Published online Aug 15, 2016. doi: 10.4291/wjgp.v7.i3.256
Peer-review started: March 7, 2016
First decision: April 15, 2016
Revised: April 22, 2016
Accepted: May 10, 2016
Article in press: May 11, 2016
Published online: August 15, 2016
Processing time: 159 Days and 2 Hours
In the field of gastroenterology, breath tests (BTs) are used intermittently as diagnostic tools that allow indirect, non-invasive and relatively less cumbersome evaluation of several disorders by simply quantifying the appearance in exhaled breath of a metabolite of a specific substrate administered. The aim of this review is to have an insight into the principles, methods of analysis and performance parameters of various hydrogen, methane and carbon BTs which are available for diagnosing gastrointestinal disorders such as Helicobacter pylori infection, small intestinal bacterial overgrowth, and carbohydrate malabsorption. Evaluation of gastric emptying is routinely performed by scintigraphy which is however, difficult to perform and not suitable for children and pregnant women, this review has abridged the 13C-octanoic acid test in comparison to scintigraphy and has emphasized on its working protocol and challenges. A new development such as electronic nose test is also highlighted. Moreover we have also explored the limitations and constraints restraining the wide use of these BT. We conclude that breath testing has an enormous potential to be used as a diagnostic modality. In addition it offers distinct advantages over the traditional invasive methods commonly employed.
Core tip: The aim of this review is to have an insight into the principles, methods of analysis and performance parameters of various breath tests available for diagnosing gastrointestinal disorders. Furthermore we have also explored the limitations and constraints restricting the wide use of these tests.
- Citation: Siddiqui I, Ahmed S, Abid S. Update on diagnostic value of breath test in gastrointestinal and liver diseases. World J Gastrointest Pathophysiol 2016; 7(3): 256-265
- URL: https://www.wjgnet.com/2150-5330/full/v7/i3/256.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v7.i3.256
Composition of human breath is a blend of various inert gases as well as nitrogen, oxygen and carbon dioxide (CO2). In addition, researchers have also revealed several other trace volatile organic compounds (VOCs) in breath with concentrations varying from parts per million (ppm) to trillion (ppt)[1,2]. Commonly present VOCs in breath include, ethane, hydrogen, and methanol which are harvests of primary metabolic processes in the body and can play a pivotal role for various medical diagnostics[3].
In the current era of advanced human diagnostics, breath analysis is widely gaining attentiveness of clinicians and laboratories as a noninvasive diagnostic option. Gas analysis sensors and sensor systems are now available, as a product of rapid development in micro and nanotechnology. These tools are being progressively amended for laboratory testing and the more recent discovery of new gas volatile compound biomarkers have opened new horizons for researchers[4].
Speaking from an analytical point of view composition of breath is less complex than serum and urine thus making it a preferable matrix for a comprehensive analysis. Furthermore, these procedures can be easily repeated if the need arises for a recheck.
To identify the disease processes occurring in the gastrointestinal (GI) tract the use of endoscopy and colonoscopy are commonly on the rise, however these modalities are not only invasive and costly but the patients are also more at risk of suffering from complications with significant morbidities. Breath testing provides a solution to some of the practical issues faced in GI testing, although suffers from its own limitations.
We selected articles from the PubMed database and Google scholar by using the search terms “breath test” (BT), “Helicobacter pylori” (H. pylori), “carbon breath test” and “urea breath test” (UBT). Inclusion criteria were articles published in English, in peer-reviewed journals, between 1966 and 2011. The articles were further filtered in a team meeting, keeping in view the ideology behind this mini review, i.e., the current practices, the new advancements and factors limiting the wide use of BTs.
BTs are based on the consumption of numerous substrates that undergo processing at different points in the GI tract. The concept revolves around the fact that the metabolized substrate leads to the production of gases (e.g., CO2, H2) that become part of the blood stream, are expelled and measured in exhaled breath via the different analyzers available.
Moreover hydrogen and carbon BTs are the most widely known and practice, methane BT are also gaining popularity based on the fact that its production is prevalent in 36%-50% of healthy subjects in comparison with hydrogen which is more pervasive. Literature review has shown that a noticeable amount of subjects do not produce hydrogen in spite of having small intestinal bacterial overgrowth (SIBO) because of the presence of the bacterium Methanobrevibacter smithii (M. smithii) which converts hydrogen into methane.
There is a significant rise in the utility of breath testing since their development considering the fact that they are non-invasive and relatively simpler and safer tools for the diagnosis of various disorders of GI tract such as H. pylori infection, gastric motility, SIBO, and sugar malabsorption. Different available BT are summarized in (Table 1).
| Indications |
| Tests for small intestinal bacterial overgrowth |
| Glucose hydrogen breath test |
| Lactulose hydrogen breath test |
| 13C-glycocholate breath test |
| 13C-xylose breath test |
| Tests for carbohydrate malabsorption |
| Fructose hydrogen breath test |
| Lactose hydrogen breath test |
| Saccharose hydrogen breath test |
| 13C-lactose breath test |
| 13C-fructose breath test |
| 13C-saccharose breath test |
| Methane breath test |
| Tests for Helicobacter pylori infection |
| 13C-urea breath test |
| 14C-urea breath test |
| Tests for the evaluation of gastric emptying |
| 13C-octanoic acid breath test |
| Tests for the evaluation of exocrine pancreatic insufficiency |
| 13C-mixed triglycerides breath test |
| 13C-starch breath test |
| 13C-egg protein breath test |
| Tests for the evaluation of hepatocellular function |
| 13C-aminopyrine breath test |
| 13C-ethacetin breath test |
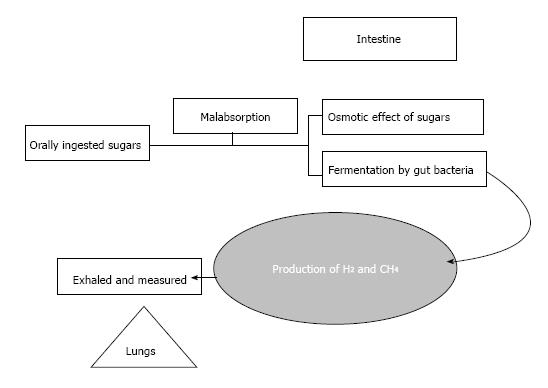
Hydrogen is a product of the intestinal bacterial overgrowth when dietary carbohydrates encounter malabsorption in the small intestine. Hydrogen producing bacteria chiefly reside in the colon. A quantifiable amount of this colonic hydrogen is absorbed into the bloodstream and is exhaled and eventually detected by breath testing[5] (Figure 1).
Hydrogen concentrations are commonly measured using gas chromatography or electrochemical cells. With the rising entity of point of care testing (POCT), portable even pocket sized breath analyzers are now being developed which enable a reliable direct measurement in practice or at bedside[6].
Hydrogen BTs lack standardization in laboratories worldwide which renders the comparison of test results difficult. The dosage of the carbohydrate, the volume of the dissolving fluid, the duration of the test period, the interval of breath samples collection as well as the optimal cut-offs used for reporting differs among test providers.
Hydrogen breath testing for SIBO: Glucose is a preferred substrate to detect SIBO as it follows a prompt reabsorption in the proximal small bowel. The recommended cut-off point diverges between 10 and 20 ppm. In the presence of bacteria in the small intestine, glucose get fermented and liberated in the high quantity and can be detected easily in breath.
Protocol: Subjects are made to undergo an overnight fast. Prerequisites of the test include teeth brushing and use of disinfecting mouth wash and gargles, keeping in mind the fact that oral bacteria can lead to false increment on hydrogen peaks. With the commencement of breath hydrogen sampling basal breath hydrogen is recorded. In circumstance when basal values of breath hydrogen are recorded in excess of 16 ppm, substrates are not given and test is abandoned as according to few researchers high basal hydrogen values are diagnostic of SIBO but this finding remains contentious. A diagnosis of SIBO is made on glucose hydrogen BT if there is an upsurge in breath hydrogen by 12 ppm above the base line levels. Reportedly sensitivity and specificity of this test are 62% and 83% respectively, when compared with culture from jejunal aspirate[7].
Some studies have also suggested lactulose BT for making a diagnosis of SIBO but it was found to be less specific compared to the glucose BT[8].
Lactose hydrogen BT: Four variants of lactase deficiency have been identified, i.e., primary, secondary, developmental and congenital lactase deficiency. Statistics suggest that primary lactase deficiency predominates affecting more than 50% of the world’s population[9,10]. Ethnicity and amount of dairy consumption are the contributing factors, whereas risk is reportedly higher in Asian and American Indian people compared to Europeans[11,12].
Protocol: Baseline hydrogen measurements are taken in expired breath. Fasting subjects are given 50 g lactose orally mixed with water. Further samples to detect the hydrogen quantity are taken at 15-30 min time intervals continued over a period of 4 h. Detection of more than 10-20 ppm over the baseline hydrogen value (detected in at least 2 breath samples) indicates lactose malabsorption.
Improvement is sensitivity have been reported by studies if the test is extended for a period of 6 h with hourly sample collection from 3 to 6 h. However, this is not yet extensively applied as standard clinical practice protocol[13].
False-positive results are seen with recent smoking or inadequate pre-test fasting (high carbohydrate load). False-negative results may arise following recent use of antibiotics, in patients with lung disorders, or in approximately 10% to 20% of patients who are hydrogen non-producers.
Other tests for carbohydrate metabolism use fructose or saccharose as substrate but are not popular for clinical use[14-16].
The addition of methane to hydrogen measurement has improved the diagnostic accuracy of these BTs by capturing the 20% to 30% of the general population which produces methane as a main byproduct of carbohydrate fermentation[17]. Furthermore, Methane testing has also potentially contributed towards an increment in sensitivity of lactose BT[18].
Methane production is prevalent in 36%-50% of healthy subjects in comparison with hydrogen which is more pervasive[19-21]. M. smithii are the chief producers of methane in humans. This process takes place chiefly in the left colon.
Methane production is more disease specific as suggested by different studies, for example: Methane excretion is not found in diarrheal states such as ulcerative colitis or Crohn’s disease and on the other hand it is more frequently observed in diverticulosis[22] and encoparesis[23] related with constipation.
Furthermore literature review revealed significant association between delayed gut motility and CH4. Reportedly mean of transit time in CH4 producers was 84.6 h and in non-producers was 48.6 h[24].
More or less follows the same protocol as hydrogen breath testing. The only difference is established while sample analysis is done for methane. Gas chromatography equipped with range of detectors based on flame ionization[25-28], thermal conductivity[29], pulsed helium discharge ionization[30] and mass spectrometry[31] are available for methane analysis. Furthermore, selective ion flow transfer mass spectrometry (SIFT-MS) methane analysis is also practiced which is relatively a more convenient technique[32].
Carbon exists in various isotopic forms; the most well-known forms being the 12C, 13C and 14C isotopes. 14C is a radioactive isotope and is instable. It has a half time decay of 5730 years, whereas only 12C and 13C are stable forms.
This technique is based on the use of either the radioactive isotope of carbon, 14C or the safer and preferable nonradioactive 13C isotope[33,34]. 13C differs by only one neutron from the naturally more common 12C-atom. The detection of 13C-carbondioxide (13CO2) in breath is the time limiting step from ingestion of the substrate to its complete metabolism, till the final outbreath of the end product 13CO2.
Breath samples are collected at intervals ranging from 4 to 24 h after ingestion of the substrate[31,35]. Most centers utilize the high resolution isotope ratio mass spectrometers (IRMS) for the differentiation of 13CO2 and 12CO2. The introduction of non-dispersive isotope selective infrared spectrometers (NDIRS) has simplified the use of 13C-BTs and have paved the way for analysis in small centers as well[36-38].
This technique has got an edge in favor of non-hydrogen-producers. Furthermore lesser quantity of substrate is required compared with other tests. However, the costs of some substrates still limits the wide spread use. Endogenous CO2 production, which fluctuated extensively in the numerous diseases, has resulted in declining diagnostic accuracy.
UBT for H. pylori infection: A meta-analysis by Ferwana et al[39] has reported pooled sensitivity and specificity of UBT to be 96% and 93% respectively. Similar results were also the outcome of a multicenter Japanese study conducted in 2002, making UBT a reliable test for H. pylori infections[40]. Study from developing world also suggest that UBT is a highly accurate and reliable diagnostic modality as reflected by another study form Egypt that revealed a sensitivity and specificity of UBT to be 98% and 89% respectively[41].
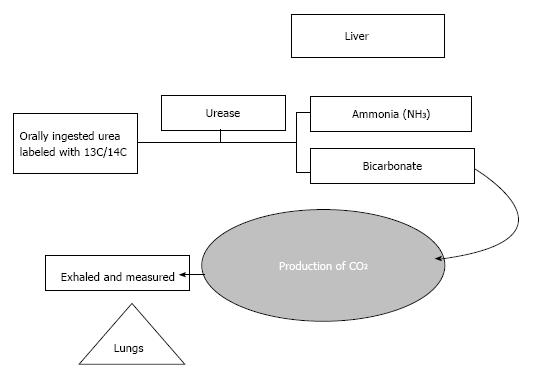
Principle: Begins with the oral administration of 13C or 14C labeled urea. H. pylori produce the urea splitting enzyme Urease, which ultimately cleaves the labeled urea to ammonia and bicarbonate. Bicarbonate is the precursor of CO2 that is incorporated into breath (Figure 2).
Owing to the radioactive hazard of 14C, here also 13C UBT is the preferred method of detection. A large multicenter study evaluated the accuracy of 13C-UBT in children taking biopsy as gold standard and stated a sensitivity ranging from 96%-98% and specificity 96%-99%[42].
Analysis: The test underwent various reforms regarding substrate dose, fasting state, test meal and breath sample intervals[43]. Commonly used protocol uses 75 mg 13C-urea administered to fasting subjects mixed with 200 mL citric acid solution. Breath samples are taken at baseline, followed by re-sampling at 20 or 30 min after ingestion of the substrate. A delta over baseline in breath 13C-enrichment above 3.5%-5% is considered positive.
Beginning of the 21th century has marked the advancement of UBT with the introduction of bench top analyzers based on the principle of molecular correlation spectrometry pooled with infrared spectrometer[44,45]. Campuzano-Maya et al[46] developed a simplified 13C-UBT protocol which when evaluated yielded an accuracy of 100% for the diagnosis of H. pylori. This version required only 50 mg 13C-urea, no prior test meal, and more importantly a single breath sample collected at 10 min[46].
Points to consider: High cost of substrate is a drawback of this test. The use of bismuth-based preparations, drugs including proton pump inhibitors several antibiotics can effects the results of this test[47]. Gargles or mouth wash are routinely advised before the commencement of the test as oral contamination could lead to false positive results.
The 14C-UBT owing to its radioactivity potential has not been promoted for use in children and women of reproductive age group. However, the amount of radioactivity delivered to the patient is low, arising the question of its prescription to the pediatric and pregnant population by some researchers[48]. On the brighter side of the picture, the 13C-UBT can be safely used in these patient groups[49]. A comparison of 13C and 14C UBT is summarized in (Table 2).
| 14C-UBT | 13C-UBT | |
| Test performed at | Nuclear medicine department | No specific location required |
| Analysis | Specialized nuclear medicine department and β-scintillation counters | Mass spectrometry analysis (in a hospital or mailed to the manufacturer) |
| Radioactive hazard | Yes | No |
| Patient selection | Not suitable for children or pregnant women | Safer for children or pregnant women |
The gold standard test to assess gastric emptying is Scintigraphy using radioactive tracers. The other alternative available is 13C-BT that uses the 13C-octanoate to label the solid components of test meals and the 13C-acetate which is utilized to label fluids.
Principle: This test is established on the fact that time taken up by the transport of the tracer substance is considered the rate limiting state together with the ingested food from the stomach into the duodenum, while the remaining processes till the elimination of CO2 follows at a constant rate[50].
Protocol: Egg yolk is used as a test meal labeled with 13C-octanoic acid. 13C-octanoic acid is absorbed upon its passage through the duodenum, and eventually oxidized by the liver to 13CO2. Gastric emptying of the egg yolk into the duodenum serves as a rate limiting step which in turn influences the detection of 13C in exhaled breath samples.
Most studies have validated 13C-octanoic acid test against scintigraphy and have found acceptable correlation. However scintigraphy itself suffers from lack of standardization. Differences in test meals, position of the patient, rate, and extent of imaging are the factors that affect test results.
Choi et al[51] in 1997 evaluated the performance of simultaneous OBT and scintigraphy in 15 healthy participants and revealed that these tests do not significantly correlate with each other. However acceptable reproducibility was obtained with a mean coefficient of variation of t1/2 of 20% between individuals and 12% within the same individual[51]. They put forward that OBT is only a reliable tool for intra-individual comparisons. However in the coming years this study faced immense criticism and findings were not adopted[52].
There is abundance of data that has compared breath testing with scintigraphy, however as magnetic resonance imaging (MRI) is potentially the most valid method; stable isotope BT should preferably be compared with MRI. In respect to this a study by Haans et al[53] evaluated MRI against breath testing for gastric emptying and revealed a strong correlation between the two techniques for liquid emptying compared for solid gastric emptying.
Advantages: Provides a better alternative test to scintigraphy as it is free from radioactive hazards, particularly in children, women in reproductive age group and subjects requiring repetitive testing. It overcomes certain limitations such as operator dependence and time constraint of ultrasonography. Furthermore it’s less costly compared to magnetic resonance imaging.
Limitations of 13C-octanoic acid BT: There are certain limitations of 13C-octanoic acid breath test (OBT). Firstly it suffers from lack of standardization. Furthermore protocol followed at different centers has certain variations. Literature review further highlighted that there also exists a difference in gastric emptying time taken as part of the methodology. Moreover collections of breath samples every fifteen minutes for four hours while the patients with minimal physical activities is difficult.
Methods of analysis: In most instances mass spectrophotometry (MS) is the technique of choice for measuring sampled 13CO2. Potential hindrances associated with MS are high cost and large operating units. Based on the convenience of its application Non-dispersive infrared isotope spectrometry (NDIRS) is potential substitute of MS[54].
13C-spirulina platensis (S. platensis) is also reported to be used as a marker of gastric emptying by some researchers[54,55]. S. platensis belongs to the family of algae used as a food product in distinct locations worldwide including the United States. It’s a blend of protein, starch and lipids. Egg yolk is used as a test meal mixed with 13C-labeled S. platensis. After undergoing the process of emptying in the stomach and finally absorption, 13C enriched CO2 is exhaled from the 13C-labeled S. platensis. This phenomenon is aimed at assessing the solid phase of gastric emptying.
Amylase, Lipase and secretin pancreozymin tests are the more reliable used in evaluation of patients with pancreatic disorders. The other testing modalities that can be included in practice are.
13C-triglyceride BT: This test utilizes the substrate using Lipase activity. The thoroughly investigated triglyceride BT is the “mixed” triglyceride test using 1,3-distearyl, 2-[13C-carboxyl] octanoyl glycerol; demonstrating a sensitivity and specificity of 89% and 81% respectively[56].
Literature review recommends 200-300 mg of the mixed triglycerides is the predominantly used doses for adults (15 mg/kg body weight for children). Before administration of test meal breath samples are collected, followed by successive sampling at 30 min intervals for 5 h[57].
13C-starch BT and 13C egg protein BT which are based on Amylase and proteases activity respectively.
13C-BTs to evaluate hepatocellular function: 13C Aminopyrine BT for hepatocellular microsomal enzyme function: This test explores hepatic microsomal enzyme function. Literature review suggests a protocol that is based on the ingestion of 2 mg/kg aminopyrine with water. The recovery of the tracer after 60 or 120 min can be used as a diagnostic marker[58].
13C-methacetin BT for assessment of microsomal liver function: 13C-methacetin is a combination of a methyl group labeled with the non-radioactive isotope 13CN-demethylase, which is cytochrome P450 dependent enzyme responsible for de-methylation of 13C-Methacetin after oral absorption. The magnitude of appearance of 13C in breath analysis is correlated with the process of de-methylation. This test has been reported to be a reliable marker for the differentiation between early cirrhotic (Child A) and noncirrhotic patients but its performance for the detection of liver fibrosis remains questionable[59].
13C D-xylose was suggested as a marker of SIBO in the 1980s. Orally ingested D-xylose labeled with 13C, after modification by gut flora yields labeled CO2 measured in the breath[35]. Reportedly D-xylose is a poor metabolic substrate for common coliform bacteria including Eschrichia coli, enterococci, and clostridia, increasing the risk of false-negative results. Due to variation found in literature in sensitivities and specificities in comparison to hydrogen BT its use still remains controversial[34].
Glycocholate BT which is considered the forerunner of BTs for the evaluation of suspected SIBO used glycocholic acid labeled with 14C[60]. Owing to its inability to distinguish small bowel from colonic bacterial deconjugation and high risk of radiation hazard this test has been mostly out of practice[61].
Though the substrates required for 13C-BTs are costly compared to the hydrogen BT, but doses required are much less and the problem of non-hydrogen producers does not exist.
Specific intestinal enzyme activities can be tested using the appropriate 13C-substrate.
Lactase: 13C-lactose[62], saccharase: 13C-saccharose[62], carbohydrate absorption: 13C-fructose[63].
Though breath testing provides a near perfect alternative, it writhes from its own limitations. The lack of standardization of various analytical methodologies adopted by laboratories worldwide is a major issue reflected in literature search when it comes to breath testing. Similarly there is extensive variation in results and cut offs reported.
When it comes to the question of availability of resources, instruments for breath analysis are expensive and comprehensive operator training is an important requirement.
Even the most thoroughly proven 13C UBT has certain limitations. Especially in a resource limited areas, the availability of mass spectrometry based analyzers to evaluate the breath samples and computing a ratio of 12C to 13C are major hindrances as these instruments are quite expensive[64]. In most cases when 13C UBT is undertaken, samples are sent to a central processing laboratory for analysis which further adds to the test cost and increases the timeline for rapid delivery of results.
Mukhopadhyay[3] reported that lack of proven associations between the various elements detected in breath and disease the testing is aimed at. As in certain instances the analyte detected in breath is exhaled in response to various other metabolic processes rather than the disease for which the test is being conducted[3].
Various available BTs which are in use in clinical practice with their reported sensitivities and specificities are listed in (Table 3).
| Test | Indication | Sensitivity and specificity |
| Glucose hydrogen breath test | SIBO | 62% and 83%[7] |
| Lactulose hydrogen breath test | SIBO | 31% and 86%[5] |
| 13C-glycocholate breath test | SIBO | 76% and 33%[69] |
| 13C-xylose breath test | SIBO | 89% and 30%[69] |
| Fructose hydrogen breath test | Carbohydrate malabsorption | 98% and 86%[65] |
| Lactose hydrogen breath test | Carbohydrate malabsorption | 80% and 100%[66] |
| 13C-lactose breath test | Carbohydrate malabsorption | 84% and 96%[70] |
| 13C-urea breath test | H. pylori infection | 96% and 93%[39] |
| 13C-aminopyrine breath test | Evaluation of liver function | 85.7% and 67.5%[58] |
| 13C-methacetin breath test | Evaluation of liver function | 92.6% and 94.1%[67] |
| 13C-phenacetin breath test | Evaluation of liver function | 98% and 60%[68] |
| 13C-mixed triglycerides breath test | Evaluation of exocrine pancreatic insufficiency | 89% and 81%[56] |
| 13C-octanoic acid breath test | Assessment of gastric emptying | 67% and 80%[71] |
Contemporary bench top analyzers are being quickly replaced by POCT systems. One such development in the breath testing technology is the introduction of electronic nose (E-nose) test[4]. These instruments are designed on a highly specific sensor technology based on diverse micro sensor arrays. Each sensor is aimed at detecting a specific chemical exhaled in breath.
A specific pattern of response in recorded on to the sensor array when odor molecules from a breath sample are passed through. This signal yield is then sorted out utilizing the power of artificial neural networks to generate a specific output pattern, aimed at a particular diagnosis.
Though various researchers have studies the use of E-nose test and have suggested its role as a highly efficient diagnostic technique, further studies and validation studies concerning this entity are still required.
We conclude that BTs are useful modalities which are currently underutilized. With the advancement of new diagnostic tools especially desktop equipment for gas analysis, use of BTs is going to rise in near future.
The most frequently utilized BTs in GI disorders worldwide are UBT and Hydrogen BT. Breath testing remains underutilized due to the widespread belief that these test requires expensive instrumentation involving a complex analytic process and highly skilled operators. There is a perception that though these tests are less invasive, they possess radioactive hazards. In cases when samples are sent to reference labs for analysis it leads to elongation of the turnaround time of results.
13C carbon test are highlighted in this review, mostly based on mass spectrometry. Such compounds are safe because they are non-radioactive. Advent of Bench top analyzers and point of care testing will further pave the way for utilization of breath testing, leading to rapid production of results. Future development is aimed at development of specific sensor based hand held analyzers like the E-nose tests. Thus to conclude these diagnostic modality can be effectively used as it is relatively safer and noninvasive compared to the contemporary tests in use and tests can be easily repeated if need arises.
| 1. | Phillips M, Herrera J, Krishnan S, Zain M, Greenberg J, Cataneo RN. Variation in volatile organic compounds in the breath of normal humans. J Chromatogr B Biomed Sci Appl. 1999;729:75-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 454] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 2. | Schubert JK, Miekisch W, Geiger K, Nöldge-Schomburg GF. Breath analysis in critically ill patients: potential and limitations. Expert Rev Mol Diagn. 2004;4:619-629. [PubMed] |
| 3. | Mukhopadhyay R. Don’t waste your breath. Researchers are developing breath tests for diagnosing diseases, but how well do they work? Anal Chem. 2004;76:273A-276A. [PubMed] |
| 4. | Arasaradnam RP, Covington JA, Harmston C, Nwokolo CU. Review article: next generation diagnostic modalities in gastroenterology--gas phase volatile compound biomarker detection. Aliment Pharmacol Ther. 2014;39:780-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Ghoshal UC. How to interpret hydrogen breath tests. J Neurogastroenterol Motil. 2011;17:312-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Braden B. Methods and functions: Breath tests. Best Pract Res Clin Gastroenterol. 2009;23:337-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Corazza GR, Menozzi MG, Strocchi A, Rasciti L, Vaira D, Lecchini R, Avanzini P, Chezzi C, Gasbarrini G. The diagnosis of small bowel bacterial overgrowth. Reliability of jejunal culture and inadequacy of breath hydrogen testing. Gastroenterology. 1990;98:302-309. [PubMed] |
| 8. | Rhodes JM, Middleton P, Jewell DP. The lactulose hydrogen breath test as a diagnostic test for small-bowel bacterial overgrowth. Scand J Gastroenterol. 1979;14:333-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 140] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Kretchmer N. Lactose and lactase--a historical perspective. Gastroenterology. 1971;61:805-813. [PubMed] |
| 10. | Kretchmer N. On the homology between human development and pediatrics. Pediatr Res. 1968;2:283-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Sahi T. Genetics and epidemiology of adult-type hypolactasia. Scand J Gastroenterol Suppl. 1994;202:7-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 163] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Heyman MB. Lactose intolerance in infants, children, and adolescents. Pediatrics. 2006;118:1279-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 269] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 13. | Matthews SB, Waud JP, Roberts AG, Campbell AK. Systemic lactose intolerance: a new perspective on an old problem. Postgrad Med J. 2005;81:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Beaugerie L, Flourié B, Lémann M, Achour L, Franchisseur C, Rambaud JC. Sorbitol absorption in the healthy human small intestine is increased by the concomitant ingestion of glucose or lipids. Eur J Gastroenterol Hepatol. 1995;7:125-128. [PubMed] |
| 15. | Jain NK, Rosenberg DB, Ulahannan MJ, Glasser MJ, Pitchumoni CS. Sorbitol intolerance in adults. Am J Gastroenterol. 1985;80:678-681. [PubMed] |
| 16. | Perman JA, Barr RG, Watkins JB. Sucrose malabsorption in children: noninvasive diagnosis by interval breath hydrogen determination. J Pediatr. 1978;93:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Levitt MD, Furne JK, Kuskowski M, Ruddy J. Stability of human methanogenic flora over 35 years and a review of insights obtained from breath methane measurements. Clin Gastroenterol Hepatol. 2006;4:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Waud JP, Matthews SB, Campbell AK. Measurement of breath hydrogen and methane, together with lactase genotype, defines the current best practice for investigation of lactose sensitivity. Ann Clin Biochem. 2008;45:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | McKay LF, Eastwood MA, Brydon WG. Methane excretion in man--a study of breath, flatus, and faeces. Gut. 1985;26:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Peled Y, Weinberg D, Hallak A, Gilat T. Factors affecting methane production in humans. Gastrointestinal diseases and alterations of colonic flora. Dig Dis Sci. 1987;32:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Melcher EA, Levitt MD, Slavin JL. Methane production and bowel function parameters in healthy subjects on low- and high-fiber diets. Nutr Cancer. 1991;16:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Weaver GA, Krause JA, Miller TL, Wolin MJ. Incidence of methanogenic bacteria in a sigmoidoscopy population: an association of methanogenic bacteria and diverticulosis. Gut. 1986;27:698-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Fiedorek SC, Pumphrey CL, Casteel HB. Breath methane production in children with constipation and encopresis. J Pediatr Gastroenterol Nutr. 1990;10:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Lin HC, Pimentel M, Chen JH. Intestinal transit is slowed by luminal methane. Neurogastroenterol Motil. 2002;14:437. |
| 25. | Weiss RF. Determinations of carbon dioxide and methane by dual catalyst flame ionization chromatography and nitrous oxide by electron capture chromatography. J Chromatogr Sci. 1981;19:611-616. [RCA] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 136] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Loftfield N, Flessa H, Augustin J, Beese F. Automated gas chromatographic system for rapid analysis of the atmospheric trace gases methane, carbon dioxide, and nitrous oxide. J Environ Qual. 1997;26:560-564. [DOI] [Full Text] |
| 27. | Zimmermann S, Krippner P, Vogel A, Muller Jr. Miniaturized flame ionization detector for gas chromatography. Sensors and Actuators B: Chemical. 2002;83:285-289. [DOI] [Full Text] |
| 28. | Thammakhet C, Thavarungkul P, Brukh R, Mitra S, Kanatharana P. Microtrap modulated flame ionization detector for on-line monitoring of methane. J Chromatogr A. 2005;1072:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Guilbot P, Valtz A, Legendre H, Richon D. Rapid on-line sampler-injector: a reliable tool for HT-HP sampling and on-line GC analysis. Analusis. 2000;28:426-431. [DOI] [Full Text] |
| 30. | Eijkel JCT, Stoeri H, Manz A. A molecular emission detector on a chip employing a direct current microplasma. Analytical Chemistry. 1999;71:2600-2606. [RCA] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Niemann HB, Atreya SK, Bauer SJ, Carignan GR, Demick JE, Frost RL, Gautier D, Haberman JA, Harpold DN, Hunten DM. The abundances of constituents of Titan’s atmosphere from the GCMS instrument on the Huygens probe. Nature. 2005;438:779-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 207] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 32. | Dryahina K, Smith D, Spanel P. Quantification of methane in humid air and exhaled breath using selected ion flow tube mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:1296-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Stotzer PO, Kilander AF. Comparison of the 1-gram (14)C-D-xylose breath test and the 50-gram hydrogen glucose breath test for diagnosis of small intestinal bacterial overgrowth. Digestion. 2000;61:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Dellert SF, Nowicki MJ, Farrell MK, Delente J, Heubi JE. The 13C-xylose breath test for the diagnosis of small bowel bacterial overgrowth in children. J Pediatr Gastroenterol Nutr. 1997;25:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | King CE, Toskes PP. Breath tests in the diagnosis of small intestine bacterial overgrowth. Crit Rev Clin Lab Sci. 1984;21:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Braden B, Haisch M, Duan LP, Lembcke B, Caspary WF, Hering P. Clinically feasible stable isotope technique at a reasonable price: analysis of 13CO2/12CO2-abundance in breath samples with a new isotope selective-nondispersive infrared spectrometer. Z Gastroenterol. 1994;32:675-678. [PubMed] |
| 37. | Braden B, Caspary WF, Lembcke B. Nondispersive infrared spectrometry for 13CO2/12CO2-measurements: a clinically feasible analyzer for stable isotope breath tests in gastroenterology. Z Gastroenterol. 1999;37:477-481. [PubMed] |
| 38. | Koletzko S, Haisch M, Seeboth I, Braden B, Hengels K, Koletzko B, Hering P. Isotope-selective non-dispersive infrared spectrometry for detection of Helicobacter pylori infection with 13C-urea breath test. Lancet. 1995;345:961-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 99] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Ferwana M, Abdulmajeed I, Alhajiahmed A, Madani W, Firwana B, Hasan R, Altayar O, Limburg PJ, Murad MH, Knawy B. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol. 2015;21:1305-1314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (4)] |
| 40. | Kato S, Ozawa K, Konno M, Tajiri H, Yoshimura N, Shimizu T, Fujisawa T, Abukawa D, Minoura T, Iinuma K. Diagnostic accuracy of the 13C-urea breath test for childhood Helicobacter pylori infection: a multicenter Japanese study. Am J Gastroenterol. 2002;97:1668-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Frenck RW, Fathy HM, Sherif M, Mohran Z, El Mohammedy H, Francis W, Rockabrand D, Mounir BI, Rozmajzl P, Frierson HF. Sensitivity and specificity of various tests for the diagnosis of Helicobacter pylori in Egyptian children. Pediatrics. 2006;118:e1195-e1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Elitsur Y, Tolia V, Gilger MA, Reeves-Garcia J, Schmidt-Sommerfeld E, Opekun AR, El-Zimaity H, Graham DY, Enmei K. Urea breath test in children: the United States prospective, multicenter study. Helicobacter. 2009;14:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Gatta L, Ricci C, Tampieri A, Osborn J, Perna F, Bernabucci V, Vaira D. Accuracy of breath tests using low doses of 13C-urea to diagnose Helicobacter pylori infection: a randomised controlled trial. Gut. 2006;55:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (3)] |
| 44. | Israeli E, Ilan Y, Meir SB, Buenavida C, Goldin E. A novel 13C-urea breath test device for the diagnosis of Helicobacter pylori infection: continuous online measurements allow for faster test results with high accuracy. J Clin Gastroenterol. 2003;37:139-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Chen TS, Chang FY, Chen PC, Huang TW, Ou JT, Tsai MH, Wu MS, Lin JT. Simplified 13C-urea breath test with a new infrared spectrometer for diagnosis of Helicobacter pylori infection. J Gastroenterol Hepatol. 2003;18:1237-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Campuzano-Maya G. An optimized 13C-urea breath test for the diagnosis of H pylori infection. World J Gastroenterol. 2007;13:5454-5464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 837] [Article Influence: 44.1] [Reference Citation Analysis (3)] |
| 48. | Gunnarsson M, Leide-Svegborn S, Stenström K, Skog G, Nilsson LE, Hellborg R, Mattsson S. No radiation protection reasons for restrictions on 14C urea breath tests in children. Br J Radiol. 2002;75:982-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Hunt R, Fallone C, Veldhuyzan van Zanten S, Sherman P, Smaill F, Flook N, Thomson A. Canadian Helicobacter Study Group Consensus Conference: Update on the management of Helicobacter pylori--an evidence-based evaluation of six topics relevant to clinical outcomes in patients evaluated for H pylori infection. Can J Gastroenterol. 2004;18:547-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Uchida M, Shimizu K. 13C-acetic acid is more sensitive than 13C-octanoic acid for evaluating gastric emptying of liquid enteral nutrient formula by breath test in conscious rats. Biol Pharm Bull. 2007;30:487-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Choi MG, Camilleri M, Burton DD, Zinsmeister AR, Forstrom LA, Nair KS. [13C]octanoic acid breath test for gastric emptying of solids: accuracy, reproducibility, and comparison with scintigraphy. Gastroenterology. 1997;112:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 145] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Perri F, Pastore MR, Annese V. 13C-octanoic acid breath test for measuring gastric emptying of solids. Eur Rev Med Pharmacol Sci. 2005;9:3-8. [PubMed] |
| 53. | Haans JJL, Paridaans NPM, Wong C, Eilers PHC, Doornbos J, de Roos A, Masclee AAM. Magnetic Resonance Imaging for Evaluation of Gastric Motor Function. Chapter 7: Comparison of gastric emptying determined by stable isotope breath test and Magnetic Resonance Imaging during simultaneous recording. Evaluation of Gastric Motor Function 8. Available from: http://digitalarchive.maastrichtuniversity.nl/fedora/get/guid:a32b0e97-332d-41f3-8695-f3a6912ef077/ASSET1. |
| 54. | Szarka LA, Camilleri M, Vella A, Burton D, Baxter K, Simonson J, Zinsmeister AR. A stable isotope breath test with a standard meal for abnormal gastric emptying of solids in the clinic and in research. Clin Gastroenterol Hepatol. 2008;6:635-643.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 55. | Lee JS, Camilleri M, Zinsmeister AR, Burton DD, Kost LJ, Klein PD. A valid, accurate, office based non-radioactive test for gastric emptying of solids. Gut. 2000;46:768-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Kalivianakis M, Verkade HJ, Stellaard F, van der Were M, Elzinga H, Vonk RJ. The 13C-mixed triglyceride breath test in healthy adults: determinants of the 13CO2 response. Eur J Clin Invest. 1997;27:434-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 57. | Löser C, Brauer C, Aygen S, Hennemann O, Fölsch UR. Comparative clinical evaluation of the 13C-mixed triglyceride breath test as an indirect pancreatic function test. Scand J Gastroenterol. 1998;33:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Giannini EG, Fasoli A, Borro P, Botta F, Malfatti F, Fumagalli A, Testa E, Polegato S, Cotellessa T, Milazzo S. 13C-galactose breath test and 13C-aminopyrine breath test for the study of liver function in chronic liver disease. Clin Gastroenterol Hepatol. 2005;3:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Braden B, Faust D, Sarrazin U, Zeuzem S, Dietrich CF, Caspary WF, Sarrazin C. 13C-methacetin breath test as liver function test in patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2005;21:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Hofmann AF, Fromm H. New breath test for bile acid deconjugation. N Engl J Med. 1971;285:686-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Gunnarsson M, Leide-Svegborn S, Stenström K, Skog G, Nilsson LE, Thorsson O, Hellborg R, Mattsson S. Long-term biokinetics and radiation exposure of patients undergoing 14C-glycocholic acid and 14C-xylose breath tests. Cancer Biother Radiopharm. 2007;22:762-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 62. | Hiele M, Ghoos Y, Rutgeerts P, Vantrappen G. Measurement of the rate of assimilation of oligo- and polysaccharides by 13CO2 breath tests and isotope ratio mass spectrometry. Biomed Environ Mass Spectrom. 1988;16:133-135. [PubMed] |
| 63. | Hoekstra JH, van den Aker JH, Kneepkens CM, Stellaard F, Geypens B, Ghoos YF. Evaluation of 13CO2 breath tests for the detection of fructose malabsorption. J Lab Clin Med. 1996;127:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 64. | Romagnuolo J, Schiller D, Bailey RJ. Using breath tests wisely in a gastroenterology practice: an evidence-based review of indications and pitfalls in interpretation. Am J Gastroenterol. 2002;97:1113-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 65. | Götze H, Mahdi A. [Fructose malabsorption and dysfunctional gastrointestinal manifestations]. Monatsschr Kinderheilkd. 1992;140:814-817. [PubMed] |
| 66. | Rosado JL, Solomons NW. Sensitivity and specificity of the hydrogen breath-analysis test for detecting malabsorption of physiological doses of lactose. Clin Chem. 1983;29:545-548. [PubMed] |
| 67. | Schneider A, Caspary WF, Saich R, Dietrich CF, Sarrazin C, Kuker W, Braden B. 13C-methacetin breath test shortened: 2-point-measurements after 15 minutes reliably indicate the presence of liver cirrhosis. J Clin Gastroenterol. 2007;41:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Lara Baruque S, Razquin M, Jimenez I, Vazquez A, Gisbert JP, Pajares JM. 13C-phenylalanine and 13C-methacetin breath test to evaluate functional capacity of hepatocyte in chronic liver disease. Dig Liver Dis. 2000;32:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Donald IP, Kitchingmam G, Donald F, Kupfer RM. The diagnosis of small bowel bacterial overgrowth in elderly patients. J Am Geriatr Soc. 1992;40:692-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 70. | Wetzel K, Fischer H. 13C-breath tests in medical research and clinical diagnosis. Leipzig: Fischer Analysen Instrumente GmbH 2005; . |
| 71. | Delbende B, Perri F, Couturier O, Leodolter A, Mauger P, Bridgi B, Bizais Y, des Varannes SB, Andriulli A, Galmiche JP. 13C-octanoic acid breath test for gastric emptying measurement. Eur J Gastroenterol Hepatol. 2000;12:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Pakistan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Bernhardt GA, Koch TR, Quigley EMM, Rosenthal P S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ