Published online Jul 18, 2019. doi: 10.4292/wjgpt.v10.i3.57
Peer-review started: February 6, 2019
First decision: March 5, 2019
Revised: March 19, 2019
Accepted: April 8, 2019
Article in press: April 9, 2019
Published online: July 18, 2019
Processing time: 167 Days and 3.2 Hours
Monitoring ventilation accurately is an indispensable aspect of patient care in procedural settings. The current gold standard method of monitoring ventilation is by measuring exhaled carbon dioxide concentration, known as capnography. A new device utilizing thermodynamic measurement, the Linshom Respiratory Monitoring Device (LRMD), has been designed to measure respiratory rate (RR) by using the temperature of exhaled breath. We hypothesized that the temperature sensor is at least equivalent in accuracy to capnography in monitoring ventilation.
To determine if the temperature sensor is equivalent to capnography in monitoring procedural ventilation.
In this prospective study, participants were individually fitted with a face mask monitored by both LRMD and capnography. The following data were collected: gender, age, body mass index, type of procedure, and doses of medication. For each patient, we report the mean RR for each device as well as the mean difference. All analyses were performed using SAS, and a P < 0.05 was considered statistically significant.
Twelve consecutive patients undergoing endoscopic procedures at our institution were enrolled. Four patients were excluded due to incomplete data, inadequate data, patient cooperation, and capnography failure. Overall, we found that LRMD RR highly correlated to capnography RR (P < 0.001); the average capnography RR increases by 0.66 breaths for every one additional breath measured by the LRMD. In addition, apnea rates were 7.4% for the capnography and 6.4% for the LRMD (95% confidence interval: 0.92-1.10).
The LRMD correlated with the gold standard capnography with respect to respiratory rate detection and apnea events. The LRMD could be used as an alternative to capnography for measuring respiration in endoscopy.
Core tip: The current gold standard method of monitoring ventilation during procedures is capnography. A new device utilizing a thermodynamic measurement, Linshom Respiratory Monitoring Device (LRMD) has been designed to measure respiratory rate by using the temperature of exhaled breath. This study showed that the LRMD correlated with the gold standard capnography with respect to respiratory rate detection and apnea events. The LRMD could be used as an alternative to capnography for measuring respiration in endoscopy.
- Citation: Wadhwa V, Gonzalez AJ, Selema K, Feldman R, Lopez R, Vargo JJ. Novel device for monitoring respiratory rate during endoscopy-A thermodynamic sensor. World J Gastrointest Pharmacol Ther 2019; 10(3): 57-66
- URL: https://www.wjgnet.com/2150-5349/full/v10/i3/57.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v10.i3.57
As endoscopic procedures continue to increase in complexity, adequate monitoring of vital signs, specifically respiratory status and ventilation, is of utmost importance[1]. Accurate monitoring of oxygenation and ventilation status is critical during the intra-operative and post-operative period[2]. Monitoring ventilation appropriately is a crucial yet challenging aspect of patient management. The importance of monitoring respiratory rate as a key vital sign has been well defined in medicine[3]. An abrupt alteration in respiratory rate can help detect changes in the status of a patient during a crucial period where modifications in management might be needed[3]. Respiratory monitoring is important for both patients receiving general anesthesia as well as for those who require moderate sedation. Patients who do not require anesthesia but are at high risk of respiratory compromise given a multitude of co-morbidities, including underlying respiratory or cardiovascular disease, also need to be closely monitored. A recent study revealed that at least 5%-10% of patients undergoing surgery ex-perienced some type of pulmonary complications[4]. That risk more than doubles to as high as 22% in high risk patient populations[4].
Respiratory problems, including but not limited to hypercarbia, hypoxemia, apnea, and respiratory arrest, are among the most common adverse events seen in the perioperative period, especially as a complication of anesthesia used during gastrointestinal endoscopy[5]. Significant respiratory complications include pneumonia, respiratory failure, atelectasis, sepsis, and even cardiac arrest[6,7]. In addition, poor monitoring of ventilation can result in exacerbation of chronic conditions and other pulmonary diseases. Therefore, given the high risk of any endoscopic or surgical procedure for possible post-operative pulmonary sequelae, close monitoring of respiratory parameters provides clinical information that may help reduce morbidity and mortality[5,8,9]. Standards have been developed by the American Society of Anesthesiologists (ASA) that state that during the use of all anesthetics, a patient’s oxygenation, ventilation, circulation, and temperature should be continuously evaluated[10]. Continual monitoring for the presence of expired carbon dioxide with the use of capnography has been advocated as the standard of care and recommended by ASA to be utilized in any gastrointestinal endoscopic procedure utilizing moderate or deep sedation. It is important to note that, although capno-graphy has been used as the standard of care in critical care settings and operating rooms, the hardware is expensive, cumbersome to transfer, and results are influenced by secretions and moisture. In addition, when carbon dioxide insufflation is utilized, particularly of upper endoscopic procedures, the use of the CO2 gas confounds the capnography monitoring by elevating the end tidal CO2 level, leading clinicians to assume falsely that profound hypoventilation is occurring.
The Linshom Respiratory Monitor Device (LRMD) is a thermodynamic respiratory device that is novel, non-invasive, and portable and can potentially modernize the way physicians monitor respiratory activity both within critical care and ambulatory settings. The LRMD consists of two rapid-responding medical-grade thermistors (1.5 mm diameter) with a unique control-loop algorithm executed by a microcontroller (United States Patents: 8579829 and 8911380). The technology utilizes a highly sensitive thermistor-based sensor that compares the instantaneous temperature of the breath inside the mask with the ambient temperature. The sensor is comprised of an actively controlled thermistor inside the mask along with a Thermo-Electric Cooler (TEC) outside the mask. The device maintains the thermistor in thermal balance with the TEC utilizing the Peltier effect. During power-up initialization, the sensor measures the ambient temperature and uses this as a set point for the control loop. As the expired breath heats the thermistor, the thermal balance is disrupted, and this generates a feedback signal via the control loop. The generated signal is an accurate signature representation of the breath, capturing the subtle detail of the breathing cycle. After each breath, the control-loop returns the sensor to thermal balance with the ambient temperature and ready for the next breath. The aim of this study was to compare the effectiveness of apnea detection by these two devices.
In this prospective study, 12 participants were consecutively enrolled. Institutional Review Board approval was obtained prior to study initiation and consent was obtained from all participants. The study participants were collected at random to ensure there was no underlying bias and were patients already scheduled to undergo routine procedures at our endoscopy unit. The study was conducted at Cleveland Clinic Main Campus from June to July 2016. All participants were individually fitted with a standard face mask fitted with both capnography and the LRMD. The following data were collected from the medical record: Gender, age, type of procedure, type of sedation, and dose of medication administered. Vital signs, including respiratory rate, heart rate, blood pressure, temperature, and body mass index, were recorded. Patients were screened for medical issues, including cardiac and pulmonary disease, with responses noted in the data collection. Standard monitoring and sedation procedures were employed. The choice of sedation was also performed at random considering physician preference and endoscopy unit location where the patients were scheduled to undergo the procedure. Most of the procedures were performed with moderate sedation. All procedures took place in either of our two designated endoscopy suites, with most procedures occurring with nursing personnel and anesthesia if needed. Testing and data collection occurred in the same conditions with trained personnel (JJV, VW, and RF). The ambient temperature was 71°-72° F and ambient pressure 1 atmosphere.
Two different sensors were attached to the mask: A temperature sensor and CO2 detection tubing. The LRMD was connected via a cable to the main computer control station. The facemask utilized allows for passage of an upper endoscope and is the standard mask used in our practice. Throughout the entire study, particular attention was paid to ensure tubing and sensors were not in contact with devices or objects that could affect data results. After the subjects were informed about the procedure and the planned protocol, oxygen was delivered at a flow rate of 6 L/min. Subjects were permitted to breathe through either their nasal or oral passages spontaneously without regulating tidal volumes. Participants were also instructed to avoid excessive movements that would affect data gathering.
The LRMD and capnography data were collected simultaneously via the Linshom information gathering software for later comparison. For each patient, we recorded the mean respiratory rate for each device separately. We also reported the mean differences between the two devices with corresponding 95% confidence interval (CI). Each patient’s distinct characteristics were noted and plotted against one another in order to determine the mean, standard deviation, and P values of the study group. In addition, Pearson’s correlation was calculated to describe the correlation between the respiratory rate as calculated by the capnography and the LRMD. Bland-Altman Graphs were plotted to illustrate how differences in respiratory rate vary based on the rate of respiration.
For each patient, we reported the mean respiratory rate for each device as well as the mean difference between the two devices with corresponding 95% CI. In addition, we calculated Pearson’s correlation coefficient to describe the correlation between capnography and thermodynamic respiratory rate. We did not use a concordance correlation coefficient because of the known differences on how the devices measure respiratory rate. Bland-Altman graphs were plotted to illustrate how the difference in respiratory rate varies based on the magnitude of the respiratory rate. In addition, generalized estimating equations (GEE) were used to compute the difference in percentage of reports showing apnea between the two devices while accounting for the paired nature of the data. Linear mixed models were used to evaluate overall association between capnography respiratory rate and Linshom respiratory rate while accounting for within subject correction due to multiple measurements per patient. Lastly, GEE analysis was used to assess the overall difference between Linshom and capnography apnea rates taking into consideration the correlation between measures done in the same subject. All analysis were performed using SAS (version 9.4, The SAS Institute, Cary, NC, United States) and a P value of less than 0.05 was considered statistically significant.
Twelve consecutive patients scheduled to undergo endoscopic procedures at our facility in Cleveland Clinic, Ohio, were enrolled. Eight patients underwent colo-noscopy, and four underwent an upper endoscopy. One patient underwent both upper and lower endoscopy. Patients’ age ranged between 41 and 90 years. There were seven males and five females in the conducted study. Both conscious sedation and monitored anesthesia care was used during the study (Tables 1 and 2).
| Patient | Age | Sex | Weight in kg | Height in cm | BMI in kg/m2 | ASA class | Sleep apnea | Included |
| 1 | 70 | Male | 96.6 | 168 | 34.4 | III | Yes | Yes |
| 2 | 51 | Female | 79.4 | 165 | 29.1 | II | No | No |
| 3 | 61 | Female | 99.8 | 168 | 35.5 | II | Yes | No |
| 4 | 90 | Male | 72.6 | 163 | 27.5 | II | No | Yes |
| 5 | 51 | Male | 95.3 | 170 | 32.9 | III | No | Yes |
| 6 | 71 | Male | 145.2 | 191 | 40 | IV | Yes | Yes |
| 7 | 65 | Male | 108.0 | 180 | 33.2 | III | No | Yes |
| 8 | 51 | Female | 96.2 | 152 | 41.4 | III | Yes | No |
| 9 | 65 | Male | 115.7 | 175 | 37.7 | II | No | Yes |
| 10 | 47 | Male | 79.4 | 175 | 25.8 | I | No | Yes |
| 11 | 41 | Female | 64.0 | 157 | 25.8 | III | No | Yes |
| 12 | 77 | Female | 65.8 | 157 | 26.5 | III | No | No |
| Patient | Procedure | Date | RR | O2 Sat% | HR | BP inmmHg | Sedationused | Included |
| 1 | EGD | 6/13/16 | 20 | 97 | 87 | 145/78 | Conscious | Yes |
| 2 | Colonoscopy | 6/13/16 | 18 | 100 | 68 | 159/93 | Conscious | No |
| 3 | Colonoscopy | 6/13/16 | 19 | 97 | 87 | 139/73 | Conscious | No |
| 4 | Colonoscopy | 6/13/16 | 18 | 97 | 79 | 142/74 | Conscious | Yes |
| 5 | EGD | 6/14/16 | 18 | 98 | 76 | 133/80 | Conscious | Yes |
| 6 | Colonoscopy | 6/16/16 | 16 | 92 | 64 | 151/92 | MAC | Yes |
| 7 | EGD | 6/16/16 | 16 | 98 | 70 | 155/81 | MAC | Yes |
| 8 | EUS | 6/16/16 | 16 | 98 | 59 | 120/61 | MAC | No |
| 9 | Colonoscopy | 7/25/16 | 20 | 97 | 55 | 133/74 | Conscious | Yes |
| 10 | Colonoscopy | 7/25/16 | 16 | 99 | 64 | 117/67 | Conscious | Yes |
| 11 | Colonoscopy | 7/26/16 | 18 | 99 | 55 | 111/62 | MAC | Yes |
| 12 | EGD/ Colonoscopy | 7/26/16 | 16 | 98 | 77 | 177/107 | MAC | No |
After data review, 4 patients had to be excluded. Patients were subjected to exclusion for various reasons, including technical difficulties with capnography machine operations leading to incomplete data gathering due to constant calibration of capnography and respiratory mask falling off during procedure due to patient cooperation. When the mask fell, neither Linshom nor capnography data could be captured. There were no issues with the Linshom monitor capturing data. Out of the 12 patients who were selected, data was gathered over a total time period of 190 min. Approximately 3000 breaths were collected over the 190 min period given that each patient averaged 16 breaths per minute. Based on the data collection and review, we found that the LRMD was able to capture a statistically significant number of respirations compared to the conventional capnography monitor.
The LRMD respiratory rate highly correlated with capnography respiratory rate with a P value of < 0.001. In addition to being statistically comparable to the capno-graphy device in measuring respiratory rate, the LRMD was also able to detect increases in respiratory rate similar to that of the capnography device. The average capnography respiratory rate increased by 0.66 breaths for every additional one breath measured by the LRMD.
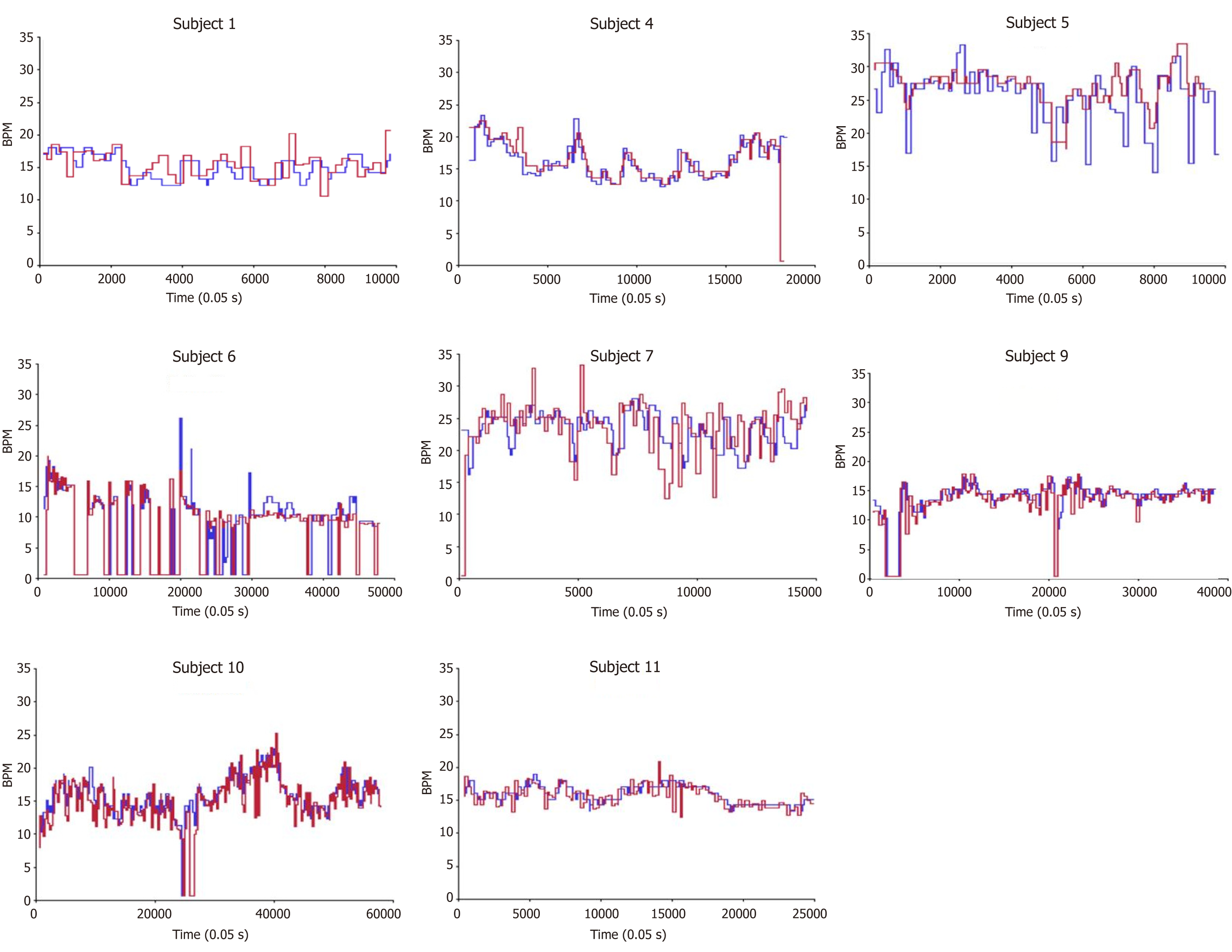
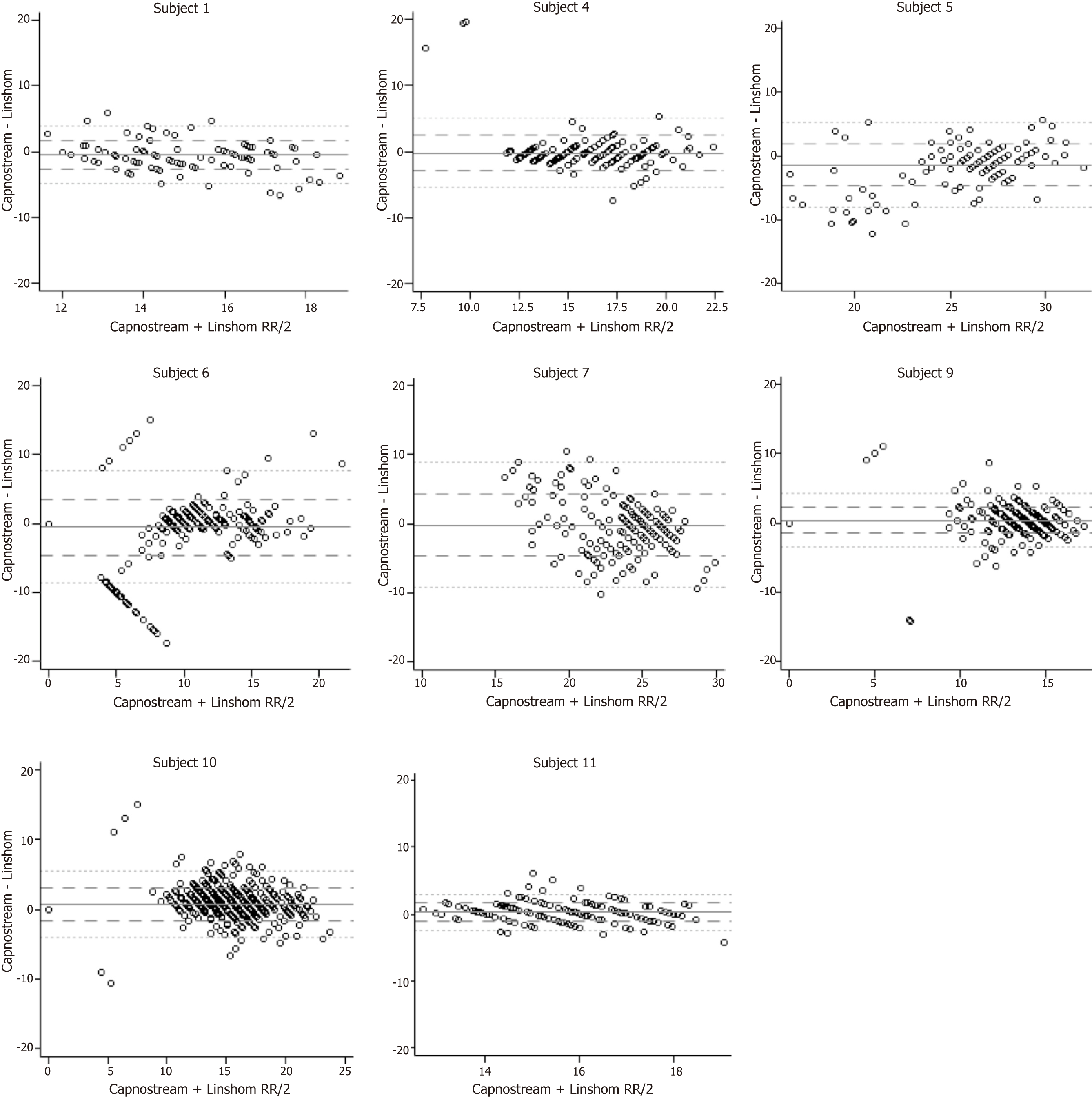
Figure 1 depicts respiratory rate (breaths per minute) vs time for each patient measured by both capnography and LRMD. Table 3 presents the mean respiratory rate for each patient as well as the average difference between the two. Note that the differences between the two devices are very small, with distinct significance seen as there is a large number of observations captured per patient to power further and detect such small differences. Figure 2 shows Bland- Altman plots. This scatterplot of measurement averages on the horizontal axis and differences in measurements plotted on the vertical axis shows the amount of disagreement between the two measurements by analyzing via means of the difference. The plots can also highlight certain types of anomalies in the measurements. Based on review of the data, it seems that there are inconsistencies between the two devices in the lower respiratory rate values as seen on the plot, especially when the respiratory rate is less than 10.
| Subject | Capnostream, mean ± SD | Linshom, mean ± SD | Capnostream–Linshom (95%CI) | Pearson’s Correlation1 (95%CI) |
| 1 | 14.9 ± 1.9 | 15.3 ± 2.1 | -0.46 (-0.50, -0.42) | 0.41 (0.40, 0.43) |
| 4 | 15.6 ± 2.6 | 15.8 ± 3.3 | -0.21 (-0.25, -0.17) | 0.62 (0.61, 0.63) |
| 5 | 25.1 ± 4.2 | 26.6 ± 2.9 | -1.4 (-1.5, -1.3) | 0.61 (0.60, 0.62) |
| 6 | 7.9 ± 5.8 | 8.4 ± 5.2 | -0.52 (-0.56, -0.48) | 0.72 (0.72, 0.73) |
| 7 | 23.1 ± 2.7 | 23.3 ± 4.4 | -0.21 (-0.28, -0.14) | 0.27 (0.25, 0.28) |
| 9 | 13.2 ± 3.3 | 12.9 ± 3.4 | 0.37 (0.35, 0.39) | 0.84 (0.84, 0.84) |
| 10 | 15.7 ± 3.0 | 15.0 ± 3.6 | 0.76 (0.74, 0.78) | 0.76 (0.75, 0.76) |
| 11 | 15.8 ± 1.5 | 15.5 ± 1.7 | 0.26 (0.24, 0.27) | 0.65 (0.64, 0.65) |
Apnea was also investigated during this study. When evaluating for apnea events, it is apparent that the two devices have similar rates of detection (< 2%). Subject 6 was the only exception to this as there were 7% more events detected by capnography compared to LRMD (Table 4).
| Subject | Capnostream | Linshom | Capnostream–Linshom (95%CI) |
| 1 | 0 | 0 | -- |
| 4 | 0 | 1.4 (1.4, 1.4) | -1.4 (-1.4, -1.4) |
| 5 | 0 | 0 | -- |
| 6 | 30.3 (29.9, 30.8) | 23.3 (23.0, 23.7) | 7.0 (6.7, 7.3) |
| 7 | 0 | 0.81 (0.81, 0.81) | -0.81 (-0.81, -0.81) |
| 9 | 4.8 (4.6, 5.0) | 5.1 (4.9, 5.3) | -0.30 (-0.40, -0.20)) |
| 10 | 0.83 (0.76, 0.91) | 1.8 (1.7, 1.9) | -0.93 (-1.03, -0.84) |
| 11 | 0 | 0 | -- |
In this prospective study, we measured the ability of LRMD to accurately and precisely detect respiration rate and variations thereof relative to commonly used capnography. Overall, the LRMD breaths per minute highly correlated to the capnography respiratory rate (P < 0.001). In addition to monitoring respirations per minute, fluctuations in respiratory rate also proved to be similar when comparing both these devices. We also found that the average capnography respiratory rate increased by 0.66 breaths for every one additional breath measured by LRMD, further demonstrating the ability of the LRMD to detect respiration just as accurately as standard capnography. When monitoring for adverse events in respiration, the LRMD was able to detect apnea rates 6.4% of the time compared to 7.4% with capnography, corresponding to an average 1% difference in reporting apnea events between the two devices.
This study demonstrated that the LRMD can be used in both critical care and ambulatory settings in place of conventional capnography for respiratory status monitoring, as has been shown previously[11]. The advent of the LRMD allows us to measure ventilation in a new, easier to use, efficient, and more affordable manner. With the results of this study, this device can potentially prove to be an effective and safe alternative to monitoring ventilation in various settings. Interestingly, the greatest inconsistencies between capnography and LRMD were noticed in respiratory rates less than 10 (Figure 2). This is clinically relevant as hypoventilation state may be a preceding factor for apnea and respiratory failure. This observation may be explained by the fact that the algorithm used by the capnography takes time to analyze the data while the LRMD is instantaneous. In this scenario, we could argue that the LRMD is a superior monitor than capnography.
Although this research was able to reach our primary endpoint, there were some unavoidable shortcomings. First, because it was unclear just how many participants would be needed to collect a substantial amount of data, the sample size was small, totaling eight participants after four were excluded. Therefore, to generalize the results for larger groups, the study should have recruited more applicants in both groups. Secondly, this study does not apply to mechanically ventilated individuals, as none of the participants in this study were monitored via mechanical ventilation. The study may also not be generalizable to patients of all ages, as no children were studied. Moreover, the study was non-randomized and open label, further leading to limitations. Researchers were aware of the aim of the study, which introduces unintentional confounders that could influence the study results.
Although there were some limitations to our study, the development of this novel apparatus has the potential to improve upon the current standards of how we monitor vital signs, specifically respiration and ventilation. The ASA aims to ensure adequate oxygen concentration in inspired gas and blood as well as adequate ventilation during all procedures[3,6,10]. The LRMD provides a minimally invasive, inexpensive, and portable means to measure respiration[11]. This device has the potential to allow medical personnel to recognize properly any deterioration in respiratory status, noted either with a decline in tidal volume or change in respiratory rate, therefore providing an increased measure for patient safety and the need to intervene more promptly if need be[3,11]. Not only is this device extremely accurate and reliable based on our data, but this easy to use respiratory monitor is independent of motion artifact and electrical interference making it user friendly[11].
Further studies are needed to demonstrate the entire magnitude of LRMD capabilities, including the ability to monitor tidal volume. One of the most profound weaknesses of capnography is the inability to measure tidal volume. The LRMD is able to measure accurately tidal volume in addition to respiration rate, as has been reported previously[11]. Unfortunately, our study was not designed to evaluate tidal volume monitoring; and hence, further studies are needed that would incorporate this while comparing with capnography.
In summary, our results demonstrate that the LRMD has the potential to be utilized as an alternative to capnography for measuring respiratory rate, specifically in the endoscopy setting, and may also be potentially useful in post-anesthesia care, critical care, ambulatory environments, and during in-hospital monitoring where similar sedation practices are applied[3,11]. The LRMD and capnography were at least equivalent in measuring respiration rate and apnea events. The implication of our results will need further studies that will include a larger sample size, both pediatric and geriatric patients, and a multitude of settings, including in-patient as well as ambulatory settings, to determine its ability to be used in these areas of care.
Monitoring ventilation appropriately during endoscopic procedures is a crucial yet challenging aspect of patient management and safety. Capnography is currently the standard of care for monitoring respiratory rate during endoscopy, but it is expensive and cumbersome to transfer. Furthermore, secretions, moisture, and carbon dioxide gas influence its results. The Linshom Respiratory Monitoring Device (LRMD) is a novel, non-invasive, and portable method for physicians to monitor respiratory activity during endoscopic procedures.
There is very limited data on how this novel and convenient method of monitoring respiratory rate, the LRMD, compares to the standard of care, capnography.
The main objective was to compare the effectiveness of apnea detection of the LRMD and capnography.
A prospective study was performed. Twelve participants scheduled to undergo routine endoscopic procedures at Cleveland Clinic Main Campus were consecutively enrolled. All participants were individually fitted with a standard facemask fitted with both capnography and the LRMD. Data were collected from the medical record (gender, age, type of procedure, type of sedation, and dose of medication) and during the procedure (vital signs and body mass index). The data for the LRMD and capnography were collected simultaneously. A biomedical statistician conducted statistical analysis. The mean difference between the two devices was calculated. Pearson’s correlation was calculated to describe the correlation between the respiratory rate as calculated by the capnography and the LRMD.
Twelve patients were enrolled. Four were excluded due to technical difficulties. Data were gathered for approximately 3000 breaths over a total period of 190 min. The LRMD respiratory rate highly correlated with the capnography respiratory rate with a P value of < 0.001. LRMD and capnography had similar rates of detecting apnea.
Our prospective study found that the respiratory rate measured by the LRMD highly correlates with the respiratory rate measured by capnography. The LRMD and capnography were also similar in detecting increases in respiratory rates and apnea episodes. This study demonstrates that the LRMD can be used in place of conventional capnography for monitoring respiratory status. This gives physicians an alternative to capnography that is new, efficient, easier to use, and more affordable. This reliable device can be an effective and safe alternative to monitoring ventilation in various settings.
Our results show that the LRMD may be used as an alternative to capnography for measuring respiratory rate in the endoscopy setting and potentially in the post-anesthesia care, critical care, and other ambulatory settings. Further studies are needed to demonstrate the entire magnitude of LRMD capabilities, including the ability to monitor tidal volume. These studies should include the use of the LRMD in other settings.
| 1. | Gaucher A, Frasca D, Mimoz O, Debaene B. Accuracy of respiratory rate monitoring by capnometry using the Capnomask(R) in extubated patients receiving supplemental oxygen after surgery. Br J Anaesth. 2012;108:316-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Taylor S, Kirton OC, Staff I, Kozol RA. Postoperative day one: A high risk period for respiratory events. Am J Surg. 2005;190:752-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Vimlati L, Gilsanz F, Goldik Z. Quality and safety guidelines of postanaesthesia care: Working Party on Post Anaesthesia Care (approved by the European Board and Section of Anaesthesiology, Union Européenne des Médecins Spécialistes). Eur J Anaesthesiol. 2009;26:715-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Chiumello D, Chevallard G, Gregoretti C. Non-invasive ventilation in postoperative patients: A systematic review. Intensive Care Med. 2011;37:918-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Porhomayon J, Pourafkari L, El-Solh A, Nader ND. Novel therapies for perioperative respiratory complications. J Cardiovasc Thorac Res. 2017;9:121-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98:1388-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 581] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 7. | Mailey J, Digiovine B, Baillod D, Gnam G, Jordan J, Rubinfeld I. Reducing hospital standardized mortality rate with early interventions. J Trauma Nurs. 2006;13:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Apfelbaum JL, Silverstein JH, Chung FF, Connis RT, Fillmore RB, Hunt SE, Nickinovich DG, Schreiner MS, Silverstein JH, Apfelbaum JL, Barlow JC, Chung FF, Connis RT, Fillmore RB, Hunt SE, Joas TA, Nickinovich DG, Schreiner MS; American Society of Anesthesiologists Task Force on Postanesthetic Care. Practice guidelines for postanesthetic care: An updated report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology. 2013;118:291-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Lawrence VA, Hilsenbeck SG, Mulrow CD, Dhanda R, Sapp J, Page CP. Incidence and hospital stay for cardiac and pulmonary complications after abdominal surgery. J Gen Intern Med. 1995;10:671-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 188] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Committee of Standards and Practice Parameters. American Society of Anesthesiologists. Standards for basic anesthesis monitoring. Schaumburg (IL). 2015. Available from: URL: https://www.asahq.org/standards-and-guidelines/standards-for-basic-anesthetic-monitoring/. |
| 11. | Preiss D, Drew BA, Gosnell J, Kodali BS, Philip JH, Urman RD. Linshom thermodynamic sensor is a reliable alternative to capnography for monitoring respiratory rate. J Clin Monit Comput. 2018;32:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Christodoulou DKK, Ogata H S-Editor: Yan JP L-Editor: Filipodia E-Editor: Xing YX