Published online Aug 6, 2013. doi: 10.4292/wjgpt.v4.i3.69
Revised: May 22, 2013
Accepted: June 19, 2013
Published online: August 6, 2013
Processing time: 106 Days and 17.4 Hours
AIM: To examine the association between statin use and the development of esophageal cancer
METHODS: We performed a systematic review and meta-analysis. Multiple databases (Pubmed, EMBASE, Cochrane Library, Web of Science, Wiley Interscience and Google Scholar) were systematically searched for studies reporting the association of statin use and the development of esophageal cancer. Literature searching and data abstraction were performed independently by two separate researchers. The quality of studies reviewed was evaluated using the Newcastle-Ottawa Quality assessment scale. Meta-analysis on the relationship between statin use and cancer incidence was performed. The effect of the combination of statin plus a cyclo-oxygenase inhibitor was also examined.
RESULTS: Eleven studies met eligibility criteria, 9 high and 2 medium quality. All were observational studies. Studies examining adenocarcinoma development in Barrett’s oesophagus included 317 cancers and 1999 controls, population-based studies examining all esophageal cancers included 371203 cancers and 6083150 controls. In the Barrett’s population the use of statins (OR = 0.57; 95%CI: 0.43-0.75) and cyclo-oxygenase inhibitors (OR = 0.59; 95%CI: 0.45-0.77) were independently associated with a reduced incidence of adenocarcinoma. Combined use of a statin plus cyclo-oxygenase inhibitor was associated with an even lower adenocarcinoma incidence (OR = 0.26; 95%CI: 0.1-0.68). There was more heterogeneity in the population-based studies but pooled adjusted data showed that statin use was associated with a lower incidence of all combined esophageal cancers (OR = 0.81; 95%CI: 0.75-0.88).
CONCLUSION: Statin use in patients with Barrett’s oesophagus is associated with a significantly lower incidence of adenocarcinoma. The chemopreventive actions of statins, especially combined with cyclo-oxygenase inhibitors deserve further exploration.
Core tip: Esophageal cancer remains a major burden upon health. The incidence of esophageal adenocarcinoma has increased dramatically in western countries. Experimental studies have suggested that statins may have useful actions against esophageal cancer cells. This systematic review and meta-analysis of observational studies shows that statin use was associated with a reduced incidence of all esophageal cancers (19% decrease). A more striking reduction in adenocarcinoma incidence in patients with Barrett’s esophagus taking statins was seen (43% decrease) and this effect was enhanced in those also taking cyclo-oxygenase inhibitors (74% decrease). This combination offers promise for chemoprevention and further interventional studies are warranted.
- Citation: Beales ILP, Hensley A, Loke Y. Reduced esophageal cancer incidence in statin users, particularly with cyclo-oxygenase inhibition. World J Gastrointest Pharmacol Ther 2013; 4(3): 69-79
- URL: https://www.wjgnet.com/2150-5349/full/v4/i3/69.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v4.i3.69
Esophageal cancer remains an important worldwide problem with high rates of incidence and death as well as considerable morbidity and burdens of treatment[1,2]. In the developed world, the incidence of esophageal adenocarcinoma (EAC) has increased dramatically over the last 30 years and now outstrips esophageal squamous cell cancer (ESC) in some countries[3-7]. Although the incidence of squamous cancer appears relatively flat in the developed world it continues to be a major health problem in many places[2]. Despite improvements in the diagnosis, screening and treatment, the mortality and morbidity of these conditions remains substantial.
Chemoprevention remains one attractive way to reduce the incidence of esophageal cancer. Most of the attention has been devoted to EAC, as this appears to develop in most cases from a pre-malignant phenotype, metaplastic (columnar-lined) esophagus (Barrett’s esophagus), providing both a means to study, and intervene in cancer development[8,9]. At present there are no proven chemotherapeutic agents, although aspirin appears to offer the most attractive combination of risks and benefits, and the results of the large United Kingdom (ASPECT) trial are awaited with interest[10].
Experimental laboratory studies have suggested that statins, hydroxymethylglutaryl-CoA reductase inhibitors (HMG-CoA), might have useful anti-cancer effects against the progression of Barrett’s esophagus and EAC. In our laboratory we have shown that 4 different statins (simvastatin, pravastatin, lovastatin and rosuvastatin) inhibit the proliferation and induce apoptosis in both malignant EAC cell lines (OE33 and Flo-1) and non-malignant QhERT Barrett’s cells[11,12]. These effects appear to be due to inhibition of HMG-CoA reductase, which not only reduces the intermediates which are required for the subsequent formation of cholesterol but also limits the availability for other metabolic intermediates that are required for the prenylation of signalling G-proteins. This prenylation of G-proteins, localises them to the cell membrane where there are key players in pro-proliferative and anti-apoptotic signalling[13]. We have shown than statins inhibit signalling via the ERK and Akt cascades in Barrett’s cells, which contribute to the anti-proliferative and pro-apoptotic effects[11]. Similar effects, albeit with less detailed characterization, have also been reported in other EAC cells (simvastatin in OE19 cells[14] and simvastatin, and less so atorvastatin, in FLO-1 cells[15]). Experiments using pharmacological inhibitors and RNA interference have shown that the anti-cancer effect of statins in Barrett’s cells seems to be separate from, but additive to, the effects produced by inhibition of the cyclooxygenase (COX)-2/prostaglandin E2 pathway[11,12]. There is a single laboratory study showing that lovastatin has some modest anti-proliferative and pro-apoptotic effects in TE-8 and SKGT-4 esophageal squamous cancer cell lines[16].
Although these experimental studies are clearly promising, it is important that these are correlated with clinical outcomes before embarking on either significant change in practice or even an adequately powered randomised trial to further explore these effects.
Although several studies have attempted to explore the association of statin use and esophageal cancer incidence: individually these have often been relatively small and underpowered[17,18]. To place this in context, a prospective study in patients with Barrett’s esophagus would require approximately 4000 subjects followed up 5 for years, assuming a statin use rate of 40% and a cancer incidence of 0.5% per annum, to have 80% power to detect a 50% reduction in cancer incidence. A proportionately larger study would be needed based on the latest and more conservative rates (0.1%-0.3% per annum) of malignant progression in Barrett’s esophagus[19,20]. No individual study has come close to this recruitment. Therefore to further explore the potential cancer-protective effects of statins in esophageal cancer we have performed a systematic review and meta-analysis of published literature examining the association of statin use and esophageal cancer, following the MOOSE guidelines[21]. Our review of the literature demonstrated two distinct categories of studies: those examining statin use in relation to malignant progression to EAC in Barrett’s oesophagus and those examining statin use on a population scale which either combined or did not differentiate between EAC and ESC. We have analysed these separately.
The Pubmed, EMBASE, Cochrane Library, Web of Science, Wiley Interscience and Google Scholar databases were searched for relevant publications, published in English up to February 1st 2013 using the search terms “esophageal neoplasm,”“Barrett’s esophagus,”“esophageal adenocarcinoma,”“statin” and “Hydroxymethyg-lutaryl-CoA reductase inhibitor.” The reference lists of these papers were then hand searched for any additional publications. Randomised controlled studies, case-control studies and prospective cohort studies were eligible for inclusion. Two investigators (Beales ILP, Hensley A) independently reviewed the articles and extracted the data, differences were clarified by discussion and mutual agreement.
The following information was abstracted from the publications: type of study, numbers of participants, raw data for ever or never use statins and unadjusted and adjusted risk estimates for statins (where available).
A pre-specified protocol was used to record the following from the eligible studies: authors, journal, participant source, selection criteria, drug exposures of interest, ascertainment of drug exposure and outcome, and confounding factors adjusted for.
We checked the validity of the included studies based on possibility of confounding and potential for misclassification of tumour pathology and/or drug exposure. Risk of bias assessment was focused on the selection of participants, comparability of cases and controls (with any adjustments for confounding), and methods used in ascertaining drug exposure and outcomes. The quality of all studies was assessed using the Newcastle-Ottawa Quality Assessment Scales for cohort and case-control studies using the star rated system as previously described[22]. In brief, this scores studies in each of 9 categories, with a star rating awarded for high quality in separate areas related to the selection of subjects, comparability of groups, reliability of outcomes and exposures. We regarded 9 stars as high-quality studies, 7-8 stars as medium quality, 5-6 stars as low quality and < 4 stars as very low quality.
Review Manager (RevMan) version 5.023 (Nordic Cochrane Center, Copenhagen, Denmark) was used to calculate the pooled risk ratio (based on ORs or hazard ratios from individual studies) using the inverse variance method, random effects model. Statistical heterogeneity was assessed using the Cochrane I2 statistic, with I2 > 25% indicating moderate statistical heterogeneity, and I2 > 50% indicating a substantial level of heterogeneity[23]. A sensitivity analysis was performed by separately omitting one study at a time to assess if the pooled estimate had changed significantly compare to the results of all pooled studies.
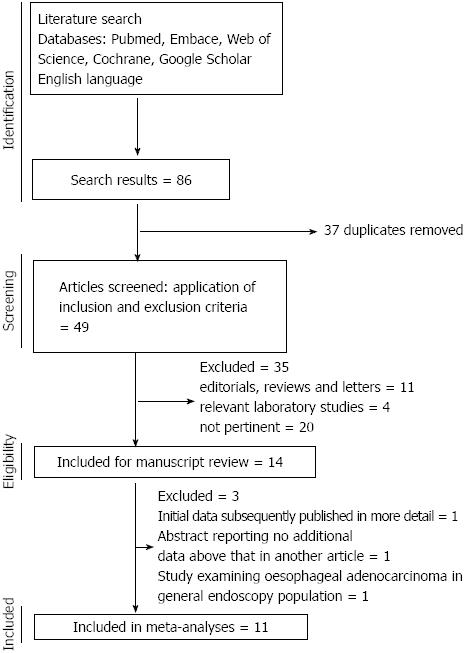
The search yielded 146 potentially eligible publications, after exclusion of experimental and animal studies, reviews, editorials and other papers irrelevant to the current study, 14 relevant papers were reviewed and 11 were eligible for inclusion in the meta-analysis. The flow chart of study selection is shown in Figure 1. Of these 11 papers, 10 were published in full[17,18,24-31] and one only published in abstract form[32]. Of those excluded from the final analysis, one paper reported initial data[12] that were subsequently published in full with larger cohorts in 2 subsequent papers and one abstract reported on essentially the same cohort reported in another abstract but with insufficient extra information to be utilised in the meta-analysis (we attempted to clarify the data with the author but received no response)[33]. One further study was the only one which examined the association between statin use in EAC patients compared to all-comers without cancer[34], as all other studies examined either EAC in the Barrett’s esophagus population or all esophageal cancers in general population, this paper was not analysed in the meta-analysis but the data were extracted for review. Two of the studies included in the meta-analysis involved different methodologies of interrogating the same research database and generated different data sets, hence both were included[28,31]. The studies reviewed are summarised in Table 1. No randomised studies were identified; 6 case-control studies and 5 cohort studies were included. There was heterogeneity in the methods of presentation of the results between the studies with reference to the adjustment for risk factors; therefore we performed separate meta-analyses on the adjusted and unadjusted ORs. Data on individual statins, dose or duration of exposure were reported too variably to be analysed robustly in the meta-analysis. Overall 9 of the papers were rated as high quality (9 stars out of a possible 9) and 2 of medium quality (7-8 stars out of a possible 9) using the Newcastle-Ottawa scale.
| Study | Setting | Studies | Participants | Ascertainment of statin use for inclusion | Risk estimates for statins (vs no statin) and factors adjusted for | Limitations, notes and quality |
| Kastelein et al[25] | Netherlands, hospital based | Prospective cohort | Cohort of 570 BO, 38 developed EAC or HGD | All statins, statin use during study period, patient interview and questionnaire, pharmacy records | Statin use = 1 mo HR = 0.46 (95%CI: 0.21-0.99); statin use = 5 yr HR = 0.51 (018-0.1.47); statin use = 5 yr HR = 0.49 (95%CI: 0.22-0.85); statin plus aspirin HR = 0.22 (95%CI: 0.06-0.85); adjusted for age, sex, length of BO, baseline histology and aspirin use | No adjustment for BMI or smoking; limited categorisation of duration-, and dose-relationship; Newcastle-Ottawa 9 stars |
| Nguyen et al[24] | United States, hospital based, veterans administration | Case-control | 116 EAC, 696 BO | All statins, at least 1 filled statin prescription in study period, pharmacy database | At least 1 statin prescription HR = 0.56 (95%CI: 0.36-0.87); statin use < 12 mo HR = 0.63 (95%CI: 0.38-1.06); statin use > 12 mo HR = 0.52 (95%CI: 0.30-0.91); adjusted for race, out-patient encounters, non-cancer co-morbidity, use of other medications | 97% male, veterans’ population; Not adjusted for BMI, alcohol, smoking; no categorisation of dose-relationship; Newcastle-Ottawa 9 stars |
| Beales et al[26] | United Kingdom, hospital based | Case-control | 85 EAC, 170 BO | All statins, statin use for > 6 mo prior to cancer diagnosis, questionnaire and clinical and prescribing records | Statin use OR = 0.57 (0.28-0.94); statin and aspirin combined OR = 0.31 (95%CI: 0.04-0.69); adjusted for age, sex, smoking, aspirin, NSAIDs, proton pump inhibitors, BMI, diabetes mellitus, metformin, alcohol; significant negative associations with statin dose and duration. | Cancers were a mix of de novo and screening-detected cancers; Newcastle-Ottawa 8 stars |
| Beales et al[34] | United Kingdom, hospital based | Case-control | 112 EAC, 448 cancer negative gastroenterology outpatients | All statins, statin use for > 6 mo prior to cancer diagnosis, questionnaire and clinical and prescribing records | Statin use OR = 0.52 (95%CI: 0.27-0.92); statin and aspirin combined OR 0.27 (95%CI: 0.05-0.67); adjusted for age, sex, smoking, aspirin, NSAIDs, proton pump inhibitors, BMI, alcohol, diabetes mellitus, metformin; United Kingdom, population based | Controls were hospital outpatients; Newcastle-Ottawa 8 stars |
| Fang et al[12] | United Kingdom, hospital based | Case-control | EAC 63, cancer-negative gastroenterology outpatients 252 | All statins, statin use for > 6 mo prior to cancer diagnosis, questionnaire and clinical and prescribing records | Unadjusted statin OR = 0.42 (95%CI: 0.19-0.89); unadjusted statin plus aspirin OR = 0.11 (95%CI: 0.01-0.82) | Controls were hospital outpatients. Unadjusted for any risk factors; not included in meta-analysis as more extensive dataset published subsequently; not quality rated |
| Kantor et al[18] | United States | Prospective cohort | BO 411 in cohort, EAC developed in 56 | All statins, any statin use during study period, questionnaire | Statin use OR = 0.68 (95%CI: 0.30-1.54); adjusted for sex, age, smoking, NSAIDs | No adjustment for BMI; no data on dose or duration relationship. Included any use of statin, Relatively low incidence of statin use in BO population; Newcastle-Ottawa 9 stars |
| Nguyen et al[17] | United States, hospital based, veterans administration | Retrospective cohort | BO 344 in cohort, EAC or HGD developed in 33 | All statins, any statin prescription during the period of study, pharmacy and clinical records | Statin use OR = 0.73 (95%CI: 0.30-1.78), unadjusted | 94% male, veterans population. Incomplete adjustment for potential confounding factors; Newcastle-Ottawa 8 stars |
| Bhutta et al[32] | United Kingdom, population based | Case-control | 4242 cancers, 17233 controls | All statins, statin prescription for 10 mo in the year preceding diagnosis of cancer; read codes within GPRD | Use of statins OR = 0.84 (95%CI: 0.73-0.95); adjusted for BMI, smoking, aspirin, NSAIDs, proton pump inhibitors, vasodilators | No categorisation of statin dose; related to Hippisley-Cox 2010 but different methodology to interrogate the same research database; Newcastle-Ottawa 9 stars |
| Vinograd ova et al[28] | United Kingdom, population based | Case-control | 3159 cancers, 13041 controls | All statins, statin use as defined by 2 prescriptions over a 5 year period at least 12 mo prior to cancer diagnosis; read codes within QResearch database | Use of statins OR = 0.88 (95%CI: 0.77-1.01); adjusted for Townsend score, smoking, circulatory disease, diabetes mellitus, rheumatoid arthritis, COX-2 inhibitors | Data for EAC and ESC combined; no individual confirmation of pathology. No categorisation of statin dose; related to Hippisley-Cox 2010 but different methodology to interrogate the same research database; Newcastle-Ottawa 9 stars |
| Hippisley-Cox et al[31] | United Kingdom, population based | Prospective cohort | 1809 cancers, 2004692 overall participants | All statins, new users of statins defined by a new statin prescription in the study period; read codes within QResearch database | Men, statin HR = 0.78 (95%CI: 0.66-0.91); women, statin HR 0.68 (95%CI: 0.52-0.88); adjusted for, age, BMI, smoking, townsend score, type 2 diabetes | Data for EAC and ESC combined. No individual confirmation of pathology; no adjustment for aspirin or NSAIDs; no data on duration or long term statin exposure; Newcastle-Ottawa 9 stars |
| Kaye et al[29] | United Kingdom, population based | Case-control | 100 cancers, 430 controls | All statins, current use defined as a statin prescription that started within 12 mo of cancer diagnosis; read codes within GPRD | Statin use OR = 0.80 (95%CI: 0.30-1.80); adjusted for smoking, BMI, number of GP visits | Data for EAC and ESC combined. No individual confirmation of pathology; no adjustment for aspirin or NSAIDs; no data on duration or long term statin exposure; Newcastle-Ottawa 9 stars |
| Friedman et al[30] | United States, population based | Retrospective cohort | 68 cancers, 4413032 controls | All statin, any statin use prior to cancer diagnosis, Kaiser Permanente Cancer Registry and Pharmacy management systems | Overall unadjusted statin use OR = 1.0 (95%CI: 0.77-1.27); men with > 5 yr statin use OR = 1.70 (95%CI: 1.05-12.75). | Data for EAC and ESC combined; no individual confirmation of pathology; no dose-effect relationship examined; no correction for confounding variables; small number of cancers; Newcastle-Ottawa 9 stars |
| Lai et al[27] | Taiwan, population based | Case-control | 549 cancers, 2196 controls. | All statins, statin prescription prior to cancer diagnosis; data from Taiwanese NHI programme | Statin use OR = 0.66 (0.45-0.95); atorvastatin = 12 mo OR = 0.14 (95%CI: 0.04-0.56); adjusted for esophageal diseases, H. pylori infection, alcoholism, smoking, lipid lowering drugs, proton pump inhibitors, H2RA, NSAIDs and aspirin | Data for EAC and ESC combined; no individual confirmation of pathology; no dose-effect relationship examined; Newcastle-Ottawa 9 stars |
| Bhutta et al[33] | United Kingdom, population based | Case-control | Not clearly defined | Not clearly defined; read codes within general practice research database | Statin use OR for EAC 0.61 (95%CI: 0.35-0.94), OR for ESC (95%CI: 0.21-0.80); unclear what adjustments applied | No individual confirmation of pathology; insufficient data for inclusion in meta-analysis appears to be essentially the same cohort as Bhutta 2011; no response from author when asked for further information; not quality rated or included |
Five separate studies examined the association of statin use with the development of esophageal adenocarcinoma in patients with Barrett’s esophagus[17,18,24-26]. Where high-grade dysplasia was reported as an outcome, this was included with adenocarcinoma for analysis due to the indication for intervention at that stage. Data from a total of 317 adenocarcinomas and 1999 non-cancer Barrett’s controls were included in the meta-analysis. In addition, one further study examined the association of statin use in patients with esophageal adenocarcinoma compared to cancer-negative a controls rather than just Barrett’s esophagus patients, this study was not included in the meta-analysis[34].
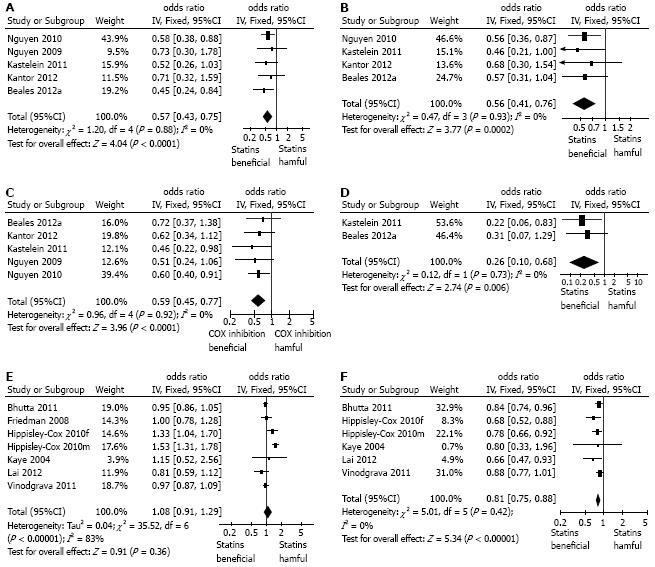
Meta-analysis of all the 5 studies providing crude, unadjusted ORs showed a significant negative association between statin use and the development of esophageal adenocarcinoma in patients with Barrett’s esophagus (OR = 0.57, 95%CI: 0.43-0.75) without any significant heterogeneity in the results (Figure 2A). Very similar results were seen the meta-analysis using pooled adjusted ORs (combined OR = 0.56; 95%CI: 0.41-0.76), again without heterogeneity (Figure 2B). Sensitivity analysis showed that omitting any single study did not demonstrably alter the results. Interestingly in the additional study examining the association of statins and esophageal adenocarcinoma compared to endoscopy negative controls, not included in the meta-analysis, the unadjusted OR was very similar (adjusted OR = 0.52; 95%CI: 0.27-0.92)[34].
We also performed a meta-analysis of the relationship between cyclo-oxygenase inhibitor use and esophageal adenocarcinoma development reported in these studies. This showed that the use of aspirin or other cyclo-oxygenase inhibitors was associated with a significantly lower incidence of adenocarcinoma in Barrett patients (OR = 0.59, 95%CI: 0.45-0.77), again without any heterogeneity (Figure 2C).
Two of the studies specifically reported the association of the combination of cyclo-oxygenase inhibitors (aspirin and/or non-steroidal anti-inflammatory drug, NSAIDs/coxibs) with a statin and esophageal adenocarcinoma in patients with Barrett’s esophagus[25,26]: as shown in Figure 2D this combination was associated with a significantly lower incidence of esophageal adenocarcinoma (OR = 0.26; 95%CI: 0.1-0.68), that seen with either statins or aspirin/NSAIDs alone (Figure 2D).
Only one of the five studies reported data on statin dose: in this one study, higher doses (greater than 40 mg simvastatin or equivalent daily) were associated with a lower incidence of EAC compared to lower doses[26].
Similarly there were inconsistencies in the reporting of duration of statin use: Kastelein et al[25] reported no difference with either more or less than 5 years use of statin (OR both approximately 0.50), whilst Nguyen et al reported that more than one year of statin was associated with a lower incidence of EAC [corrected incidence density ratio (0.52, 95%CI: 0.30-0.91)] than use for less than 12 mo [corrected incidence density ratio (0.63, 95%CI: 0.38-1.06)][24]. Beales et al[26] reported that more than 5 years of statin use (OR = 0.41; 95%CI: 0.15-0.85) was associated with lower incidence of EAC than use for less than 2 years (OR = 0.77; 95%CI: 0.29-1.87).
A total of 6 studies reported the association of statin use and all cancers of the esophagus[27-32]. These were all population-based studies utilizing databases, without any individual confirmation of the precise pathology involved. There were no studies specifically examining the relationship between statins and squamous cell cancer. One study reported separate data for men and women and these were included separately in the meta-analysis[31]. Data from a total of 371203 esophageal cancers and 6083150 controls were included in this meta-analysis. There was considerable and significant heterogeneity in the data for unadjusted OR: overall there was no association of statin use and esophageal cancer (OR = 1.08; 95%CI: 0.91-1.29, I2 = 83%) (Figure 2E). There was less heterogeneity in the pooled adjusted OR (pooled OR = 0.81; 95%CI: 0.75-0.88, I2 = 0%) which showed a significant negative association between statin use and the incidence of all esophageal cancers (Figure 2F). Sensitivity analysis of the pooled adjusted data showed that omission of any one single study did not alter the overall effects.
Again data on dose, duration and individual statins were inconsistently presented and formal meta-analysis of these data is problematical if not impossible. Vinogradova et al[28] reported that the OR for less than 12 mo statin use (OR = 0.90; 95%CI: 0.67-1.20) was similar to that in those using statins for greater than 73 mo (OR = 1.03; 95%CI: 0.07-1.52). Lai et al[27] reported that use atorvastatin but not other statins for greater than 12 mo was associated with a significantly reduced incidence of esophageal cancers, (adjusted OR = 0.14; 95%CI: 0.04-0.56). Sub-groups analysis of the study by Hippisley-Cox and Coupland[31] showed that there seemed to be a dose-response relationship but only in men: low simvastatin dose (10/20 mg), (adjusted OR = 0.91; 95%CI: 0.73-1.12), compared to high dose (40/80 mg) (adjusted OR = 0.66; 95%CI: 0.48-0.91). Statin dose-relationships were not reported in the other studies.
Our meta-analysis has confirmed a significant negative association between the use of statins and a reduced incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus. This suggests that statins may have important chemopreventive effects that should now be explored further in interventional studies. The results from all 5 studies are consistent with each other, with no statistical heterogeneity.
Our data have consistency with those previously published: the pooled adjusted OR for cyclo-oxygenase inhibitor use (combined aspirin, NSAIDs and coxibs) in the 5 studies of adenocarcinoma development in Barrett’s esophagus is 0.59 (95%CI: 0.45-0.77). This result is consistent with previous studies and meta-analysis[35-37], although other studies have failed to show a negative association between cyclooxygenase inhibitor use and adenocarcinoma development in Barrett’s esophagus[38]. Within these current studies there were sufficient data to perform a meta-analysis on the combined effects of statin and cyclooxygenase inhibitor usage and this showed that the combination was associated with a greater reduction in adenocarcinoma incidence. These findings are consistent with the laboratory data in Barrett’s cancer and non-cancer cell lines, where the anti-proliferative and pro-apoptotic effects of statins are mechanistically both separate from, and additive to, the effects of pharmacological inhibitors of the COX/prostaglandin production pathway[11,12,14]. Our data strongly support further experimental and interventional studies exploring the combination of aspirin and statin for chemoprevention. Further studies are required to define which of the various families of cyclo-oxygenase inhibitors have the greatest negative association with EAC. The available do not allow differentiation between traditional NSAIDs, coxibs and aspirin.
All the studies included in the meta-analysis were observational in nature, and despite the consistency of results the possibility of bias must still be considered. The pooled adjusted and adjusted ORs both showed that statin use is associated with a lower incidence of adenocarcinoma in Barrett’s esophagus, but it is possible that a degree of confounding by uncorrected factors remains. In general the known risk factors that direct the clinical use of statins (risk of circulatory disease, obesity, smoking etc.) also increase the risk of adenocarcinoma development, which would tend to diminish the apparent protective effects of statins[39-41]. It is possible that other factors related to the use of statins within a cohort of Barrett’s patients (perhaps some dietary factor) may have led to residual confounding. However the consistency of the results in 5 geographically distinct cohorts suggests that this is not likely to be a significant effect. Singh et al[42] recently published a similar meta-analysis examining statins and esophageal cancers, although with slightly different inclusion criteria and an earlier cut-off point for the literature review. The results are very similar to ours: in that study statin use was associated with a reduced incidence of adenocarcinoma in Barrett’s esophagus [pooled unadjusted OR = 0.57 (95%CI: 0.44-0.75), pooled adjusted OR = 0.59 (95%CI: 0.45-0.78)]. Whilst further suitably sized randomized studies are required to fully inform choices over statin and aspirin use as chemopreventive agents in Barrett’s esophagus, the currently available data do suggest that these should certainly be prescribed to Barrett’s patients with increased risk of circulatory diseases.
Despite the meta-analysis including over 300 cancers and nearly 2000 Barrett’s non-cancer controls, there are insufficient data on the dose- and duration-relationships, in the negative association between statin use and adenocarcinoma development. These areas require further investigation. There are also insufficient data on either individual statins or lipophilic versus hydrophilic statins. All 5 studies grouped all statins together and only in one were individual drugs examined. These areas also require important follow up studies. Based on available data, the most plausible mechanism underlying the chemopreventive effect of statins is inhibition of the mevalonate synthetic pathway and subsequent reduction in the availability of functional signalling mediators that promote proliferation and inhibit apoptosis within the Barrett’s epithelium[11,13]. The cell line studies suggest that these effects are mediated by statins at the level of the Barrett’s epithelial cells, but the contribution of overall reduction in mevalonate pathway synthetic function (predominantly in the liver) to any esophageal clinical effects remain to be explored. This may have some bearing as lipophilic statins (simvastatin and atorvastatin) are thought to be able to enter all cells by passive diffusion, whereas the hydrophilic statins (pravastatin and rosuvastatin) require the presence of an active transport mechanism[43]. The latter is expressed by hepatocytes and not usually in other cells (although to our knowledge has not been specifically explored in normal and pathological esophageal epithelium)[44]. In addition other mechanisms such as altered adipokine secretion or altered inflammatory responses could contribute to the possible protective effects of statins against esophageal adenocarcinoma[45] and the individual statins or their chemical properties, such as intrinsic anti-oxidants effects, could be important determinants of these effects[43].
These current clinical data are important when discussing the mechanistic cell-line studies: some of the latter are open to, (perhaps valid) criticism that the statin concentrations employed in vitro (often significantly greater than 1 mmol/L)[46] are rather higher than those generally seen with in vivo therapeutic use (in the nmol/L range)[47,48]. The correlation of positive clinical and laboratory studies is supportive of a chemopreventive effect of statins against EAC.
The data from the population-based studies examining the incidence of all esophageal cancers in relation to statin use are rather less robust than the more specific Barrett’s-adenocarcinoma data. There was considerable heterogeneity in the crude pooled data but the pooled adjusted ORs did show a significantly lower incidence of all esophageal cancers in statin users. In contrast to the Barrett’s group studies, all of these population-based studies relied on interrogation of databases and were not specifically designed to examine esophageal cancer incidence (this was one of many outcomes assessed). Data on drug exposure is probably not as complete in this set of studies as aspirin, NSAIDs and statins are all available over the counter in many of the relevant areas and non-prescription use would not have been detected in these prescribing database studies. We feel that this is unlikely to greatly affect the results but does increase the level of uncertainty. The previously mentioned meta-analysis by Singh et al[42] did not separately examine population-based (but non-Barrett’s) esophageal cancers, and included overall less subjects (9285 cases and 1132969 total patients) than our current study. However the pooled results for all studies examining statin use and esophageal cancer incidence was similar to ours [pooled unadjusted OR = 0.74 (95%CI: 0.62-0.90), pooled adjusted OR = 0.72 (95%CI: 0.60-0.86)], considering the that the Singh et al[42] results are affected by the inclusion of the Barrett’s adenocarcinoma studies, where statins seem to be associated with greater protection against cancer compared to the true population-based studies.
The major difficulty in interpreting the population-based studies is that the cancer diagnoses would have included a mixture of esophageal adenocarcinoma, gastroesophageal junction adenocarcinoma and esophageal squamous cell cancer. These have different risk factors and pathology and it is not clear whether the negative association with statin use reported reflects a similar effect against all possible esophageal cancer types or whether there is a more obvious negative association with adenocarcinoma, as suggested by the Barrett’s-cancer data and a less obvious, or indeed no, association with a reduced incidence of squamous cell cancer. This area needs further clarification. Again there were insufficient data, and what were available were too inconsistently reported to draw any conclusions regarding the dose and duration relationships between statin use and esophageal cancer incidence or whether individual statins or statin classes had different effects.
In addition to the single study showing some modest effects with lovastatin in esophageal squamous cell lines[16], anti-cancer effects of statins have been also demonstrated in vitro against non-esophageal squamous cancer cell lines, such as lung, skin or head and neck cancers[49-52]. However although some clinical studies have suggested a non-statistically significant trend to improved outcomes in statin-treated squamous cell cancer patients[50], other large studies have failed to show any benefit. Further studies are clearly required to examine the associations (if any) between statin use and esophageal squamous cell cancer[28,53]. Similarly, further studies are required to examine whether statin use has any association with the incidence of Barrett’s metaplasia, all the studies in our meta-analysis examined adenocarcinoma (or high-grade dysplasia) development in Barrett’s mucosa.
The potential cancer chemopreventive effects of statins continue to attract widespread attention: statins have been reported to be associated with reduced overall cancer-related mortality[54] but data on the clinical effects of statins on the incidence or prognosis of cancers various different sites have often been inconclusive and require that different cancers are addressed separately (reviewed by Boudreau et al[55].
Our meta-analysis has shown that statin use is consistently associated with a reduced incidence of adenocarcinoma in populations of patients with Barrett’s esophagus. The combination of a cyclo-oxygenase inhibitor and statin is associated with a greater reduction in the incidence of adenocarcinoma. In population based studies of all esophageal cancers statin use was also associated with a reduced cancer incidence. The chemopreventive actions of statins, especially in combination with aspirin/NSAIDs deserves further exploration in interventional trials.
Cancers of the esophagus are common causes of mortality and morbidity worldwide. The incidence of esophageal adenocarcinoma is increasing in the Western world and although it is accepted that most cases of esophageal adenocarcinoma arise from metaplastic Barrett’s esophagus, there are, as yet, no proven chemopreventive interventions. Laboratory-based experimental cell-line studies have shown that statins have potentially useful anti-cancer effects against esophageal cancer and that in some model systems, at least, these effects can be enhanced by combining with a cyclo-oxygenase inhibitor. At present there are only limited data on the clinical correlations of these observations.
It is not clear if the clinical use of statins is associated with a reduced incidence of esophageal cancers and equally the effects of combined use of statins and cyclo-oxygenase on the development of esophageal cancer are unclear. Several of the laboratory cell-line studies have used relatively high concentrations of statins to show anti-cancer effects, probably higher than seen in usual clinical therapeutic use, and hence it is important to determine the relationship between statin use and esophageal cancer incidence with usual clinical use of the drugs.
This is the largest systematic review and meta-analysis in this area and has included over 300 cases of Barrett’s-related adenocarcinoma, 1999 non-cancer Barrett’s controls. In addition the population-based studies included over 370000 total cases of esophageal cancer and almost 6 million controls. The results show that statin use in patients Barrett’s esophagus was associated with a 43% reducon in the incidence of adenocarcinoma. Inhibition of cyclo-oxygenase (COX) with aspirin, non-aspirin non-steroidal anti-inflammatory agents or selective COX-2 inhibitors was independently associated with a reduced adenocarcinoma incidence in Barrett’s esophagus (41% decrease). The combination of a statin plus a cyclo-oxygenase inhibitor was associated with a greater reduction in adenocarcinoma incidence than either alone (74% reduction). The data from the population-based studies are more heterogeneous, containing a mixture of esophageal cancer types but again statin use was associated with a reduced incidence of cancer development (19% reduction).
These data from observational studies suggests that statins may have useful chemopreventive effects against esophageal cancer; particularly against the development of adenocarcinoma in Barrett’s esophagus when used in combination with a cyclo-oxygenase inhibitor. Further interventional studies are warranted. As patients with Barrett’s esophagus are at increased risk of circulatory diseases, statins should not be withheld from such patients where otherwise indicated.
Statins are inhibitors of the enzyme hydroxymethylglutaryl-CoA reductase. This is the rate limiting step on cholesterol biosynthesis. These drugs are widely used to treat and prevent circulatory diseases. Intermediates of the cholesterol synthetic pathway are also essential in other cell signalling pathways which are important in controlling many functions including cell proliferation and survival.
Chemoprevention for esophageal cancers, especially in the context of Barrett’s esophagus, is an area of active interest around the world. This systematic review and meta-analysis examined the association with statin use and the incidence of esophageal cancers. The study results show a consistent and significant negative between statin use and the development of esophageal adenocarcinoma. It also showed that the combined use of statins with aspirin or other cyclooxygenase inhibitors was associated with even lower incidence of adenocarcinoma development in patients with Barrett’s esophagus. The results would further stimulate research and interest in combined chemoprevention. The findings are topical and relevant to clinical practice.
| 1. | Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology. 2011;140:e18-e52; quiz e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 901] [Cited by in RCA: 812] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 2. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11889] [Article Influence: 792.6] [Reference Citation Analysis (6)] |
| 3. | Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 956] [Cited by in RCA: 966] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 4. | Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184-1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 528] [Cited by in RCA: 536] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 5. | Wu X, Chen VW, Andrews PA, Ruiz B, Correa P. Incidence of esophageal and gastric cancers among Hispanics, non-Hispanic whites and non-Hispanic blacks in the United States: subsite and histology differences. Cancer Causes Control. 2007;18:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Bollschweiler E, Wolfgarten E, Gutschow C, Hölscher AH. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer. 2001;92:549-555. [PubMed] |
| 7. | Bosetti C, Levi F, Ferlay J, Garavello W, Lucchini F, Bertuccio P, Negri E, La Vecchia C. Trends in oesophageal cancer incidence and mortality in Europe. Int J Cancer. 2008;122:1118-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Sikkema M, de Jonge PJ, Steyerberg EW, Kuipers EJ. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2010;8:235-244; quiz e32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 258] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 9. | Yousef F, Cardwell C, Cantwell MM, Galway K, Johnston BT, Murray L. The incidence of esophageal cancer and high-grade dysplasia in Barrett’s esophagus: a systematic review and meta-analysis. Am J Epidemiol. 2008;168:237-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 10. | Das D, Chilton AP, Jankowski JA. Chemoprevention of oesophageal cancer and the AspECT trial. Recent Results Cancer Res. 2009;181:161-169. [PubMed] |
| 11. | Ogunwobi OO, Beales IL. Statins inhibit proliferation and induce apoptosis in Barrett’s esophageal adenocarcinoma cells. Am J Gastroenterol. 2008;103:825-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Fang D, Das KM, Cao W, Malhotra U, Triadafilopoulos G, Najarian RM, Hardie LJ, Lightdale CJ, Beales IL, Felix VN. Barrett’s esophagus: progression to adenocarcinoma and markers. Ann N Y Acad Sci. 2011;1232:210-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Holstein SA, Wohlford-Lenane CL, Wiemer DF, Hohl RJ. Isoprenoid pyrophosphate analogues regulate expression of Ras-related proteins. Biochemistry. 2003;42:4384-4391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Konturek PC, Burnat G, Hahn EG. Inhibition of Barret‘s adenocarcinoma cell growth by simvastatin: involvement of COX-2 and apoptosis-related proteins. J Physiol Pharmacol. 2007;58 Suppl 3:141-148. [PubMed] |
| 15. | Sadaria MR, Reppert AE, Yu JA, Meng X, Fullerton DA, Reece TB, Weyant MJ. Statin therapy attenuates growth and malignant potential of human esophageal adenocarcinoma cells. J Thorac Cardiovasc Surg. 2011;142:1152-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Ye F, Zhang GH, Guan BX, Xu XC. Suppression of esophageal cancer cell growth using curcumin, (-)-epigallocatechin-3-gallate and lovastatin. World J Gastroenterol. 2012;18:126-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Nguyen DM, El-Serag HB, Henderson L, Stein D, Bhattacharyya A, Sampliner RE. Medication usage and the risk of neoplasia in patients with Barrett‘s esophagus. Clin Gastroenterol Hepatol. 2009;7:1299-1304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Kantor ED, Onstad L, Blount PL, Reid BJ, Vaughan TL. Use of statin medications and risk of esophageal adenocarcinoma in persons with Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2012;21:456-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 1003] [Article Influence: 66.9] [Reference Citation Analysis (1)] |
| 20. | Desai TK, Krishnan K, Samala N, Singh J, Cluley J, Perla S, Howden CW. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: a meta-analysis. Gut. 2012;61:970-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 427] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 21. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 17254] [Article Influence: 663.6] [Reference Citation Analysis (0)] |
| 22. | Kimball BP, LiPreti V, Bui S, Wigle ED. Comparison of proximal left anterior descending and circumflex coronary artery dimensions in aortic valve stenosis and hypertrophic cardiomyopathy. Am J Cardiol. 1990;65:767-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 23. | Alexandre L, Broughton T, Loke Y, Beales IL. Meta-analysis: risk of esophageal adenocarcinoma with medications which relax the lower esophageal sphincter. Dis Esophagus. 2012;25:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Nguyen DM, Richardson P, El-Serag HB. Medications (NSAIDs, statins, proton pump inhibitors) and the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus. Gastroenterology. 2010;138:2260-2266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Kastelein F, Spaander MC, Biermann K, Steyerberg EW, Kuipers EJ, Bruno MJ. Nonsteroidal anti-inflammatory drugs and statins have chemopreventative effects in patients with Barrett’s esophagus. Gastroenterology. 2011;141:2000-2008; quiz 2000-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Beales IL, Vardi I, Dearman L. Regular statin and aspirin use in patients with Barrett’s oesophagus is associated with a reduced incidence of oesophageal adenocarcinoma. Eur J Gastroenterol Hepatol. 2012;24:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Lai SW, Liao KF, Lai HC, Muo CH, Sung FC. Atorvastatin correlates with decreased risk of esophageal cancer: a population-based case-control study from Taiwan. Libyan J Med. 2012;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Vinogradova Y, Coupland C, Hippisley-Cox J. Exposure to statins and risk of common cancers: a series of nested case-control studies. BMC Cancer. 2011;11:409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Kaye JA, Jick H. Statin use and cancer risk in the General Practice Research Database. Br J Cancer. 2004;90:635-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 217] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 30. | Friedman GD, Flick ED, Udaltsova N, Chan J, Quesenberry CP, Habel LA. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol Drug Saf. 2008;17:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 31. | Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 388] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 32. | Bhutta HY, Clark A, Holt S, Lewis MPN, Hart AR. Oesophageal cancer - an aetiological investigation into the potential protective effect of statins in the UK general practice research database (GPRD). Gut. 2011;60:A36-A37. [DOI] [Full Text] |
| 33. | Bhutta HY, Alexandre L, Clark A. Do statins prevent the histological subtypes of oesophageal cancer Prospective data from the UK General Practice Database (GPRD). Gut. 2012;61:A299-A300. [DOI] [Full Text] |
| 34. | Beales IL, Vardi I, Dearman L, Broughton T. Statin use is associated with a reduction in the incidence of esophageal adenocarcinoma: a case control study. Dis Esophagus. 2012;Sep 18; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Vaughan TL, Dong LM, Blount PL, Ayub K, Odze RD, Sanchez CA, Rabinovitch PS, Reid BJ. Non-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett’s oesophagus: a prospective study. Lancet Oncol. 2005;6:945-952. [RCA] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 36. | Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003;124:47-56. [PubMed] |
| 37. | Abnet CC, Freedman ND, Kamangar F, Leitzmann MF, Hollenbeck AR, Schatzkin A. Non-steroidal anti-inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta-analysis. Br J Cancer. 2009;100:551-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 38. | Gatenby PA, Ramus JR, Caygill CP, Winslet MC, Watson A. Aspirin is not chemoprotective for Barrett’s adenocarcinoma of the oesophagus in multicentre cohort. Eur J Cancer Prev. 2009;18:381-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | van der Burgh A, Dees J, Hop WC, van Blankenstein M. Oesophageal cancer is an uncommon cause of death in patients with Barrett’s oesophagus. Gut. 1996;39:5-8. [PubMed] |
| 40. | Solaymani-Dodaran M, Logan RF, West J, Card T. Mortality associated with Barrett’s esophagus and gastroesophageal reflux disease diagnoses-a population-based cohort study. Am J Gastroenterol. 2005;100:2616-2621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Moayyedi P, Burch N, Akhtar-Danesh N, Enaganti SK, Harrison R, Talley NJ, Jankowski J. Mortality rates in patients with Barrett’s oesophagus. Aliment Pharmacol Ther. 2008;27:316-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Singh S, Singh AG, Singh PP, Murad MH, Iyer PG. Statins Are Associated With Reduced Risk of Esophageal Cancer, Particularly in Patients With Barrett’s Esophagus: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2013;11:620-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 43. | Mason RP. Molecular basis of differences among statins and a comparison with antioxidant vitamins. Am J Cardiol. 2006;98:34P-41P. [PubMed] |
| 44. | Nezasa K, Higaki K, Takeuchi M, Nakano M, Koike M. Uptake of rosuvastatin by isolated rat hepatocytes: comparison with pravastatin. Xenobiotica. 2003;33:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Jalving M, Koornstra JJ, De Jong S, De Vries EG, Kleibeuker JH. Review article: the potential of combinational regimen with non-steroidal anti-inflammatory drugs in the chemoprevention of colorectal cancer. Aliment Pharmacol Ther. 2005;21:321-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Beales IL. Letter: Potential chemopreventive effects of statins in oesophageal adenocarcinoma. Aliment Pharmacol Ther. 2012;36:1105; author reply 1105-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 47. | Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther. 1999;84:413-428. [PubMed] |
| 48. | Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol. 2004;94:1140-1146. [PubMed] |
| 49. | Takeda I, Maruya S, Shirasaki T, Mizukami H, Takahata T, Myers JN, Kakehata S, Yagihashi S, Shinkawa H. Simvastatin inactivates beta1-integrin and extracellular signal-related kinase signaling and inhibits cell proliferation in head and neck squamous cell carcinoma cells. Cancer Sci. 2007;98:890-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Zhao TT, Le Francois BG, Goss G, Ding K, Bradbury PA, Dimitroulakos J. Lovastatin inhibits EGFR dimerization and AKT activation in squamous cell carcinoma cells: potential regulation by targeting rho proteins. Oncogene. 2010;29:4682-4692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Pelaia G, Gallelli L, Renda T, Fratto D, Falcone D, Caraglia M, Busceti MT, Terracciano R, Vatrella A, Maselli R. Effects of statins and farnesyl transferase inhibitors on ERK phosphorylation, apoptosis and cell viability in non-small lung cancer cells. Cell Prolif. 2012;45:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Chen J, Hou J, Zhang J, An Y, Zhang X, Yue L, Liu J, Li X. Atorvastatin synergizes with IFN-γ in treating human non-small cell lung carcinomas via potent inhibition of RhoA activity. Eur J Pharmacol. 2012;682:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Dore DD, Lapane KL, Trivedi AN, Mor V, Weinstock MA. Association between statin use and risk for keratinocyte carcinoma in the veterans affairs topical tretinoin chemoprevention trial. Ann Intern Med. 2009;150:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2013;368:576-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 808] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 55. | Boudreau DM, Yu O, Johnson J. Statin use and cancer risk: a comprehensive review. Expert Opin Drug Saf. 2010;9:603-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 224] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
P- Reviewers Deans C, Ma JY, Nishida T, Tseng YL, Xu XC S- Editor Gou SX L- Editor A E- Editor Lu YJ