Published online Nov 5, 2024. doi: 10.4292/wjgpt.v15.i6.95532
Revised: August 25, 2024
Accepted: September 19, 2024
Published online: November 5, 2024
Processing time: 195 Days and 7.3 Hours
Inflammatory bowel disease (IBD), with its rising prevalence rates is associated with an increased risk of cardiovascular and thromboembolic events. Antiplatelets and/or anticoagulants agents are often prescribed but the literature on the impact of long-term anticoagulation and/or antiplatelet use among patients hospitalized with IBD is scarce. The aim of this study is to assess the outcomes of patients hospitalized with IBD on antiplatelet and/or anticoagulant agents.
To investigate the effects of long-term use of antiplatelets/anticoagulants on clinical outcomes in patients hospitalized with IBD.
We conducted a retrospective cohort study using the Nationwide Inpatient Sample database, including all adult IBD patients hospitalized in the United States from 2016 to 2019. Patient cohorts were stratified based on antiplatelet/anticoagulant therapy status. Multivariate regression analysis was done to assess outcomes, adjusting for potential confounders. The primary outcome was mortality, whereas length of stay (LOS), total parenteral nutrition, acute kidney injury, sepsis, shock, gastrointestinal bleeding, need for colonoscopy/sigmoidoscopy, abdominal surgery and total hospitalization charges were secondary outcomes.
Among 374744 hospitalized IBD patients, antiplatelet or anticoagulant therapy alone was associated with significantly lower in-hospital mortality and reduced healthcare utilization, including shorter LOS and decreased hospitalization costs. Combined therapy was associated with a protective effect on mortality, but did not reach statistical significance. Notably, therapy did not exacerbate disease severity or complications, although higher odds of gastrointestinal bleeding were observed.
Our study highlights the potential benefits of long-term anticoagulation/antiplatelet therapy in hospitalized IBD patients, with improved mortality outcomes and healthcare utilization. While concerns regarding gastrointestinal bleeding exist, the overall safety profile suggests a role for these agents in mitigating thromboembolic risks without exacerbating disease severity. Further research is needed to look at optimal treatment strategies and addressing limitations to guide clinical decision-making in this population.
Core Tip: In this retrospective study, hospitalized inflammatory bowel disease (IBD) patients on long-term antiplatelet or anticoagulant therapy demonstrated benefits in terms of inpatient mortality, length of stay, and total hospitalization charges compared to those not on such therapy. While the use of anticoagulation alone or in combination with antiplatelet agents was linked to higher odds of lower gastrointestinal bleeding, there was no significant difference in the development of acute kidney injury, sepsis, or the use of total parenteral nutrition. Further research is needed to optimize treatment strategies and inform clinical decision-making in the IBD population.
- Citation: Changela M, Pandey S, Bahirwani J, Patel N, Kaneriya M, Basida SD, Shah A, Thakur R, Bodrya K, Jai Kumar Ahuja S, Schneider Y. Protective effects of long term antiplatelet and anticoagulant therapy in hospitalized patients with inflammatory bowel disease. World J Gastrointest Pharmacol Ther 2024; 15(6): 95532
- URL: https://www.wjgnet.com/2150-5349/full/v15/i6/95532.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v15.i6.95532
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract (GIT), comprised of ulcerative colitis (UC) and Crohn’s disease (CD). CD can affect the entire GIT with transmural inflammation whereas UC is limited to mucosal layers of the rectum and colon[1]. In a systematic analysis for global burden of disease study 2019, age standardized prevalence rate of IBD was reported 59.25 per 100000 population[2].
In a systematic review of studies reporting cerebrovascular accidents (CVA) and ischemic heart disease (IHD) incidents among patients with IBD, findings indicate a higher risk of CVA and IHD in this population[3]. Yarur et al[4], conducted a prospective longitudinal study revealing an increased incidence of coronary artery disease(CAD) events in IBD patients, despite having a lower prevalence of traditional risk factors for CAD. Similarly, a large Danish nationwide study con
Findings of a large database study by Nelson et al[8] revealed that patients with IBD were found to have higher odds of associated thromboembolic disorders compared to those without IBD[8]. Long term systemic anticoagulation is therefore oftentimes a part of the medication regimen in patients with IBD with history of venous thromboembolism (in addition to those with AF, mechanical heart valves, etc.). Limited data available on use of aspirin on patents hospitalized with IBD shows aspirin use was associated with lower risk of death, sepsis and shock in an analysis of Nationwide Inpatient Sample (NIS) database[9]. Similar studies on the outcomes of hospitalization among patients with IBD who are on antiplatelet agents vs anticoagulation vs combined antiplatelets and anticoagulation are lacking.
There is paucity of literature on the impact of long-term anticoagulation and/or antiplatelet use among patients hospitalized with IBD[9]. Therefore, we conducted this study to assess outcomes among patients on long term anticoagulation and/or antiplatelets agents hospitalized with IBD.
We conducted a retrospective cohort study with adult patients hospitalized with IBD in the United States from the year 2016 to 2019. Patient data was obtained from NIS database which is a part of databases developed for Healthcare-Cost and Utilization Project (HCUP). The NIS is largest publicly available all-payer inpatient healthcare database which estimates United States regional and national inpatient utilization, access, cost, quality and outcomes from all nonfederal acute care hospitals nationwide. NIS approximates a 20-percent stratified sample of all discharges from United States community hospitals, excluding rehabilitation and long-term acute care hospitals. The NIS contains anonymized clinical and resource-use information that is included in a typical discharge with safeguards to protect the privacy of patients, physicians and hospitals. Unweighted, NIS contains data from around 7 million hospital stays and around 35 million weighted estimates of hospitalizations nationwide. Clinical and non-clinical information obtained from NIS include International Classification of Diseases, ninth revision, clinical modification (ICD-10-CM)/procedure coding system diagnosis, procedure, and external causes of morbidity codes from 2016-2019, anonymized patient demographic characteristics (like age, sex, race, median household income for Zip code), hospital characteristics (like ownership), expected payment source, total charges, length of stay, severity and comorbidity measures, etc.[10,11].
The study was conducted in compliance with the set of ethical principles of Declaration of Helsinki. NIS database contained anonymized clinical and resource-use information with safeguards to protect the privacy of patients, physicians and hospitals. As patient data was de-identified, institutional review board approval and written consent was not required before proceeding with the study.
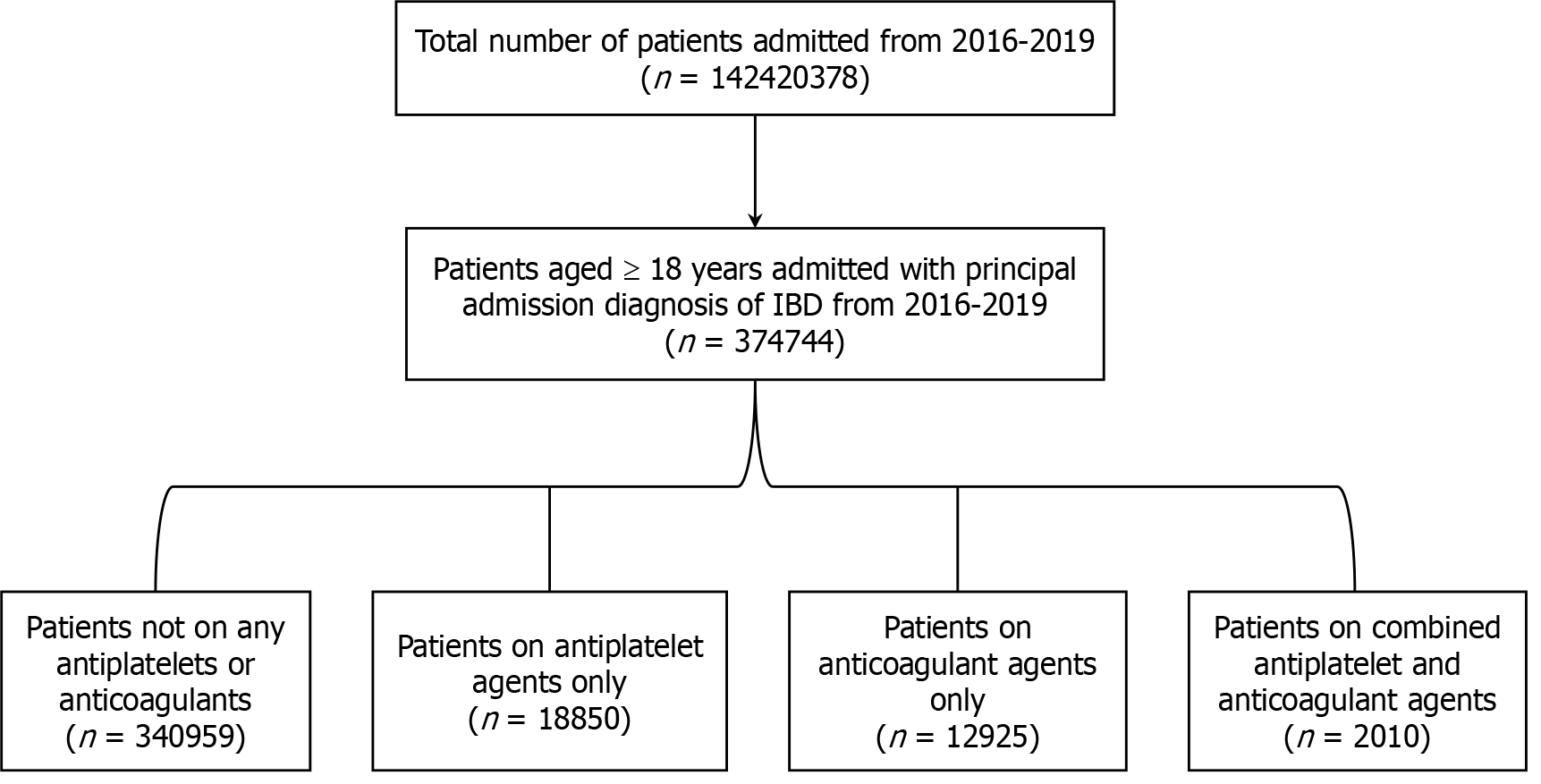
Patients aged 18 years or more years, admitted from 2016 to 2019 with principal admission diagnosis of IBD were identified using ICD-10-CM coding system from NIS database. Patients aged < 18 years were excluded from the study. ICD-10 code K50 and K51 was used to query the NIS database 2016-2019 to identify patients admitted with principal diagnosis of CD and UC respectively. Patients admitted with principal admission diagnosis of IBD (CD/UC) were then stratified into four groups: Patients not on any antiplatelet or anticoagulants, patients on long term antiplatelet agent only, patient on long term anticoagulant agents only and patients on both long term antiplatelet and anticoagulant agents. Patients taking long term anticoagulants/antiplatelets on daily outpatient basis were identified using appropriate ICD-10 codes. ICD-10 code Z7902 and Z7982 were used to identify patients on long term antiplatelet agents whereas ICD-10 code Z7901 was used to identify patients on long term anticoagulant agents[9,12,13]. Combination of ICD-10 codes for patients on long term antiplatelet agents and anticoagulant agents were used to query the patient subgroup on both antiplatelet and anticoagulant agents. A total of 374744 hospitalizations with principal admission diagnosis of IBD were included in the study. Flow diagram depicting the distribution of study participants is presented in Figure 1 below.
The study variables encompass patient demographic details, hospital characteristics, and the Charlson Comorbidity Index (CCI). Demographic information collected from the database included age, sex, race, insurance provider, and the median income of the patient’s zip code. Hospital characteristics considered in the study are the hospital's region, teaching status, bed size, and location. The patient's comorbidity status was categorized using the Deyo adaptation of the CCI, which is specifically designed for research that utilizes ICD diagnosis and procedure codes. The Deyo adaptation of the CCI comprises 19 categories of clinical comorbid conditions, each assigned a predetermined score. The CCI is used to predict a patient's 10-year mortality risk based on their CCI score[14].
The primary outcome of the study was mortality where secondary outcomes included length of stay, total parenteral nutrition (TPN), acute kidney injury (AKI), sepsis, shock, lower GI bleeding, non-variceal upper GI bleeding, colo
We used Stata® Version 18 software (StataCorp, Texas, United States) for data analysis and all analyses were conducted using the weighted samples for national estimates in adjunct with HCUP regulations for using the NIS database. HCUP rules and regulations were thoroughly followed in utilizing the NIS database. Hospitalization characteristics, including the mean age and the distribution of variables such as race, gender, hospital location, teaching status, and bed capacity, were gathered from the database. Continuous variables were presented as mean ± SD and analyzed using Student’s t-test, while categorical variables were reported as frequencies and percentages and evaluated using the Pearson chi-square method. To identify potential confounders, we conducted a comprehensive review of existing literature on IBD, antiplatelets, and anticoagulation, sought expert opinions from specialists in these fields, and carried out a univariate screening. All the variables with P value less than 0.2 in the univariate screening were included in multivariate regression model to adjust for confounders for calculation of primary and secondary outcomes. During multivariate logistic and linear regressions, outcomes were adjusted for age, race, Charlson comorbidity groups, hospital location, hospital region, hospital bed size, hospital teaching status, insurance status, quartile of household income, as well as additional comorbidities not included for in the Charlson index including AF, DVT, and Pulmonary Embolism. To compare the outcomes across different groups, Multivariate Linear regression analysis was used for continuous variables and Multivariate Logistic regression analysis was used for binary/dichotomous variables. All P-values <0.05 were considered statistically significant. The statistical methods of this study were reviewed by Suruchi Jai-Kumar-Ahuja from Depart
A total of 374744 patients hospitalized with principal diagnosis of IBD were included in the study which were further stratified into four groups based upon use of antiplatelet/anticoagulant agents. Figure 1 depicts the distribution of study population into those four groups. Although numerically small difference, patients not receiving drug therapy were more likely to be females (53.9%) compared to the other three groups. Patients on combination antiplatelet and anticoagulant agents were more likely to have Medicare (72.47%) and higher CCI (3 or more, 37.81%) than rest of the three groups. Numerically small but statistically significant differences were also noted among patient in four groups in terms of median income, hospital region, hospital teaching status, hospital bed size, race and hospital location. Table 1 demon
| Variable | No drug therapy | Antiplatelet therapy | Anticoagulant therapy | Antiplatelet and anticoagulant therapy | P value |
| Female | 53.9 | 52.06 | 52.73 | 43.28 | < 0.001 |
| Male | 46.1 | 47.94 | 47.27 | 56.72 | < 0.001 |
| Mean age in years | 44.02 | 65.55 | 58.8 | 67.97 | < 0.001 |
| Insurance provider | < 0.001 | ||||
| Medicare | 23.61 | 63.1 | 53.73 | 72.47 | |
| Medicaid | 20.27 | 7.6 | 13.19 | 8.67 | |
| Private | 50.01 | 27.9 | 30.94 | 17.09 | |
| Uninsured | 6.11 | 1.41 | 02.14 | 0.77 | |
| Charlson comorbidity index | < 0.001 | ||||
| 0 | 69.24 | 29.97 | 41.47 | 17.16 | |
| 1 | 18.68 | 26.87 | 22.51 | 24.13 | |
| 2 | 6.36 | 18.62 | 15.82 | 20.9 | |
| 3 or more | 5.72 | 24.54 | 20.19 | 37.81 | |
| Median income in patients zip code | < 0.0178 | ||||
| $1-51999 | 25.8 | 25.15 | 23.8 | 22.65 | |
| $52000-65999 | 25.1 | 27.3 | 25.85 | 30.79 | |
| $66000-87999 | 25.73 | 25.18 | 26.59 | 23.92 | |
| $88000+ | 23.38 | 22.37 | 23.76 | 22.65 | |
| Hospital region | < 0.001 | ||||
| Northwest | 21.42 | 19.89 | 22.13 | 19.65 | |
| Midwest | 23.93 | 27.93 | 28.43 | 29.35 | |
| South | 38.18 | 36.47 | 32.84 | 37.56 | |
| West | 16.46 | 15.7 | 16.6 | 13.43 | |
| Hospital teaching status | 0.0001 | ||||
| Non-teaching | 26.15 | 29.81 | 26.27 | 25.37 | |
| Teaching | 73.85 | 70.19 | 73.73 | 74.63 | |
| Hospital bed size | 0.0224 | ||||
| Small | 19.27 | 21.22 | 19.3 | 22.39 | |
| Medium | 27.89 | 27.24 | 28.67 | 31.59 | |
| Large | 52.84 | 51.54 | 52.03 | 46.02 | |
| Race | < 0.001 | ||||
| White | 72.46 | 82.39 | 79.82 | 84.62 | |
| Black | 14.39 | 9.66 | 11.89 | 10.77 | |
| Hispanic | 08.42 | 4.33 | 5.23 | 2.82 | |
| Asian or Pacific Islander | 1.5 | 1.12 | 0.96 | 1.03 | |
| Native American | 0.39 | 0.52 | 0.2 | 0.26 | |
| Other | 2.85 | 1.99 | 1.91 | 0.51 | |
| Hospital location | < 0.001 | ||||
| Rural | 6.7 | 8.91 | 6.73 | 9.7 | |
| Urban | 93.3 | 91.09 | 93.27 | 90.3 | |
The incidence of in-hospital mortality was highest among patients using combined antiplatelet and anticoagulant agents (0.75%) followed by those using anticoagulant (0.62%) and antiplatelet agents alone (0.42%). After adjusting for patient and hospital level confounders on multivariate analysis, use of combined antiplatelet and anticoagulant agents was not found to be associated as an independent predictor of in-hospital mortality, however there was a trend toward a protective benefit [adjusted odds ratio (aOR): 0.23 with P value = 0.052]. However, use of either antiplatelet or anticoagulant agents was found to be associated with lower in-hospital mortality by 60% [aOR: 0.40 (0.23-0.70) with P value = 0.001] and 59% [aOR: 0.41 (0.22-0.75) with P value = 0.004] respectively compared to those on no drug therapy on multivariate analysis.
Hospital length of stay and total hospitalization charge were used as surrogate measures of healthcare utilization. The mean length of stay (5.87 days) and total hospitalization charges ($54912) were reported higher among the patients taking anticoagulant therapy compared to all other groups. The mean length of stay was lowest among control group of patients not receiving antiplatelet or antiplatelet agents (4.91 days) and mean total hospitalization charges were lowest among patients receiving antiplatelet agents ($45978). The incidence of AKI (21.14%), shock (4.48%), lower GI bleeding (6.72%), colonoscopy (33.33%) was higher among the group using both antiplatelet and anticoagulant agents. TPN use (3.33%) and abdominal surgery (9.13%) was found to highest among group using anticoagulant agents along. On multivariate analysis, use of either antiplatelet or anticoagulant agent alone or in combination was not found to be independent predictor for development of AKI, sepsis, shock, or use of TPN except use of anticoagulant alone which was associated with reduced odds of developing shock by 36 % [aOR: 0.64 (0.43-0.94) with P value = 0.025]. Use of anticoagulant agent alone or combined use of antiplatelet and anticoagulant agents was associated with higher odds of lower GI bleeding [aOR: 1.34 (1.09-1.65) with P value = 0.004 and aOR: 1.56 (1.03-1.65) with P value = 0.035 respectively] compared to those not on any drug therapy on multivariate analysis. However, odds of colonoscopy were higher among groups using antiplatelet agent alone [aOR: 1.16 (1.07-1.26) with P value = < 0.001] and those using combined antiplatelet and anticoagulant agents [aOR: 1.31 (1.04-1.66) with P value = 0.019] compared to those not on any drug therapy. The cohorts of patients on antiplatelet, anticoagulant and combined antiplatelet and anticoagulant were associated with higher like
| Outcomes | No drug therapy | Antiplatelet therapy | Anticoagulant therapy | Antiplatelet and anticoagulant therapy | P value |
| Mortality | 0.27 | 0.42 | 0.62 | 0.75 | 0.0016 |
| LOS (days) | 4.91 | 5.01 | 5.87 | 5.61 | < 0.001 |
| TPN | 2.23 | 1.59 | 3.33 | 2.74 | 0.0001 |
| AKI | 7.33 | 16.13 | 15.2 | 21.14 | < 0.001 |
| Sepsis | 2.0 | 1.27 | 1.47 | 1.24 | 0.0025 |
| Shock | 0.7 | 1.22 | 1.55 | 4.48 | < 0.001 |
| Colonoscopy | 23.37 | 27.8 | 25.96 | 33.33 | < 0.001 |
| Lower GI bleed | 3.04 | 3.95 | 5.34 | 6.72 | < 0.001 |
| Non variceal Upper GI bleed | 0.81 | 0.95 | 1.04 | 1.49 | 0.1905 |
| Abdominal surgery | 10.05 | 7.53 | 9.13 | 5.47 | < 0.001 |
| Total charge ($) | 46800 | 45978 | 54912 | 50569 | < 0.001 |
| Home or home health care | 92.84 | 88.17 | 86.69 | 82.84 | < 0.001 |
| Short term hospital | 1.55 | 1.03 | 1.70 | 1.53 | < 0.001 |
| SNF or other facility | 2.98 | 9.52 | 9.48 | 13.93 | < 0.001 |
| Outcome | Unadjusted OR/coefficient (95%CI) | P value | Adjusted OR/coefficient (95%CI) | P value |
| Mortality (OR) | ||||
| Antiplatelet | 1.55 (0.93-2.60) | 0.09 | 0.40 (0.23-0.70) | 0.001 |
| Anticoagulant | 2.27 (1.36-3.80) | 0.002 | 0.41 (0.22-0.75) | 0.004 |
| Antiplatelet and anticoagulant | 2.74 (0.87-8.67) | 0.085 | 0.23 (0.05-1.01) | 0.052 |
| LOS days (coefficient) | ||||
| Antiplatelet | 0.096 (-0.06-0.25) | 0.249 | -0.46 (-0.63 to -0.28) | < 0.001 |
| Anticoagulant | 0.96 (0.71-1.20) | 0.000 | -0.502 (-0.78 to -0.22) | < 0.001 |
| Antiplatelet and anticoagulant | 0.698 (0.06-1.32) | 0.030 | -0.95 (-1.62 to -0.27) | < 0.001 |
| AKI (OR) | ||||
| Antiplatelet | 2.43 (2.21-2.66) | < 0.001 | 0.95 (0.85-1.05) | 0.354 |
| Anticoagulant | 2.26 (2.02-2.53) | < 0.001 | 0.99 (0.86-1.14) | 0.942 |
| Antiplatelet and anticoagulant | 3.38 (2.66-4.31) | < 0.001 | 0.92 (0.69-1.22) | 0.586 |
| Sepsis (OR) | ||||
| Antiplatelet | 0.63 (0.47-0.84) | 0.002 | 0.78 (0.57-1.06) | 0.117 |
| Anticoagulant | 0.73 (0.52-1.00) | 0.057 | 0.77 (0.54-1.10) | 0.154 |
| Antiplatelet and anticoagulant | 0.62 (0.25-1.48) | 0.279 | 0.78 (0.32-1.94) | 0.586 |
| Shock (OR) | ||||
| Antiplatelet | 1.76 (1.29-2.39) | < 0.001 | 0.75 (0.53-1.05) | 0.103 |
| Anticoagulant | 2.24 (1.62-3.09) | < 0.001 | 0.64 (0.43-0.94) | 0.025 |
| Antiplatelet and anticoagulant | 6.68 (4.14-10.76) | < 0.001 | 1.52 (0.88-2.63) | 0.129 |
| TPN (OR) | ||||
| Antiplatelet | 0.7 (0.54-0.92) | 0.01 | 0.76 (0.57-1.01) | 0.064 |
| Anticoagulant | 1.5 (1.21-1.87) | < 0.001 | 1.07 (0.83-1.40) | 0.569 |
| Antiplatelet and anticoagulant | 1.23 (0.64-2.33) | 0.522 | 1.05 (0.53-2.077) | 0.877 |
| Lower GI bleed (OR) | ||||
| Antiplatelet | 1.31 (1.10-1.55) | 0.002 | 1.05 (0.87-1.26) | 0.578 |
| Anticoagulant | 1.79 (1.50-2.14) | < 0.001 | 1.34 (1.09-1.65) | 0.004 |
| Antiplatelet and anticoagulant | 2.29 (1.58-3.40) | < 0.001 | 1.56 (1.03-2.37) | 0.035 |
| Non-variceal upper GI bleed (OR) | ||||
| Antiplatelet | 1.18 (0.84-1.66) | 0.325 | 1.13 (0.78-1.65) | 0.505 |
| Anticoagulant | 1.29 (0.87-1.91) | 0.189 | 1.03 (0.64-1.64) | 0.891 |
| Antiplatelet and anticoagulant | 1.86 (0.82-4.18) | 0.132 | 1.47 (0.62-3.46) | 0.376 |
| Colonoscopy (OR) | ||||
| Antiplatelet | 1.26 (1.17-1.36) | < 0.001 | 1.16 (1.07-1.26) | < 0.001 |
| Anticoagulant | 1.14 (1.04-1.26) | 0.003 | 0.99 (0.90-1.26) | 0.95 |
| Antiplatelet and anticoagulant | 1.63 (1.32-2.03) | < 0.001 | 1.31 (1.04-1.66) | 0.019 |
| Abdominal surgery (OR) | ||||
| Antiplatelet | 0.73 (0.64-0.82) | < 0.001 | 0.836 (0.72-0.96) | 0.014 |
| Anticoagulant | 0.89 (0.78-1.02) | 0.123 | 0.879 (0.74-1.03) | 0.118 |
| Both antiplatelet and anticoagulant | 0.51 (0.33-0.79) | 0.03 | 0.623 (0.39-0.97) | 0.037 |
| Total charge ($) (coefficient) | ||||
| Antiplatelet | -822.2 (-2777-1132) | 0.41 | -4889 (-7126 to -2652) | < 0.001 |
| Anticoagulant | 8112 (5015-11208) | < 0.001 | -7094 (-10979 to -3209) | < 0.001 |
| Antiplatelet and anticoagulant | 3768 (-3105-10643) | 0.283 | -11546 (-19475 to -3617) | < 0.001 |
| Discharge to home or home health care (OR) | ||||
| Antiplatelet | 0.57 (0.51-0.63) | < 0.0001 | 1.23 (1.10-1.37) | < 0.001 |
| Anticoagulant | 0.50 (0.44-0.56) | < 0.0001 | 1.32 (1.14-1.15) | < 0.001 |
| Antiplatelet and anticoagulant | 0.37 (0.28-0.48) | < 0.0001 | 1.47 (1.07-2.01) | 0.015 |
| Discharge to short term hospital (OR) | ||||
| Antiplatelet | 0.66 (0.47-0.92) | 0.015 | 0.56 (0.39-0.81) | 0.02 |
| Anticoagulant | 1.10 (0.81-1.49) | 0.535 | 0.84 (0.59-1.12) | 0.362 |
| Antiplatelet and anticoagulant | 1.12 (0.53-2.38) | 0.756 | 0.76 (0.33-1.74) | 0.521 |
| Discharge to SNF or other facility (OR) | ||||
| Antiplatelet | 3.42 (0.05-3.08) | < 0.001 | 0.94 (0.83-1.07) | 0.403 |
| Anticoagulant | 3.40 (2.96-3.92) | < 0.001 | 0.80 (0.66-0.95) | 0.016 |
| Antiplatelet and anticoagulant | 5.26 (3.93-7.05) | < 0.001 | 0.78 (0.55-1.10) | 0.169 |
This retrospective cohort study of patients with IBD who were admitted to the hospital found to have significantly lower inpatient mortality associated with the use of either antiplatelet or anticoagulant agents alone on daily basis. Patients receiving daily combined antiplatelet and anticoagulant therapy on an outpatient basis had a trend towards benefit with regard to inpatient mortality, although this did not reach statistical significance. In addition, use of antiplatelet or anticoagulant agents alone or in combination was associated with significantly decreased burden of healthcare utilization as measured by length of stay and total hospitalization charge. Furthermore, use of antiplatelet or anticoagulant agents alone or in combination was not associated with more severe disease course measured in terms of development of AKI, sepsis, shock or use of TPN (rather use of anticoagulant alone was associated with lower odds of developing shock). Conversely, use of long-term anticoagulant agent alone or in combination with antiplatelet agents was associated with significant higher odds of GI bleeding. Also, use of antiplatelet or anticoagulant alone or in combination was found to be associated with higher likelihood of discharge to home or receive home health care compared to patients not on any drug therapy. Among all IBD patients, nearly one-fourth of the subgroups received care in non-teaching hospitals, while the rest were managed in teaching hospitals. Regarding insurance providers, most patients not receiving long-term therapy had private insurance, whereas those on long-term antiplatelet, anticoagulant, or both therapies were predominantly covered by Medicare.
Venous thromboembolism (VTE) is a common extraintestinal complication in hospitalized IBD patients particularly during active disease flares, with the risk significantly higher compared to the general population, especially in hospital settings[15,16]. Moderate to severe IBD is therefore considered a risk factor for the development of VTE and routine anticoagulant thromboprophylaxis is recommended during IBD hospitalization[15]. Additionally, risk of CVA and IHD is also higher among patients with IBD[3]. In a study reporting utilization of anticoagulant thromboprophylaxis among patients admitted with IBD (from Southern Health Network Database, Australia, 1998-2009), it was found that half of the hospitalized patients suffering venous thromboembolic episodes did not receive thromboprophylaxis. Subsequently, a significantly higher mortality and prolonged length of stay was reported among those patients with a significant proportion requiring ICU admission[17]. Although this study is done among patients with IBD who were on long term therapeutic anticoagulation, findings from this study underscore the importance of anticoagulation/antiplatelet therapy in IBD for reducing mortality and healthcare burden.
Several factors have been implicated behind the increased risk for VTE. Increased plasma levels of factor V, VII, VIII and fibrinogen as acute phase reactants in response to underlying chronic inflammation create a prothrombotic en
Studies have been conducted in an attempt to evaluate the risk of bleeding among patients with IBD on anticoagulation. Scharrer et al[23] in a multicentric cohort study evaluated the risk of major bleeding events among patients with IBD on therapeutic anticoagulation via telephone interview and chart review. Major bleeding was defined as fatal bleeding or symptomatic bleeding in critical area/organ or leading to more than equal to 2 gm/dL drop in hemoglobin or leading to transfusion of more than equal to 2 units of whole blood or red cells. The risk for major but non-fatal bleeding was found increased in patients with IBD on anticoagulation. However, majority of the patients had active disease with mucosal lesion on endoscopy at the time of major bleeding[23]. This is in contrast to a study by Lobo et al[24] where no difference in major bleeding were reported among patients with IBD who developed VTE during acute flare, during remission and patients without IBD. Therapeutic anticoagulation in IBD was therefore, considered as effective and safe in patients without IBD[24]. In our study, with a larger cohort of IBD patients, we observed higher odds of lower GI bleeding among patients on anticoagulant agent alone or on combined antiplatelet and anticoagulant therapy. However, use of antiplatelet or anticoagulant agents alone or in combination was not associated with more severe disease course despite higher odds of lower GI bleeding. There was no significant difference in association with regards to non-variceal upper GI bleeding. Given the diagnostic and therapeutic benefits of colonoscopy, it remains the preferred intervention for lower gastrointestinal bleeding in hospitalized patients[25]. Our study also found higher odds of colonoscopy among patients on antiplatelet therapy alone and those on combined antiplatelet and anticoagulant therapy, which further supports the likelihood of colonoscopic interventions in this subgroup.
Among hospitalized IBD population, patients with UC are more prone to develop life-threatening complications like colonic perforation, gastrointestinal hemorrhage, and toxic megacolon which requires immediate abdominal surgery[26]. We reported lower odds of requirement of emergent abdominal surgery among patients on antiplatelet agents alone and those on combined antiplatelet and anticoagulant agents. Lower odds of requirement of abdominal surgery translates to lower chances of post-surgical complications and subsequent morbidity among patients hospitalized with IBD. Our study demonstrated higher odds of routine discharge to home with or without home health care in all the three groups including antiplatelet, anticoagulant and combined antiplatelet and anticoagulant group compared to patients not receiving either of these drugs. Also, patients taking antiplatelet agents were associated with lower odds of transfer to short term hospital compared to patients not on it. There was a significantly lower association of transfer to skilled nursing facility or any other intermediate care facility among patients taking long term anticoagulant agents compared to those not taking it. These statistically significant discharge disposition results favor the association of beneficiary effects of these long-term agents on overall safety, reduced morbidity and healthcare burden among the patients admitted with IBD.
The study has some inherent limitations. We used the NIS database to obtain information on study population. NIS being an administrative database, has a potential for misclassification of disease using ICD-10 codes or missing codes. However, previous studies have used similar ICD-10 codes to obtain information regarding long term use of antiplatelet/anticoagulant agents used[9,12,13]. The ICD-10 codes were used to query for long term antiplatelets/anticoagulant agents use. However, information regarding indication for antiplatelets agents use i.e. primary vs secondary prevention, single vs dual antiplatelets agents use, compliance could not be obtained. Similarly, information regarding type of the anticoagulant or antiplatelets used, dosage of anticoagulant agents used, specific regimens of anticoagulants (low molecular weight heparin vs direct oral anticoagulants) their indications, duration of use, and compliance could not be obtained which can be potential limitations for this study. The severity of IBD at hospitalization, clinical details about outcomes like GI bleed (occult bleeding vs major bleeding vs fatal bleeding, etc.) could not be obtained due to the administrative nature of the NIS database which can be another limitation. Lastly, information about use of proton pump inhibitors, non-steroidal anti-inflammatory agents, objective laboratory parameters at hospitalization could not be obtained from the database which may influence the outcomes.
Despite the limitations of the study, this is one of the first population-based studies done using the largest publicly available national database to evaluate the outcomes of patients with IBD on long term anticoagulation/antiplatelet therapy. The findings of the study demonstrated the benefits in terms of mortality, length of stay and total charges of hospitalization. Although anticoagulation use alone or combined anticoagulation and antiplatelet agent use was associated with higher odds of lower GI bleeding, it was not associated with increased severity of disease course like development of AKI, sepsis, shock or use of TPN. The overall safety profile suggests a role for these agents in mitigating thromboembolic risks without exacerbating disease severity. Further research is needed to look at optimal treatment strategies and addressing limitations to guide clinical decision-making in this population.
| 1. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2254] [Article Influence: 132.6] [Reference Citation Analysis (10)] |
| 2. | Wang R, Li Z, Liu S, Zhang D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: a systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open. 2023;13:e065186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 350] [Reference Citation Analysis (35)] |
| 3. | Singh S, Singh H, Loftus EV Jr, Pardi DS. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12 382-93.e1:quiz e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 4. | Yarur AJ, Deshpande AR, Pechman DM, Tamariz L, Abreu MT, Sussman DA. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroenterol. 2011;106:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 5. | Rungoe C, Basit S, Ranthe MF, Wohlfahrt J, Langholz E, Jess T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: a nationwide Danish cohort study. Gut. 2013;62:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | Kristensen SL, Lindhardsen J, Ahlehoff O, Erichsen R, Lamberts M, Khalid U, Torp-Pedersen C, Nielsen OH, Gislason GH, Hansen PR. Increased risk of atrial fibrillation and stroke during active stages of inflammatory bowel disease: a nationwide study. Europace. 2014;16:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Kumbhani DJ, Cannon CP, Beavers CJ, Bhatt DL, Cuker A, Gluckman TJ, Marine JE, Mehran R, Messe SR, Patel NS, Peterson BE, Rosenfield K, Spinler SA, Thourani VH. 2020 ACC Expert Consensus Decision Pathway for Anticoagulant and Antiplatelet Therapy in Patients With Atrial Fibrillation or Venous Thromboembolism Undergoing Percutaneous Coronary Intervention or With Atherosclerotic Cardiovascular Disease: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77:629-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 8. | Nelson AD, Fluxá D, Caldera F, Farraye FA, Hashash JG, Kröner PT. Thromboembolic Events in Hospitalized Patients with Inflammatory Bowel Disease. Dig Dis Sci. 2023;68:2597-2603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (34)] |
| 9. | Iqbal H, Arora GS, Singh I, Kohli I, Chaudhry H, Sohal A, Prajapati D. The impact of aspirin use on outcomes in patients with inflammatory bowel disease: Insights from a national database. Int J Colorectal Dis. 2023;39:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (33)] |
| 10. | HCUP-US NIS. Overview. Available from: https://hcup-us.ahrq.gov/nisoverview.jsp. |
| 11. | Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP). Available from: https://www.ahrq.gov/data/hcup/index.html. |
| 12. | Huang YM, Shih HJ, Chen YC, Hsieh TY, Ou CW, Su PH, Chen SM, Zheng YC, Hsu LS. Systemic Anticoagulation and Inpatient Outcomes of Pancreatic Cancer: Real-World Evidence from U.S. Nationwide Inpatient Sample. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (34)] |
| 13. | Kröner PT, Wallace MB, Raimondo M, Antwi SO, Ma Y, Li Z, Ji B, Bi Y. Systemic anticoagulation is associated with decreased mortality and morbidity in acute pancreatitis. Pancreatology. 2021;21:1428-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (29)] |
| 14. | Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7532] [Cited by in RCA: 8778] [Article Influence: 258.2] [Reference Citation Analysis (0)] |
| 15. | Nguyen GC, Bernstein CN, Bitton A, Chan AK, Griffiths AM, Leontiadis GI, Geerts W, Bressler B, Butzner JD, Carrier M, Chande N, Marshall JK, Williams C, Kearon C. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146:835-848.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 16. | Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375:657-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 572] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 17. | Dwyer JP, Javed A, Hair CS, Moore GT. Venous thromboembolism and underutilisation of anticoagulant thromboprophylaxis in hospitalised patients with inflammatory bowel disease. Intern Med J. 2014;44:779-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am J Gastroenterol. 2007;102:174-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 288] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 19. | Lentz SR. Thrombosis in the setting of obesity or inflammatory bowel disease. Hematology Am Soc Hematol Educ Program. 2016;2016:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Senchenkova E, Seifert H, Granger DN. Hypercoagulability and Platelet Abnormalities in Inflammatory Bowel Disease. Semin Thromb Hemost. 2015;41:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 21. | Kuenzig ME, Bitton A, Carroll MW, Kaplan GG, Otley AR, Singh H, Nguyen GC, Griffiths AM, Stukel TA, Targownik LE, Jones JL, Murthy SK, McCurdy JD, Bernstein CN, Lix LM, Peña-Sánchez JN, Mack DR, Jacobson K, El-Matary W, Dummer TJB, Fung SG, Spruin S, Nugent Z, Tanyingoh D, Cui Y, Filliter C, Coward S, Siddiq S, Benchimol EI. Inflammatory Bowel Disease Increases the Risk of Venous Thromboembolism in Children: A Population-Based Matched Cohort Study. J Crohns Colitis. 2021;15:2031-2040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (33)] |
| 22. | Dhaliwal G, Patrone MV, Bickston SJ. Venous Thromboembolism in Patients with Inflammatory Bowel Disease. J Clin Med. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (34)] |
| 23. | Scharrer S, Primas C, Eichinger S, Tonko S, Kutschera M, Koch R, Blesl A, Reinisch W, Mayer A, Haas T, Feichtenschlager T, Fuchssteiner H, Steiner P, Ludwiczek O, Platzer R, Miehsler W, Tillinger W, Apostol S, Schmid A, Schweiger K, Vogelsang H, Dejaco C, Herkner H, Novacek G; Austrian IBD Study Group. Inflammatory Bowel Disease and Risk of Major Bleeding During Anticoagulation for Venous Thromboembolism. Inflamm Bowel Dis. 2021;27:1773-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (33)] |
| 24. | Lobo JL, Garcia-Fuertes JA, Trujillo-Santos J, Merah A, Blanco-Molina MÁ, Casado I, Hirmerova J, De Miguel J, Salgado E, Monreal M; RIETE Investigators. Anticoagulant therapy for venous thromboembolism in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2018;30:526-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Sengupta N, Feuerstein JD, Jairath V, Shergill AK, Strate LL, Wong RJ, Wan D. Management of Patients With Acute Lower Gastrointestinal Bleeding: An Updated ACG Guideline. Am J Gastroenterol. 2023;118:208-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 111] [Article Influence: 37.0] [Reference Citation Analysis (33)] |
| 26. | Andersson P, Söderholm JD. Surgery in ulcerative colitis: indication and timing. Dig Dis. 2009;27:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/