Published online Oct 25, 2018. doi: 10.4291/wjgp.v9.i3.63
Peer-review started: August 3, 2018
First decision: August 24, 2018
Revised: September 17, 2018
Accepted: October 11, 2018
Article in press: October 11, 2018
Published online: October 25, 2018
Processing time: 84 Days and 3 Hours
Eosinophilic oesophagitis (EoE) and gastro-oesophageal reflux disease (GORD) are the most common causes of chronic oesophagitis and dysphagia associated with oesophageal mucosal eosinophilia. Distinguishing between the two is imperative but challenging due to overlapping clinical and histological features. A diagnosis of EoE requires clinical, histological and endoscopic correlation whereas a diagnosis of GORD is mainly clinical without the need for other investigations. Both entities may exhibit oesophageal eosinophilia at a similar level making a histological distinction between them difficult. Although the term proton-pump inhibitor responsive oesophageal eosinophilia has recently been retracted from the guidelines, a relationship between EoE and GORD still exists. This relationship is complex as they may coexist, either interacting bidirectionally or are unrelated. This review aims to outline the differences and potential relationship between the two conditions, with specific focus on histology, immunology, pathogenesis and treatment.
Core tip: The relationship between gastro-oesophageal reflux disease and eosinophilic oesophagitis is complex as they may coexist, either interacting bidirectionally or are unrelated. This review aims to outline the differences and potential relationship between the two conditions, with specific focus on histology, immunology, pathogenesis and treatment.
- Citation: Wong S, Ruszkiewicz A, Holloway RH, Nguyen NQ. Gastro-oesophageal reflux disease and eosinophilic oesophagitis: What is the relationship? World J Gastrointest Pathophysiol 2018; 9(3): 63-72
- URL: https://www.wjgnet.com/2150-5330/full/v9/i3/63.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v9.i3.63
Eosinophilic oesophagitis (EoE) is a clinicopathological condition characterised by an antigen-driven immunologic process that manifests clinically with symptoms of oesophageal dysfunction and histologically by eosinophilic inflammation[1]. The first case report of oesophageal eosinophilia can be traced back as far as 1962 by Schreiber[2], followed by the first published case series of EoE as a distinct clinicopathological condition in 1993 by Attwood et al[3] In 2007, the first consensus recommendation by an international expert panel for the diagnosis and treatment of EoE was published[4]. This consensus was recently updated in 2017[5].
The recognition of EoE has increased so swiftly that it is now thought to be the most frequent eosinophilic gastrointestinal disorder as well as the second most common cause of chronic oesophagitis and dysphagia after gastro-oesophageal reflux disease (GORD)[6]. Although it is still an uncommon disease, the prevalence has been increasing over the past few years with an estimated prevalence in the general population of 13-49 cases/100000 persons[5,7]. This is also in keeping with an increasing incidence of EoE estimated at 1-20 cases/100000 persons[5,7]. Various hypotheses have been considered for this phenomenon particularly that of an increase in the recognition of the disease and an increase in volume of endoscopies performed[8-10]. However, two population-based studies have shown that the incidence and cumulative prevalence of EoE has indeed increased more than the rate of annual endoscopies during the observation period[11,12]. This, therefore, argues in favour of a true rise in the incidence and prevalence of the disease.
Attwood et al[3] first characterized EoE as a distinct entity from GORD in 1993 where patients with more than 20 eosinophils per high power field and dysphagia in the absence of endoscopic oesophagitis and a normal 24-h pH testing were proposed to have EoE. According to the diagnostic criteria for EoE, other diseases associated with oesophageal eosinophilia must be excluded before a diagnosis of EoE is made (Table 1), with the main differential being GORD[1,13,14]. It is important to distinguish between EoE and GORD as their pathogenesis, natural history, monitoring and treatment differ[15]. This is challenging as many of their clinical and histological features overlap[15,16]. Given the prevalence of GORD in the general population is approximately 20%, it is inevitable that there will be a high probability for EoE to co-exist with GORD[16].
| GORD |
| Eosinophilic gastrointestinal diseases |
| Atopy |
| Coeliac disease |
| Crohn’s disease |
| Oesophageal infections |
| Hypereosinophilic syndrome |
| Achalasia |
| Drug hypersensitivity |
| Vasculitis |
| Pemphigoid vegetans |
| Connective tissue disease |
| Graft-versus-host-disease |
| Oesophageal atresia |
Prior to the 2017 consensus, a lack of response to a 2-mo course of a proton-pump inhibitor (PPI) was required exclude PPI-responsive oesophageal eosinophilia (PPI-REE) and confirm the diagnosis of EoE[1]. Patients with PPI-REE presented symptomatically like a typical EoE patient, had GORD diagnostically excluded and exhibited a clinicopathologic response to PPI therapy[1]. Recent evidence, however, indicate that differentiating PPI-REE from EoE is counterintuitive as their phenotypic, molecular, mechanistic and therapeutic features cannot be reliably distinguished[15,17-20]. Also, there was no definition regarding the extent of clinical and histological response required to diagnose PPI-REE[13,15]. Thus, the most recent consensus has retracted the term PPI-REE and considers PPI therapy as a therapeutic agent, rather than a diagnostic criterion[5]. The term “PPI-responsive EoE” has been proposed to replace the now defunct PPI-REE[20].
Despites the fact that PPI responders are now considered to be within the EoE continuum, a relationship between EoE and GORD still exists[5]. Studies have suggested that up to 30%-40% of EoE patients may be PPI responsive, either due to a reduction in acid secretion in patients with co-existent GORD or by means of other still unknown anti-inflammatory mechanisms[21,22]. PPI therapy may also be helpful in patients with EoE as the altered oesophagus may be predisposed and more sensitive to acid exposure[23]. This review aims to outline the factors that differentiate between EoE and GORD as well as to evaluate the complex relationship between the two entities in term of pathophysiology and immunology.
The main pathogenic mechanism of GORD is increased transient lower oesophageal sphincter (LOS) relaxations (TLOSRs), leading to excessive reflux of gastric acid to the lower oesophageal mucosa[24]. Other potential mechanistic factors that can increase acid reflux to the oesophagus are impaired LOS resting pressure, impaired oesophageal acid clearance, delayed gastric emptying and anatomical factors, such as a hiatus hernia[24]. More recently, impaired mucosal resistance and increased visceral hypersensitivity to acid have also been reported to predispose to GORD[24]. Histologically, it was thought that erosive changes in the distal oesophagus developed due to direct chemical-induced injury of the oesophageal mucosa and death of surface cells[25]. Such injury has been shown to provoke a T-helper Type 1 (Th1) inflammatory response, activating mostly granulocytes and lymphocytes[25]. Thus, it is intriguing that oesophageal eosinophilia can occasionally be seen in GORD, and the underlying mechanism remains unclear[26]. A study showing that GORD may also be a cytokine-mediated disease lead to the discovery that oesophageal squamous cells from EoE and GORD patients exhibit similar levels of eotaxin-3 (a chemokine that attracts eosinophils) when stimulated by T-helper Type 2 (Th2) cytokines; production of which is typical of an allergic disorder[10,15,22,26,27]. This suggests that GORD may be driven to a Th2 inflammatory response when the appropriate stimulus is present leading to oesophageal eosinophilia[26]. Low intraluminal baseline impedance has been shown to be associated with dilatation of intercellular spaces and increased acid exposure in patients with GORD[28]. However, whether this damage can lead to exposure of food allergens and subsequently a Th2 response is unknown[26,29,30].
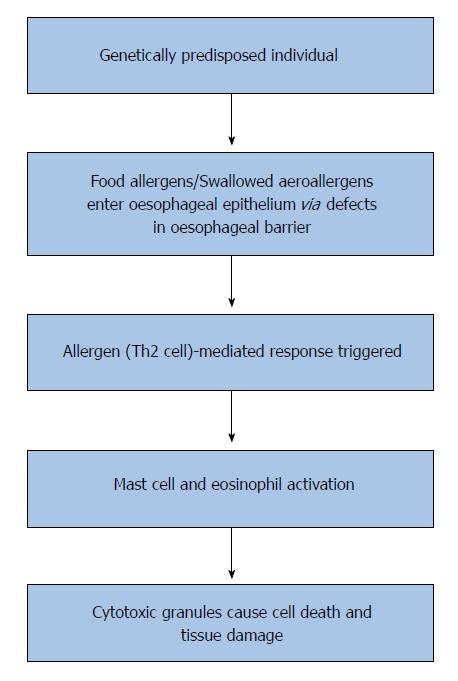
Although the exact pathophysiology of EoE is not fully understood, substantial evidence exists to show that EoE is an allergen (Th2 cell)-mediated response in genetically predisposed individuals (Figure 1)[10,31,32]. Defects in the oesophageal barrier are thought to facilitate the entry of food allergens or swallowed aeroallergens into the oesophageal epithelium which trigger a Th2 response and lead to mast cell activation and release of mediators such as interleukin (IL)-5, which is a known eosinophil activator[10,22]. Activated eosinophils then release cytotoxic granules which contribute to cell death and tissue damage in these patients[10,33,34]. The gene coding for eotaxin-3, CCL26 is overexpressed in the oesophagus of patients with EoE compared to healthy controls, which correlates with the increased levels of IL-5 and IL-13 in the oesophagus and blood of EoE patients[35,36]. The development of EoE may also be associated with a genetic predisposition[10]. Hereditary collagen disorders such as Marfan and Ehlers-Danlos syndromes are the most frequent associations of EoE with an incidence of about one percent[21]. In patients with atopic dermatitis, a loss of function mutation in the gene filaggrin (2282del4) is overexpressed in EoE patients compared with healthy controls[37]. Filaggrin is a key structural, keratin-binding protein that plays an important role in the maturation of skin as an epithelial barrier by preventing keratin proteolysis[37]. EoE has been shown in paediatric patients to be associated with variants at chromosome 5q22 encompassing the gene TSLP (thymic stromal lymphopoetin), which encodes a cytokine that controls dendritic cell-mediated Th2-cell responses[21,38]. More recently, EoE susceptibility locus was found at 2p23 which encodes CAPN14, which is upregulated on exposure to IL-13[39]. However, the exact impact of these genetic abnormalities on the pathogenesis of EoE is uncertain.
A few epidemiological differences exist between GORD and EoE. GORD is typically diagnosed in the second to fifth decade of life[20]. In contrast, EoE has a bimodal age presentation, with one peak in childhood and the second in the third and fourth decade with the mean age of diagnosis of 38 years[1,33,40]. Furthermore, whilst there is no gender preponderance in GORD, EoE affects males three times more than females[1,41,42]. Both conditions have been more frequently reported in Caucasians compared with other ethnicities[1,8,41,43]. It should be noted that the prevalence of GORD is much higher than that of EoE, ranging between 10%-20% in the Western population as compared to less than 1% for EoE[8,9,40,41]. Obesity has been shown to associated with GORD whereas EoE is strongly associated with atopic diseases such as asthma, food allergy, eczema, environmental allergies and chronic rhinitis[1,8,10,31,44].
GORD has been defined by the Montreal Classification as a condition that occurs due to retrograde flow of gastric contents into the oesophagus that lead to troublesome symptoms, which are typically heartburn and regurgitation[45,46]. Other less common symptoms include chest pain, dysphagia, dyspepsia, epigastric pain, nausea, bloating, belching, chronic cough, asthma, laryngitis and other respiratory symptoms[45-48]. Whilst dysphagia is infrequent in GORD, it is the most common presenting symptom for EoE along with food bolus impaction[1,10,49]. Approximately 50% of patients who present with food bolus impaction and up to 15% of patients who undergo endoscopy for non-obstructive dysphagia will have EoE[6,50]. Although some EoE patients report GORD symptoms, they may respond poorly to PPIs[51]. Fifty to eighty percent of EoE patients have a prior history of atopic symptoms[21]. Other non-specific symptoms include chest pain, heartburn, regurgitation, dyspepsia, nausea and vomiting, odynophagia, abdominal pain and non-specific throat symptoms[1,10,31,33,49,52].
A diagnosis of GORD is usually based on clinical symptoms, typically heartburn and regurgitation, in a patient who is responsive to PPI therapy[46]. Thus, upper endoscopy, routine biopsies from the distal oesophagus and ambulatory pH testing are not usually required in a patient with typical GORD symptoms in the absence of alarm symptoms such as dysphagia, odynophagia and weight loss[16,44,46]. The diagnosis of EoE on the other hand, relies on a correlation between clinical symptoms, endoscopic and histological features as there is no one pathognomonic feature of EoE[10,13]. According to the most recent consensus, it requires the presence of ≥ 15 intraepithelial eosinophils per high power field in one or more oesophageal mucosal biopsies in combination with symptoms of oesophageal dysfunction[5]. However, this definition may be too simplified as the diagnosis of EoE may be established with a lower intraepithelial eosinophil count if there is strong clinical suspicion and other histological features associated with eosinophilic inflammation are present[1,10]. Given that excessive accumulation of eosinophils in tissues is a common finding in numerous gastrointestinal disorders, other causes of oesophageal eosinophilia (Table 1) should also be excluded, particularly GORD[1,14]. The following diagnostic features that may be found in GORD and EoE and may help distinguish between the two entities are summarised in Table 2.
| GORD | EoE | |
| Endoscopic | Erosive oesophagitis | Trachealization |
| Peptic strictures | Felinization | |
| Hiatus hernia | Whitish exudates | |
| Barrett’s oesophagus | Longitudinal furrows | |
| Oedema | ||
| Diffuse oesophageal narrowing | ||
| Narrow-calibre oesophagus | ||
| Oesophageal lacerations | ||
| Loss of mucosal vascular pattern | ||
| Histological | Eosinophilia < 10/hpf | Eosinophilia ≥ 15/hpf |
| Eosinophilic microabscesses | ||
| Eosinophil degranulation | ||
| Basal cell hyperplasia | ||
| Papillary lengthening | ||
| Superficial layering of eosinophils | ||
| Extracellular eosinophil granules | ||
| Intracytoplasmic keratinocyte vacuolation | ||
| Dilated intracellular spaces | ||
| Lamina propria fibrosis | ||
| Positive intrasqamous IgG4 | ||
| Motor function | Non-specific | Non-specific |
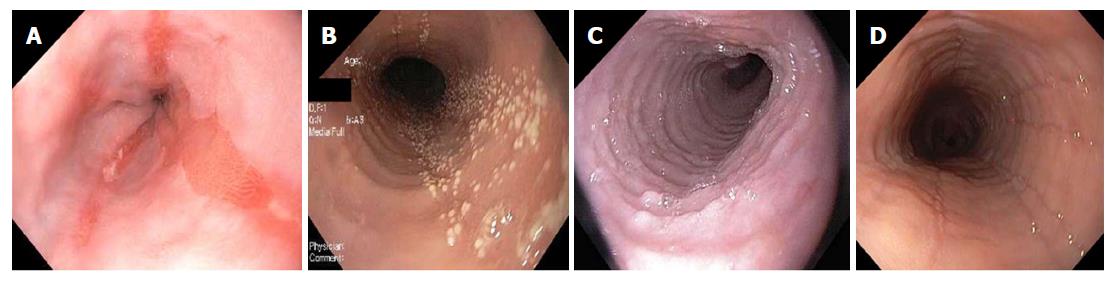
Relevant endoscopic findings of GORD are erosive oesophagitis, peptic strictures, a hiatus hernia and Barrett’s oesophagus[15,16,46]. Endoscopy has a high specificity for diagnosing GORD particularly when erosive oesophagitis is seen and the Los Angeles classification is used[53]. However, most patients with GORD will have normal endoscopies[15,16]. In contrast, endoscopic oesophageal features of EoE patients are trachealization, felinization, whitish exudates, longitudinal furrows, oedema, diffuse oesophageal narrowing, narrow-calibre oesophagus and oesophageal lacerations secondary to passage of the endoscope[1,10,13,16,54] (Figure 2). Loss of mucosal vascular pattern has also been reported[55]. These features however, are not pathognomonic for EoE and thus histological correlation is required[1,10]. Normal endoscopic findings have been reported in up to 30% of patients with EoE[10,13].
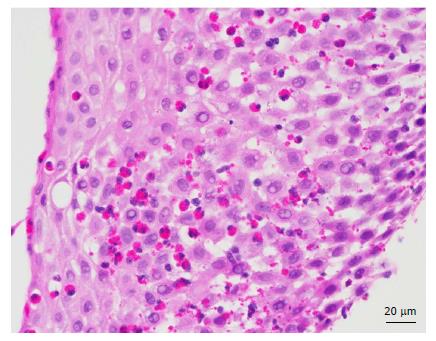
Patients with GORD may exhibit oesophageal eosinophilia, typically less than 10 per high power field as compared to ≥ 15 per high power field for EoE[1,10,15,56] (Figure 3). The presence of additional histological features of eosinophilic microabscesses, eosinophil degranulation, basal cell hyperplasia, papillary lengthening, superficial layering of eosinophils, extracellular eosinophil granules, intracytoplasmic keratinocyte vacuolation, dilated intracellular spaces or lamina propria fibrosis are more supportive of a diagnosis of EoE[1,10,13,16,57]. Although some of these additional histological features have been reported in biopsy specimens of patients with GORD, they are less commonly found as compared to EoE[10,13,16,57]. Recently, Zukerberg et al[17] showed that immunohistochemical staining of oesophageal tissue with IgG4 could help distinguish EoE from GORD, given that 76% of EoE cases were positive for intrasqamous IgG4 and none of the GORD cases were positive. The distribution of oesophageal eosinophilia may also be helpful in distinguishing the two conditions, with diffuse oesophageal eosinophilia more suggestive of EoE and distal oesophageal eosinophilia of GORD[16]. Thus, it is important to biopsy at least 2 regions of the oesophagus and accurately label the site of oesophageal biopsies.
Oesophageal manometry is of limited use in the diagnosis of GORD and EoE given that findings have so far been non-specific[1,13,58]. Oesophageal motility disorders found in patients with GORD have a similar type and prevalence to patients with EoE ranging between 4%-87%[14,21,33]. However, in cases where dysphagia is the main symptom, it is important to perform manometric assessment to exclude major and minor disorders of peristalsis which can sometimes mimic symptoms of GORD and EoE[18,33]. The duration of EoE has been shown to be longer in those with abnormal oesophageal motility[59].
The initial management of GORD usually involves a combination of lifestyle interventions and medical therapy with the aim of eliminating symptoms, repairing any existing oesophageal mucosal injury and preventing further inflammatory injury[46,60]. Lifestyle interventions of weight loss (particularly if BMI > 25 or recent weight gain) and head of bed elevation have been proven to reduce symptoms and improve oesophageal pH values[61,62]. Other lifestyle interventions such as avoidance of late evening meals and cessation of alcohol, tobacco, chocolate, caffeine, spicy foods, citrus and carbonated drinks lack evidence and are not routinely recommended[46]. Medical therapy such as antacids, histamine-receptor antagonists (H2RA) or PPI therapy should then be considered in patients failing lifestyle interventions alone[46,60]. PPI therapy is effective in 70%-80% of patients and has been shown to be superior to H2RAs in regard to healing rates and decreased relapse rates[63]. Surgical therapy is as effective as medical therapy and may be contemplated in GORD patients who wish to discontinue medications, are non-compliant, have side-effects associated with medications, have a large hiatus hernia or have refractory oesophagitis and symptoms despite optimal medical therapy[46].
The choice of initial treatment for EoE patients on the other hand is made on an individualized basis as PPI therapy, topical steroids and dietary therapy can all be considered as first-line therapeutic options[5]. All EoE patients should receive treatment to improve quality of life, prevent oesophageal remodelling secondary to active eosinophilic inflammation and prevent oesophageal injury due to the disease or endoscopic intervention[64]. 30%-40% of EoE patients may be responsive to PPIs, either due to a reduction in acid secretion in patients with co-existent GORD or by means of other still unknown anti-inflammatory mechanisms[21,22]. EoE patients can also be treated with topical steroids as it has been shown to improve symptoms and reduces oesophageal eosinophilia[21,65]. Viscous steroids have been shown to be more effective than nebulized steroids possibly due to greater mucosal contact time compared with the latter[66]. A recent meta-analysis of seven randomized controlled trials concluded that although there was an increased risk of asymptomatic oesophageal candidiasis with topical steroid therapy, it is considered safe with no evidence of adrenal suppression[67]. Dietary therapy is based on the fact that the majority of EoE patients have food allergies that may contribute to the pathogenesis of the disease[22,68]. There are 3 strategies of dietary therapy: An amino acid-based formula/elemental diet, a targeted elimination diet guided by allergy testing, and an empiric elimination diet[22,65,68]. All diets should be followed for a minimum of 6 wk and its efficacy evaluated via symptoms as well and oesophageal biopsies[65,69].
Oesophageal dilation, either via through-the-scope balloons or by Savary bougies can lead to long-lasting symptom improvement in EoE patients with structuring disease or impaired oesophageal distensibility due to subepithelial fibrosis[21,22]. Clinical improvement post dilation occurred in 75% of patients[70]. A meta-analysis evaluating the clinical efficacy and safety of oesophageal dilation in these patients showed that it is a safe procedure with a < 1% rate of serious complications[70]. However, it does not result in a decreased in eosinophil infiltration or histologic improvement and thus should not be used as a sole therapeutic option in these patients[5,71]. Several other treatment options for EoE have been assessed namely Montelukast (leukotriene receptor antagonist), Infliximab (anti-tumour necrosis factor), Mepolizumab (anti-IL-5), Azathioprine or 6-mercaptopurine, Reslizumab (IL-5 neutralizing antibody), Omalizumab (anti-IgE), QAX576 (anti-IL-13) and OC000459 (prostaglandin D2 receptor antagonist)[34,64,72-80] Although studies of these agents have shown changes in the biological behaviour of EoE disease markers, they have not yet displayed sufficient clinical benefit for widespread use[81].
The interaction between EoE and GORD is complex and may be bidirectional[5]. An approximate prevalence of GORD in the general population of 20% is sufficiently high enough to make the coexistence of EoE and GORD plausible[16]. In patients with refractory GORD symptoms, EoE was found in approximately 4%[10,56]. Four hypotheses to account for interactions between oesophageal eosinophilia and GORD have been proposed: Eosinophilia as a marker of GORD; GORD and EoE coexist but are unrelated, EoE contributes or causes GORD; and GORD contributes to or causes EoE[16,20,82,83].
GORD is thought to cause a mild eosinophilia in the absence of EoE[16,82]. Acid exposure was thought to cause oesophageal injury which results in chronic inflammation, including the presence of oesophageal eosinophils that are recruited via an increase in expression of adhesion molecules, release of chemokines that attract eosinophils and increase in blood flow[16]. However, the role of these adhesion molecules and chemokines in the pathogenesis of GORD is yet unclear[16]. A study also showed that dense oesophageal eosinophilia in GORD was uncommon[3].
As mentioned above, due to a high prevalence of GORD in the general population, the coexistence of EoE and GORD due to chance alone is plausible[16,83]. Oesophageal pH studies have shown that 25%-50% of EoE patients have increased oesophageal acid exposure, thus supporting the notion that the two entities can coexist[1,16].
This hypothesis is based on the fact that eosinophils secrete a number of agents that affect the integrity of the mucosal barrier and the function of oesophageal smooth muscle as well as producing a direct cytotoxic effect on the mucosa[16,20]. Remodelling effect in EoE may contribute to increased acid exposure due to effects on the LOS or impaired oesophageal clearance of refluxed contents[16,20].
The relationship between EoE and GORD is complex as they are different entities that may coexist. Distinguishing between the two remains challenging given that it has multiple overlapping features. At present, the combination of clinical, endoscopic and histological features, as well as response to PPI therapy, may help to differentiate the two conditions. Further studies into the immuno-pathophysiology are needed to elucidate more objective diagnostic testing that can reliably differentiate between the two disease processes.
| 1. | Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, Burks AW, Chehade M, Collins MH, Dellon ES. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3-20.e6; quiz 21-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1605] [Cited by in RCA: 1521] [Article Influence: 101.4] [Reference Citation Analysis (1)] |
| 2. | Schreiber MH. Granuloma of the esophagogastric junction with eosinophilic infiltration. Gastroenterology. 1962;43:206-211. [PubMed] |
| 3. | Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 623] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 4. | Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, Bonis P, Hassall E, Straumann A, Rothenberg ME; First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1175] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 5. | Lucendo AJ, Molina-Infante J, Arias Á, von Arnim U, Bredenoord AJ, Bussmann C, Amil Dias J, Bove M, González-Cervera J, Larsson H, Miehlke S, Papadopoulou A, Rodríguez-Sánchez J, Ravelli A, Ronkainen J, Santander C, Schoepfer AM, Storr MA, Terreehorst I, Straumann A, Attwood SE. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J. 2017;5:335-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 773] [Article Influence: 85.9] [Reference Citation Analysis (1)] |
| 6. | Arias Á, Pérez-Martínez I, Tenías JM, Lucendo AJ. Systematic review with meta-analysis: the incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. 2016;43:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 218] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 7. | Shaheen NJ, Mukkada V, Eichinger CS, Schofield H, Todorova L, Falk GW. Natural history of eosinophilic esophagitis: a systematic review of epidemiology and disease course. Dis Esophagus. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43:201-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 9. | Mahesh VN, Holloway RH, Nguyen NQ. Changing epidemiology of food bolus impaction: is eosinophilic esophagitis to blame? J Gastroenterol Hepatol. 2013;28:963-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Ali MA, Lam-Himlin D, Voltaggio L. Eosinophilic esophagitis: a clinical, endoscopic, and histopathologic review. Gastrointest Endosc. 2012;76:1224-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology. 2003;125:1660-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 12. | Giriens B, Yan P, Safroneeva E, Zwahlen M, Reinhard A, Nydegger A, Vavricka S, Sempoux C, Straumann A, Schoepfer AM. Escalating incidence of eosinophilic esophagitis in Canton of Vaud, Switzerland, 1993-2013: a population-based study. Allergy. 2015;70:1633-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Schoepfer A. Diagnostic approach to eosinophilic oesophagitis: Pearls and pitfalls. Best Pract Res Clin Gastroenterol. 2015;29:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Lucendo AJ. Disease associations in eosinophilic oesophagitis and oesophageal eosinophilia. Best Pract Res Clin Gastroenterol. 2015;29:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Molina-Infante J, Bredenoord AJ, Cheng E, Dellon ES, Furuta GT, Gupta SK, Hirano I, Katzka DA, Moawad FJ, Rothenberg ME, Schoepfer A, Spechler SJ, Wen T, Straumann A, Lucendo AJ; PPI-REE Task Force of the European Society of Eosinophilic Oesophagitis (EUREOS). Proton pump inhibitor-responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut. 2016;65:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 16. | Kia L, Hirano I. Distinguishing GERD from eosinophilic oesophagitis: concepts and controversies. Nat Rev Gastroenterol Hepatol. 2015;12:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Zukerberg L, Mahadevan K, Selig M, Deshpande V. Oesophageal intrasquamous IgG4 deposits: an adjunctive marker to distinguish eosinophilic oesophagitis from reflux oesophagitis. Histopathology. 2016;68:968-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Santander C, Chavarría-Herbozo CM, Becerro-González I, Burgos-Santamaría D. Impaired esophageal motor function in eosinophilic esophagitis. Rev Esp Enferm Dig. 2015;107:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Jiao D, Ishimura N, Maruyama R, Ishikawa N, Nagase M, Oshima N, Aimi M, Okimoto E, Mikami H, Izumi D. Similarities and differences among eosinophilic esophagitis, proton-pump inhibitor-responsive esophageal eosinophilia, and reflux esophagitis: comparisons of clinical, endoscopic, and histopathological findings in Japanese patients. J Gastroenterol. 2017;52:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Molina-Infante J, van Rhijn BD. Interactions between gastro-oesophageal reflux disease and eosinophilic oesophagitis. Best Pract Res Clin Gastroenterol. 2015;29:749-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Guarino MP, Cicala M, Behar J. Eosinophilic esophagitis: New insights in pathogenesis and therapy. World J Gastrointest Pharmacol Ther. 2016;7:66-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Schoepfer A, Safroneeva E, Straumann A. Eosinophilic Esophagitis: Impact of Latest Insights Into Pathophysiology on Therapeutic Strategies. Dig Dis. 2016;34:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 23. | Krarup AL, Villadsen GE, Mejlgaard E, Olesen SS, Drewes AM, Funch-Jensen P. Acid hypersensitivity in patients with eosinophilic oesophagitis. Scand J Gastroenterol. 2010;45:273-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | De Giorgi F, Palmiero M, Esposito I, Mosca F, Cuomo R. Pathophysiology of gastro-oesophageal reflux disease. Acta Otorhinolaryngol Ital. 2006;26:241-246. [PubMed] |
| 25. | Molina-Infante J, Gonzalez-Cordero PL, Lucendo AJ. Proton pump inhibitor-responsive esophageal eosinophilia: still a valid diagnosis? Curr Opin Gastroenterol. 2017;33:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Cheng E, Zhang X, Huo X, Yu C, Zhang Q, Wang DH, Spechler SJ, Souza RF. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. 2013;62:824-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 268] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 27. | Dunbar KB, Agoston AT, Odze RD, Huo X, Pham TH, Cipher DJ, Castell DO, Genta RM, Souza RF, Spechler SJ. Association of Acute Gastroesophageal Reflux Disease With Esophageal Histologic Changes. JAMA. 2016;315:2104-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 182] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 28. | Kandulski A, Weigt J, Caro C, Jechorek D, Wex T, Malfertheiner P. Esophageal intraluminal baseline impedance differentiates gastroesophageal reflux disease from functional heartburn. Clin Gastroenterol Hepatol. 2015;13:1075-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 29. | Tobey NA, Carson JL, Alkiek RA, Orlando RC. Dilated intercellular spaces: a morphological feature of acid reflux--damaged human esophageal epithelium. Gastroenterology. 1996;111:1200-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 332] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 30. | Katzka DA. The complex relationship between eosinophilic esophagitis and gastroesophageal reflux disease. Dig Dis. 2014;32:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 32. | Ronkainen J, Talley NJ, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E, Vieth M, Stolte M, Walker MM. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut. 2007;56:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 33. | Weiss AH, Iorio N, Schey R. Esophageal motility in eosinophilic esophagitis. Rev Gastroenterol Mex. 2015;80:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Straumann A. Eosinophilic esophagitis: emerging therapies and future perspectives. Gastroenterol Clin North Am. 2014;43:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Blanchard C, Stucke EM, Rodriguez-Jimenez B, Burwinkel K, Collins MH, Ahrens A, Alexander ES, Butz BK, Jameson SC, Kaul A. A striking local esophageal cytokine expression profile in eosinophilic esophagitis. J Allergy Clin Immunol. 2011;127:208-217, 217.e1-217.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 36. | Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, Jameson SC, Kirby C, Konikoff MR, Collins MH. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 682] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 37. | Kawasaki H, Nagao K, Kubo A, Hata T, Shimizu A, Mizuno H, Yamada T, Amagai M. Altered stratum corneum barrier and enhanced percutaneous immune responses in filaggrin-null mice. J Allergy Clin Immunol. 2012;129:1538-46.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 38. | Blanchard C, Stucke EM, Burwinkel K, Caldwell JM, Collins MH, Ahrens A, Buckmeier BK, Jameson SC, Greenberg A, Kaul A. Coordinate interaction between IL-13 and epithelial differentiation cluster genes in eosinophilic esophagitis. J Immunol. 2010;184:4033-4041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 39. | Kottyan LC, Davis BP, Sherrill JD, Liu K, Rochman M, Kaufman K, Weirauch MT, Vaughn S, Lazaro S, Rupert AM. Genome-wide association analysis of eosinophilic esophagitis provides insight into the tissue specificity of this allergic disease. Nat Genet. 2014;46:895-900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 241] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 40. | Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology. 2008;134:1316-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 41. | El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1322] [Article Influence: 110.2] [Reference Citation Analysis (2)] |
| 42. | Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1256] [Cited by in RCA: 1272] [Article Influence: 60.6] [Reference Citation Analysis (1)] |
| 43. | Gonsalves N. Distinct features in the clinical presentations of eosinophilic esophagitis in children and adults: is this the same disease? Dig Dis. 2014;32:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Antunes C, Curtis SA. Gastroesophageal Reflux Disease. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing 2018; . [PubMed] |
| 45. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920; quiz 1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2368] [Cited by in RCA: 2523] [Article Influence: 126.2] [Reference Citation Analysis (2)] |
| 46. | Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-28; quiz 329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1136] [Cited by in RCA: 1148] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 47. | Gerson LB, Kahrilas PJ, Fass R. Insights into gastroesophageal reflux disease-associated dyspeptic symptoms. Clin Gastroenterol Hepatol. 2011;9:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Badillo R, Francis D. Diagnosis and treatment of gastroesophageal reflux disease. World J Gastrointest Pharmacol Ther. 2014;5:105-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 115] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 49. | Sá CC, Kishi HS, Silva-Werneck AL, Moraes-Filho JP, Eisig JN, Barbuti RC, Hashimoto CL, Navarro-Rodriguez T. Eosinophilic esophagitis in patients with typical gastroesophageal reflux disease symptoms refractory to proton pump inhibitor. Clinics (Sao Paulo). 2011;66:557-561. [PubMed] |
| 50. | Kerlin P, Jones D, Remedios M, Campbell C. Prevalence of eosinophilic esophagitis in adults with food bolus obstruction of the esophagus. J Clin Gastroenterol. 2007;41:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 51. | Menard-Katcher P, Marks KL, Liacouras CA, Spergel JM, Yang YX, Falk GW. The natural history of eosinophilic oesophagitis in the transition from childhood to adulthood. Aliment Pharmacol Ther. 2013;37:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 52. | Kinoshita Y, Furuta K, Ishimaura N, Ishihara S, Sato S, Maruyama R, Ohara S, Matsumoto T, Sakamoto C, Matsui T. Clinical characteristics of Japanese patients with eosinophilic esophagitis and eosinophilic gastroenteritis. J Gastroenterol. 2013;48:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 53. | Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1518] [Cited by in RCA: 1697] [Article Influence: 62.9] [Reference Citation Analysis (2)] |
| 54. | Potter JW, Saeian K, Staff D, Massey BT, Komorowski RA, Shaker R, Hogan WJ. Eosinophilic esophagitis in adults: an emerging problem with unique esophageal features. Gastrointest Endosc. 2004;59:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 203] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 55. | Straumann A, Spichtin HP, Bucher KA, Heer P, Simon HU. Eosinophilic esophagitis: red on microscopy, white on endoscopy. Digestion. 2004;70:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 56. | García-Compeán D, González González JA, Marrufo García CA, Flores Gutiérrez JP, Barboza Quintana O, Galindo Rodríguez G, Mar Ruiz MA, de León Valdez D, Jaquez Quintana JO, Maldonado Garza HJ. Prevalence of eosinophilic esophagitis in patients with refractory gastroesophageal reflux disease symptoms: A prospective study. Dig Liver Dis. 2011;43:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 57. | Naik RD, Vaezi MF. Recent advances in diagnostic testing for gastroesophageal reflux disease. Expert Rev Gastroenterol Hepatol. 2017;11:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Kahrilas PJ, Shaheen NJ, Vaezi MF, Hiltz SW, Black E, Modlin IM, Johnson SP, Allen J, Brill JV; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391, 1391.e1-1391.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 399] [Article Influence: 22.2] [Reference Citation Analysis (35)] |
| 59. | van Rhijn BD, Oors JM, Smout AJ, Bredenoord AJ. Prevalence of esophageal motility abnormalities increases with longer disease duration in adult patients with eosinophilic esophagitis. Neurogastroenterol Motil. 2014;26:1349-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 60. | Patti MG. An Evidence-Based Approach to the Treatment of Gastroesophageal Reflux Disease. JAMA Surg. 2016;151:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 61. | Pollmann H, Zillessen E, Pohl J, Rosemeyer D, Abucar A, Armbrecht U, Bornhofen B, Herz R. Effect of elevated head position in bed in therapy of gastroesophageal reflux. Z Gastroenterol. 1996;34 Suppl 2:93-99. [PubMed] |
| 62. | Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA Jr. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;354:2340-2348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 435] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 63. | Labenz J, Malfertheiner P. Treatment of uncomplicated reflux disease. World J Gastroenterol. 2005;11:4291-4299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 64. | Straumann A. Medical therapy in eosinophilic oesophagitis. Best Pract Res Clin Gastroenterol. 2015;29:805-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA; American College of Gastroenterology. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108:679-692; quiz 693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 864] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 66. | Dellon ES, Sheikh A, Speck O, Woodward K, Whitlow AB, Hores JM, Ivanovic M, Chau A, Woosley JT, Madanick RD. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology. 2012;143:321-324.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 260] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 67. | Chuang MY, Chinnaratha MA, Hancock DG, Woodman R, Wong GR, Cock C, Fraser RJ. Topical Steroid Therapy for the Treatment of Eosinophilic Esophagitis (EoE): A Systematic Review and Meta-Analysis. Clin Transl Gastroenterol. 2015;6:e82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 68. | Warners MJ, Vlieg-Boerstra BJ, Bredenoord AJ. Elimination and elemental diet therapy in eosinophilic oesophagitis. Best Pract Res Clin Gastroenterol. 2015;29:793-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | Molina-Infante J, Gonzalez-Cordero PL, Arias A, Lucendo AJ. Update on dietary therapy for eosinophilic esophagitis in children and adults. Expert Rev Gastroenterol Hepatol. 2017;11:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 70. | Moawad FJ, Cheatham JG, DeZee KJ. Meta-analysis: the safety and efficacy of dilation in eosinophilic oesophagitis. Aliment Pharmacol Ther. 2013;38:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 71. | Schoepfer AM, Gonsalves N, Bussmann C, Conus S, Simon HU, Straumann A, Hirano I. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol. 2010;105:1062-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 72. | Attwood SE, Lewis CJ, Bronder CS, Morris CD, Armstrong GR, Whittam J. Eosinophilic oesophagitis: a novel treatment using Montelukast. Gut. 2003;52:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 232] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 73. | Lucendo AJ, De Rezende LC, Jiménez-Contreras S, Yagüe-Compadre JL, González-Cervera J, Mota-Huertas T, Guagnozzi D, Angueira T, González-Castillo S, Arias A. Montelukast was inefficient in maintaining steroid-induced remission in adult eosinophilic esophagitis. Dig Dis Sci. 2011;56:3551-3558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 74. | Straumann A, Bussmann C, Conus S, Beglinger C, Simon HU. Anti-TNF-alpha (infliximab) therapy for severe adult eosinophilic esophagitis. J Allergy Clin Immunol. 2008;122:425-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 75. | Assa’ad AH, Gupta SK, Collins MH, Thomson M, Heath AT, Smith DA, Perschy TL, Jurgensen CH, Ortega HG, Aceves SS. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141:1593-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 335] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 76. | Straumann A, Conus S, Grzonka P, Kita H, Kephart G, Bussmann C, Beglinger C, Smith DA, Patel J, Byrne M. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 444] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 77. | Netzer P, Gschossmann JM, Straumann A, Sendensky A, Weimann R, Schoepfer AM. Corticosteroid-dependent eosinophilic oesophagitis: azathioprine and 6-mercaptopurine can induce and maintain long-term remission. Eur J Gastroenterol Hepatol. 2007;19:865-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 78. | Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G 3rd, O’Gorman MA, Abonia JP, Young J, Henkel T, Wilkins HJ, Liacouras CA. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129:456-463, 463.e1-463.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 423] [Article Influence: 28.2] [Reference Citation Analysis (1)] |
| 79. | Rocha R, Vitor AB, Trindade E, Lima R, Tavares M, Lopes J, Dias JA. Omalizumab in the treatment of eosinophilic esophagitis and food allergy. Eur J Pediatr. 2011;170:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 80. | Straumann A, Hoesli S, Bussmann Ch, Stuck M, Perkins M, Collins LP, Payton M, Pettipher R, Hunter M, Steiner J, Simon HU. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy. 2013;68:375-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 81. | Attwood S, Sabri S. Historical aspects of eosinophilic esophagitis: from case reports to clinical trials. Dig Dis. 2014;32:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 82. | Cheng E, Souza RF, Spechler SJ. Eosinophilic esophagitis: interactions with gastroesophageal reflux disease. Gastroenterol Clin North Am. 2014;43:243-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 83. | Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007;102:1301-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 84. | van Rhijn BD, Weijenborg PW, Verheij J, van den Bergh Weerman MA, Verseijden C, van den Wijngaard RM, de Jonge WJ, Smout AJ, Bredenoord AJ. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2014;12:1815-1823.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Abdel-Hamid SMM, Akiho H, Mann O, Venu RP S- Editor: Ji FF L- Editor: A E- Editor: Huang Y