Published online Sep 5, 2024. doi: 10.4292/wjgpt.v15.i5.97350
Revised: July 28, 2024
Accepted: July 31, 2024
Published online: September 5, 2024
Processing time: 96 Days and 24 Hours
Pain is the predominant symptom troubling patients. Pain management is one of the most important aspects in the management of surgical patients leading to early recovery from surgical procedures or in patients with chronic diseases or malignancy. Various groups of drugs are used for dealing with this; however, they have their own implications in the form of adverse effects and dependence. In this article, we review the concerns of different pain-relieving medicines used postoperatively in gastrointestinal surgery and for malignant and chronic diseases.
Core Tip: Pain is the most common symptom encountered by patients and their physicians. In the present era, there has been a change in the understanding of pain, its causes, assessment and management. Patient education and preoperative intervention are an integral part of pain management. Post-operative pain management is also an integral part of the enhanced recovery after surgery protocols in today's era. Management of pain can be pharmacological or non-pharmacological.
- Citation: Shukla A, Chaudhary R, Nayyar N, Gupta B. Drugs used for pain management in gastrointestinal surgery and their implications. World J Gastrointest Pharmacol Ther 2024; 15(5): 97350
- URL: https://www.wjgnet.com/2150-5349/full/v15/i5/97350.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v15.i5.97350
Pain is the most common symptom with which patients usually present to the emergency room[1]. Previously, it was thought to be a subjective term that could only be quantified by the patient experiencing the pain. As the 20th century advanced, there has been a change in the understanding of pain, its causes, assessment and management. The International Association for the study of pain defines it as,” An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage”[2]. Acute pain that was once thought to be of short duration is a more complex and unpleasant experience having cognitive, emotional and sensory response to tissue trauma caused after surgery[3], which is reported by nearly 80% people in the post-operative period. Most of these patients report moderate pain, and severe or extreme pain is reported by about 20%-40% patients[4,5]. There was a time when the surgeons were afraid to conduct surgeries because of the pain it caused patients, making them apprehensive, so much so that patients feared the scalpels and avoided the dreadful yet sometimes life-saving surgeries.
As modern scientific knowledge brought improvement in the surgical techniques, pain management also became an area of interest. For thousands of years hashish, mandrake, opium and alcohol have been used to produce analgesia during surgical procedures. In a failed attempt in 1845, Horace Wells brought to the limelight the use of Nitrous Oxide as an anaesthetic agent. William TH Warren successfully removed a soft tissue tumour without pain from a young man’s neck in 1846, making use of Ether. Soon, chloroform and ether were being used worldwide for painless surgeries. William Halsted made use of regional anaesthesia to produce field blocks for painless surgery, which was followed by the development of spinal and epidural anaesthesia by 1920. Sodium Thiopental was used to produce a similar pain-free experience by 1934. The progress in pain management became more and more significant in the coming times, making surgery a painless and pleasant experience to the patients.
Despite the advances in this field and numerous studies showing the superiority of one approach over the other, pain management in surgical patients is still not absolute, but evolving[6,7]. Acute pain must subside once the obnoxious stimulus is stopped and healing occurs, but poorly managed pain can lead to loss of function, poor mobility & recovery, increased risk of post operative complications and chronicity. Evidence based studies have led to formulation of guidelines that have been periodically updated; the guidelines include preoperative planning and patient education and perioperative pain management using pharmacological and nonpharmacological methods, while emphasizing that pain management is a complex process with significant implications over the patient’s quality of life[8]. Despite all the advancements in this field, the incidence of severe acute postoperative pain has remained unchanged over the last 30 years, at about 20%[9]. Opioid use for pain management has led to an opioid abuse epidemic such as chronic pancreatitis, malignancy etc, thus making it even more important to make use of alternative multiple modalities in pain management. This can include non-opioid medications, neuraxial analgesic techniques, and intravenous lignocaine. Besides this, minimally invasive techniques are supposed to cause less pain compared to traditional open surgery. But the studies have not produced any consistent results favouring a particular approach[10]. The World Health Organisation has developed an analgesic step ladder where non-opioid plus optional adjuvant is used for mild pain; weak opioid plus non-opioid and adjuvant analgesic is used for mild to moderate pain; a strong opioid plus non-opioid and adjuvant analgesic is used for moderate to severe pain. It advises to move one step up when pain is intense.
The authors performed an online search on PubMed, Google Scholar and Cochrane Database for relevant articles. Further, the articles’ reference lists were also searched for additional appropriate studies. The keywords used for searching were “post operative pain”; “pain management”; “Analgesia”; “acute pain”; “pain score”; “pain after GI surgery”; “spinal anaesthesia”; “epidural anaesthesia”; “intra venous anaesthesia”; “regional anaesthesia”. The search was limited to publications in English. All authors agreed that the articles selected for the minireview were relevant.
The central nervous system receives the information about tissue damage after the nociceptors are activated due to trauma[11]. The classical mechanism of pain involves conversion of the energy from noxious stimulus into sensory receptors called signal transduction[11]. These signals are then transmitted to the spinal cord and brain where these signals are perceived as pain[12]. This results in modulation of the nociceptive response at the spinal cord level through the inhibitory or facilitatory response from the brain[13]. The neurotransmitters released, such as enkephalins and endorphins, inhibit the release of neurotransmitters involved in pain transmission. The pharmacological agents act at these different steps to produce the analgesic effects[14]. Pain management should begin before surgery with a thorough assessment of the patients, allowing the optimal pain management techniques to be used and to help to alleviate the patient’s anxiety and fears about post-operative pain, as some of them might be using opioids preoperatively for complex pain syndromes[15].
Thorough patient education and perioperative intervention (Tables 1 and 2) allows advanced planning, especially for patients with comorbidities, which could subject the patients to significant side effects of the drugs used[16]. It allays the fear of post-operative pain in the patient and allows healthcare professionals to predict the types of patients who are about to have significant pain problems after surgery. Young females who smoke and have anxiety or depression and people already using opioids before surgery are particularly at risk of having significant post-operative pain[17]. Major emergency abdominal surgeries are also a risk factor for significant post-operative pain[18]. Studies have demonstrated that all of these factors are associated with persistent post-operative pain[19].
| Establish good relationship with patients’ relatives |
| Take pain history |
| Teach patient about pain assessment and management plan |
| Inspect concerns and handle misinterpretations regarding pain medications, adverse effects and dependence |
| Create a plan for postoperative analgesia in alliance with patient depending on type of surgery, anticipated severity of postoperative pain, co morbidities, the risk-benefit and expense of techniques, patient’s preferences |
| Patient selected appropriate pain assessment tool (e.g., Numeric Rating Scale, Visual Analog Scale) |
| Mention the patient’s selected pain assessment tool |
| Teach patient and relatives about their responsibilities |
| Examine multiple indicators of pain, including (1) Patient perceptions; (2) Cognitive attempts to address pain; (3) Behavioural responses (e.g., reduced mobility, sleeplessness, anxiety, depression); and (4) Physiological changes (vital signs: Tachycardia, hypertension) |
| Accept patients self-report, and only replace behaviour and/or physiological changes when he is unable to communicate |
| Assess pain at rest and during activity (e.g., moving, coughing) |
| Measure pain frequently during the early post operative period: At regular intervals, consistent with type of surgery and severity |
| Document pain intensity and its response to any interventions and adverse effects |
| Promptly assess instances of sudden intense pain |
| Think of reasons or any disparities between patients’ self-report of pain and behaviour. As patient may be denying pain due to casualness or worry of inadequate pain relief |
| Special attention to special populations, and be aware of hurdles of effective communication (e.g., language issues, mental, cognitive or hearing impairments, etc) |
| Revisit the management plan, if needed |
| Before discharging patient, review interventions implied and their efficiency; give specific and detailed discharge instructions for pain management at home |
Underassessment of the pain has been found to be the leading cause of undertreatment of pain. Therefore, the American Pain Society has included, “Pain as the 5th vital sign”[20,21]. Thus, it is as important to assess pain as the other four vital signs. Pain has also been included as the 5th vital sign in the National Pain Management Strategy by the Veterans Health Administration[22]. Various one-dimensional and multidimensional tools for pain assessment have been developed to be used in different situations. One-dimensional tools like Numeric Rating Scale, Visual Analog Scale and Categorical Scales using simple visual or verbal descriptors of pain are good for assessing the acute pain of fixed origins like post-operative pain[23]. Multidimensional tools like Initial Pain Assessment tool, Brief Pain Inventory[24] and McGill Pain Questionnaire are important tools for assessing more complex and chronic pain[25]. Clinically Aligned Pain Assessment includes comfort, change in pain, pain control, functioning and sleep, and thus can be used in the perioperative period. Pain assessment in mentally disabled people, people suffering from dementia and those unable to verbalize can be assessed with Pain in Advanced Dementia, Dolopus-2, Critical care Pain Observation Tool and Behavioural Pain Scale[26-28]. Patients should be continuously monitored for any pain worse than mild, which needs priority treatment.
Post-operative pain management is an integral part of the enhanced recovery after surgery (ERAS) protocols. Traditionally for abdominal surgery, Epidural Analgesia (EA) or Intravenous Patient-Controlled Analgesia (IVPCA) based on opioids has been used. It has good pain control but has a significant drug associated morbidity, which hampers the achievement of the goal of early Drinking, Eating and Moving (DrEaMing)[29]. There is no single drug with the ideal pain management properties, hence a multimodal approach towards reaching a perfect analgesia is favoured. It involves the use of various drugs acting at different levels of the pain pathway to achieve better control[30]. As in evidence-based PROcedure-SPECific Pain ManagemenT (PROSPECT) guidelines, these different drugs can be used to effectively lower the total analgesic dose and the associated side effects[31].
Pain treatment can be pharmacological or nonpharmacological in the multimodal treatment strategy[32]. Commonly used pharmacological agents are as follows:
Non-steroidal anti-inflammatory drugs (NSAIDs), including aspirin and other salicylic acid derivatives, work by inhibiting prostaglandin production responsible for pain and inflammation[33]. They could be selective cyclo-oxygenase-2 inhibitors like celecoxib or, nonspecific cyclooxygenase inhibitors like aspirin, Ibuprofen and Naproxen[34-36]. The major concerns are renal toxicity and gastritis. Paracetamol is another NSAID used for mild to moderate pain management. It has a significant opioid sparing effect in multimodal analgesia approach. When used intravenously for pain prophylaxis, it lowers the incidence of pain-associated nausea and vomiting. It has been proven safe at the therapeutic dosage in different studies[37,38]. A major concern is hepatotoxicity at higher dosages[39].
Mu opioid agonists (morphine like agonists) and agonist-antagonist opioids are the cornerstone of treating moderate to severe pain. They can be further classified as natural, synthetic or semi-synthetic opioids. Many people report opioid-related adverse effects (ORADE) in the immediate post-operative period, like dizziness, vomiting, nausea, constipation, dry mouth, dependence, pruritus, etc. Development of ORADE leads to a prolonged hospital stay. When drugs are prescribed beyond the recommended post-operative period, they can be misused and sold to other people[40]. These patients need continuous monitoring as they are liable to develop respiratory depression, drug tolerance, drug dependence and addiction when used over a long period. Patients who have a preoperative history of prolonged pain, use of benzodiazepines, anxious personality and history of drug addiction are more liable to develop drug dependence and addiction[41]. Such types of patients are liable to have withdrawal effects in the post-operative period so they should be maintained on minimal opioid dosage and supplemented with other types of analgesics and use of regional anaesthesia techniques[42].
Adjuvant analgesics or co-analgesics include a wide variety of drugs mainly used for purposes other than pain relief, but with some analgesic properties. Commonly used ones are gabapantenoids, magnesium, lignocaine IV, Ketamine, antidepressants like Selective Serotonin Reuptake Inhibitors and anti-epileptic drugs[32].
Ketamine is a dissociative anaesthetic, which when used for acute pain relief in perioperative settings may reduce morphine consumption and pain intensity[43]. It prevents the development of persistent post-surgical pain in patients. Although it is not a part of the ERAS protocols, it may reduce morphine consumption when used in multimodal analgesia[44]. Adverse effects include amnesia, psychosis, hypertension, depression, impaired coordination and judgement, depression, respiratory complications, etc.
Gabapantenoids act on the ascending as well as descending pathway of pain perception by decreasing nociception[45]. They have been found to be effective in post-operative pain management and have a morphine sparing effect in multimodal analgesia[43,46]. They prevent the development of persistent post-surgical pain but they have an abuse potential and can lead to addiction and death. Various other side effects include ataxia, angioedema, suicidal tendency, viral infections, nystagmus, constipation, weight gain, etc. They should be used with caution in patients with previous history of drug abuse or addiction[47].
Drugs like Clonidine and Dexmedetomidine can be used to decrease opiate use in the perioperative period either orally, intravenously, intrathecally, or as a transdermal patch[48]. When they are used for nerve blocks, they produce prolonged analgesic effect, but they are associated with hypotension and sedation. Therefore, patients require strict perioperative monitoring when these drugs are used[49].
Although the current ERAS guidelines included intravenous lignocaine infusion for post-operative pain relief in colorectal surgery, there has not been sufficient evidence to support this practice anymore[50]. Studies have found insufficient evidence that it helps in post-operative pain, ileus, nausea or vomiting[51].
Intravenous magnesium has been found to be useful in post-operative pain management and has a morphine sparing effect under multimodal post-operative analgesia protocols[52]. It has been demonstrated to prolong the effect of nerve blocks and spinal anaesthesia[53].
EA uses local anaesthetics along with adjuncts, such as morphine, buprenorphine, tramadol, fentanyl, hydroxymorphine, clonidine, dexmedetomidine or diamorphine. Drugs like clonidine and dexmedetomidine prolong the effect of the nerve block[54]. EA provides better analgesia after GI surgery with low incidence of ileus, pulmonary complications and analgesic requirements. It improves ileus and promotes food tolerance by reducing nausea and vomiting, thus helping the patient to achieve an early state of DrEaMing[55]. EA is associated with high failure rate as compared to IVPCA and has a high complication rates like hematoma formation, hypotension, permanent harm in about 17.4 per 100000 patients with death reported in about 6.1 per 100000 patients[56,57]. ERAS guidelines also support the use of EA in esophage
A process in which the local anaesthetic agent, sometimes mixed with adjuncts, is instilled into the subarachnoid space to produce anaesthetic/analgesic effect, which can last up to 24 hours. It has high efficacy and a low complication rate compared to EA with permanent damage in about 2.2 per 100000 and death in about 1.2 per 100000 patients[57]. It reduces the opioid consumption and has low pain scores in laparoscopic colorectal surgery[58]. It can lead to respiratory depression, hence will require strict monitoring. It has been included in the ERAS protocols for colorectal surgery[60].
They provide analgesia in abdominal surgery. Previously blind techniques were used but now ultrasonogram (USG) guidance has increased their popularity. They avoid the adverse effects of epidural and spinal analgesia like hypotension, motor block and the risk of neurological damage. USG should eliminate the complications, but studies have failed to demonstrate this[61]. They could be of particular importance when the neuraxial blockade is contraindicated like in sepsis, coagulopathy, preexisting neurological deficit or when the patients decide against it. Catheters for infusion can be used to prolong the blockade.
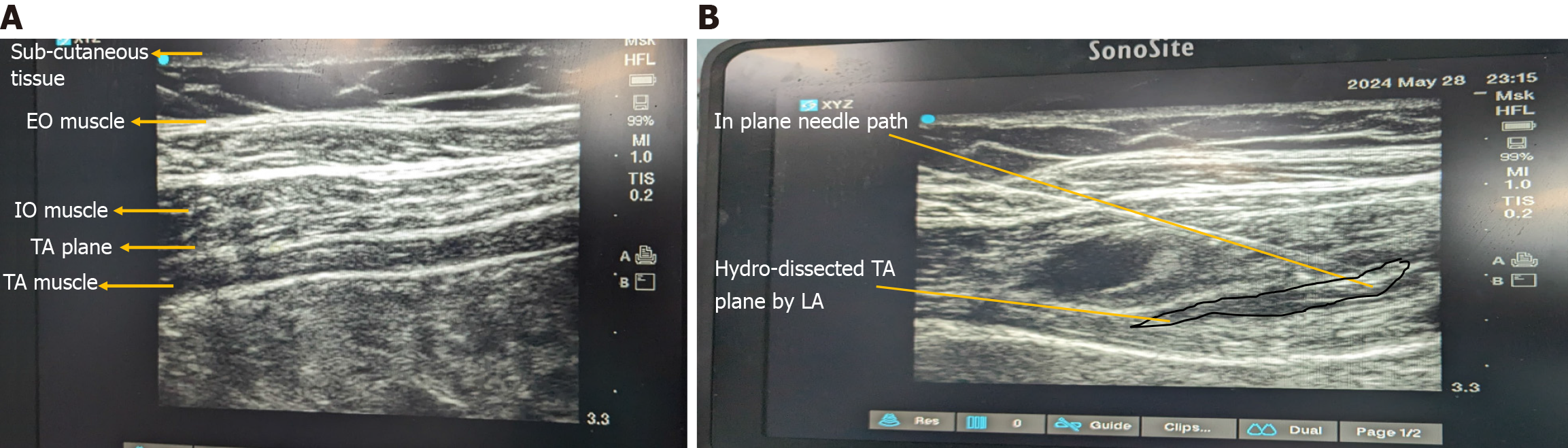
Transversus Abdominis Plane block (TAP) can be performed blindly but USG guidance increases the precision (Figure 1A and B). It can be used in a wide variety of surgeries including abdominal, urological, gynaecological, and obstetric surgeries. It has the opioid sparing effect in multimodal analgesic approach[62]. It blocks the T7-L1 nerves. The subcostal TAP block aims to block the lower thoracic nerves including T6-T9. A study has found the USG-guided posterior TAP block to be superior compared to the lateral TAP block in lower abdomen incisions[63].
Quadratus lumborum block: USG-guided quadratus lumborum block has been used in providing analgesia in midline laparotomy and laparoscopic procedures. It is a newer technique about which there is little evidence about efficacy in abdominal surgery[64].
Transversalis fascia plane: It blocks the lateral cutaneous branches of T12-L1, which are commonly missed by the TAP block. It has been successfully used in open appendectomy and inguinal hernia surgeries[65].
Erector Spinae Plane block: ESP is a new technique where the LA is injected around the tip of T5 transverse process level depositing the drug deep to the erector spinae muscle. It can have analgesic effect in laparotomies. Recently it has been reported to be used successfully in laparoscopic ventral hernia repair[66].
USG guided blocks are supposed to be safe, as the needle tip can be directly visualised while injecting the drug, but studies have found peritoneal breech while the needle tip was being visualised during the attempt to infiltrate around the nerve bundles. Thus, the needle should aim for the fascial planes rather than the nerve bundles. Systemic LA toxicity is a concern due to a large volume of the drug being used to infiltrate. The abdomen is well vascularised, so absorption is fast. Thus, less cardiotoxic alternatives should be used[67].
They have been used successfully for the management of chronic pain but recently they have been used in the acute postoperative period also. Cognitive behavioural therapy distraction techniques like music, aromatherapy, canine therapy and virtual reality have been used effectively in perioperative pain management. They decrease anxiety and help the patients in self-management[9,67]. These measures can be an area of future research for developing better methods of pain relief.
Pain is the main symptom troubling patients and its management is one of the most important aspect for better outcomes and early recovery after surgery. Numerous drugs and procedures are used for the purpose of managing pain. The World Health Organisation has advocated simple and valuable use of analgesic step ladder for pain management. However, despite advances in this field and various studies, pain management in surgical patients is still not absolute and is still evolving.
| 1. | Swieboda P, Filip R, Prystupa A, Drozd M. Assessment of pain: types, mechanism and treatment. Ann Agric Environ Med. 2013;Spec no. 1:2-7. [PubMed] |
| 2. | Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, Benoliel R, Cohen M, Cruccu G, Davis KD, Evers S, First M, Giamberardino MA, Hansson P, Kaasa S, Korwisi B, Kosek E, Lavand'homme P, Nicholas M, Nurmikko T, Perrot S, Raja SN, Rice ASC, Rowbotham MC, Schug S, Simpson DM, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ, Barke A, Rief W, Treede RD; Classification Committee of the Neuropathic Pain Special Interest Group (NeuPSIG). The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019;160:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 741] [Article Influence: 105.9] [Reference Citation Analysis (0)] |
| 3. | Ashburn MA, Ready LB. Postoperative pain. In: Loeser JD, Butler SH, Chapman CR, et al, eds. Bonica's Management of Pain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2001: 765-779. |
| 4. | Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1276] [Cited by in RCA: 1358] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 5. | Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118:934-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 1107] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 6. | Rutkow IM. Origins of Modern Surgery. Surgery. 2008;. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Collier R. A short history of pain management. CMAJ. 2018;190:E26-E27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, Carter T, Cassidy CL, Chittenden EH, Degenhardt E, Griffith S, Manworren R, McCarberg B, Montgomery R, Murphy J, Perkal MF, Suresh S, Sluka K, Strassels S, Thirlby R, Viscusi E, Walco GA, Warner L, Weisman SJ, Wu CL. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1346] [Cited by in RCA: 1922] [Article Influence: 192.2] [Reference Citation Analysis (0)] |
| 9. | Small C, Laycock H. Acute postoperative pain management. Br J Surg. 2020;107:e70-e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 10. | Pirie K, Traer E, Finniss D, Myles PS, Riedel B. Current approaches to acute postoperative pain management after major abdominal surgery: a narrative review and future directions. Br J Anaesth. 2022;129:378-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (1)] |
| 11. | Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010;120:3760-3772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 870] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 12. | Ahmad AH, Abdul Aziz CB. The brain in pain. Malays J Med Sci. 2014;21:46. |
| 13. | Hill RG. Molecular basis for the perception of pain. Neuroscientist. 2001;7:282-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Pasero C, Paice JA, McCaffery M. Basic mechanisms underlying the causes and effects of pain. In: McCaffery M, Pasero C, eds. Pain Clinical Manual. 2nd ed. St. Louis, MO: Mosby Inc; 1999: 15-34. |
| 15. | Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 592] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 16. | Sultana A, Torres D, Schumann R. Special indications for Opioid Free Anaesthesia and Analgesia, patient and procedure related: Including obesity, sleep apnoea, chronic obstructive pulmonary disease, complex regional pain syndromes, opioid addiction and cancer surgery. Best Pract Res Clin Anaesthesiol. 2017;31:547-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Tumber PS. Optimizing perioperative analgesia for the complex pain patient: medical and interventional strategies. Can J Anaesth. 2014;61:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 769] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 19. | Chapman CR, Vierck CJ. The Transition of Acute Postoperative Pain to Chronic Pain: An Integrative Overview of Research on Mechanisms. J Pain. 2017;18:359.e1-359.e38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 275] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 20. | Practice guidelines for acute pain management in the perioperative setting. A report by the American Society of Anesthesiologists Task Force on Pain Management, Acute Pain Section. Anesthesiology. 1995;82:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 145] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Jacox AK, Carr DB, Chapman CR, Ferrell B, Field HL, Heidrich G. Acute Pain Management: Operative or Medical Procedures and Trauma Clinical Practice Guideline No. 1. Rockville, MD: US Department of Health and Human Services, Agency for Health Care Policy and Research; 1992. AHCPR publication 92-0032. DIANE Publishing; 1997. |
| 22. | Kerns RD, Philip EJ, Lee AW, Rosenberger PH. Implementation of the veterans health administration national pain management strategy. Transl Behav Med. 2011;1:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, Kvarstein G, Stubhaug A. Assessment of pain. Br J Anaesth. 2008;101:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1019] [Cited by in RCA: 1346] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 24. | Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23:129-138. [PubMed] |
| 25. | Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2935] [Cited by in RCA: 3099] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 26. | Twining J, Padula C. Pilot Testing the Clinically Aligned Pain Assessment (CAPA) Measure. Pain Manag Nurs. 2019;20:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Schofield P. The Assessment of Pain in Older People: UK National Guidelines. Age Ageing. 2018;47:i1-i22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 28. | Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, Watson PL, Weinhouse GL, Nunnally ME, Rochwerg B, Balas MC, van den Boogaard M, Bosma KJ, Brummel NE, Chanques G, Denehy L, Drouot X, Fraser GL, Harris JE, Joffe AM, Kho ME, Kress JP, Lanphere JA, McKinley S, Neufeld KJ, Pisani MA, Payen JF, Pun BT, Puntillo KA, Riker RR, Robinson BRH, Shehabi Y, Szumita PM, Winkelman C, Centofanti JE, Price C, Nikayin S, Misak CJ, Flood PD, Kiedrowski K, Alhazzani W. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018;46:e825-e873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 2430] [Article Influence: 347.1] [Reference Citation Analysis (0)] |
| 29. | Levy N, Mills P, Mythen M. Is the pursuit of DREAMing (drinking, eating and mobilising) the ultimate goal of anaesthesia? Anaesthesia. 2016;71:1008-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Hughes MJ, Ventham NT, McNally S, Harrison E, Wigmore S. Analgesia after open abdominal surgery in the setting of enhanced recovery surgery: a systematic review and meta-analysis. JAMA Surg. 2014;149:1224-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 31. | Lee B, Schug SA, Joshi GP, Kehlet H; PROSPECT Working Group. Procedure-Specific Pain Management (PROSPECT) - An update. Best Pract Res Clin Anaesthesiol. 2018;32:101-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Alorfi NM, Ashour AM, Algarni AS, Alsolami FA, Alansari AM, Tobaiqy M. Assessment of the Community Pharmacists' Knowledge and Attitudes Toward Pain and Pain Management in Saudi Arabia. Int J Gen Med. 2022;15:8527-8537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 33. | Ghlichloo I, Gerriets V. Nonsteroidal Anti-inflammatory Drugs (NSAIDs). In: Treatment of Chronic Pain Conditions: A Comprehensive Handbook. Springer; 2023: 77-79. |
| 34. | Pountos I, Theodora Georgouli, Howard Bird, Giannoudis P. Nonsteroidal anti-inflammatory drugs: prostaglandins, indications, and side effects. IJICMR. 2011;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Green GA. Understanding NSAIDs: from aspirin to COX-2. Clin Cornerstone. 2001;3:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 191] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Labianca R, Sarzi-Puttini P, Zuccaro SM, Cherubino P, Vellucci R, Fornasari D. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin Drug Investig. 2012;32 Suppl 1:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 37. | Maund E, McDaid C, Rice S, Wright K, Jenkins B, Woolacott N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: a systematic review. Br J Anaesth. 2011;106:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 311] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 38. | Moore RA, Derry S, Aldington D, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2015;2015:CD008659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 39. | Dart RC, Bailey E. Does therapeutic use of acetaminophen cause acute liver failure? Pharmacotherapy. 2007;27:1219-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Shafi S, Collinsworth AW, Copeland LA, Ogola GO, Qiu T, Kouznetsova M, Liao IC, Mears N, Pham AT, Wan GJ, Masica AL. Association of Opioid-Related Adverse Drug Events With Clinical and Cost Outcomes Among Surgical Patients in a Large Integrated Health Care Delivery System. JAMA Surg. 2018;153:757-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 41. | Callinan CE, Neuman MD, Lacy KE, Gabison C, Ashburn MA. The Initiation of Chronic Opioids: A Survey of Chronic Pain Patients. J Pain. 2017;18:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 42. | Kumar K, Kirksey MA, Duong S, Wu CL. A Review of Opioid-Sparing Modalities in Perioperative Pain Management: Methods to Decrease Opioid Use Postoperatively. Anesth Analg. 2017;125:1749-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 229] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 43. | Brinck EC, Tiippana E, Heesen M, Bell RF, Straube S, Moore RA, Kontinen V. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst Rev. 2018;12:CD012033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 44. | Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev. 2013;2013:CD008307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 45. | Chincholkar M. Analgesic mechanisms of gabapentinoids and effects in experimental pain models: a narrative review. Br J Anaesth. 2018;120:1315-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 46. | Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg. 2012;115:428-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 270] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 47. | Mayor S. Pregabalin and gabapentin become controlled drugs to cut deaths from misuse. BMJ. 2018;363:k4364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Grape S, Kirkham KR, Frauenknecht J, Albrecht E. Intra-operative analgesia with remifentanil vs. dexmedetomidine: a systematic review and meta-analysis with trial sequential analysis. Anaesthesia. 2019;74:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 49. | McEvoy MD, Scott MJ, Gordon DB, Grant SA, Thacker JKM, Wu CL, Gan TJ, Mythen MG, Shaw AD, Miller TE; Perioperative Quality Initiative (POQI) I Workgroup. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on optimal analgesia within an enhanced recovery pathway for colorectal surgery: part 1-from the preoperative period to PACU. Perioper Med (Lond). 2017;6:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 50. | Weibel S, Jelting Y, Pace NL, Helf A, Eberhart LH, Hahnenkamp K, Hollmann MW, Poepping DM, Schnabel A, Kranke P. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev. 2018;6:CD009642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 51. | Kranke P, Jokinen J, Pace NL, Schnabel A, Hollmann MW, Hahnenkamp K, Eberhart LH, Poepping DM, Weibel S. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery. Cochrane Database Syst Rev. 2015;CD009642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 52. | Kumar M, Dayal N, Rautela RS, Sethi AK. Effect of intravenous magnesium sulphate on postoperative pain following spinal anesthesia. A randomized double blind controlled study. Middle East J Anaesthesiol. 2013;22:251-256. [PubMed] |
| 53. | Kahraman F, Eroglu A. The effect of intravenous magnesium sulfate infusion on sensory spinal block and postoperative pain score in abdominal hysterectomy. Biomed Res Int. 2014;2014:236024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Nimmo SM, Harrington LS. What is the role of epidural analgesia in abdominal surgery? BJA Education. 2014;14:224-229. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 55. | Emelife PI, Eng MR, Menard BL, Myers AS, Cornett EM, Urman RD, Kaye AD. Adjunct medications for peripheral and neuraxial anesthesia. Best Pract Res Clin Anaesthesiol. 2018;32:83-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 56. | Guay J, Nishimori M, Kopp S. Epidural local anaesthetics versus opioid-based analgesic regimens for postoperative gastrointestinal paralysis, vomiting and pain after abdominal surgery. Cochrane Database Syst Rev. 2016;7:CD001893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 57. | Cook TM, Counsell D, Wildsmith JA; Royal College of Anaesthetists Third National Audit Project. Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth. 2009;102:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 537] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 58. | Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, Rockall TA, Young-Fadok TM, Hill AG, Soop M, de Boer HD, Urman RD, Chang GJ, Fichera A, Kessler H, Grass F, Whang EE, Fawcett WJ, Carli F, Lobo DN, Rollins KE, Balfour A, Baldini G, Riedel B, Ljungqvist O. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS(®)) Society Recommendations: 2018. World J Surg. 2019;43:659-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1071] [Cited by in RCA: 1380] [Article Influence: 197.1] [Reference Citation Analysis (0)] |
| 59. | Low DE, Allum W, De Manzoni G, Ferri L, Immanuel A, Kuppusamy M, Law S, Lindblad M, Maynard N, Neal J, Pramesh CS, Scott M, Mark Smithers B, Addor V, Ljungqvist O. Guidelines for Perioperative Care in Esophagectomy: Enhanced Recovery After Surgery (ERAS(®)) Society Recommendations. World J Surg. 2019;43:299-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 413] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 60. | Wongyingsinn M, Baldini G, Stein B, Charlebois P, Liberman S, Carli F. Spinal analgesia for laparoscopic colonic resection using an enhanced recovery after surgery programme: better analgesia, but no benefits on postoperative recovery: a randomized controlled trial. Br J Anaesth. 2012;108:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 61. | Onwochei DN, Børglum J, Pawa A. Abdominal wall blocks for intra-abdominal surgery. BJA Educ. 2018;18:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Baeriswyl M, Kirkham KR, Kern C, Albrecht E. The Analgesic Efficacy of Ultrasound-Guided Transversus Abdominis Plane Block in Adult Patients: A Meta-Analysis. Anesth Analg. 2015;121:1640-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 63. | Abdallah FW, Laffey JG, Halpern SH, Brull R. Duration of analgesic effectiveness after the posterior and lateral transversus abdominis plane block techniques for transverse lower abdominal incisions: a meta-analysis. Br J Anaesth. 2013;111:721-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 64. | Chin KJ, McDonnell JG, Carvalho B, Sharkey A, Pawa A, Gadsden J. Essentials of Our Current Understanding: Abdominal Wall Blocks. Reg Anesth Pain Med. 2017;42:133-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 245] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 65. | Chin KJ, Chan V, Hebbard P, Tan JS, Harris M, Factor D. Ultrasound-guided transversalis fascia plane block provides analgesia for anterior iliac crest bone graft harvesting. Can J Anaesth. 2012;59:122-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Chin KJ, Adhikary S, Sarwani N, Forero M. The analgesic efficacy of pre-operative bilateral erector spinae plane (ESP) blocks in patients having ventral hernia repair. Anaesthesia. 2017;72:452-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 295] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 67. | Komann M, Weinmann C, Schwenkglenks M, Meissner W. Non-Pharmacological Methods and Post-Operative Pain Relief: An Observational Study. Anesth Pain Med. 2019;9:e84674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/