Published online Aug 15, 2016. doi: 10.4291/wjgp.v7.i3.266
Peer-review started: April 22, 2016
First decision: June 6, 2016
Revised: June 27, 2016
Accepted: July 20, 2016
Article in press: July 22, 2016
Published online: August 15, 2016
Processing time: 111 Days and 3.8 Hours
Fibrosis represents a major challenge in Crohn’s disease (CD), and many CD patients will develop fibrotic strictures requiring treatment throughout their lifetime. There is no drug that can reverse intestinal fibrosis, and so endoscopic balloon dilatation and surgery are the only effective treatments. Since patients may need repeated treatments, it is important to obtain the diagnosis at an early stage before strictures become symptomatic with extensive fibrosis. Several markers of fibrosis have been proposed, but most need further validation. Biomarkers can be measured either in biological samples obtained from the serum or bowel of CD patients, or using imaging tools and tests. The ideal tool should be easily obtained, cost-effective, and reliable. Even more challenging is fibrosis occurring in ulcerative colitis. Despite the important burden of intestinal fibrosis, including its detrimental effect on outcomes and quality of life in CD patients, it has received less attention than fibrosis occurring in other organs. A common mechanism that acts via a specific signaling pathway could underlie both intestinal fibrosis and cancer. A comprehensive overview of recently introduced biomarkers of fibrosis in CD is presented, along with a discussion of the controversial areas remaining in this field.
Core tip: Fibrosis occurs in a disturbingly large proportion of patients suffering from Crohn’s disease (CD), and invasive procedures may be required for both its diagnosis and treatment. Several biomarkers of intestinal fibrosis have recently been proposed. Most of them still need to be validated, but they could be useful for obtaining an early diagnosis of fibrosis, thereby allowing timely treatment and delaying or even avoiding surgery. A comprehensive overview of recently introduced biomarkers of fibrosis in CD is presented, along with a discussion of the controversial areas remaining in this field.
- Citation: Pellino G, Pallante P, Selvaggi F. Novel biomarkers of fibrosis in Crohn’s disease. World J Gastrointest Pathophysiol 2016; 7(3): 266-275
- URL: https://www.wjgnet.com/2150-5330/full/v7/i3/266.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v7.i3.266
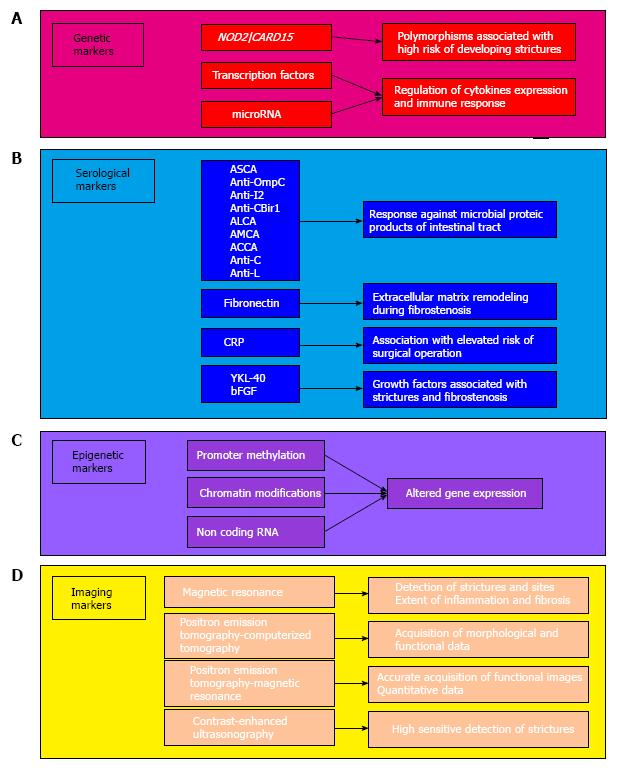
Fibrosis represents a major challenge in Crohn’s disease (CD). Approximately 50% of patients suffering from CD will develop penetrating or fibrotic strictures, and up to 75% of them will eventually need surgery[1-4]. However, fibrosis occurring in the bowel in inflammatory bowel diseases (IBD) is a problem that has been largely neglected by the scientific community, particularly compared with fibrosis occurring in other organs, such as the liver, lung, kidney, and heart[5]. Insufficient resources are allocated for research in intestinal fibrosis, and there is currently no available medical treatment for preventing or reversing fibrosis. All current efforts are focused on improving the ability to obtain an early diagnosis and apply timely treatment, ideally with the aid of noninvasive biomarkers of fibrosis (Table 1, Figure 1).
| Biomarkers | Alteration/finding | Ref. |
| Genetic markers | ||
| NOD2/CARD15 | Polymorphisms | Barrett et al[8], 2008, Lesage et al[9] 2002, Yamazaki et al[10] 2002, Buhner et al[11] 2006, Abreu et al[12] 2002, Jürgens et al[13] 2010, Alvarez-Lobos et al[14] 2005 |
| Epigenetic markers | ||
| MD-2 | Demethylation | Vamadevan et al[39] 2010 |
| IFN-γ | Methylation | Gonsky et al[40] 2011 |
| TH1 | ???? | Gonsky et al[40] 2011 |
| miR-200b | Increase | Chen et al[41] 2012 |
| miR-29a | Decrease | Nijhuis et al[42] 2014 |
| Serological markers | ||
| ASCA | High concentration | Vasiliauskas et al[17] 2000, Forcione et al[18] 2004, Mow et al[20] 2004, Ferrante et al[21] 2007, Rieder et al[22] 2010, Seow et al[23] 2009, Arnott et al[25] 2004, Papp et al[26] 2008, Simondi et al[24] 2008 |
| Anti-OmpC | High concentration | Mow et al[20] 2004, Arnott et al[25] 2004, Ferrante et al[21] 2007, Papp et al[26] 2008 |
| Anti-I2 | High concentration | Mow et al[20] 2004, Arnott et al[25] 2004 |
| Anti-CBir1 | High concentration | Targan et al[19] 2005 |
| ALCA | High concentration | Ferrante et al[21] 2007, Rieder et al[22] 2010, Seow et al[23] 2009, Papp et al[26] 2008, Simondi et al[24] 2008 |
| AMCA | High concentration | Ferrante et al[21] 2007, Rieder et al[22] 2010, Seow et al[23] 2009, Papp et al[26] 2008 |
| ACCA | High concentration | Ferrante et al[21] 2007, Seow et al[23] 2009, Papp et al[26] 2008 |
| Anti-C | High concentration | Rieder et al[22] 2010, Seow et al[23] 2009 |
| Anti-L | High concentration | Rieder et al[22] 2010, Seow et al[23] 2009 |
| CRP | Increase | Henriksen et al[29] 2008 |
| Laminin | Increase | Koutroubakis et al[30] 2003a |
| Collagen IV | Decrease | Koutroubakis et al[30] 2003a |
| Fibronectin | Decrease | Allan et al[28] 1989 |
| YKL-40 | Increase | Koutroubakis et al[33] 2003b |
| bFGF | Increase | Di Sabatino et al[34] 2004 |
| Radiological markers | ||
| MRI | Enhancement patterns | Rimola et al[46] 2015 |
| PET-MRI | Quantitative-qualitative analysis | Catalano et al[53] 2016, Pellino et al[49] 2016 |
Several problems should be considered when investigating biomarkers of intestinal fibrosis in CD[1]. No validated quantitative or qualitative scores are currently available for diagnosing the presence of fibrosis and its extent. There is also no agreement on how to perform biopsies in strictured bowel segments, and the number and depth of samples have varied among the published studies. Furthermore, no standard anatomopathological scoring system has been developed, which increases the difficulties of data interpretation. Lastly, no medical treatment is currently able to reverse intestinal fibrosis once it has occurred.
The pathogenesis of CD is complex, involving interactions between host-predisposing factors and environmental agents. Genetic factors controlling the immune system and the intestinal microbiome are likely to be involved, given that several genetic polymorphisms and variations have been associated with an increased susceptibility to IBD. However, genetic variations are present in fewer than one-quarter of CD patients[6]. Rather than considering chromosomes and genes themselves, it might be better to investigate the mechanisms that control their expression in order to understand and potentially modulate the pathways leading to fibrosis in CD.
There is accumulating evidence that circulating single-stranded, noncoding RNA molecules (microRNA) modulate adaptive immune responses[7]. This is potentially highly significant since it may make it possible to diagnose those patients who are more likely to develop fibrotic strictures at an earlier stage, or to monitor the response to treatment.
NOD2/CARD15 gene polymorphisms are the most widely investigated in intestinal fibrogenesis. They have been associated with a higher risk of developing stricturing CD[8,9], and their expression could be influenced by race[10]. The underlying mechanism could be impairment of barrier function by such genetic mutations[11]. It has been suggested that more than half of the patients carrying an NOD2/CARD15 mutation will develop stricturing CD, with the findings being similar for patients from Europe[9] and North America[12]. One large study investigated the presence of the SNP13 polymorphism of NOD2/CARD15 in patients with ulcerative colitis (UC) and CD, and found that homozygosis was only observed in the latter[12]. Most studies have suggested that the risk of developing strictures increases with the number of mutations[13]. Although clinical decision-making in this field is almost completely unexplored, NOD2 mutations have been associated with a greater need for the resection of strictures and with surgical recurrence[14]. The detection of genetic biomarkers in asymptomatic patients may therefore lead to changes in the management of such patients.
Other genetic and epigenetic factors may also play a role in intestinal fibrogenesis, and they have recently been investigated thoroughly. Genes controlling the expression of several cytokines - particularly interleukin (IL)-10[15] and IL-23[8] - have been associated with an increased risk of bowel stricture, but the evidence is conflicting, and so relying on these genes cannot be recommended for routine clinical practice[13]. Other molecules that are involved in maintaining the homeostasis between profibrotic and antifibrotic mechanisms have been proposed as candidates for diagnosing fibrotic CD at an early stage [e.g., transforming growth factor (TGF) and metalloproteinase][1,5,16], but their possible role as biomarkers needs to be further elucidated.
Obtaining more reliable findings requires prospective, collaborative studies involving several centers across multiple countries aimed at identifying genetic factors underlying fibrosis. Such an approach would lower the costs for each participating unit, make the results more consistent, facilitate the inclusion of a large patient sample, and allow the application of genome-wide analysis to a wide spectrum of genes simultaneously. Moreover, population-based cohorts would be easier to establish and would allow comparison with non-CD individuals.
Probably one of the first and best-characterized class of serological biomarkers of fibrosis in CD pathology is antibody molecules directed against microbial proteic products of the intestinal tract. These mainly comprise the following antibodies: Anti-Saccaromyces-cerevisiae (ASCA), anti-Escherichia-coli outer membrane porine C (anti-OmpC), anti-Pseudomonas-associated sequence-I2 antibodies (anti-I2), antibacterial flagellin antibodies, antilaminaribioside carbohydrate antibodies (ALCA), antimannobioside carbohydrate antibodies, antichitobioside carbohydrate antibodies, antichitin antibody, and antilaminarin antibody. In detail, high levels of ASCA have been found to be associated with fibrostenosis and penetrating disease and, more generally, with a greater need for surgery within 3 years from diagnosis compared with ASCA-negative patients[17-20].
On the other hand, with the exception of ALCA, glycan markers have been associated with complicated CD manifestations (fistulae and strictures) and surgery[21-24]. However, none of these markers has been specifically associated with fibrostenosis or penetrating disease[21-24]. In addition, it has been inferred that the intensity of immune responses influences the CD manifestations and need for surgery[19-22]. However, despite high levels of these markers being found in serum, none of them is currently recommended for use in following the course of the disease[19,20,22].
The antibodies directed against products of Escherichia coli (anti-OmpC and anti-flagellin) and Pseudomonas fluorescens (anti-I2) have been also successfully associated with the presence of fibrosis in CD[19-21,25,26]. It has been also reported that positivity for these three markers is associated with a higher frequency of fibrostenosis or penetrating disease[27].
Other markers have been also described, but diagnostically they are less useful than gut microbial markers. C-reactive protein (CRP) and proteins of the extracellular matrix (ECM; fibronectin) have been associated with fibrostenosis[28-30]. In particular, a prospective study found that the CRP level at diagnosis is closely associated with the subsequent risk of surgery in patients with CD[29].
The ECM plays a key role in disease processes, which has led to fibrinogenesis products related to its accumulation being considered as possible markers. While collagen types I and III are important in the intestinal fibrinogenesis process, the serum levels of direct procollagen precursors were not elevated in CD patients[1,5,16,31,32]. One study of the basement membrane found that while levels of laminin were increased, levels of collagen IV were decreased in CD patients relative to controls, and there was no association with fibrostenosis[30]. Lastly, like collagen IV, the levels of fibronectin were decreased in CD patients relative to controls, but higher levels of fibronectin were associated with the presence of stricture formations in the intestine[28].
Another class of markers is growth factor molecules, the best representatives of which are chitinase-like glycoprotein (YKL-40) and basic fibroblast growth factor (bFGF). YKL-40 has been associated with CD patients affected by strictures[33], and bFGF serum levels are strictly associated with a fibrostenosis phenotype[34].
Biological information is encoded in the genetic pool of each individual, but gene expression can be altered by the environment, making variations inheritable via a mechanism called epigenetics[35]. These epigenetic variations that culminate in the modulation of gene expression are mainly due to a tightly regulated and complex mechanism based on the methylation of DNA, modification of chromatin, and regulation on noncoding RNA, with the last mechanism mainly involving microRNAs. This complex mechanism is also observed in IBD, but its functioning relative to inflammation is not well understood. Nevertheless, it is known that it generally relies on the suppression of gene transcription through methylation of the promoter region. Epigenetic alteration during inflammation could be directly reflected in the activation, tolerance regulation, and regulation of T-cells[36].
Methylation of the promoter DNA and modification of histone proteins represent the main alterations observed in IBD. Several types of DNA methylation typical of intestinal disease that are observed in IBD are likely to be responsible for the altered expression of crucial genes that, in turn, induce the onset and progression of IBD. In this view, an altered methylation profile could be associated with CD. It has also been reported that at least seven CpG islands are differentially methylated in IBD patients[37] and that the pattern of DNA methylation affecting the IL-12 and IL-23 pathways in IBD is subtype-specific[38].
However, little is known about the mechanism through which methylation alters the gene expression and progression of IBD. It has been reported that demethylation of the MD-2 promoter is sufficient to induce the expression of this gene, which is crucial for the Toll-like receptor 4/MD-2 complex[39]. Additionally, it has been observed that methylation alters the levels of interferon-γ in IBD patients, resulting in them being correlated with immune responses[40]. Moreover, the expression profiles of cytokine TH1 have been associated with the epigenome setup of IBD patients[40]. It is therefore clear that genetic factors are involved in the etiology of IBD.
There is compelling evidence for the involvement of microRNAs in the regulation of immune responses in autoimmune pathologies, including CD[7]. This single-stranded noncoding class of RNA is able - via base complementarity - to regulate mRNA translation and stability. In particular, microRNAs belonging to the miR-200 family have been associated with different pathologies via their ability to modulate the key genes involved in the epithelial-to-mesenchymal transition (EMT).
More-promising candidate biomarkers for the pathology of fibrosis are currently represented by the class of noncoding molecules of circulating microRNAs. However, few studies have exploited microRNAs as fibrosis biomarkers. One study found that miR-200b levels were higher in CD patients with fibrosis than in their counterparts without fibrosis, and that TGF-β1 was able to induce the expression of miR-200b[41]. On the other hand, another study found that miR-29a was down-regulated in the serum of CD patients with stricture formations[42]. These two studies are valuable since they demonstrate that some microRNAs can be used to precisely discriminate between inflammatory and fibrotic disease, and thereby aid decisions about the use of therapy or surgery. It would therefore be very useful to find an accurate microRNA biomarker for monitoring the outcome during the course of therapy. Five microRNAs were found to be up-regulated in CD patients after 6 wk of treatment with infliximab, and two of them (let-7d and let-7e) were particularly elevated in patients exhibiting complete clinical remission[43]. These findings clearly suggest the usefulness of microRNA monitoring as a biomarker of the response to therapy in CD patients with intestinal fibrosis.
Because of the complexity of the disease and the continuous characterization of novel biomarkers, it is necessary to create structured collaboration networks for collecting and cataloging the findings of biopsies (including liquid biopsies), and to allocate appropriate resources for translational research. The results obtained on the laboratory bench could rapidly be applied to patients in hospital beds. Networks of this type have already been realized, and new ones focusing on colorectal cancer are being implemented.
Almost all IBD patients need cross-sectional imaging for guiding their clinical management. Magnetic resonance imaging (MRI) is currently considered the ideal tool for identifying fibrosis in CD[44-47]. Studies have found significant variations in the sensitivity and specificity of MRI in detecting strictures. The clinical relevance of detecting stricture is intuitive, but it might be even more important for (1) detecting pathological sites where the stricture of the lumen is too minor to be detected using current MRI technologies; and (2) assessing the extent of inflammation and fibrosis at each detected stricture.
Since there is currently no treatment method for reversing fibrosis once it has settled in the bowel wall in humans, it would be useful to be able to detect asymptomatic sites. This would allow the physician to start medical treatment or modulate an existing treatment based on other indexes of CD activity and clinical parameters.
Inflammation and fibrosis represent two sides of the same coin in CD, and most strictures show both features but to different extents[1,47]. A certain degree of inflammation can be observed even in strictures with an extensive fibrotic component, and vice versa[1]. It is consequently more likely that strictures are predominantly fibrotic or inflammatory, rather than showing features of only inflammation or fibrosis. The latter can occur in CD, but this is extremely rare in practice. This observation has significant clinical implications, because it is now well known that strictures with active inflammation - irrespective of a fibrotic component - can be effectively managed with anti-inflammatory medications[1,48,49]. Knowing the exact proportions of these two components could delay or even avoid surgery in selected patients. At the same time this could identify those patients who are very likely to not respond to medical treatment because they have no active inflammation, and should instead be treated immediately with surgery to avoid unnecessary exposure to drugs.
The focus of recent studies has moved toward quantifying fibrosis in CD using imaging, with MRI showing promise[46]. Such quantification requires both morphological and functional data to be obtained during cross-sectional imaging, which means combining conventional cross-sectional imaging with examinations that are able to detect sites of active inflammation. The most-used cross-sectional hybrid test is the 18fluorodeoxiglucose positron-emission tomography (PET) combined with computed tomography (CT). The method can provide both functional images, provided by PET, and anatomic images, provided by CT. Studies have shown that PET-CT offers advantages over PET alone in CD[50,51]. However, CT involves exposure to ionizing radiation, and most of the information provided by PET-CT can be obtained with current MRI scanning technologies in experienced hands.
PET-MRI is a new hybrid tool that was recently tested in patients with cancers[52,53]. It was shown that a machine that can perform PET and MRI scans simultaneously changed the management of cancer patients[52]. This has led to PET-MRI being used to assess patients with CD. PET-MRI is superior to both MRI alone and PET-CT because it provides functional images that are not available with conventional MRI, and the quality of images is significantly higher for PET-MRI than for PET-CT[49]. PET-MRI is more accurate than PET-CT at detecting extraluminal disease, and may be used to identify patients who are more likely to need fecal diversion during surgery. Moreover, PET-MRI can more reliably identify distant CD sites, hence aiding the selection of patients in whom surgery should either start open or with hand-assisted laparoscopy, reducing the intraoperative time that would be associated with starting with minimally invasive surgery that would eventually need to be converted to open surgery[49]. PET-MRI has been reported to detect fibrosis more accurately than both PET-CT and MRI alone. The use of PET-MRI to select patients suitable for a trial with rescue medical treatment before surgery found that over 70% of them did not require surgery[49]. Even more importantly, PET-MRI can produce quantitative data[53]. A direct correlation has been observed between PET standard uptake values and the degree of inflammation, by testing simultaneously each stricture detected with PET-MRI. Furthermore, some variables can be used to grade the extent of fibrosis quantitatively, ultimately resulting in a more reliable and reproducible way to diagnose CD patients[53]. This results in better patient management, based on agreed criteria, thereby also reducing interobserver and intraobserver variability.
Questions could be raised about the safety of PET-MRI, due to it involving exposure to radioactive nuclides, especially in young patients. However, a PET-MRI examination can be effective even at low radiation doses, and MRI alone can involve exposing patients to higher radiation levels during the reconstruction phase[54].
Shortcomings of PET-MRI are the high costs of individual tests, the requirement for a hybrid machine, and the long acquisition times, which make this technique unsuitable for patients needing immediate treatment and those who cannot tolerate long examinations[49,51,52].
A novel tool that is now being used frequently is contrast-enhanced ultrasonography (US), which has been associated with good sensitivity in detecting strictures[55,56]. However, concerns exist about the ability of US to discriminate between inflammation and fibrosis, and the implementation of a US scan with elastography has not yet been validated. Moreover, the physical shape of a patient can significantly influence the efficacy of US, which may be relevant given that many CD patients show mesenteric obesity (often drug-related), and the capabilities of US are affected by the operator’s ability.
While fibrotic strictures are key features of CD, recent studies have suggested that UC patients can also develop fibrosis. This occurs in the large bowel and raises the concern of malignancy, justifying a surgical approach. In addition to concerns about cancer, thickening and increased stiffness of the large bowel cause several types of dysfunction since they affect intestinal motility[57,58].
Fibrosis in UC was neglected until very recently[57,58], and investigating it further would improve our understanding of the mechanisms underlying fibrosis development. The fibrosis that occurs in UC is particularly intriguing since it might occur without mucosal inflammation[58]. It is known that fibrosis results from chronic inflammation, but cases with a fibrostenosis pattern of CD are often observed. This could reflect a link between CD and UC fibrogenesis. Serological markers of fibrosis in the diagnosis of these patients could also be applied to medical treatments, assessing responses, and the following up of these patients. As an example, mucosal healing is currently used to assess the effectiveness of treatment in IBD, but this pathway might not be involved in patients who progress to fibrosis without significant mucosal involvement.
In addition, investigating UC-related fibrosis could highlight possible differences among CD and UC patients who develop fibrosis and facilitate the identification of biomarkers of fibrosis specific to each of these two entities. In UC patients it could be important to look beyond the mucosal surface[58], and this represents a further challenge in IBD patients that researchers involved in intestinal fibrosis development should take into account.
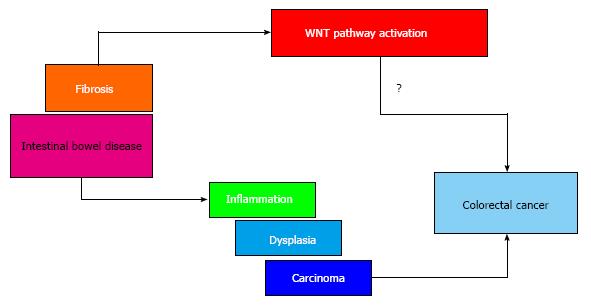
The aberrant activation of the Wnt pathway has recently been associated with the pathogenesis of IBD[59]. It has also been reported that several molecules involved in Wnt signaling are down-regulated in CD tissues: Wnt2, Wnt5a, Wnt5b, Fzd2, Fzd4, Fzd6, LRP6, Dvl, and SFRP1[60,61]. In addition, it has been reported that attenuation of the Wnt pathway by alteration of TCF4 and LRP6 is directly responsible for the diminished production of alpha-defensins by Paneth cells[62] and the dysfunction of the barrier[63]. This represents a hallmark of CD, and hence could potentially represent a therapeutic target[64]. Furthermore, activation of the Wnt/β-catenin pathway is able to induce the EMT and is important for fibrogenesis mediated by TGF-β[65]. In particular, TGF-β is able to stimulate Wnt signaling by suppressing the expression of DKK-1, a Wnt inhibitor. In addition, activation of the Wnt/β-catenin pathway is able to increase ECM synthesis and regulate several MMP genes[66]. It is also reported that blocking the Wnt pathway can reverse the fibrosis, thus representing a useful therapeutic target[67].
The Wnt pathway represents an important signaling pathway during development[68]. Wnt ligands are able to activate either canonical or noncanonical pathways, with the former based on the crucial role of the β-catenin protein[69]. The Wnt pathway is of course also important in adult tissue homeostasis, and it is down-regulated in cancer. In particular, about 80% of colorectal cancer patients carry mutations in key components of the pathway, and in particular activating mutations in β-catenin and inactivating mutations in the adenomatous polyposis coli gene[70]. The Wnt pathway could be activated either directly through mutations of its components, or indirectly through the secretion of triggering ligands or the depletion of inhibitors.
It is well known that the lifetime susceptibility to colorectal cancer is higher among IBD patients than the rest of the population[71,72]. This specific type of carcinogenesis is characterized by the well-defined progression from inflammation to dysplasia to carcinoma, without the appearance of adenoma, as observed in sporadic cancer[72-74], and with several differences in the pathogenesis with respect to the sporadic counterpart. p53 mutations are frequently found in colitis-associated carcinogenesis, whereas in sporadic cases they are only found in advanced disease[75,76]. Moreover, these kinds of mutations are also found in the colonic mucosa adjacent to the area with dysplastic colitis[77,78]. It has also been found that the Wnt pathway is frequently activated very early in colitis-associated carcinogenesis[59], as well in the adjacent nondysplastic mucosa. This clearly suggests that early activation of the Wnt pathway in the area surrounding a dysplastic or malignant lesion in colitis could represent a link between fibrosis and the future development of colorectal cancer (Figure 2).
In recent years several biomarkers of fibrosis have been proposed, tested, and verified in patients with CD. However, validating studies are still needed to confirm the reliability of these markers. This would eventually allow for the development of noninvasive tools to detect them and perform early diagnoses of incipient fibrosis, and hence implement prompt treatment. Moreover, such researches could lead to a better understanding of the mechanisms underlying intestinal fibrogenesis and clarify the potential links between CD fibrosis and cancer.
| 1. | Rieder F, Latella G, Magro F, Yuksel ES, Higgins PD, Di Sabatino A, de Bruyn JR, Rimola J, Brito J, Bettenworth D. European Crohn’s and Colitis Organisation Topical Review on Prediction, Diagnosis and Management of Fibrostenosing Crohn’s Disease. J Crohns Colitis. 2016; Feb 29; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 2. | Latella G, Papi C. Crucial steps in the natural history of inflammatory bowel disease. World J Gastroenterol. 2012;18:3790-3799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg. 2000;231:38-45. [PubMed] |
| 4. | Siassi M, Weiger A, Hohenberger W, Kessler H. Changes in surgical therapy for Crohn’s disease over 33 years: a prospective longitudinal study. Int J Colorectal Dis. 2007;22:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Latella G, Rogler G, Bamias G, Breynaert C, Florholmen J, Pellino G, Reif S, Speca S, Lawrance IC. Results of the 4th scientific workshop of the ECCO (I): pathophysiology of intestinal fibrosis in IBD. J Crohns Colitis. 2014;8:1147-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2110] [Cited by in RCA: 2033] [Article Influence: 127.1] [Reference Citation Analysis (0)] |
| 7. | Dalal SR, Kwon JH. The Role of MicroRNA in Inflammatory Bowel Disease. Gastroenterol Hepatol (NY). 2010;6:714-722. [PubMed] |
| 8. | Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40:955-962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2114] [Cited by in RCA: 2066] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 9. | Lesage S, Zouali H, Cézard JP, Colombel JF, Belaiche J, Almer S, Tysk C, O’Morain C, Gassull M, Binder V. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am J Hum Genet. 2002;70:845-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 725] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 10. | Yamazaki K, Takazoe M, Tanaka T, Kazumori T, Nakamura Y. Absence of mutation in the NOD2/CARD15 gene among 483 Japanese patients with Crohn’s disease. J Hum Genet. 2002;47:469-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Buhner S, Buning C, Genschel J, Kling K, Herrmann D, Dignass A, Kuechler I, Krueger S, Schmidt HH, Lochs H. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut. 2006;55:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 263] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 12. | Abreu MT, Taylor KD, Lin YC, Hang T, Gaiennie J, Landers CJ, Vasiliauskas EA, Kam LY, Rojany M, Papadakis KA. Mutations in NOD2 are associated with fibrostenosing disease in patients with Crohn’s disease. Gastroenterology. 2002;123:679-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 331] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Jürgens M, Brand S, Laubender RP, Seiderer J, Glas J, Wetzke M, Wagner J, Pfennig S, Tillack C, Beigel F. The presence of fistulas and NOD2 homozygosity strongly predict intestinal stenosis in Crohn’s disease independent of the IL23R genotype. J Gastroenterol. 2010;45:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 14. | Alvarez-Lobos M, Arostegui JI, Sans M, Tassies D, Plaza S, Delgado S, Lacy AM, Pique JM, Yagüe J, Panés J. Crohn’s disease patients carrying Nod2/CARD15 gene variants have an increased and early need for first surgery due to stricturing disease and higher rate of surgical recurrence. Ann Surg. 2005;242:693-700. [PubMed] |
| 15. | Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3533] [Cited by in RCA: 3472] [Article Influence: 192.9] [Reference Citation Analysis (0)] |
| 16. | Lawrance IC, Rogler G, Bamias G, Breynaert C, Florholmen J, Pellino G, Reif S, Speca S, Latella G. Cellular and Molecular Mediators of Intestinal Fibrosis. J Crohns Colitis. 2015; Nov 2; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Vasiliauskas EA, Kam LY, Karp LC, Gaiennie J, Yang H, Targan SR. Marker antibody expression stratifies Crohn’s disease into immunologically homogeneous subgroups with distinct clinical characteristics. Gut. 2000;47:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 232] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Forcione DG, Rosen MJ, Kisiel JB, Sands BE. Anti-Saccharomyces cerevisiae antibody (ASCA) positivity is associated with increased risk for early surgery in Crohn’s disease. Gut. 2004;53:1117-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Targan SR, Landers CJ, Yang H, Lodes MJ, Cong Y, Papadakis KA, Vasiliauskas E, Elson CO, Hershberg RM. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005;128:2020-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 355] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 20. | Mow WS, Vasiliauskas EA, Lin YC, Fleshner PR, Papadakis KA, Taylor KD, Landers CJ, Abreu-Martin MT, Rotter JI, Yang H. Association of antibody responses to microbial antigens and complications of small bowel Crohn’s disease. Gastroenterology. 2004;126:414-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 372] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 21. | Ferrante M, Henckaerts L, Joossens M, Pierik M, Joossens S, Dotan N, Norman GL, Altstock RT, Van Steen K, Rutgeerts P. New serological markers in inflammatory bowel disease are associated with complicated disease behaviour. Gut. 2007;56:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 229] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 22. | Rieder F, Schleder S, Wolf A, Dirmeier A, Strauch U, Obermeier F, Lopez R, Spector L, Fire E, Yarden J. Association of the novel serologic anti-glycan antibodies anti-laminarin and anti-chitin with complicated Crohn’s disease behavior. Inflamm Bowel Dis. 2010;16:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Seow CH, Stempak JM, Xu W, Lan H, Griffiths AM, Greenberg GR, Steinhart AH, Dotan N, Silverberg MS. Novel anti-glycan antibodies related to inflammatory bowel disease diagnosis and phenotype. Am J Gastroenterol. 2009;104:1426-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Simondi D, Mengozzi G, Betteto S, Bonardi R, Ghignone RP, Fagoonee S, Pellicano R, Sguazzini C, Pagni R, Rizzetto M. Antiglycan antibodies as serological markers in the differential diagnosis of inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Arnott ID, Landers CJ, Nimmo EJ, Drummond HE, Smith BK, Targan SR, Satsangi J. Sero-reactivity to microbial components in Crohn’s disease is associated with disease severity and progression, but not NOD2/CARD15 genotype. Am J Gastroenterol. 2004;99:2376-2384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 173] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Papp M, Altorjay I, Dotan N, Palatka K, Foldi I, Tumpek J, Sipka S, Udvardy M, Dinya T, Lakatos L. New serological markers for inflammatory bowel disease are associated with earlier age at onset, complicated disease behavior, risk for surgery, and NOD2/CARD15 genotype in a Hungarian IBD cohort. Am J Gastroenterol. 2008;103:665-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Dubinsky MC, Kugathasan S, Mei L, Picornell Y, Nebel J, Wrobel I, Quiros A, Silber G, Wahbeh G, Katzir L. Increased immune reactivity predicts aggressive complicating Crohn’s disease in children. Clin Gastroenterol Hepatol. 2008;6:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 28. | Allan A, Wyke J, Allan RN, Morel P, Robinson M, Scott DL, Alexander-Williams J. Plasma fibronectin in Crohn’s disease. Gut. 1989;30:627-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Henriksen M, Jahnsen J, Lygren I, Stray N, Sauar J, Vatn MH, Moum B; IBSEN Study Group. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57:1518-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 306] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 30. | Koutroubakis IE, Petinaki E, Dimoulios P, Vardas E, Roussomoustakaki M, Maniatis AN, Kouroumalis EA. Serum laminin and collagen IV in inflammatory bowel disease. J Clin Pathol. 2003;56:817-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Rieder F, Fiocchi C. Intestinal fibrosis in IBD--a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 32. | Loeschke K, Kaltenthaler P. [Procollagen-III-peptide in the serum of patients with Crohn disease]. Z Gastroenterol. 1989;27:137-139. [PubMed] |
| 33. | Koutroubakis IE, Petinaki E, Dimoulios P, Vardas E, Roussomoustakaki M, Maniatis AN, Kouroumalis EA. Increased serum levels of YKL-40 in patients with inflammatory bowel disease. Int J Colorectal Dis. 2003;18:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Di Sabatino A, Ciccocioppo R, Armellini E, Morera R, Ricevuti L, Cazzola P, Fulle I, Corazza GR. Serum bFGF and VEGF correlate respectively with bowel wall thickness and intramural blood flow in Crohn’s disease. Inflamm Bowel Dis. 2004;10:573-577. [PubMed] |
| 35. | Bonasio R, Tu S, Reinberg D. Molecular signals of epigenetic states. Science. 2010;330:612-616. [PubMed] [DOI] [Full Text] |
| 36. | Scarpa M, Stylianou E. Epigenetics: Concepts and relevance to IBD pathogenesis. Inflamm Bowel Dis. 2012;18:1982-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Lin Z, Hegarty JP, Cappel JA, Yu W, Chen X, Faber P, Wang Y, Kelly AA, Poritz LS, Peterson BZ. Identification of disease-associated DNA methylation in intestinal tissues from patients with inflammatory bowel disease. Clin Genet. 2011;80:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 38. | Lin Z, Hegarty JP, Yu W, Cappel JA, Chen X, Faber PW, Wang Y, Poritz LS, Fan JB, Koltun WA. Identification of disease-associated DNA methylation in B cells from Crohn’s disease and ulcerative colitis patients. Dig Dis Sci. 2012;57:3145-3153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Vamadevan AS, Fukata M, Arnold ET, Thomas LS, Hsu D, Abreu MT. Regulation of Toll-like receptor 4-associated MD-2 in intestinal epithelial cells: a comprehensive analysis. Innate Immun. 2010;16:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Gonsky R, Deem RL, Landers CJ, Derkowski CA, Berel D, McGovern DP, Targan SR. Distinct IFNG methylation in a subset of ulcerative colitis patients based on reactivity to microbial antigens. Inflamm Bowel Dis. 2011;17:171-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Chen Y, Ge W, Xu L, Qu C, Zhu M, Zhang W, Xiao Y. miR-200b is involved in intestinal fibrosis of Crohn’s disease. Int J Mol Med. 2012;29:601-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 42. | Nijhuis A, Biancheri P, Lewis A, Bishop CL, Giuffrida P, Chan C, Feakins R, Poulsom R, Di Sabatino A, Corazza GR. In Crohn’s disease fibrosis-reduced expression of the miR-29 family enhances collagen expression in intestinal fibroblasts. Clin Sci (Lond). 2014;127:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 43. | Fujioka S, Nakamichi I, Esaki M, Asano K, Matsumoto T, Kitazono T. Serum microRNA levels in patients with Crohn’s disease during induction therapy by infliximab. J Gastroenterol Hepatol. 2014;29:1207-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Panes J, Bouhnik Y, Reinisch W, Stoker J, Taylor SA, Baumgart DC, Danese S, Halligan S, Marincek B, Matos C. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7:556-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 496] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 45. | Ha CY, Kumar N, Raptis CA, Narra VR, Ciorba MA. Magnetic resonance enterography: safe and effective imaging for stricturing Crohn’s disease. Dig Dis Sci. 2011;56:2906-2913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Rimola J, Planell N, Rodríguez S, Delgado S, Ordás I, Ramírez-Morros A, Ayuso C, Aceituno M, Ricart E, Jauregui-Amezaga A. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol. 2015;110:432-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 47. | Rieder F, de Bruyn JR, Pham BT, Katsanos K, Annese V, Higgins PD, Magro F, Dotan I. Results of the 4th scientific workshop of the ECCO (Group II): markers of intestinal fibrosis in inflammatory bowel disease. J Crohns Colitis. 2014;8:1166-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Yaffe BH, Korelitz BI. Prognosis for nonoperative management of small-bowel obstruction in Crohn’s disease. J Clin Gastroenterol. 1983;5:211-215. [PubMed] |
| 49. | Pellino G, Nicolai E, Catalano OA, Campione S, D’Armiento FP, Salvatore M, Cuocolo A, Selvaggi F. PET/MR Versus PET/CT Imaging: Impact on the Clinical Management of Small-Bowel Crohn’s Disease. J Crohns Colitis. 2016;10:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 50. | Lenze F, Wessling J, Bremer J, Ullerich H, Spieker T, Weckesser M, Gonschorrek S, Kannengiesser K, Rijcken E, Heidemann J. Detection and differentiation of inflammatory versus fibromatous Crohn’s disease strictures: prospective comparison of 18F-FDG-PET/CT, MR-enteroclysis, and transabdominal ultrasound versus endoscopic/histologic evaluation. Inflamm Bowel Dis. 2012;18:2252-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 51. | Maccioni F, Patak MA, Signore A, Laghi A. New frontiers of MRI in Crohn’s disease: motility imaging, diffusion-weighted imaging, perfusion MRI, MR spectroscopy, molecular imaging, and hybrid imaging (PET/MRI). Abdom Imaging. 2012;37:974-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Catalano OA, Rosen BR, Sahani DV, Hahn PF, Guimaraes AR, Vangel MG, Nicolai E, Soricelli A, Salvatore M. Clinical impact of PET/MR imaging in patients with cancer undergoing same-day PET/CT: initial experience in 134 patients--a hypothesis-generating exploratory study. Radiology. 2013;269:857-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 53. | Catalano OA, Gee MS, Nicolai E, Selvaggi F, Pellino G, Cuocolo A, Luongo A, Catalano M, Rosen BR, Gervais D. Evaluation of Quantitative PET/MR Enterography Biomarkers for Discrimination of Inflammatory Strictures from Fibrotic Strictures in Crohn Disease. Radiology. 2016;278:792-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 54. | Jones T, Budinger TF. The potential for low-dose functional studies in maternal-fetal medicine using PET/MR imaging. J Nucl Med. 2013;54:2016-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Castiglione F, de Sio I, Cozzolino A, Rispo A, Manguso F, Del Vecchio Blanco G, Di Girolamo E, Castellano L, Ciacci C, Mazzacca G. Bowel wall thickness at abdominal ultrasound and the one-year-risk of surgery in patients with Crohn’s disease. Am J Gastroenterol. 2004;99:1977-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Castiglione F, Testa A, Rea M, De Palma GD, Diaferia M, Musto D, Sasso F, Caporaso N, Rispo A. Transmural healing evaluated by bowel sonography in patients with Crohn’s disease on maintenance treatment with biologics. Inflamm Bowel Dis. 2013;19:1928-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 57. | Gordon IO, Agrawal N, Goldblum JR, Fiocchi C, Rieder F. Fibrosis in ulcerative colitis: mechanisms, features, and consequences of a neglected problem. Inflamm Bowel Dis. 2014;20:2198-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 58. | Latella G, Rieder F. Time to Look Underneath the Surface: Ulcerative Colitis-Associated Fibrosis. J Crohns Colitis. 2015;9:941-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Claessen MM, Schipper ME, Oldenburg B, Siersema PD, Offerhaus GJ, Vleggaar FP. WNT-pathway activation in IBD-associated colorectal carcinogenesis: potential biomarkers for colonic surveillance. Cell Oncol. 2010;32:303-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 60. | Tuller T, Atar S, Ruppin E, Gurevich M, Achiron A. Common and specific signatures of gene expression and protein-protein interactions in autoimmune diseases. Genes Immun. 2013;14:67-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 61. | Hughes KR, Sablitzky F, Mahida YR. Expression profiling of Wnt family of genes in normal and inflammatory bowel disease primary human intestinal myofibroblasts and normal human colonic crypt epithelial cells. Inflamm Bowel Dis. 2011;17:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 62. | Koslowski MJ, Kübler I, Chamaillard M, Schaeffeler E, Reinisch W, Wang G, Beisner J, Teml A, Peyrin-Biroulet L, Winter S. Genetic variants of Wnt transcription factor TCF-4 (TCF7L2) putative promoter region are associated with small intestinal Crohn’s disease. PLoS One. 2009;4:e4496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 63. | Beisner J, Teltschik Z, Ostaff MJ, Tiemessen MM, Staal FJ, Wang G, Gersemann M, Perminow G, Vatn MH, Schwab M. TCF-1-mediated Wnt signaling regulates Paneth cell innate immune defense effectors HD-5 and -6: implications for Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2014;307:G487-G498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 64. | Koslowski MJ, Teltschik Z, Beisner J, Schaeffeler E, Wang G, Kübler I, Gersemann M, Cooney R, Jewell D, Reinisch W. Association of a functional variant in the Wnt co-receptor LRP6 with early onset ileal Crohn’s disease. PLoS Genet. 2012;8:e1002523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 65. | Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun. 2012;3:735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 532] [Cited by in RCA: 710] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 66. | Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26:227-239. [PubMed] |
| 67. | Henderson WR, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci USA. 2010;107:14309-14314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 400] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 68. | Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001;1:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 673] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 69. | Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. EMBO J. 2012;31:2714-2736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 1317] [Article Influence: 94.1] [Reference Citation Analysis (0)] |
| 70. | White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142:219-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 403] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 71. | Annese V, Beaugerie L, Egan L, Biancone L, Bolling C, Brandts C, Dierickx D, Dummer R, Fiorino G, Gornet JM. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. J Crohns Colitis. 2015;9:945-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 336] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 72. | Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 688] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 73. | Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 926] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 74. | Egan L, D’Inca R, Jess T, Pellino G, Carbonnel F, Bokemeyer B, Harbord M, Nunes P, Van der Woude J, Selvaggi F. Non-colorectal intestinal tract carcinomas in inflammatory bowel disease: results of the 3rd ECCO Pathogenesis Scientific Workshop (II). J Crohns Colitis. 2014;8:19-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 75. | Risques RA, Rabinovitch PS, Brentnall TA. Cancer surveillance in inflammatory bowel disease: new molecular approaches. Curr Opin Gastroenterol. 2006;22:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [PubMed] [DOI] [Full Text] |
| 77. | Braakhuis BJ, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730. [PubMed] |
| 78. | Brentnall TA, Crispin DA, Rabinovitch PS, Haggitt RC, Rubin CE, Stevens AC, Burmer GC. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology. 1994;107:369-378. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cario E, Cheifetz AS, Seicean A S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ