodgers C, McElnay JC. Pharmacogenomic studies of the anticancer and immunosuppressive thiopurines mercaptopurine and azathioprine. Br J Clin Pharmacol. 2008;66:517-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 159. | Jorgensen AL, FitzGerald RJ, Oyee J, Pirmohamed M, Williamson PR. Influence of CYP2C9 and VKORC1 on patient response to warfarin: a systematic review and meta-analysis. PLoS One. 2012;7:e44064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 160. | Dickens D, Yusof SR, Abbott NJ, Weksler B, Romero IA, Couraud PO, Alfirevic A, Pirmohamed M, Owen A. A multi-system approach assessing the interaction of anticonvulsants with P-gp. PLoS One. 2013;8:e64854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 161. | Vilar FJ, Naisbitt DJ, Park BK, Pirmohamed M. Mechanisms of drug hypersensitivity in HIV-infected patients: the role of the immune system. J HIV Ther. 2003;8:42-47. [PubMed] |
| 162. | Egan LJ, Derijks LJ, Hommes DW. Pharmacogenomics in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 163. | Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980;32:651-662. [PubMed] |
| 164. | Man M, Farmen M, Dumaual C, Teng CH, Moser B, Irie S, Noh GJ, Njau R, Close S, Wise S. Genetic variation in metabolizing enzyme and transporter genes: comprehensive assessment in 3 major East Asian subpopulations with comparison to Caucasians and Africans. J Clin Pharmacol. 2010;50:929-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 165. | Corominas H, Domènech M, del Río E, Gich I, Domingo P, Baiget M. Frequency of thiopurine S-methyltransferase alleles in different ethnic groups living in Spain. Med Clin (Barc). 2006;126:410-412. [PubMed] |
| 166. | Lowenthal A, Meyerstein N, Ben-Zvi Z. Thiopurine methyltransferase activity in the Jewish population of Israel. Eur J Clin Pharmacol. 2001;57:43-46. [PubMed] |
| 167. | Lennard L. Implementation of TPMT testing. Br J Clin Pharmacol. 2014;77:704-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 168. | Yip JS, Woodward M, Abreu MT, Sparrow MP. How are Azathioprine and 6-mercaptopurine dosed by gastroenterologists? Results of a survey of clinical practice. Inflamm Bowel Dis. 2008;14:514-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 169. | Colletti RB, Baldassano RN, Milov DE, Margolis PA, Bousvaros A, Crandall WV, Crissinger KD, D’Amico MA, Day AS, Denson LA. Variation in care in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2009;49:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 170. | Colombel JF, Ferrari N, Debuysere H, Marteau P, Gendre JP, Bonaz B, Soulé JC, Modigliani R, Touze Y, Catala P. Genotypic analysis of thiopurine S-methyltransferase in patients with Crohn’s disease and severe myelosuppression during azathioprine therapy. Gastroenterology. 2000;118:1025-1030. [PubMed] |
| 171. | Larussa T, Suraci E, Lentini M, Nazionale I, Gallo L, Abenavoli L, Imeneo M, Costanzo FS, Cuda G, Luzza F. High prevalence of polymorphism and low activity of thiopurine methyltransferase in patients with inflammatory bowel disease. Eur J Intern Med. 2012;23:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 172. | Loit E, Tricco AC, Tsouros S, Sears M, Ansari MT, Booth RA. Pre-analytic and analytic sources of variations in thiopurine methyltransferase activity measurement in patients prescribed thiopurine-based drugs: A systematic review. Clin Biochem. 2011;44:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 173. | Booth RA, Ansari MT, Loit E, Tricco AC, Weeks L, Doucette S, Skidmore B, Sears M, Sy R, Karsh J. Assessment of thiopurine S-methyltransferase activity in patients prescribed thiopurines: a systematic review. Ann Intern Med. 2011;154:814-23, W-295-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 174. | Marinaki AM, Ansari A, Duley JA, Arenas M, Sumi S, Lewis CM, Shobowale-Bakre el-M, Escuredo E, Fairbanks LD, Sanderson JD. Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPase). Pharmacogenetics. 2004;14:181-187. [PubMed] |
| 175. | Modén O, Zhang W, Mannervik B. Mutational analysis of human glutathione transferase A2-2 identifies structural elements supporting high activity with the prodrug azathioprine. Protein Eng Des Sel. 2012;25:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 176. | Mazor Y, Koifman E, Elkin H, Chowers Y, Krivoy N, Karban A, Efrati E. Risk factors for serious adverse effects of thiopurines in patients with Crohn’s disease. Curr Drug Saf. 2013;8:181-185. [PubMed] |
| 177. | Smith MA, Marinaki AM, Arenas M, Shobowale-Bakre M, Lewis CM, Ansari A, Duley J, Sanderson JD. Novel pharmacogenetic markers for treatment outcome in azathioprine-treated inflammatory bowel disease. Aliment Pharmacol Ther. 2009;30:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 178. | Ban H, Andoh A, Imaeda H, Kobori A, Bamba S, Tsujikawa T, Sasaki M, Saito Y, Fujiyama Y. The multidrug-resistance protein 4 polymorphism is a new factor accounting for thiopurine sensitivity in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2010;45:1014-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 179. | Gisbert JP, Luna M, González-Lama Y, Pousa ID, Velasco M, Moreno-Otero R, Maté J. Liver injury in inflammatory bowel disease: long-term follow-up study of 786 patients. Inflamm Bowel Dis. 2007;13:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 180. | Ardizzone S, Cassinotti A, Manes G, Porro GB. Immunomodulators for all patients with inflammatory bowel disease? Therap Adv Gastroenterol. 2010;3:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
P- Reviewers: Beales ILP, Saha L S- Editor: Ma YJ L- Editor: A E- Editor: Wu HL