Published online Mar 5, 2022. doi: 10.4292/wjgpt.v13.i2.23
Peer-review started: August 2, 2021
First decision: October 15, 2021
Revised: October 31, 2021
Accepted: January 22, 2022
Article in press: January 22, 2022
Published online: March 5, 2022
Processing time: 211 Days and 8.4 Hours
Eosinophilia and related organ damage are extensively studied hot topics among rare disorders. Any addition to the cohort of available case reports of the same will be adding knowledge for better management of this less known entity.
In this article, we describe a 27-year-old Indo-Aryan man who presented with abdominal pain, abdominal distension, and loose stools for variable days. He had splinter hemorrhages in the majority of fingernails. He was diagnosed with predominant eosinophilic gastrointestinal involvement with bowel obstruction and ascites, and was managed with intravenous immunoglobulin. He was subsequently treated with oral low dose steroid therapy and responded completely.
Our experience is evidence that prompt management of this hypereosinophilic lethal gastrointestinal (all three layers) infiltrative disease provides a cure and avoids complications. Splinter nail hemorrhages may be seen in the same disease.
Core Tip: This case reveals the wide spectrum of atypical manifestations of hyper
- Citation: Bhasi A, Patnaik I, Panda PK, Singh A. Hypereosinophilic syndrome presenting as eosinophilic gastroenteritis disorder and splinter hemorrhages: A case report. World J Gastrointest Pharmacol Ther 2022; 13(2): 23-29
- URL: https://www.wjgnet.com/2150-5349/full/v13/i2/23.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v13.i2.23
Hypereosinophilic syndrome (HES) is an idiopathic, less defined, less known, primary leuco-proliferative disorder associated with peripheral blood eosinophilia and organ damage. The term HES was first used by Anderson[1] in 1968 in a case series, and since then, there have been considerable improvements in the understanding and hence changes in the definition and criteria for the diagnosis of this syndrome. The old definition (1975, Chusid et al[2]) included persistent blood eosinophilia ≥ 1.5 × 109/L lasting for > 6 mo, associated with eosinophil-mediated organ damage. However, the new definition (2010, Simon et al[3]) includes more than peripheral blood eosinophilia or duration of symptoms, and the evidence of eosinophil-mediated organ damage is enough for diagnosis of HES.
Presentations may vary, and can be organ-specific, may present as a myeloproliferative disorder, or may be asymptomatic. Treatment guidelines are not yet defined. Patients mostly present with organ damage in organ-specific eosinophilia syndrome and thus require an immediate intensive phase treatment to avoid organ failure and then a continuous phase of immunomodulator or steroids to avoid recurrences.
Eosinophilic gastroenteritis is a term reserved for an entity, where there is eosinophilic inflammation of more than one histologically distinct anatomic location in the gut[4]. Clinical presentation depends on the anatomical site and histological layer of the gut. The presentation can be as mild and chronic as dyspepsia, nausea, diarrhea, or as a differential for protein-losing enteropathy with mucosal layer involvement to as severe as crampy abdominal pain and intestinal obstruction with muscular layer involvement. The least common serosal type presents with eosinophilic ascites and abundant peripheral eosinophilia or rarely in severe cases as perforation[5].
Here we report a case of HES who presented with abdominal pain, distension, diarrhea, and splinter hemorrhages.
A 27-year-old Indo-Aryan man, resident of North-India, an electrician by profession, without addictions or co-morbidities presented with abdominal pain of 20 d, abdominal distension of 10 d, and loose stools of 3 d.
Abdominal pain was a continuous dull ache around the umbilicus. The pain became intermittent associated with the non-passage of flatus and feeling of fullness in the abdomen that was relieved with passage of flatus after some time. In the previous 10 d he had abdominal distension, which started around the left flank, and progressed to involve the whole abdomen. He had no vomiting, fever, jaundice, significant weight loss, urinary problems, chest problems, or lower limb swelling. Reports from outside our hospital showed eosinophilia (absolute count, 11400/μL).
He had no allergic tendency, pruritus ani, any recent intake of drugs, or B symptoms.
No history of atopy in his family and no history suggestive of the same.
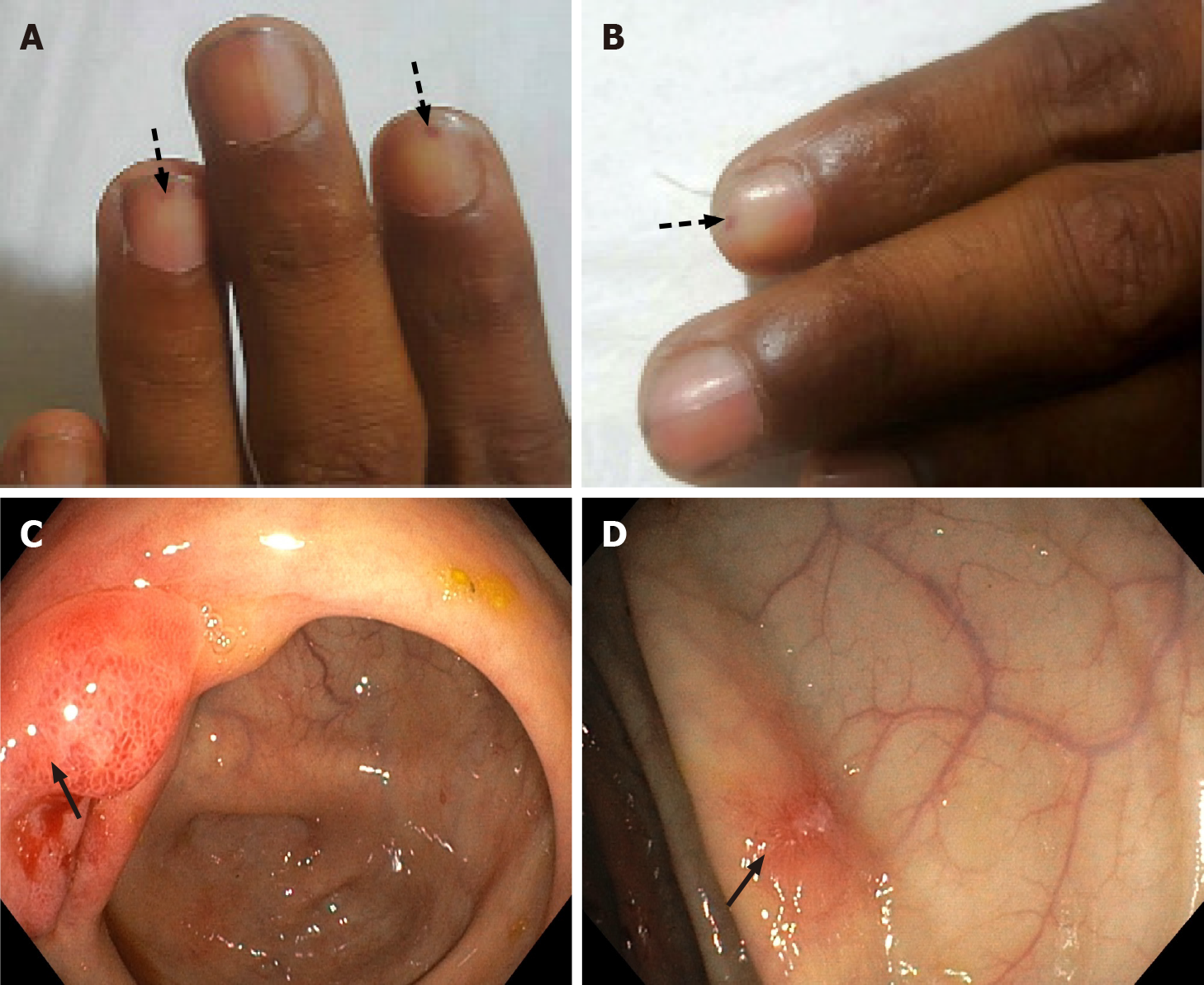
On examination, he was of average built (body mass index, 22 kg/m2) with stable vitals. His local examination revealed splinter hemorrhages in digital nails (Figure 1A and B); however, fundoscopy did not show any Roth spots. Abdominal examination showed a distended abdomen with shifting dullness and sluggish bowel sounds without organomegaly or any stigmata of chronic liver disease. His systemic examination was non-contributory.
His initial hemogram showed a leucocyte count of 33000/μL (absolute eosinophil count, 25000/μL) and normal hemoglobin and platelet count. Peripheral smear confirmed eosinophilic leukocytosis without any blasts or hemiparasites. Midnight diethyl carbamazine provocation test was negative (diethyl carbamazine single dose 300 mg at midnight to increase yield). His kidney and liver functions were essentially normal. Total IgE was 830 kUA/L (normal range, < 64 kUA/L). The ascitic fluid study revealed low SAAG ascites (ascitic albumin: 2.9 g/dL and SAAG: 0.7 g/dL) with raised leukocyte count (6000 cells/mm3) and numerous eosinophils (95% polymorphs and 5% monomorphs) and reactive mesothelial cells. Multiple stool microscopy samples were obtained, however no evidence of parasites was observed.
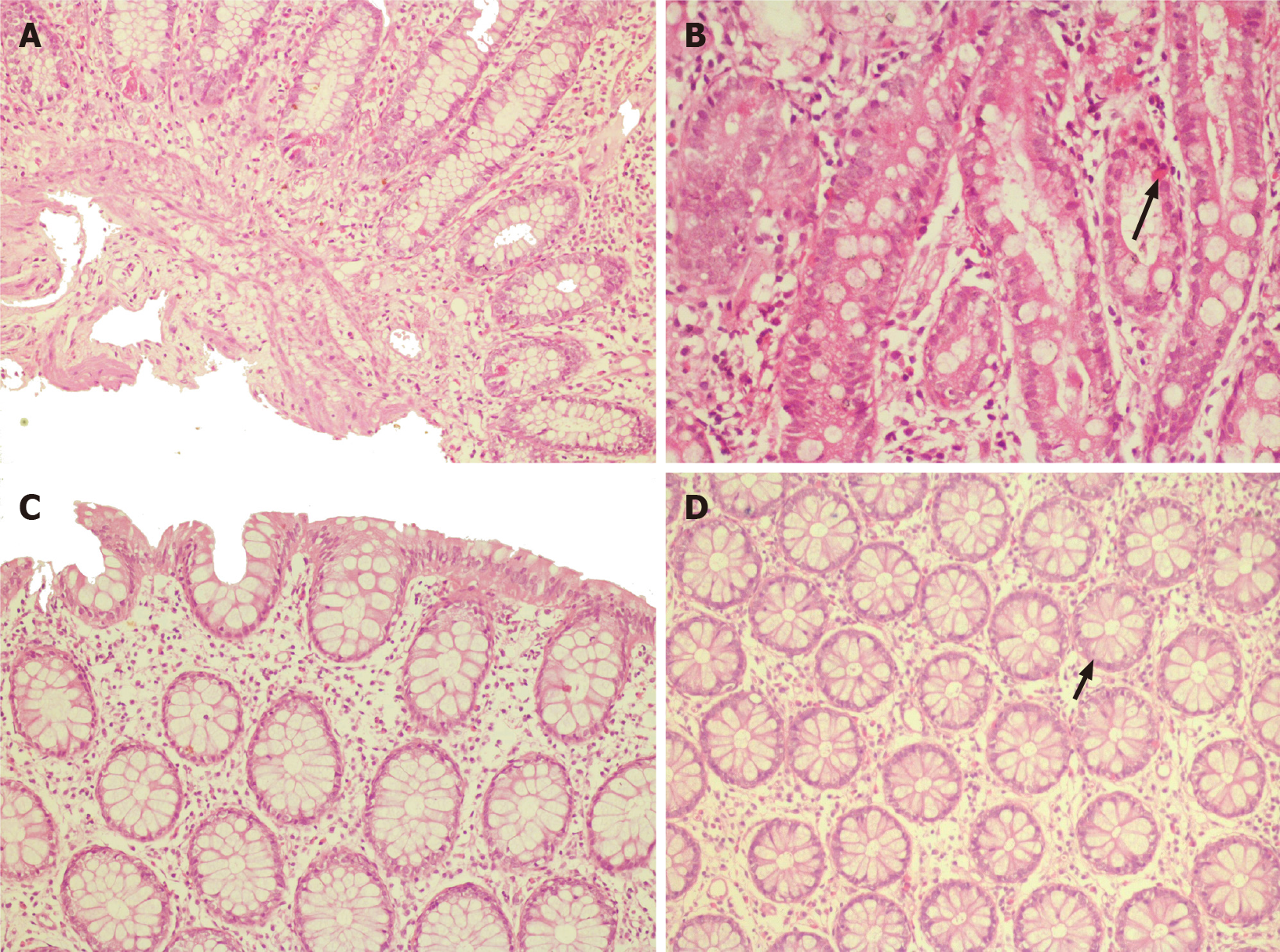
Ultrasonography of the abdomen showed ascites with multiple echogenic foci (septated) and few subcentimetric mesenteric lymph nodes. ECG and chest X-ray were normal. A contrast-enhanced computed tomography (CT) of the abdomen was performed to rule out any occult abdominal pathology, which revealed diffuse bowel wall thickening and submucosal edema. Both upper gastrointestinal (GI) endoscopy and colonoscopy were performed and showed erythematous mucosa (Figure 1C and D). The biopsy from multiple sites (antrum, duodenum, ileum, cecum, rectum) revealed eosinophilic infiltration in the lamina propria with dense infiltration of muscularis mucosa at the terminal ileum (Figure 2). His esophageal biopsies were essentially normal.
At initial evaluation, differentials were parasitic infiltrations with secondary eosinophilia vs hyper-eosinophilic gastroenteritis vs hyper IgE syndrome. However, after all the investigations, the patient was confirmed to have hypereosinophilic syndrome presenting as eosinophilic gastroenteritis disorder (EGD) with atypical splinter hemorrhages.
He was started on intravenous immunoglobulin (IVIG) at a total dose of 2 gm/kg divided over 5 d, after which he showed significant symptom improvement. He was started on a six food elimination diet (cow’s milk, soy, wheat, egg, peanut, seafood/shellfish). His abdominal girth had reduced from 96 cm to 81 cm at discharge. His eosinophilic count also reduced to 1200/μL.
He was discharged on oral prednisolone 30 mg/d and planned to taper off.
In his last follow-up after 2-mo, there was no evidence of ascites or splinter hemorrhages. He has resumed work with normal eosinophil counts on low-dose steroid (prednisolone 10 mg/d) and plans to stop the steroid after 6-mo with a wait and watch policy.
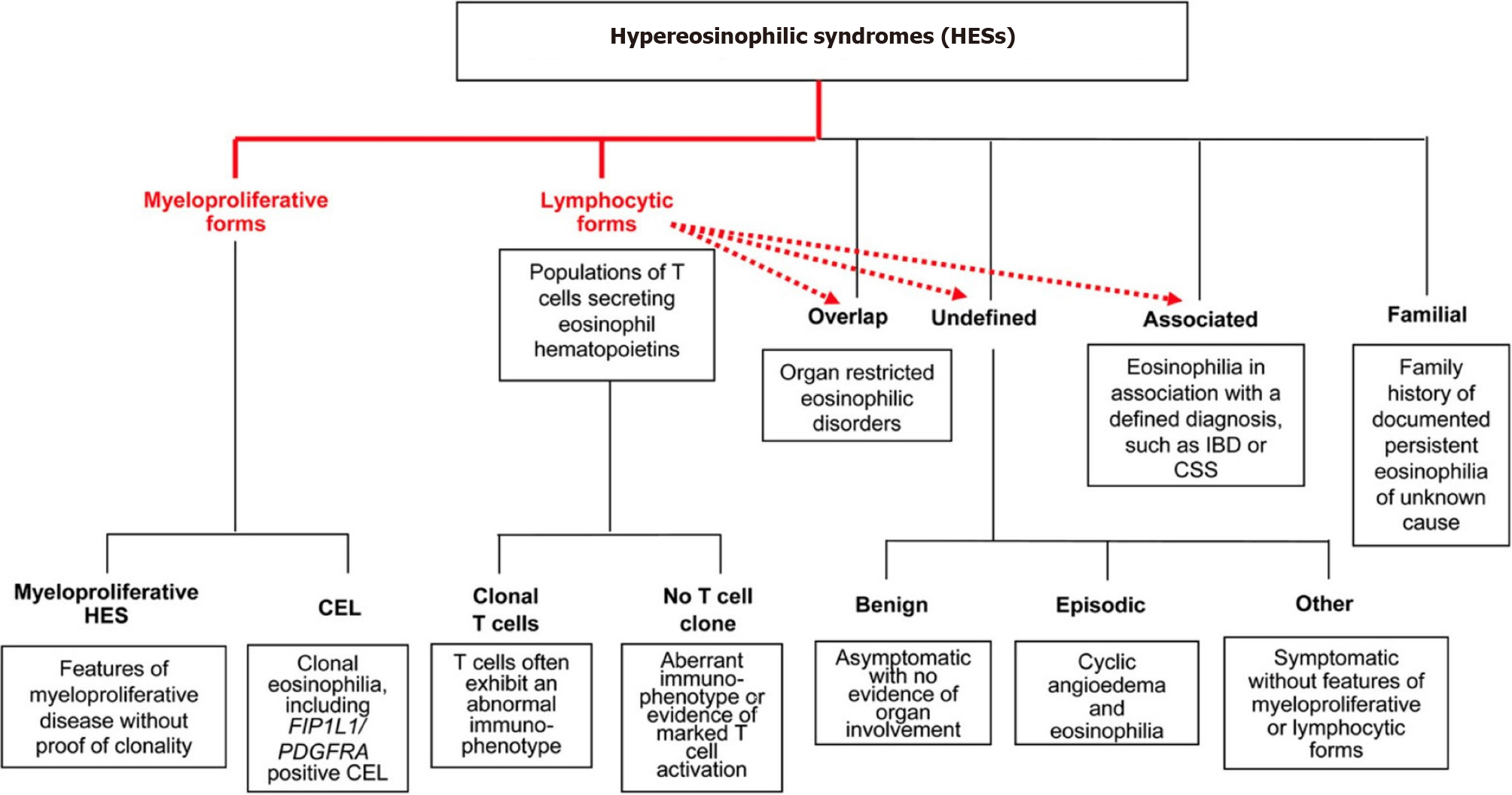
This particular case revealed two major findings concerning HES. One is EGD and the other is splinter hemorrhages. EGD is proposed to have a centrifugal eosinophilic infiltrate starting from the mucosa to serosa evidenced by the fact that the disease with serosal and muscular types always has mucosal involvement[6]. This case had symptoms suggestive of the involvement of all the three layers of the GI tract: Diarrhea and hypoalbuminemia suggestive of mucosal involvement, intestinal obstruction, and related symptoms suggestive of muscular involvement, and exudative ascites suggestive of serosal involvement. Such extensive symptomatology, whether reported in a single case or not is to our knowledge unknown. The classification or therapeutic guideline for HES is still limited as most review articles are based on case reports and case series. To date, no randomized studies have been carried out in the adult population regarding management. Simon et al[3] classified HES into multiple independent categories based on clinical manifestations and pathogenesis (Figure 3). Lymphoproliferative HES is associated with increased production of eosinophil hematopoietin (mainly IL-5) by activated T-lymphocytes[7]. Another feature associated with the same is increased levels of serum IgE, dermatologic manifestations, and gastrointestinal symptoms. Hence our case could be a type of this variety. Other types such as undefined and organ-specific variants are also associated with a similar pathogenesis, lymphocyte-mediated increased eosinophil hematopoietin production, and related eosinophilic infiltration and organ damage[8]. These variants are responsive to immunosuppressive therapy, both to steroids and steroid-sparing immunomodulatory agents.
Eosinophilic gastroenteritis was first reported in 1937; however, the pathogenesis and management are unknown. There are two main reasons for this apparent contradiction. Firstly, it presents just like any other GI disorder with heterogeneous and nonspecific symptoms and is thus under-diagnosed. Secondly, it is a less prevalent disease[4]. The pathogenesis of EGD is complex and multifactorial. A trigger factor such as a food allergen, helminth, or bacterial infection is often involved, leading to and IL-5 mediated eosinophilic recruitment to the GI tract. The eosinophil-mast cell axis is a known factor involved in functional GI disorder[4]. Activated mast cells secrete histamine and serotonin, which results in visceral sensitivity and motility disorder. The same axis is proposed to function in EGD. EGD can be associated with or without peripheral eosinophilia and diagnosis thus remains tricky. In the presence of ascites, a diagnostic tap showing exudative ascites with eosinophilia is suggestive of the disease[9]. Endoscopy guided biopsy is a useful test; however, patchy involvement of the GI tract means that diagnosis can be missed in 25% of cases[10]. In such cases, CT imaging is useful to identify thickened bowel areas where a surgical biopsy can be attempted[11]. Small bowel MRI, as well as CT scanning, are nonspecific in diagnosing eosinophilic gastroenteritis and use of imaging is to guide biopsies. Presently, no studies comparing the diagnostic accuracy of these procedures are available. Capsule endoscopy[12] may be used to detect eosinophilic gastroenteritis as it may provide clues for lesions in the small bowel which may be difficult to diagnose with conventional endoscopy; however, studies pertaining to the same are not available.
Subungual splinter hemorrhages are vertical hemorrhages occurring in the capillary loops of the nail. Their association with an eosinophilic syndrome was provided in a case report of eosinophilic endomyocarditis[13]. Their association with HES or EGD has not been reported to date. The pathogenesis is questionable as cutaneous manifestations of HES are related to dermal microthrombosis. Usual cutaneous manifestations are angioedema, erythematous pruritic papules and nodules, generalized erythroderma, and blistering skin lesions in the form of vesiculobullous lesions[14]. In infective endocarditis, splinter hemorrhages are thought to be due to the embolic phenomenon and subsequent hemorrhagic changes[15]. Thus, hemorrhagic changes on microthrombosis can be the reason for splinter hemorrhages in HES but further research and clarification are needed.
EGD is still a novel disease requiring extensive research for further knowledge expansion. Until then treatment is based primarily on clinician experience. Treatment strategies involve immunosuppression with corticosteroids, leukotriene receptor antagonists, mast cell stabilizers, antihistamines, or treatment with immunomodulators such as 6-mercaptopurine and azathioprine. Novel therapies include biologicals like Reslizumab (IL5 antagonist), Infliximab (TNF alpha inhibitor), Omalizumab (a monoclonal antibody against IgE), or IVIG[4]. Our patient presented with alarming symptoms of bowel obstruction and exudative ascites, hence we preferred to start him on IVIG therapy, to which he responded, potentially avoiding surgical intervention, and then started him on oral prednisolone therapy. Hence, we suggest the use of IVIG therapy in patients with alarming symptoms for rapid response. However, this was a single case report and hence requires blinded, prospective and randomized studies to determine the efficacy of IVIG for HES.
Our experience provides evidence that prompt management of this infiltrative disease offers a cure and avoids organ complications. Clinicians must be vigilant in order to diagnose primary instead of secondary eosinophilia as the former can cause diffuse organ infiltration and widespread tissue damage. EGD can present with the involvement of all three layers of the GI tract. HES can also present with splinter hemorrhages.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Allergy
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferrarese A S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH
| 1. | Anderson RE. The hypereosinophilic syndromes. Aun Intern Med. 1968;68:1220. |
| 2. | Chusid MJ, Dale DC, West BC, Wolff SM. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine (Baltimore). 1975;54:1-27. [PubMed] |
| 3. | Simon HU, Rothenberg ME, Bochner BS, Weller PF, Wardlaw AJ, Wechsler ME, Rosenwasser LJ, Roufosse F, Gleich GJ, Klion AD. Refining the definition of hypereosinophilic syndrome. J Allergy Clin Immunol. 2010;126:45-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 4. | Sunkara T, Rawla P, Yarlagadda KS, Gaduputi V. Eosinophilic gastroenteritis: diagnosis and clinical perspectives. Clin Exp Gastroenterol. 2019;12:239-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Chen MJ, Chu CH, Lin SC, Shih SC, Wang TE. Eosinophilic gastroenteritis: clinical experience with 15 patients. World J Gastroenterol. 2003;9:2813-2816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 154] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Chang JY, Choung RS, Lee RM, Locke GR 3rd, Schleck CD, Zinsmeister AR, Smyrk TC, Talley NJ. A shift in the clinical spectrum of eosinophilic gastroenteritis toward the mucosal disease type. Clin Gastroenterol Hepatol. 2010;8:669-75; quiz e88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Roufosse F, Cogan E, Goldman M. Lymphocytic variant hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27:389-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Simon D, Simon HU. Eosinophilic disorders. J Allergy Clin Immunol. 2007;119:1291-300; quiz 1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Harmon WA, Helman CA. Eosinophilic gastroenteritis and ascites. J Clin Gastroenterol. 1981;3:371-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut. 1990;31:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 524] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 11. | Wiesner W, Kocher T, Heim M, Bongartz G. CT findings in eosinophilic enterocolitis with predominantly serosal and muscular bowel wall infiltration. JBR-BTR. 2002;85:4-6. [PubMed] |
| 12. | Herrera Quiñones G, Scharrer SI, Jiménez Rodríguez AR, García Compean D, Borjas Almaguer OD, Martínez Segura JA, Jáquez Quintana JO, González González JA, Maldonado Garza HJ. Diagnosis of Eosinophilic Enteritis With Video Capsule Endoscopy and Double Balloon Enteroscopy With Favorable Response to Corticosteroids. ACG Case Rep J. 2019;6:e00127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Usui S, Dainichi T, Kitoh A, Miyachi Y, Kabashima K. Janeway Lesions and Splinter Hemorrhages in a Patient With Eosinophilic Endomyocarditis. JAMA Dermatol. 2015;151:907-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Weller PF, Bubley GJ. The idiopathic hypereosinophilic syndrome. Blood. 1994;83:2759-2779. [PubMed] |
| 15. | Kilpatrick ZM, Greenberg PA, Sanford JP. Splinter hemorrhages--their clinical significance. Arch Intern Med. 1965;115:730-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 33] [Article Influence: 0.5] [Reference Citation Analysis (0)] |