Published online Aug 8, 2020. doi: 10.4292/wjgpt.v11.i3.40
Peer-review started: January 14, 2020
First decision: April 8, 2020
Revised: May 11, 2020
Accepted: July 18, 2020
Article in press: July 18, 2020
Published online: August 8, 2020
Processing time: 203 Days and 19.1 Hours
Neovascularisation is common to a variety of gastrointestinal (GI) disorders with differing aetiologies and presentations; usually affecting adults above 60 years. Shared angiogenic factors modulated by disease specific elements could be a common denominator and represent novel diagnostic and therapeutic targets. As yet, assessment of angiogenic factors across several GI vascular disorders associated with recurrent bleeding and anaemia has not been reported.
To assess serum levels of angiogenic factors in several intestinal vascular disorders.
A case control study was performed in Tallaght University Hospital in patients with endoscopically proven small bowel angiodysplasia (SBA), portal hypertensive gastropathy (PHG), gastric antral vascular ectasia (GAVE) and non-bleeding, non-anaemic controls. Using enzyme-linked immunosorbent assay, concentrations of Angiopoietin 1 (Ang-1), Ang-2 and vascular endothelial growth factor (VEGF) were measured from 2 serum tubes of blood following informed consent. The relative expression of Ang-1 and Ang-2 and Ang-1/2 ratio was calculated and compared between groups. Statistical analysis was applied using a t-test, and a P value of < 0.05 was considered significant.
To date 44 samples were tested: 10 SBA, 11 PHG, 8 GAVE and 15 controls. Mean age 60 (range 20-85) years and 20 (45%) were males. Controls were significantly younger (49 years vs 66 years, P = 0.0005). There was no difference in VEGF levels between the groups (P = 0.6). SBA, PHG and GAVE Ang-1 levels were similar and were significantly lower than controls, (P = 0.0002, 95%CI: 241 to 701). Ang-2 levels were statistically higher in PHG and GAVE groups compared to controls (P = 0.01, 95%CI: 77.8 to 668) and as a result, also had a lower Ang-1/2 ratios compared to controls. While SBA Ang-2 levels were higher than controls, this did not reach statistical significance. Neither age nor haemoglobin level, which was similar between disease groups, could explain the difference. In addition, the median Ang-1/Ang-2 ratio for all patients was found to be significantly lower compared to controls, 8 vs 28 respectively, P = 0.001, 95%CI: -27.55 to -7.12.
Our novel pilot study suggests common alterations in Ang-1 and Ang-2 levels across several GI vascular disorders. Differences in Ang-1/Ang-2 ratios among vascular disorders compared to controls suggest disease-specific modulation.
Core tip: This is the first study to look at key angiogenic factors across several distinct intestinal vascular disorders. Our novel study suggests a common alteration in Angiopoietin 1 (Ang-1) levels, a vascular factor associated with vessel stabilization and maturation, across a variety of gastrointestinal vascular disorders. VEGF appears not to play a significant role in these conditions. Serum elevation in Ang-2 levels and lower than normal Ang-1 levels are associated with clinically significant disease and warrant further investigation as potential biomarkers and therapeutic targets. This offers a potential final common pathway which could be of use diagnostically and therapeutically across several vascular conditions.
- Citation: Douglas AR, Holleran G, Smith SM, McNamara D. Shared changes in angiogenic factors across gastrointestinal vascular conditions: A pilot study. World J Gastrointest Pharmacol Ther 2020; 11(3): 40-47
- URL: https://www.wjgnet.com/2150-5349/full/v11/i3/40.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v11.i3.40
Vascular abnormalities can affect any part of the gastrointestinal (GI) tract and present with bleeding and anaemia[1]. Abnormal neovascularization, with friable, dilated superficial blood vessels, is common to a variety of GI disorders with differing etiologies and presentations including small bowel angiodysplasia (SBA), gastral antral vascular ectasia (GAVE) and portal hypertensive gastropathy (PHG)[2]. While associated with different clinical conditions and different locations these lesions are all common causes of recurrent or chronic intestinal bleeding especially in adults above 60 years. Reported rates of significant bleeding are; SBA (30%-50%), GAVE (2%-4%) and PHG (3%-26%)[3-5]. GI vascular malformations and bleeding in SBA have been associated with varying disturbances in angiogenesis, however, the precise mechanisms underlying these conditions remain unclear[3]. Our hypothesis is that localised aberrant neovascularisation develops in different conditions in response to a variety of upstream triggers including portal hypertension, hypoxia and inflammation, which may be regulated at a local level by common angiogenic regulators.
Angiogenesis is mediated by several angiogenic factors including the two major angiopoietin factors: Angiopoietin-1 (Ang-1) and Ang-2 and vascular endothelial growth factor (VEGF), a sub-family of the platelet-derived growth factor (PDGF). Ang-1 is involved in the maturation of blood vessels and has vascular protective properties, whereas Ang-2 promotes endothelial permeability. Both angiopoietins utilize their angiogenic regulatory effects via the tyrosine-kinase receptor (Tie-2)[6]. A more recent study has confirmed an imbalance in Ang-1/Ang-2 ratios in SBA patients with bleeding[3]. VEGF appears to play an essential role in promoting angiogenesis and vascular development; however, it can also cause vascular permeability and tissue oedema by working in synergy with Ang-2[2]. To date, the exact aetiology and pathophysiology of these conditions and the role of angiogenic factors is not yet fully established.
Due to a limited understanding of the role of angiogenic factors in common non-hereditary GI vascular disorders, no specific treatments targeting the angiogenic pathway are currently available. The management of these conditions is primarily endoscopic which can both diagnose and treat the source of bleeding. Despite this, these conditions frequently recur, and patients are often hospitalized for management of bleeding episodes, which has a significant health and financial impact. There is a need for identification of biomarkers as potential diagnostic and novel therapeutic targets, which could dramatically improve disease outcome for affected patients[7]. As yet, assessment of angiogenic factors across common GI vascular disorders has not been reported. Shared angiogenic factors modulated by disease specific elements could be a common denominator and represent novel diagnostic and therapeutic targets.
The aim of this study is to assess serum levels of angiogenic factors in different intestinal vascular disorders associated with recurrent bleeding and anaemia.
Ethical approval was obtained from our institutions research and ethics committee and any patient over the age of 18 years undergoing an endoscopic procedure with a known diagnosis of SBA, PHG or GAVE and confirmed on endoscopy were invited to participate. Gender matched controls with negative surveillance colonoscopies, with two sequential negative faecal immunological tests were identified and invited to participate. Controls with significant dyspepsia, or evidence of GI bleeding or anaemia defined as haemoglobin (Hb) of < 11.5 g/dL in females and < 13 g/dL in males along with a serum ferritin of < 14, known chronic liver disease or non-liver related portal hypertension were excluded. Similarly, cases and controls with chronic renal failure, active malignancy or severe cardiac or respiratory failure were excluded. Recruitment continued until a minimum of 8 patients was included in each group.
Following informed consent, 2 serum separator tubes (3.5 mL each) of blood were taken on the same day using standard phlebotomy techniques. All relevant clinical data including basic demographics and relevant tests were recorded and filed on an encrypted database. Plasma samples were analysed routinely for Hb level and serum samples were left to clot for 30 min before undergoing centrifugation for 15 min at 1000 rpm. The resultant supernatant was extracted and stored in aliquots at -80 °C for batch analysis.
Serum levels of Ang-1, Ang-2 and VEGF were measured using commercially available solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) kits (R and D systems, Minneapolis, MN, United States). Samples were prepared in duplicate and results were read at 450 nm absorbance. The intra-assay coefficients of variation (CV) were calculated as an average of all of the individual CVs for the sample concentration duplicates analysed by ELISA.
Categorical data was compared with a Chi2 test. Serum levels of angiogenic factors were expressed as a mean and compared between groups using the Student t-test. Results were controlled for patient demographics including age, gender and haemoglobin level. In addition, the relative expression of Ang-1 and Ang-2, expressed as a ratio, per individual was calculated and compared between groups. A P value of < 0.05 was considered significant.
Overall, 44 patients were recruited, 45% (n = 20) were males, with a mean age of 60 years (range 20-85), 15 controls, 10 with SBA, 11 PHG and 8 with GAVE. Subjects in the control group were significantly younger, 49 years (range 20-74) than cases, 65 years (range 38-85), P = 0.0005, 95%CI: 8 to 26. There was no difference in mean age among patient groups; 68 years (53-79) SBA, 60 years (38-81) PHG and 68 years (58-85) GAVE. There was no statistical difference in gender between controls and cases overall or by group with 47% (n = 7/15), 50% (n = 5/10), 27% (n = 3/11) and 50% (n = 4/8) being male respectively. A current haemoglobin level was available in 29 /44 (66%) subjects and was similar among cases. Mean Hb levels by group were; SBA 11.4 g/dL (range 7.2-13.8), PHG 11.2 g/dL (9.5-12.2), GAVE 11.5 g/dL (8.3-15.3). While as expected the mean haemoglobin for controls was higher, this did not reach statistical significance 12.5g/dL (11.5 -13.2). However, more patients in the disease groups were anaemic compared to controls 1/5 (20%) vs 14/24 (59%), P < 0.05 (Table 1).
Ang-1/Ang-2: Mean Ang-1 levels were significantly higher in controls 53115 ± 17506 ng/mL than patients 29559 ± 18084, P = 0.0002, 95%CI: -35040 to -12072. While mean serum SBA, PHG and GAVE Ang-1 levels were all similar; 35696 ng/mL (range 12466-54338), 23111 ng/mL (range 1950-72445), 30753 ng/mL (range 9773-72609) respectively. As with Ang-1, mean Ang-2 levels were also similar among SBA, PHG and GAVE groups; 2803 ng/mL (range 125-13141), 4298 ng/mL (range 1299-8702) and 4232 ng/mL (range 1253-6081) respectively. Contrary to Ang-1 findings which were lower, the mean Ang-2 levels, 3764 ± 2763 ng/mL for patients were higher than controls, 1899 ± 772 ng/mL, P < 0.01, 95%CI: 389 to 3342.
The relative expression of Ang-1 and Ang-2 was calculated for each group. Controls as expected had a higher ratio; 35696/2803 ng/mL = 13 for SBA, 23111/4298 ng/mL = 5 for PHG, 30753/4232 ng/mL = 7 for GAVE vs 53114/1899 ng/mL = 28 for controls. However, the difference between SBA and control Ang-1/Ang-2 ratios did not reach statistical significance which was not unexpected as SBA Ang-2 levels while higher, was not statistically different from controls (Table 2).
| SBA | PHG | GAVE | Control | |
| Ang-1 (ng/mL), mean (range) | 35696 (12466-54338) | 23111 (1950-72445) | 30753 (9773-72609) | 53115 (28624-82066) |
| P value vs controls | 0.01 | 0.0003 | 0.01 | |
| Ang-2 (ng/mL), mean (range) | 2803 (125-13141) | 4298 (1299-8702) | 4232 (1253-6081) | 1899 (742-3693) |
| P value vs controls | 0.4 | 0.0008 | 0.003 | |
| VEGF (ng/mL), mean (range) | 443 (273-964) | 316 (51-600) | 435 (288-637) | 421 (250-678) |
| P value vs controls | 0.8 | 0.08 | 0.8 | |
| Ang-1/Ang-2 ratios by group | 13 | 5 | 7 | 28 |
| Median Ang-1/Ang-2 ratios | 8 | 28 | ||
| P value vs controls | 0.001 | |||
In addition, the median Ang-1/Ang-2 ratio for all patients was found to be significantly lower compared to controls, 8 vs 28 respectively, P = 0.001, 95%CI: -27.55 to -7.12.
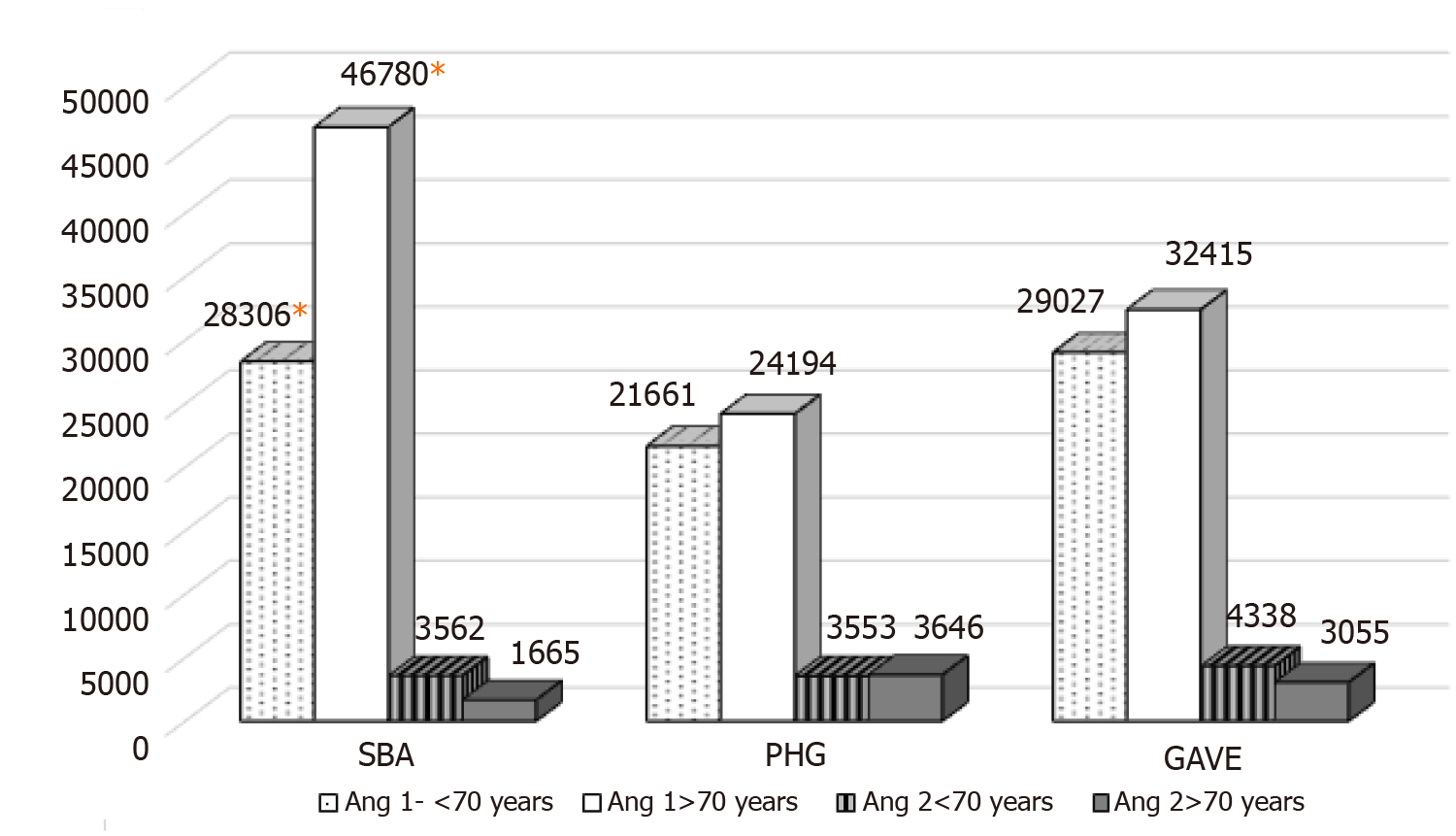
Age has been previously reported to affect angiopoietin levels[8]. In our cohort only SBA cases showed a variation in Ang-1 levels by age, with older age (> 70 years) associated with higher levels. Mean Ang-1 SBA < 70 years = 28306 ± 11479 ng/mL vs > 70 years = 46780 ± 5251 ng/mL, P = 0.02, 95%CI: 4142 to 32805. While mean Ang-1 levels by age for PHG and GAVE were 21661 ng/mL vs 24194 ng/mL, P = 0.9 and 29027 ng/mL vs 32415 ng/mL, P = 0.8 respectively. There was no difference in Ang-2 levels among cases by age. SBA 3562 ng/mL vs 1665 ng/mL, PHG 3555 ng/mL vs 3646 ng/mL and GAVE 4338 ng/mL vs 3055 ng/mL (Figure 1). Of note neither haemoglobin level or the presence of anaemia affected our findings.
VEGF: Again, mean serum VEGF levels were similar among cases; SBA = 443 ng/mL (range 273-964), PHG = 316 ng/mL (range 51-600) and GAVE = 435 ng/mL (range 288-637). Unlike angiopoietin levels, VEGF did not differ significantly between cases and controls. Mean VEGF for the control group was 421 ng/mL (range 250-678) (Table 2).
To our knowledge, this is the first study to look at key angiogenic factors across several distinct intestinal vascular disorders. In our pilot study subjects with endoscopically documented sporadic small bowel angiodysplasia, portal hypertensive gastropathy and gastric antral vascular ectasia all shared a common dysregulation in serum angiopoietin profile, with reduced levels of Ang-1 and higher levels of Ang-2 and resultant lower Ang-1/Ang-2 ratios. Adding weight to the hypothesis that dysregulated neovascularisation driven by Ang-2 excess is a key factor in the development of these conditions irrespective of upstream drivers. This offers a potential final common pathway which could be of use diagnostically and therapeutically across several vascular conditions.
Ang-2 is a circulating antagonist of the endothelial-specific Tie-2 receptor and has been identified as an important modulator of angiogenesis. While Ang-1 acts as a growth and maturation factor promoting development and stabilization of mature normal vessels in vivo by mediating endothelial cell interactions. Ang-2 antagonises Ang-1 and prevents Tie-2 receptor activation by competitively binding Tie-2. Ang-2 is known to be expressed at the site of vascular remodelling and its antagonism of Ang-1 actions therefore promotes vessel destabilization, possibly leading to the development of abnormal friable, leaky immature vessels, a hallmark of all these conditions[8,9].
Of interest, in contrast to previous studies which have documented elevated VEGF levels in GI disease and bleeding, VEGF levels were similar among controls and all disease groups in our cohort[10,11]. These findings mirror those we have previously demonstrated in a larger sporadic SBA cohort[8]. The role of VEGF and angiopoietins in blood vessel development and maturation is complex and their specific roles may explain our findings. VEGF is a central mediator of early phase angiogenesis[12]. The initial phase of angiogenesis is characterized by VEGF-dependent formation and sprouting of blood vessels. Local concentrations of VEGF determine whether normal or aberrant angiogenesis is induced[13]. High local concentrations of VEGF result in vascular malformations and lesions resembling the chaotic architecture of angiodysplasias, thin-walled fragile vessels lacking smooth muscle cells that are susceptible to rupture[14,15]. To mature, primitive vascular complexes require remodelling during which vessels acquire a smooth muscle layer, and become less permeable and friable, a process dependant on other angiogenic factors including Ang-1[16]. As such, it remains a possibility that high local tissue levels of VEGF are responsible for initiation of angiogenesis triggered by a variety of pathological processes. While high circulating levels of Ang-2 interferes with vessel remodelling and maturation, resulting in the abnormal friable, permeable vasculature, with a propensity for bleeding, common to these conditions. Confirmatory studies exploring local tissue VEGF expression would be required to confirm this hypothesis, which is beyond the scope of this pilot study.
While our initial results are of interest and warrant further investigation, there are significant limitations with our study. This is only a small, pilot study and findings will need to be confirmed in a larger cohort. Our sample size prevents effective subgroup analysis including the potential effects of age and other potential confounding factors including recent active bleeding events, although haemoglobin level did not affect expression in our cohort. We did control for gender and excluded conditions known to be associated with disturbances in angiogenic levels, which adds weight to our findings. In addition to a larger sample size, repeated testing at different times would be advantageous and help to establish whether these factors are predictive of disease severity, bleeding events and long-term outcomes. Similarly, our small cohort included patients with symptomatic disease only requiring endoscopic intervention which could represent a bias as potentially less severe forms may have a different angiogenic profile. However, clinical application is likely only to be relevant to just such a symptomatic cohort.
As mentioned previously, circulating angiogenic factors may not reflect local tissue activity and should be investigated to confirm the potential key role of angiopoietins and possibly VEGF across several GI vascular conditions. However, as a potential diagnostic or prognostic biomarker serum sampling is preferable and would be more useful clinically.
In conclusion, this is the first study to show common changes in angiogenic factors across several distinct intestinal vascular disorders. Serum elevation in Ang-2 levels and lower than normal Ang-1 levels are associated with clinically significant disease and warrant further investigation as potential biomarkers and therapeutic targets.
Neovascularisation is a common feature of gastrointestinal (GI) vascular disorders with differing aetiologies and presentations; including small bowel angiodysplasia (SBA), gastral antral vascular ectasia and portal hypertensive gastropathy. These lesions are all common causes of recurrent or chronic intestinal bleeding especially in adults above 60 years. GI vascular malformations and bleeding in SBA have been associated with varying disturbances in angiogenesis, however, the precise mechanisms underlying these conditions remain unclear. Our hypothesis is that response to a variety of upstream triggers including portal hypertension, hypoxia and inflammation, which may be regulated at a local level by common angiogenic regulators including Angiopoietin 1 (Ang-1), Ang-2 and vascular endothelial growth factor (VEGF) may be responsible for these conditions.
At present, the precise mechanisms underlying these conditions remain unclear and assessment of angiogenic factors across several GI vascular disorders associated with recurrent bleeding and anaemia has not been reported. In addition, there is currently no specific treatment for these vascular conditions, the development of which is limited by a deficient knowledge of the underlying pathophysiology.
The overarching aim of our work is to identify angiogenic factors associated with the condition which may be useful both as diagnostic and prognostic markers and as future treatment targets.
Using enzyme-linked immunosorbent assay, concentrations of Ang-1, Ang-2 and VEGF were measured from 2 serum tubes of blood using standard phlebotomy techniques. Categorical data was compared with a Chi2 Test. Serum levels of angiogenic factors were expressed as a mean and compared between groups using the Student t-test. Results were controlled for patient demographics including age, gender and haemoglobin level. A P value of < 0.05 was considered significant.
We observed a common reduction in Ang-1 levels and elevation in Ang-2 levels across several GI vascular disorders compared to controls. Differences in Ang-1/Ang-2 ratios among vascular disorders compared to controls suggest disease-specific modulation. This warrants further investigation as potential biomarkers and therapeutic targets.
Our novel pilot study shows the common alteration in Ang-1 and Ang-2 levels across a variety of GI disorders. This suggests that the modulation of these angiogenic factors may play a vital role in these GI vascular conditions. This shows the value of these factors as potential biomarkers and therapeutic targets.
Targeting these angiogenic factors could potentially serve as diagnostic or prognostic biomarkers and therapeutic targets in a clinical setting.
We thank all the volunteers and medical staff who agreed to participate in this study.
| 1. | Gordon FH, Watkinson A, Hodgson H. Vascular malformations of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2001;15:41-58. |
| 2. | Randi AM, Laffan MA, Starke RD. Von Willebrand factor, angiodysplasia and angiogenesis. Mediterr J Hematol Infect Dis. 2013;5:e2013060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Holleran G, Hussey M, Smith S, McNamara D. Assessment of serum angiogenic factors as a diagnostic aid for small bowel angiodysplasia in patients with obscure gastrointestinal bleeding and anaemia. World J Gastrointest Pathophysiol. 2017;8:127-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Nguyen H, Le C, Nguyen H. Gastric antral vascular ectasia (watermelon stomach)-an enigmatic and often-overlooked cause of gastrointestinal bleeding in the elderly. Perm J. 2009;13:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (3)] |
| 5. | Gjeorgjievski M, Cappell MS. Portal hypertensive gastropathy: A systematic review of the pathophysiology, clinical presentation, natural history and therapy. World J Hepatol. 2016;8:231-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 6. | Linares PM, Chaparro M, Gisbert JP. Angiopoietins in inflammation and their implication in the development of inflammatory bowel disease. A review. J Crohns Colitis. 2014;8:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Swanson E, Mahgoub A, MacDonald R, Shaukat A. Medical and endoscopic therapies for angiodysplasia and gastric antral vascular ectasia: a systematic review. Clin Gastroenterol Hepatol. 2014;12:571-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Holleran G, Hall B, O'Regan M, Smith S, McNamara D. Expression of Angiogenic Factors in Patients With Sporadic Small Bowel Angiodysplasia. J Clin Gastroenterol. 2015;49:831-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Thurston G, Daly C. The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold Spring Harb Perspect Med. 2012;2:a006550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 10. | Junquera F, Saperas E, de Torres I, Vidal MT, Malagelada JR. Increased expression of angiogenic factors in human colonic angiodysplasia. Am J Gastroenterol. 1999;94:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 115] [Article Influence: 4.3] [Reference Citation Analysis (3)] |
| 11. | Fujita H, Momoi M, Chuganji Y, Tomiyama J. Increased plasma vascular endothelial growth factor levels in patients with angiodysplasia. J Intern Med. 2000;248:268-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (3)] |
| 12. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 7049] [Article Influence: 306.5] [Reference Citation Analysis (0)] |
| 13. | Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, McDonald DM, Blau HM. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113:516-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 391] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 14. | Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102:898-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 516] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 15. | Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511-2514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1047] [Cited by in RCA: 1014] [Article Influence: 37.6] [Reference Citation Analysis (3)] |
| 16. | Uemura A, Ogawa M, Hirashima M, Fujiwara T, Koyama S, Takagi H, Honda Y, Wiegand SJ, Yancopoulos GD, Nishikawa S. Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. J Clin Invest. 2002;110:1619-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (3)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Ireland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Gangl A, Niu ZS, Tanaka N S-Editor: Wang JL L-Editor: A E-Editor: Li JH