Published online Sep 5, 2025. doi: 10.4292/wjgpt.v16.i3.108190
Revised: May 6, 2025
Accepted: July 7, 2025
Published online: September 5, 2025
Processing time: 150 Days and 0.4 Hours
Hepatitis B virus (HBV) is one of the major micro-pathogens in the progression of chronic liver infections worldwide. Despite a vaccine and anti-virus drugs used in the management of HBV infection, the prognosis and outcomes of this chronic infection remain complex and the infection can easily recur. Several parameters such as host age, viral mutations and genotypes, regional distributions, etc. have an effect on the outcome of hepatitis B infection following preventive measures and therapy around viral life cycle in the clinic. In addition, the economic status in different regions and groups of patients also affect disease progression. A cost-effectiveness analysis is considered to play a critical role in the management of chronic HBV infection. This mini-review investigates the above-mentioned aspects and provides a perspective viewpoint for the management of HBV infection in the future.
Core Tip: Hepatitis B virus (HBV) infection remains the major obstacle in the management of chronic liver disease worldwide, despite vaccination and anti-viral therapy. The life cycle of HBV replication in hepatocytes has a critical role in the escape of virus under the management strategy. It is important to summarize the associated factors between the host and virus in the development of HBV-mediated chronic progression using a cost-effectiveness analysis. This mini-review highlights both vaccination and anti-viral therapy interruption of the associated stages of the HBV life cycle as well as the corresponding cost-effectiveness methods applied to evaluate the diagnostic strategies, vaccination and treatment in HBV management.
- Citation: Zhu W. Therapeutic and preventive treatment on outcomes of hepatitis B virus induced chronic liver disease with a cost-effectiveness analysis. World J Gastrointest Pharmacol Ther 2025; 16(3): 108190
- URL: https://www.wjgnet.com/2150-5349/full/v16/i3/108190.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v16.i3.108190
Approximately 300 million people were estimated to be hepatitis B virus (HBV) carriers up to 2015 with hundreds of thousands of deaths per year with a rising trend worldwide[1,2]. Despite the application of vaccination and anti-viral therapy to reduce viral load in the global population, chronic infection with HBV has been seen in patients with different pathogenic phases in the clinic[3]. In patients with chronic HBV infection, a series of ongoing hepatic diseases including inflammation, fibrosis, cirrhosis and even hepatocellular carcinoma (HCC) have been observed during viral recovery[4]. To confirm the evolved history of HBV-mediated chronic hepatitis, it is necessary to investigate the structure and function of the virus and the distinct effective factors in susceptible hosts during the interaction process.
HBV was initially discovered by Blumberg et al[5] in 1965 who received a Nobel Prize in 1976. The pathogenesis of chronic HBV infection is an attractive topic with the hypothesis that overproduction of HBV replication and viral products result in liver damage[6]. HBV is classified as an enveloped DNA virus with a unique life cycle which can convert the partial relaxed-circular DNA (rcDNA) of genomic DNA into covalently closed circular DNA (cccDNA) of template DNA[7]. The template cccDNA has resistance to anti-viral therapy and is considered the ideal goal in the cure of patients with chronic HBV infection[8]. In addition, the amplification of viral RNA intermediate was produced by reverse-transcription from viral RNA into DNA. It was previously reported that the above RNA transcripts-encoded viral proteins had a negative role on the immune microenvironment in hepatocytes with live HBV[9]. Hence, the complex life cycle of HBV in hepatocytes represents the interaction between virus and host, and indicates the difficulty of prevention and therapy in patients with HBV infection.
Currently, the serum parameter, hepatitis B surface antigen (HBsAg) of HBV has a prevalence rate of 3.8% based on a calculation by the World Health Organization (WHO), and is unevenly distributed throughout the world with most infections in the Western Pacific Region with 116 million humans and in African Regions with 81 million people[10].
A previous study supported that socio-economic factors could affect the clinical outcomes of chronic HBV infection after treatment which differed between developing and developed countries or regions[11]. Furthermore, the role of government in reducing mortality was considered to support several interventions such as adequate services to cover clinical diagnosis and treatment, decrease the financial cost in people and adjustments in the management of patients with chronic HBV infection[12]. For example, a universal screening strategy in adults for HBV infection could be a good cost-effective way of preventing HBV in China[13]. Nevertheless, the models used in cost-effectiveness analysis are based on the development of progression. One combined method of cost-effectiveness analysis was used as an intervention in public health. The outcomes of metabolic dysfunction-associated steatotic liver disease (MASLD) were evaluated by quality-adjusted life years (QALY) while the total cost of the management strategy per QALY was estimated by the incremental cost-effectiveness ratio[14]. In addition, there are several other methods used including years of life lost, years lived with disability, disability adjusted life years, etc., which could replace QALY to achieve distinct aspects in the assessment of public health using systematic analysis[15]. Therefore, the social economics in public health issues can have a critical role in the management of HBV infection besides traditional prevention and therapy such as vaccines and anti-viral agents.
Historically, the recent economic order in the world has resulted from the colonial age, where quality goods and services were produced by significant skilled labor in the southern hemisphere in view of mobile investment and technology dissemination in the northern hemisphere[16]. This transitional phenomenon can be explained by the cross-border flow between goods and capital due to trade without barriers from different countries which occurred under globalization after World War II[17]. Global capitalism around globalization provided several factors such as economics, politics and cultures to advance our knowledge of social factors in public health issues[18]. Similar to the positive effect of globalization on the improvement of economics in previous years, the international coalition was introduced to establish the elimination of HBV in view of enough funding and strong government policy in the management of HBV infection[19]. Hence, this mini-review describes the management of HBV-mediated chronic diseases. Several factors such as host age, viral diversity, and distribution are considered to affect the chronic progression of patients after HBV vaccination and anti-viral therapy; thus, a cost-effectiveness analysis was conducted.
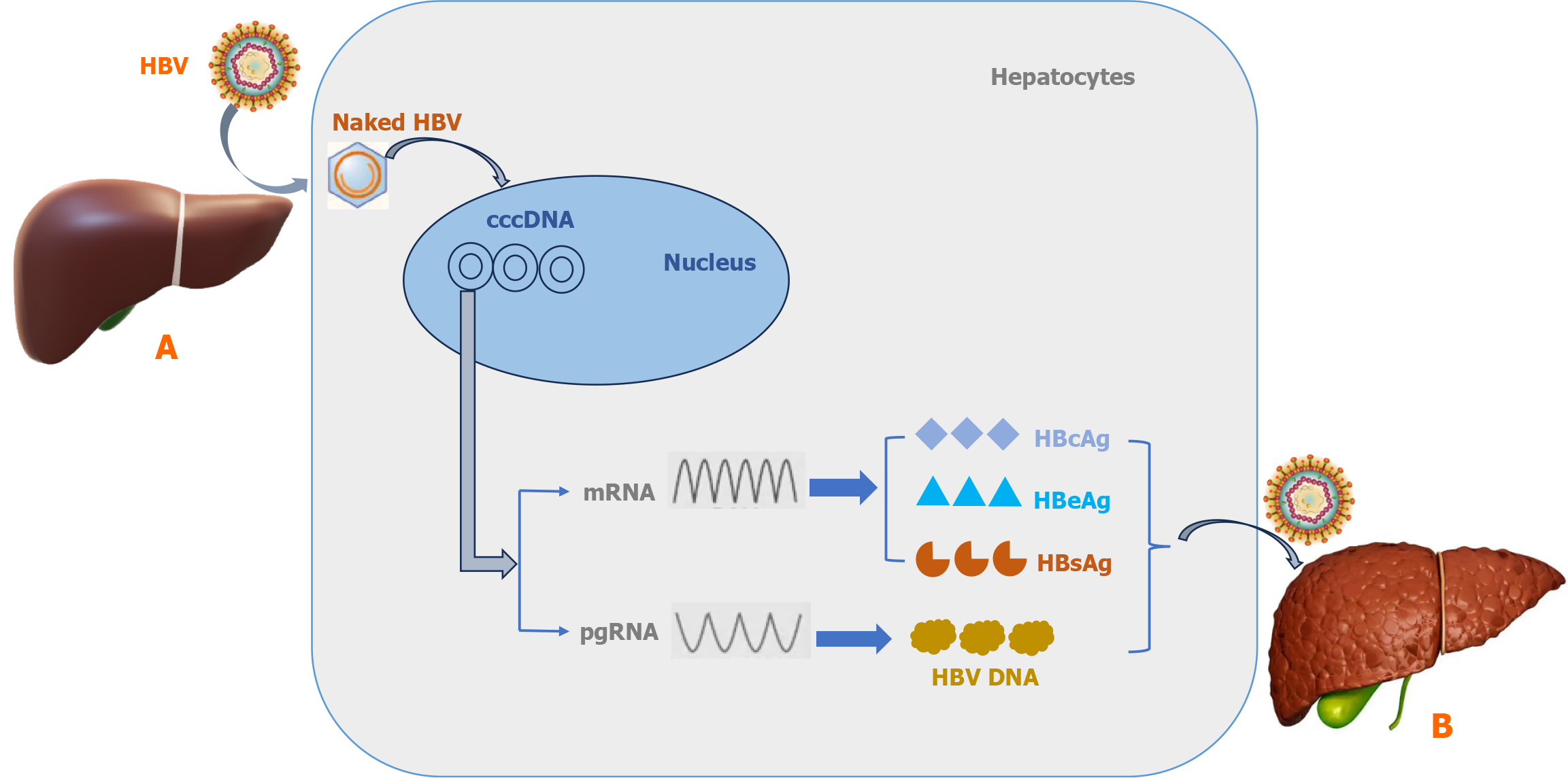
It is known that HBV includes reverse-transcription in its life cycle in spite of it being identified as a DNA virus. Acute HBV infections easily initiate chronic progression from hepatitis to HCC after viral entry into hepatocytes due to resistance and host immunity escape[20]. This survival capacity of HBV may be the result of genome combination and viral structure. There are four overlapping open reading frames (ORFs) including viral polymerase, surface polypeptides, core protein, and X protein to construct the compact and limited coding genome structure[21]. These ORFs encode the functional fragments such as viral replication, packaging, capsid and regulation of genome transcription in the HBV life cycle. In particular, HBV surface polypeptides have a crucial role in binding with hepatocyte receptors including heparin sulfate proteoglycans and bile acid transporter sodium-taurocholate co-transporting polypeptide to mediate endocytosis[22,23]. Following endocytosis-induced HBV internalization, the viral capsid is liberated in the cytoplasm and then converts its rcDNA into cccDNA in the nucleus of hepatocytes[24]. In addition, rcDNA displays integrated fragments without replication competence in the host genome in the early period of HBV infection[25]. Different to these integrated rcDNA fragments, cccDNA is located outside the host genome DNA in the nucleus which operates as a template for viral replication[7]. The nucleus cccDNA can form episomes which are stably maintained under host immune factors[4]. Several host nucleus factors such as histones H3, H4, H2A, H2B, H1 as well as viral production including HBV X protein, and hepatitis B core (HBc) protein can be gathered to surround cccDNA in the development of episomes[26,27]. It was reported that a low level of cccDNA also had a high risk of acute hepatitis with liver failure in occult HBV patients due to latent cccDNA in the nucleus[28]. In addition, the fundamental role of cccDNA also dominated transcription of all viral RNA and the generation of new virions as well as persistent damage to host hepatocytes after HBV infection[29]. It is considered that cccDNA is a critical obstacle and difficult to eradicate in the development of HBV-mediated liver pathogenesis[30]. Despite cccDNA in the nucleus of hepatocytes infected with HBV it could not be disrupted by general anti-viral treatment in the clinic, thus, cccDNA may potentially be considered a target in the management of patients with chronic HBV infection[31]. Based on these factors, the concise life cycle of HBV entering hepatocytes is shown in Figure 1. It can be seen from this Figure 1 that the viral envelope was excluded in the cell membrane and then naked virus was transferred by the viral nucleocapsid into the nucleus to form the cccDNA structure in hepatocytes. In addition, the cccDNA template has a critical role in the formation of mRNA encoding proteins including HBc antigen, hepatitis B e antigen (HBeAg) and HBsAg as well as pregenomic RNA to undertake HBV DNA using reverse transcription. Finally, these viral components are constructed into new virions to enter other hepatocytes which could initiate chronic HBV infection in the liver of patients.
The HBV vaccine was recommended in national immunization programs during childhood by the WHO to prevent the occurrence of infectious hepatitis B and related chronic liver diseases worldwide[32]. It was reported that the common routes of HBV transmission in families was mother-to-child at birth or breastfeeding and close contact with infected family members during childhood[33]. Corresponding to this situation, three immunization strategies including perinatal, infant and adolescent administrations were used in the public sector for a clinical effect analysis of HBV vaccination[34]. During 2000-2019, the 3 HBV vaccine doses rose by at least 50% coverage in persons worldwide which had a critical role in the reduction of HBV infection and associated chronic diseases[35]. The primary immune capacity of HBV vaccine is the neutralizing antibody to target amino acids 124-149 of HBsAg against all HBV genotypes (A-H) as previously described[36]. Secondly, it was reported that CD8+ T cells as well as CD4+ T cells had a crucial role in clearance of the viral load in an HBV-mediated model[37,38]. Nevertheless, efficacy of the HBV vaccine can be threatened by breakthrough in view of S gene mutation, epitope mutants of B/T-lymphocytes and HBV variants[39]. For example, one case not protected by the HBV vaccine was reported to show the substitution of Q129H in the preS-S region which altered the viral antigenicity resulting in acute hepatitis B in the 55-year-old Caucasian male patient[40]. In upper Egypt, several point mutations with a total prevalence of 16.3% (20/123) including S143L, T115S, P120T/S, G145R/A, and D144E/A, etc., were observed in the S region of genotype D/E and commonly observed in patients with chronic HBV infection after vaccine administration[41]. In addition, HBV vaccine escaped mutations had an average global prevalence of 1.3% (37/2837) vs a higher prevalence in genotype C with 2.2% (24/1102) and in Asia with 1.6% (34/2109) based on the HBV database[42]. In view of these findings, it is easy to understand that viral mutations of the S region cause dysfunction of anti-HBsAg neutralization induced by HBV vaccines. Moreover, diversity of the HBV core (18-27) epitope under selected pressure by human leucocyte antigen-I molecules can play a crucial role in substitutions in the escape of HBV-mediated CD8+ T cells therapy[43]. One explanation for this is that dysfunction of HBV-activated CD8+ T cells damage their associated immunity due to CD4+ T cell exhaustion resulting in the development of chronic HBV infection[44]. Despite viral resistance against the vaccine, the development of chronic HBV infection can be prevented in addition to mother-to-child transmission, and is a more effective method of HBV elimination accompanied by a series of strategies such as maternal screening, neonate vaccination or anti-viral therapy[45].
As previously mentioned, patients treated with HBV vaccines can develop chronic liver diseases such as fibrosis, cirrhosis and even HCC due to viral mutational escape. More importantly, birth dose HBV vaccination did not achieve more than 70% of the delivery rate in many regions as mother-to-infant transmission was not controlled until 2019 as previously demonstrated[46]. Hence, it is critical to treat patients with chronic HBV infection according to public health policy. It was demonstrated that viral hepatitis should be eliminated by 2030 under the global health strategy of the 69th World Health Assembly[47]. Countries need to increase the capacity of both viral detection and treatment in humans with HBV infection. Specifically, HBV cure is defined as continuous examination without HBsAg and HBV DNA in serum after a period of treatment with nucleos(t)ide analogues (NAs) and interferon (IFN)[48]. Long-term anti-viral therapy including polyethylene glycol IFN and NAs has dominated the outcome of HBV-mediated chronic progression with or without HBV vaccine administration[49]. Anti-viral therapy could prevent chronic progression including decompensation of cirrhosis to HCC in order to reduce mortality in patients with HBV infection[50]. IFN including type I IFN-α, IFN-β, type II IFN-γ, type III IFN-λ1, IFN-λ2, and IFN-λ3 are glycoproteins which interact with their corresponding receptors on the cell surface and initiate the Janus kinase/signal transducer and activator of the transcription signaling pathway to produce cytokines in the first line of host immunity against viral infection[51]. Moreover, IFN has been used as an alternative treatment to cure HBV infection in the past, but its low efficacy and severe side effects have an unsatisfactory role in the management of patients with chronic HBV-mediated liver diseases, IFN has also been combined with NAs in the clinic[52,53]. It was reported that NAs treatment such as entecavir (ETV), lamivudine (LAM) or tebivudine plus adefovir dipivoxil, etc., can result in the alleviation of ascites and decrease the levels of alanine aminotransferase, and HBV DNA in the serum of patients with HBV-related cirrhosis[54]. A previous meta-analysis indicated that the NAs, ETV and LAM, were well-tolerated and reduced short-term mortality following therapy in patients with acute-on-chronic liver failure after HBV reactivation[55]. Although NAs are considered to be an effective anti-viral therapy for HBV infection, the key cccDNA template in the nucleus could not be eliminated and S gene mutation-mediated drug resistance was also activated by long-term application of NAs in patients[56]. Furthermore, it was demonstrated that viral mutations-mediated drug resistance including A194T, L180M + M204V, S202I, M204I, and A181S in the reverse transcriptase region of the HBV genome were observed in 6% of 189 chronic HBV-infected patients naïve to treatment from the Northeastern region of Brazil and these mutations were not found in patients from the Northern region of Brazil[57]. This diversity of drug-resistant mutations indicates the complex presentations of HBV which is associated with regional distribution or other factors.
The majority of HBV-induced morbidity and mortality during childhood was found to occur in low- and middle-income regions and countries despite the administration of HBV vaccine and anti-viral therapy[58]. To determine the prevalence of chronic HBV infection-mediated liver diseases, a meta-analysis investigated the national, regional, and global prevalence of chronic progression in liver disease, and found that related HBsAg seroprevalence was estimated to be 3.61% in the world, 8.83% in African regions and 5.26% in the Western Pacific region[59]. It was recently reported that several liver diseases such as alcoholic fatty liver disease, MASLD and HCC similar to the prevalence of hepatitis B/C virus infections have increased based on data from the Global Burden of Disease study[60-62]. In addition, it was proved that the socio-demographic index displayed a negative association with mortality due to these chronic liver diseases[62]. Moreover, the medical costs for HBV infection were evaluated using generalized estimating equations and regression models in a retrospective cohort study, which found that the cost was 54.61% for 65175 outpatients vs 6.17% for 12649 inpatients, indicating that the economic burden in the management of HBV was affected by modes of both payment and treatment[63]. In terms of HBV vaccination, the world level of coverage using three doses was considered to be 75%, but the vaccination coverage in developed countries such as the United States was higher than the rate in developing countries including Africa and South-East Asia[64]. In particular, Africa has the highest prevalence of HBV infections with a positive rate of 5.4% of HBsAg, posing a major economic burden in Africa in the management of HBV infections[65]. In addition, the existing models of cost-effectiveness evaluation using distinct parameters such as risk factors, settings, intervention methods, outcomes, etc., had a different role in the management of HBV infections among associated studies[66].
Table 1 shows the economic assessment in the management of HBV-associated diseases[13,67-70]. Early screening using five-tests for HBsAg, HBs antibody (Ab), HBeAg, HBeAb, and HBcAb was used to prevent HBV-mediated diseases to aid viral hepatitis elimination with a cost-effectiveness analysis in China[13]. The global burden of HBV-associated diseases was estimated by a Bayesian meta-regression model from the global burden of diseases database and showed a decline in the ratio of all-age prevalence of 31.3% vs a greater reduction in children under 5 years of 76.8% between 1990 and 2019[67]. Five HBsAg screening projects were conducted to examine the cost-effectiveness in neonates and to determine the screening cost in a limited population of migrants in the United States, but requires more participants in future screening to ascertain cost-effectiveness in the management of HBV infection[68]. Declining trends in both incidence and prevalence of acute and chronic HBV as well as stabilized mortality due to HBV infection conducted in four countries including Greece, Italy, Portugal, and Spain in south-west Europe were observed during the period of 2010-2019 following economic austerity in 2008[69]. In addition, the strategy of HBV screening in community and treatment projects had a beneficial role in monitoring HBV infection in Gambia, which was cost-effective[70]. From these studies on the cost-effectiveness of HBV management, it was revealed that the economic status of countries or regions had a significant effect on the trend of chronic HBV infection in view of specific strategies such as vaccination and NAs treatment administration.
| Ref. | Period/age | Data/region | Management | Disease/case | Model | Index | Effect |
| Su et al[13] | 2000-2021; 18-70 years | PubMed, EMBASE, Web of Science; China | The serum five-test screening | HBV-hepatitis; per 100000 | Markov cohort | ICER/quality-adjusted life-years: $18295 | Prevent 3.46 million deaths due to HBV |
| GBD 2019 Hepatitis B Collaborators[67] | 1990-2019; all ages | GBD; global | WHO-GHSS; WHO interim guidance | Chronic HBV infection; per 100000 | DisMod-MR 2.1 (a Bayesian mixed-effects meta-regression tool) | Mortality-to-incidence ratios for chronic HBV infection | Prevalence/no: 4.1%/316 million; decline ratio (1990 to 2019): 31.3% |
| Rein et al[68] | 2008-2009; neonate with six months observation | Centers for Disease Control and Prevention; Asia migrant of the United States | Serum HBsAg screening | 5.6%-6.6% HBsAg of 1623 participants | Community clinic model; community outreach model; partnership and contract model | $40-$280 per general screen; $609-$4657 per positive screen | Limited screening |
| Palladino et al[69] | 2010-2019; all ages | GBD; Southern Western Europe | WHO-GHSS; WHO interim guidance | Acute and chronic HBV infection; per 100000 | Time series model | Prevalence, incidence, mortality, years lived with disability, years of life lost, DALY | Declined prevalence and incidence; stable mortality |
| Schmit et al[70] | Up to 2020; 15-45 years | Medline database; Gambia | HBV vaccine; tenofovir disoproxil fumarate treatment | Chronic HBV infection; per 100000 | Bayesian network | ICER/DALY: $338-$404 | Reduced mortality due to HBV |
In addition to the above-mentioned factors, there were at least ten genotypes (A-J) with more than 8% nucleotide difference in the diversity among intergroups of the HBV genome, which had a critical role in the outcome of chronic HBV infection under anti-viral therapy[71,72]. Furthermore, genotypes F and H of HBV showed frequent prevalence in the American continent and are considered to be associated with severe progression of liver disease, particularly genotype F[72]. Also, genotype D of HBV is distributed throughout the world, as well as other genotypes and show distinct geographic features including genotype A in Europe, north America, and Africa, genotypes B, C in the Asia-Pacific area and genotype E in most regions of Africa[73]. Based on the diversity of HBV genotypes in distinct regions worldwide, it can be speculated that there is an interesting association between viral prevalence and social-communication in the development of HBV-mediated chronic liver disease. This topic needs further investigation to support this type of communication in the transmission of HBV worldwide.
Recently, several tools such as Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR associated genes (CRISPR/Cas) and Chimeric Antigen Receptor T-cell (CAR-T) therapy have been developed to overcome existing problems in the management of chronic HBV-mediated disease[74,75]. These developments can be considered potential candidates for addition to HBV vaccination and NAs treatment. Nevertheless, sufficient investment from formal or informal sources is needed for this public health project by the WHO. However, there are several challenges including off-target capacity, delivery barriers and immune resistance which can have a negative effect on the specificity, efficacy and safety of CRISPR/Cas against HBV infection[76]. In addition, the limitations of CAR-T therapy include several aspe
The diversity of HBV due to viral mutational capacity significantly promotes viral escape and the function of vaccination or anti-viral therapy and continuously infects hepatocytes in the host to develop chronic HBV-mediated disease. Cost-effectiveness analysis can perform a critical role in strategy designs for the management for chronic HBV infection worldwide. More efforts are necessary to develop novel agents as well as widespread vaccination or NAs treatment in the management of chronic HBV infection.
| 1. | Glebe D, Goldmann N, Lauber C, Seitz S. HBV evolution and genetic variability: Impact on prevention, treatment and development of antivirals. Antiviral Res. 2021;186:104973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Mnyandu NZ, Limani SW, Ely A, Wadee R, Arbuthnot P, Maepa MB. Long-term inhibition of Hepatitis B virus gene expression by a primary microrna expressing ancestral adeno-associated viral vector. Virol J. 2025;22:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Yuen MF, Chen DS, Dusheiko GM, Janssen HLA, Lau DTY, Locarnini SA, Peters MG, Lai CL. Hepatitis B virus infection. Nat Rev Dis Primers. 2018;4:18035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 579] [Article Influence: 72.4] [Reference Citation Analysis (1)] |
| 4. | Hu J, Protzer U, Siddiqui A. Revisiting Hepatitis B Virus: Challenges of Curative Therapies. J Virol. 2019;93:e01032-e01019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Blumberg BS, Alter HJ, Visnich S. A "new" antigen in leukemia sera. JAMA. 1965;191:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 736] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 6. | Zhang YY, Hu KQ. Rethinking the pathogenesis of hepatitis B virus (HBV) infection. J Med Virol. 2015;87:1989-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Tsukuda S, Watashi K. Hepatitis B virus biology and life cycle. Antiviral Res. 2020;182:104925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 275] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 8. | Wang J, Huang H, Liu Y, Chen R, Yan Y, Shi S, Xi J, Zou J, Yu G, Feng X, Lu F. HBV Genome and Life Cycle. Adv Exp Med Biol. 2020;1179:17-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Rapicetta M, Ferrari C, Levrero M. Viral determinants and host immune responses in the pathogenesis of HBV infection. J Med Virol. 2002;67:454-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Hsu YC, Huang DQ, Nguyen MH. Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nat Rev Gastroenterol Hepatol. 2023;20:524-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 366] [Reference Citation Analysis (1)] |
| 11. | Zampino R, Sagnelli C, Boemio A, Sagnelli E, Coppola N. Treatment of chronic HBV infection in developing countries. Ann Hepatol. 2016;15:816-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Liu J, Liang W, Jing W, Liu M. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ. 2019;97:230-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 353] [Article Influence: 50.4] [Reference Citation Analysis (1)] |
| 13. | Su S, Wong WC, Zou Z, Cheng DD, Ong JJ, Chan P, Ji F, Yuen MF, Zhuang G, Seto WK, Zhang L. Cost-effectiveness of universal screening for chronic hepatitis B virus infection in China: an economic evaluation. Lancet Glob Health. 2022;10:e278-e287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 14. | Pochopien M, Dziedzic JW, Aballea S, Clay E, Zerda I, Toumi M, Borissov B. Cost-Effectiveness Analysis of Innovative Therapies for Patients with Non-Alcoholic Fatty Liver Disease. J Mark Access Health Policy. 2024;12:35-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, Li X, Wang L, Wang L, Liu Y, Liu J, Zhang M, Qi J, Yu S, Afshin A, Gakidou E, Glenn S, Krish VS, Miller-Petrie MK, Mountjoy-Venning WC, Mullany EC, Redford SB, Liu H, Naghavi M, Hay SI, Wang L, Murray CJL, Liang X. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394:1145-1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2594] [Cited by in RCA: 2610] [Article Influence: 372.9] [Reference Citation Analysis (2)] |
| 16. | Jakovljevic M, Péntek M, Wijeratne T, Kockaya G, Pau LF. Editorial: Accelerated Globalization and Its Impact to the World's Health Care Achievement. Front Public Health. 2021;9:690239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 17. | Labonté R. Trade, investment and public health: compiling the evidence, assembling the arguments. Global Health. 2019;15:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Flynn MB. Global capitalism as a societal determinant of health: A conceptual framework. Soc Sci Med. 2021;268:113530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Revill P, Testoni B, Locarnini S, Zoulim F. Global strategies are required to cure and eliminate HBV infection. Nat Rev Gastroenterol Hepatol. 2016;13:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 20. | Kostyusheva A, Brezgin S, Glebe D, Kostyushev D, Chulanov V. Host-cell interactions in HBV infection and pathogenesis: the emerging role of m6A modification. Emerg Microbes Infect. 2021;10:2264-2275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 21. | Wei L, Ploss A. Mechanism of Hepatitis B Virus cccDNA Formation. Viruses. 2021;13:1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 22. | Schulze A, Gripon P, Urban S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology. 2007;46:1759-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 363] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 23. | Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1280] [Cited by in RCA: 1649] [Article Influence: 117.8] [Reference Citation Analysis (1)] |
| 24. | Diogo Dias J, Sarica N, Neuveut C. Early Steps of Hepatitis B Life Cycle: From Capsid Nuclear Import to cccDNA Formation. Viruses. 2021;13:757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Pollicino T, Caminiti G. HBV-Integration Studies in the Clinic: Role in the Natural History of Infection. Viruses. 2021;13:368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 26. | Bock CT, Schranz P, Schröder CH, Zentgraf H. Hepatitis B virus genome is organized into nucleosomes in the nucleus of the infected cell. Virus Genes. 1994;8:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 158] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | He P, Zhang P, Fang Y, Han N, Yang W, Xia Z, Zhu Y, Zhang Z, Shen J. The role of HBV cccDNA in occult hepatitis B virus infection. Mol Cell Biochem. 2023;478:2297-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Wong DK, Fung J, Lee CK, Seto WK, Leung J, Huang FY, Lin CK, Lai CL, Yuen MF. Intrahepatic hepatitis B virus replication and liver histology in subjects with occult hepatitis B infection. Clin Microbiol Infect. 2016;22:290.e1-290.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64:1972-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 730] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 30. | Allweiss L, Dandri M. The Role of cccDNA in HBV Maintenance. Viruses. 2017;9:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 31. | Zhu A, Liao X, Li S, Zhao H, Chen L, Xu M, Duan X. HBV cccDNA and Its Potential as a Therapeutic Target. J Clin Transl Hepatol. 2019;7:258-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | World Health Organization. Hepatitis B vaccines: WHO position paper, July 2017 - Recommendations. Vaccine. 2019;37:223-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 33. | Nguwoh PS, Ngounouh CT, Essomba RG, Olinga PZ, Likeng JLN, Nguepidjo G, Douyong SCT, Tchoffo D, Nlend AEN, Assoumou MCO, Fokam J. Effect of hepatitis B vaccination on HBV-infection among school children in Yaounde; ten years after the introduction of HBV vaccine into routine Immunization Program in Cameroon. Pan Afr Med J. 2024;47:169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Margolis HS, Coleman PJ, Brown RE, Mast EE, Sheingold SH, Arevalo JA. Prevention of hepatitis B virus transmission by immunization. An economic analysis of current recommendations. JAMA. 1995;274:1201-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 140] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Pattyn J, Hendrickx G, Vorsters A, Van Damme P. Hepatitis B Vaccines. J Infect Dis. 2021;224:S343-S351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 173] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 36. | Romanò L, Paladini S, Galli C, Raimondo G, Pollicino T, Zanetti AR. Hepatitis B vaccination. Hum Vaccin Immunother. 2015;11:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Yang PL, Althage A, Chung J, Maier H, Wieland S, Isogawa M, Chisari FV. Immune effectors required for hepatitis B virus clearance. Proc Natl Acad Sci U S A. 2010;107:798-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 38. | Baudi I, Kawashima K, Isogawa M. HBV-Specific CD8+ T-Cell Tolerance in the Liver. Front Immunol. 2021;12:721975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 39. | Qin Y, Liao P. Hepatitis B virus vaccine breakthrough infection: surveillance of S gene mutants of HBV. Acta Virol. 2018;62:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Luongo M, Critelli R, Grottola A, Gitto S, Bernabucci V, Bevini M, Vecchi C, Montagnani G, Villa E. Acute hepatitis B caused by a vaccine-escape HBV strain in vaccinated subject: sequence analysis and therapeutic strategy. J Clin Virol. 2015;62:89-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | El-Mokhtar MA, Hetta HF, Mekky MA, Abd El-Kareem DM, Ramadan M, Salah M, Mohamed NA, El-Masry EA, Adel S, Sayed IM. Characterization of Antigen Escape Mutations in Chronic HBV-Infected Patients in Upper Egypt. Infect Drug Resist. 2021;14:2419-2427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Mokaya J, Vasylyeva TI, Barnes E, Ansari MA, Pybus OG, Matthews PC. Global prevalence and phylogeny of hepatitis B virus (HBV) drug and vaccine resistance mutations. J Viral Hepat. 2021;28:1110-1120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Walker A, Schwarz T, Brinkmann-Paulukat J, Wisskirchen K, Menne C, Alizei ES, Kefalakes H, Theissen M, Hoffmann D, Schulze Zur Wiesch J, Maini MK, Cornberg M, Kraft AR, Keitel V, Bock HH, Horn PA, Thimme R, Wedemeyer H, Heinemann FM, Luedde T, Neumann-Haefelin C, Protzer U, Timm J. Immune escape pathways from the HBV core(18-27) CD8 T cell response are driven by individual HLA class I alleles. Front Immunol. 2022;13:1045498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Hoogeveen RC, Boonstra A. Checkpoint Inhibitors and Therapeutic Vaccines for the Treatment of Chronic HBV Infection. Front Immunol. 2020;11:401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 45. | Matthews PC, Ocama P, Wang S, El-Sayed M, Turkova A, Ford D, Torimiro J, Garcia Ferreira AC, Espinosa Miranda A, De La Hoz Restrepo FP, Seremba E, Mbu R, Pan CQ, Razavi H, Dusheiko G, Spearman CW, Hamid S. Enhancing interventions for prevention of mother-to-child- transmission of hepatitis B virus. JHEP Rep. 2023;5:100777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 46. | Thomas DL, Kiser JJ, Baum MM. Long-Acting Treatments for Hepatitis B. Clin Infect Dis. 2022;75:S517-S524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Cui F, Blach S, Manzengo Mingiedi C, Gonzalez MA, Sabry Alaama A, Mozalevskis A, Séguy N, Rewari BB, Chan PL, Le LV, Doherty M, Luhmann N, Easterbrook P, Dirac M, de Martel C, Nayagam S, Hallett TB, Vickerman P, Razavi H, Lesi O, Low-Beer D. Global reporting of progress towards elimination of hepatitis B and hepatitis C. Lancet Gastroenterol Hepatol. 2023;8:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 208] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 48. | Wong GLH, Gane E, Lok ASF. How to achieve functional cure of HBV: Stopping NUCs, adding interferon or new drug development? J Hepatol. 2022;76:1249-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 201] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 49. | Qi WQ, Zhang Q, Wang X, Xu Y, Zhao P, Guo HH, Zhou CY, Sun Y, Liu L, Wang JB. Long-term clinical benefit of Peg-IFNα and NAs sequential anti-viral therapy on HBV related HCC. Neoplasma. 2021;68:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 50. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, Heathcote EJ. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1410] [Article Influence: 108.5] [Reference Citation Analysis (1)] |
| 51. | Lin FC, Young HA. Interferons: Success in anti-viral immunotherapy. Cytokine Growth Factor Rev. 2014;25:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 52. | Zoulim F, Lebossé F, Levrero M. Current treatments for chronic hepatitis B virus infections. Curr Opin Virol. 2016;18:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 53. | Boni C, Barili V, Acerbi G, Rossi M, Vecchi A, Laccabue D, Penna A, Missale G, Ferrari C, Fisicaro P. HBV Immune-Therapy: From Molecular Mechanisms to Clinical Applications. Int J Mol Sci. 2019;20:2754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Li M, Zong Z, Xiong X, Fan J, Zhong H, Liu N, Ye W, Jing J. Ascites re-compensation in HBV-related first decompensated cirrhosis after anti-viral therapy. Front Cell Infect Microbiol. 2022;12:1053608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 55. | Yu S, Jianqin H, Wei W, Jianrong H, Yida Y, Jifang S, Liang Y, Zhi C, Hongyu J. The efficacy and safety of nucleos(t)ide analogues in the treatment of HBV-related acute-on-chronic liver failure: a meta-analysis. Ann Hepatol. 2013;12:364-372. [PubMed] [DOI] [Full Text] |
| 56. | Wang ML, Tang H. Nucleos(t)ide analogues causes HBV S gene mutations and carcinogenesis. Hepatobiliary Pancreat Dis Int. 2016;15:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Pacheco SR, Dos Santos MIMA, Stocker A, Zarife MAS, Schinoni MI, Paraná R, Dos Reis MG, Silva LK. Genotyping of HBV and tracking of resistance mutations in treatment-naïve patients with chronic hepatitis B. Infect Drug Resist. 2017;10:201-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Nannini P, Sokal EM. Hepatitis B: changing epidemiology and interventions. Arch Dis Child. 2017;102:676-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 59. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 2054] [Article Influence: 186.7] [Reference Citation Analysis (4)] |
| 60. | Zhu W. Roles of olive oil and physical exercise in non-alcoholic fatty liver disease after ultrasound-based evaluation. World J Hepatol. 2025;17:100243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 61. | Paik JM, Kabbara K, Eberly KE, Younossi Y, Henry L, Younossi ZM. Global burden of NAFLD and chronic liver disease among adolescents and young adults. Hepatology. 2022;75:1204-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 179] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 62. | Xiao J, Wang F, Yuan Y, Gao J, Xiao L, Yan C, Guo F, Zhong J, Che Z, Li W, Lan T, Tacke F, Shah VH, Li C, Wang H, Dong E. Epidemiology of liver diseases: global disease burden and forecasted research trends. Sci China Life Sci. 2025;68:541-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 63. | Yang S, Chen G, Li Y, Li G, Liang Y, Zhou F, Zhou S, Yang Y, Jia W, Gao Y, Chen Y. The trend of direct medical costs and associated factors in patients with chronic hepatitis B in Guangzhou, China: an eight-year retrospective cohort study. BMC Med Inform Decis Mak. 2021;21:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | La Torre G, Mannocci A, Saulle R, Colamesta V, Meggiolaro A, Mipatrini D, Sinopoli A. Economic evaluation of HBV vaccination: A systematic review of recent publications (2000-2013). Hum Vaccin Immunother. 2016;12:2299-2311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Voeller AS, Johannessen A, Abebe ZZ, Adugna W, Gamkrelidze I, Seyoum E, Gebremedhin LT, Meselu MG, Nigussie SA, Silesh A, Razavi H, Razavi-Shearer D, Tirsite G, Desalegn H. The Disease and Economic Burden of HBV and HCV in Ethiopia. J Viral Hepat. 2025;32:e14053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 66. | Geue C, Wu O, Xin Y, Heggie R, Hutchinson S, Martin NK, Fenwick E, Goldberg D; Consortium; ECDC. Cost-Effectiveness of HBV and HCV Screening Strategies--A Systematic Review of Existing Modelling Techniques. PLoS One. 2015;10:e0145022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 67. | GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:796-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 515] [Article Influence: 128.8] [Reference Citation Analysis (1)] |
| 68. | Rein DB, Lesesne SB, Smith BD, Weinbaum CM. Models of community-based hepatitis B surface antigen screening programs in the U.S. and their estimated outcomes and costs. Public Health Rep. 2011;126:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Palladino C, Ramis R, Ezeonwumelu IJ, Biondi A, Carreras G, Fischer F, Gallus S, Golinelli D, Gorini G, Hassan S, Kabir Z, Koyanagi A, Lazarus JV, Mentis AA, Meretoja TJ, Mokdad AH, Monasta L, Mulita F, Postma MJ, Tabarés-Seisdedos R, Thiyagarajan A, Taveira N, Briz V; GBD 2019 Southern Europe Hepatitis B & C Collaborators. Impact of the 2008 economic crisis on the burden of hepatitis B and C diseases in Southern European countries. BMC Public Health. 2024;24:1642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 70. | Schmit N, Nayagam S, Lemoine M, Ndow G, Shimakawa Y, Thursz MR, Hallett TB. Cost-effectiveness of different monitoring strategies in a screening and treatment programme for hepatitis B in The Gambia. J Glob Health. 2023;13:04004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Chen J, Li L, Yin Q, Shen T. A review of epidemiology and clinical relevance of Hepatitis B virus genotypes and subgenotypes. Clin Res Hepatol Gastroenterol. 2023;47:102180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 72. | Pujol F, Jaspe RC, Loureiro CL, Chemin I. Hepatitis B virus American genotypes: Pathogenic variants? Clin Res Hepatol Gastroenterol. 2020;44:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 73. | Dopico E, Vila M, Tabernero D, Gregori J, Rando-Segura A, Pacín-Ruíz B, Guerrero L, Ubillos I, Martínez MJ, Costa J, Quer J, Pérez-Garreta J, González-Sánchez A, Antón A, Pumarola T, Riveiro-Barciela M, Ferrer-Costa R, Buti M, Rodríguez-Frías F, Cortese MF. Genotyping Hepatitis B virus by Next-Generation Sequencing: Detection of Mixed Infections and Analysis of Sequence Conservation. Int J Mol Sci. 2024;25:5481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Tian Y, Fan Z, Xu L, Cao Y, Chen S, Pan Z, Gao Y, Li H, Zheng S, Ma Y, Duan Z, Zhang X, Ren F. CRISPR/Cas13a-assisted rapid and portable HBV DNA detection for low-level viremia patients. Emerg Microbes Infect. 2023;12:e2177088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 75. | Fu S, Zhang Q, Jing R, Zu C, Ni F, Lv Y, Cui J, Zheng H, Zhang Y, Zhang M, Wei G, Cen Z, Chang AH, Hu Y, Huang H. HBV reactivation in patients with chronic or resolved HBV infection following BCMA-targeted CAR-T cell therapy. Bone Marrow Transplant. 2023;58:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 76. | Nair DM, Vajravelu LK, Thulukanam J, Paneerselvam V, Vimala PB, Lathakumari RH. Tackling hepatitis B Virus with CRISPR/Cas9: advances, challenges, and delivery strategies. Virus Genes. 2024;60:592-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 77. | Zheng Z, Li S, Liu M, Chen C, Zhang L, Zhou D. Fine-Tuning through Generations: Advances in Structure and Production of CAR-T Therapy. Cancers (Basel). 2023;15:3476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 78. | Siontas O, Ahn S. Challenges in AAV-Based Retinal Gene Therapies and the Role of Magnetic Nanoparticle Platforms. J Clin Med. 2024;13:7385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 79. | Zhu W, Xie K, Xu Y, Wang L, Chen K, Zhang L, Fang J. CRISPR/Cas9 produces anti-hepatitis B virus effect in hepatoma cells and transgenic mouse. Virus Res. 2016;217:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 80. | Zhu W. Effective roles of exercise and diet adherence in non-alcoholic fatty liver disease. World J Gastroenterol. 2024;30:3456-3460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Reference Citation Analysis (10)] |
| 81. | Wang J, Qiu K, Zhou S, Gan Y, Jiang K, Wang D, Wang H. Risk factors for hepatocellular carcinoma: an umbrella review of systematic review and meta-analysis. Ann Med. 2025;57:2455539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 82. | Yang WS, Zeng XF, Liu ZN, Zhao QH, Tan YT, Gao J, Li HL, Xiang YB. Diet and liver cancer risk: a narrative review of epidemiological evidence. Br J Nutr. 2020;124:330-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/