Published online Feb 15, 2016. doi: 10.4291/wjgp.v7.i1.97
Peer-review started: May 5, 2015
First decision: July 27, 2015
Revised: September 18, 2015
Accepted: November 3, 2015
Article in press: November 4, 2015
Published online: February 15, 2016
Processing time: 278 Days and 11.9 Hours
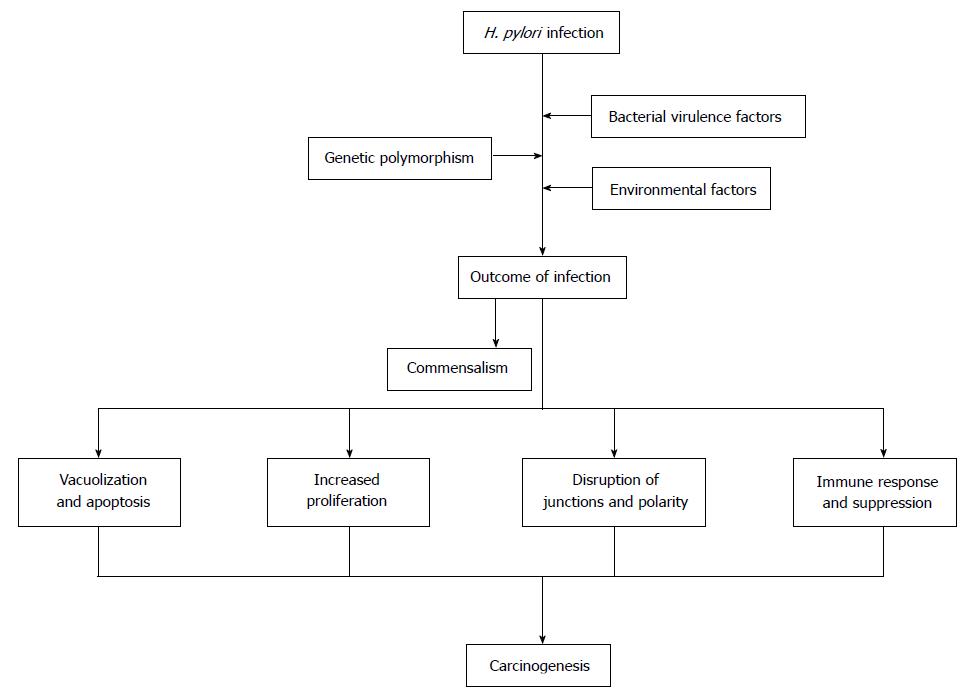
Gastric cancer (GC) is one of the most common carcinoma and the second leading cause of cancer-related deaths worldwide. Helicobacter pylori (H. pylori) infection causes a series of precancerous lesions like gastritis, atrophy, intestinal metaplasia and dysplasia, and is the strongest known risk factor for GC, as supported by epidemiological, preclinical and clinical studies. However, the mechanism of H. pylori developing gastric carcinoma has not been well defined. Among infected individuals, approximately 10% develop severe gastric lesions such as peptic ulcer disease, 1%-3% progresses to GC. The outcomes of H. pylori infection are determined by bacterial virulence, genetic polymorphism of hosts as well as environmental factors. It is important to gain further understanding of the pathogenesis of H. pylori infection for developing more effective treatments for this common but deadly malignancy. The recent findings on the bacterial virulence factors, effects of H. pylori on epithelial cells, genetic polymorphism of both the bacterium and its host, and the environmental factors for GC are discussed with focus on the role of H. pylori in gastric carcinogenesis in this review.
Core tip: It is important to gain further understanding of the pathogenesis of Helicobacter pylori (H. pylori) infection for developing more effective treatments for this common but deadly malignancy. The recent findings on the bacterial virulence factors, effects of H. pylori on epithelial cells, genetic polymorphism of both the bacterium and its host, and the environmental factors for gastric cancer are discussed with focus on the role of H. pylori in gastric carcinogenesis in this review.
- Citation: Zhang RG, Duan GC, Fan QT, Chen SY. Role of Helicobacter pylori infection in pathogenesis of gastric carcinoma. World J Gastrointest Pathophysiol 2016; 7(1): 97-107
- URL: https://www.wjgnet.com/2150-5330/full/v7/i1/97.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v7.i1.97
Gastric cancer (GC) is one of the most common malignancies globally[1]. The risk factors for GC consist of Helicobacter pylori (H. pylori) infection, genetic and environmental factors[1]. H. pylori mainly colonized in human stomach, has coexisted with humans for nearly sixty thousand years[2]. The outcome of infection is affected by the environmental and genetic factors, the infection in most individuals does not develop distinct disease or even become beneficial, leading to the hypothesis that H. pylori might be commensal[3]. However, accumulating evidences support that H. pylori infection cause a list of diseases, ranging from gastric to extra-gastric diseases, from chronic gastritis to gastric carcinoma, and thus this bacterium is recognized as the Class I carcinogenic pathogen in human with less than 3% of the infected eventually suffering GC[3].
Mechanism of H. pylori-associated gastric carcinogenesis has not been well defined. H. pylori infection commonly lasts for decades, provoking a series of histological changes including destruction of intercellular junctions, apoptosis and proliferation of epithelial cells and malignant transformation[4,5]. The genotypes of H. pylori strains, host genetic polymorphisms, environmental factors like high salt diet, smoking habit and certain gastric commensal organisms have been determined to be associated with occurrence of GC[6]. H. pylori genetic polymorphisms, effects of specific H. pylori products on gastric epithelium and cellular signaling process have been intensively investigated in recent decades[6]. This review is performed to discuss the role of H. pylori in gastric carcinogenesis.
Studies on H. pylori heterogeneity have proved that the strongest virulence factors were amongst the genes within the cag pathogenicity island (PAI).
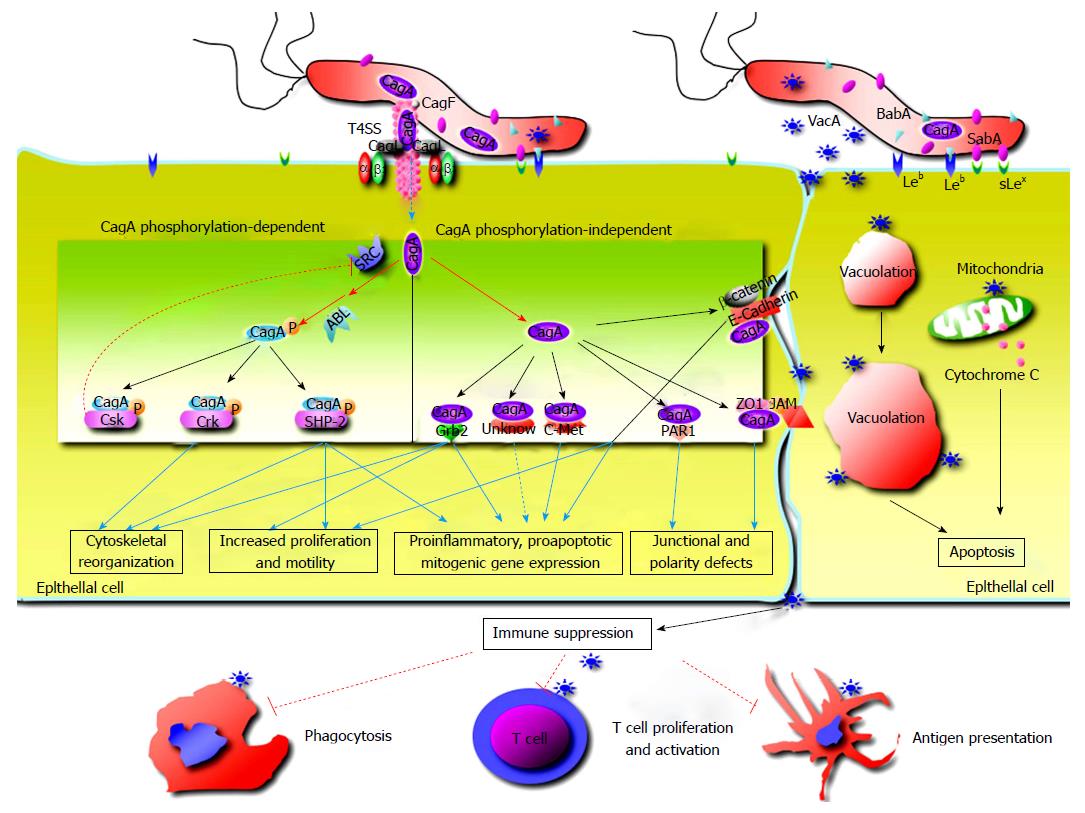
CagA, a highly immunogenic protein, is encoded at one end of the cag PAI, which encode the components to form the type IV secretion system (T4SS)[7]. As a component of T4SS, CagL protein binds to and activates the integrin α5β1 receptor on gastric epithelial cells and triggers CagA delivery into the target cells[8], CagM, along with CagX and CagT, forms an outer membrane-associated T4SS subcomplex[9], CagX and CagT interact directly[10]. As reported, CagA also facilitates its translocation into host epithelial cells by T4SS-induced externalization of phosphatidylserine from inner leaflet of the cellular membrane[8,11]. Recent studies demonstrated that fibronectinand peptidoglycan was also transported into epithelial cells by T4SS, suggesting that T4SS might have more CagA-independent functions than its ability to inject CagA[10]. CagA and CagM are important for assessing virulence of H. pylori strains.
H. pylori strains harboring the cag PAI or producing CagA are related to enhanced inflammation and risk of ulcers and carcinoma[12]. CagA contributes to myriad signaling alterations, which profoundly affects physiology of host epithelial cells. The Glu-Pro-Ile-Tyr-Ala (EPIYA) motifs in CagA are phosphorylation sites and play crucial roles in pathogenesis of H. pylori infection[13,14]. Once inside host cells, CagA is tyrosine phosphorylated by Src and Abl kinases at EPIYA motifs, and binds the SH2 domain of the SHP-2 phosphatase involved in transduction of signaling[15,16]. Phosphorylated CagA triggers the cellular signaling pathways leading to expression of proinflammatory cytokines and chemokines, and deregulates the signaling pathways that control host cell shape, adhesion and transformation[17,18]. Unphosphorylated CagA interacts with certain intracellular proteins, up-regulate production of proinflammatory cytokines, provoke mitogenic responses and disrupt intercellular junctions and epithelial cell polarity[17,19]. Additionally, CagA intoxicates dendritic cells leading to impaired activation, decreased inflammatory cytokine production and Th1 immune response[20]. Recently, it was confirmed that H. pylori infection resulted in rapid association of the virulence factor CagA with the c-Met receptor, activation of signaling and epithelial proliferation[21].
Vacuolating cytotoxin A (VacA) contributes to multiple structural and functional alterations of epithelial cells. After secretion by the bacterium through the type V secretion system, VacA binds to host cells interfering with endosomal maturation and leading to vacuolation, enhances leakage of nutrients by destruction of barrier function at tight junctions of epithelial cells, provokes mitochondrial damage and cell apoptosis, which improves H. pylori growth[22-24]. Recent studies proved that VacA could disrupt phagocytosis, interfere with antigen presentation, restrain T cell activation in vitro and inhibit T cell proliferation independing of NFAT (nuclear factor of activated T cells) activation or IL-2 expression[25-27]. These effects of VacA on the immune system may explain how H. pylori evades adaptive immune responses to establish persistent infection.
Adherence to epithelial cells is important for H. pylori colonization and delivery of virulence factors to host cells[6]. BabA and SabA are two sialic acid-binding adhesins variably expressed by H. pylori. Among babA and babB genes, only the babA2 allele possesses active function[6,28]. BabA can bind to sialyl-Lewis x/a antigens and the Lewis b ABO blood group antigen (Leb), which are mainly distributed in red blood cells and certain epithelial cells[29], and this binding activity is commonly present in CagA positive strains[30]. Adherence mediated by BabA enhances the ability of T4SS to contact host cells, thus strengthen inflammatory response[31]. BabA binding to Leb contributes to gene mutations through formation of double stranded DNA breaks in host cell lines[32]. SabA can facilitate colonization in patients lacking Leb by binding to the sialyl-Lewis antigens[33], and mediate binding of H. pylori to sialylated structures of neutrophils[34]. The data suggest that BabA and SabA might be involved in carcinogenesis as abundance of sialyl-Lewis antigens is commonly enhanced in inflamed or cancerous gastric tissues[35].
OipA is an inflammation-related outer membrane protein, and the functional oipA gene is associated with more severe clinic outcomes[36,37]. OipA is commonly expressed together with CagA in cagA positive strains, which make it rather difficult to identify the effects of oipA alone in H. pylori infected human body or animal modules[38]. OipA expression is linked to increased production of proinflammatory cytokines like IL-8, IL-1, IL-17 and TNFα[39] as well as other host effector proteins including those associated with GC[40]. OipA can activate β-catenin, and mutant H. pylori strains lacking OipA decrease nuclear translocation of β-catenin, while tumorigenesis can be depressed by inactivating oipA of H. pylori strain in experimental animals[33,37]. These data suggest that OipA might take part in gastric carcinogenesis[33].
Gamma-glutamyl transpeptidase (GGT) is mainly found in outer membrane vesicles of H. pylori, and has been proved to be related to enhanced levels of hydrogen peroxide and IL-8 production in epithelial cells and H. pylori-associated diseases[41-43]. GGT accelerates glutathione degradation, pro-oxidant compounds and reactive oxygen production[41]. GGT adjusts IL-8 expression by depletion of glutamine[41]. These findings indicate that GGT plays a significant role in H. pylori-related chronic inflammation and tissue damage.
It should be noted that interaction of the factors commonly exists in vivo, actions that a virulence factor take under the conditions with presence of other virulence factors might be different from those observed in vitro[44]. The influence of interactions between virulence factors in multifactorial pathogenesis is still uncertain. The main virulence factors in pathogenesis of H. pylori infection are shown in Figure 1[6].
Beside responsibility for digestive processes, the gastric epithelium has the function to protect underlying tissues from infection by pathogens[45]. H. pylori take specialized mechanisms to avoid host defense and adaptive immune for persistent colonization in human body, such as disruption of epithelial junctions, stimulation of cytokine production, overproliferation, DNA damage, apoptosis and cell transformation.
Intercellular apical junctions of epithelial cells are critical in keeping integrity of gastric epithelial barrier and essential cellular functions[25]. H. pylori disrupts epithelial tight junctions through binding to specific cellular receptors and stimulating the signaling pathways. As transported into epithelial cells through T4SS, CagA interacts with junction proteins like E-cadherin and ZO-1, and alters the tight or adherence junctions[25,46]. It has been confirmed that E-cadherin, a transmembrane protein, localizes at cell-to-cell junctions and interacts with β-catenin to form the E-cadherin/β-catenin complex, which play a key role in interaction of epithelial cells and stabilization of cellular architecture[46]. However, the complex is destabilized by translocated CagA in a phosphorylation independent manner during H. pylori infection[46]. As reported, CagA translocation is relevant to mislocalization of ZO-1 in epithelial cells[47,48]. Studies revealed that H. pylori altered expression and localisation of claudin-7, a cancer-associated tight junction protein, in gastroids and human epithelial cells, which was mediated by β-catenin and snail activation[49]. A recent study demonstrated that H. pylori diminished acid-induced tightening of cell junctions, affected the response of epithelial cells to acid, which took effects in inflammatory response and alteration of the barrier function[50].
H. pylori cause defect of epithelial cell polarity by targeting the epithelial adhesion receptors like E-cadherin and β1-integrin to modulate formation of cytoskeleton[51]. CagA disrupts polarity of epithelial cells through interaction with PAR1/MARK kinase[52]. As proved, an atypical protein kinase C (aPKC) contributes to disaggregation of PAR1 from tight junctions by phosphorylation of PAR1 at the junctions[52], and PAR1b binding to CagA restrains PAR1b activity and phosphorylation by aPKC to promote disruption of cellular polarity[48,52].
H. pylori not only colonize the mucus layer covering gastric mucosa, but also invade gastric epithelial cells, and even immunocytes[53]. Recent studies demonstrated that H. pylori induced autophagy of epithelial cells and phagocytes[53]. The autophagy of epithelial cells is modulated by H. pylori, and can be inducted by acute VacA exposure, and prolonged exposure to the toxin disrupts autophagy by preventing maturation of the autolysosome. The evidences support that H. pylori-suppressed autophagy facilitates intracellular survival of this bacterium and generates an environment favoring carcinogenesis[54].
Rapid turnover of epithelial cells contributes to protect the epithelium from infection. H. pylori disrupts the balance of the proliferation and turnover of gastric epithelium to facilitate its survival[55]. Apoptosis is a regulated and conserved process in tissue, and takes the key role in tissue homeostasis[56]. H. pylori regulates the balance of epithelial cell apoptosis and proliferation for its reproduction[57,58]. The mechanism for this phenomenon remains to be well defined. The damage of gastric mucosa, stimulation of inflammatory immune responses by the enzymes like urease and VacA contribute to cellular apoptosis. The elevated level of free radicals produced by neutrophils and TH1 cytokines like IFN-γ in inflammatory response can damage DNA and induce apoptosis of epithelial cells[5,59]. H. pylori adhering to the epithelial surface also stimulate cellular apoptosis[60]. Studies demonstrated that human gastric epithelial cells sensitized to H. pylori confer susceptibility to TRAIL-mediated apoptosis through regulation of cellular FLICE-inhibitory protein activity and assembly of death-inducing signaling complex[61].
The secretion of proinflammatory cytokines by gastric epithelial cells plays significant roles in pathogenesis of H. pylori-related gastric diseases. The cytokines involved in H. pylori infection include IL-8, IL-6, MCP-1, TNF-α, MIF, IL-1α, TGF-β, IL-1β and GMCSF[17]. The production of IL-8, a chemokine mediating accumulation of neutrophils, is related to the expression of CagA[17]. Further study confirmed that IL-8 and NF-κB expression was activated by urease B subunit[62], and the urease stimulates gastric epithelial cells to produce TNF-α and IL-6[63]. As reported, both cag PAI and OipA up-regulate IL-6 production in gastric epithelial cells[64]. Additionally, Th17 subsets are enriched in H. pylori infected mucosa[65]. The expression level of interleukin-17 (IL-17) has been observed to be up-regulated in gastric tissues of both human and animal during H. pylori infection, while IL-17 can enhance expression of IL-8 in epithelial cells[66]. On the other hand, elevated levels of IL-21 and IL-23 expression in gastric mucosa induce and sustain IL-17 production[66]. Recently, a new clue for the pathogenesis of H. pylori-related gastric inflammation and GC is impairment of ghrelin synthesis in H. pylori-colonized stomach. Ghrelin, the ligand of growth hormone secretagogue receptor 1a, has immunoregulatory properties and function of certain inflammatory pathways inhibitor[67]. The defective ghrelin synthesis may contribute to sustain the ongoing inflammatory response in the gastric diseases[67].
The mechanism underlying H. pylor-related gastric carcinogenesis remains unclear. CagA interacts with E-cadherin, deregulates β-catenin signal transduction and promotes gastric-to-intestinal transdifferentiation[47]. As observed, CagA translocated intracellularly binds to PAR1, destroys cellular junctions and polarity, and fosters carcinogenesis[48]. Development of gastric and hematological carcinoma has been observed in the mice that were genetically modified to express CagA[68]. Studies revealed that cagA+/vacAs1+/vacAm1+H. pylori strains promoted pathogenesis of intestinal metaplasia and gastric carcinoma[69].
Aditionally, H. pylori regulates expression of such toll-like receptors as toll-like receptor (TLR) 4 and TLR9 in epithelial cells during gastric carcinogenesis[70]. Caveolin-1 plays a protective role in immunologic injury caused by H. pylori[71]. Rapid association of the virulence factor CagA with the c-Met receptor, activation of signaling and induction of epithelial proliferation have been observed by using pluripotent stem-cell-derived gastric organoids[21].
Genetic diversity of H. pylori has major contribution in the pathogenesis[72]. Studies have been conducted focusing on polymorphism of the main virulence factors, such as cagA, vacA, oipA, iceA and hopQ.
The highly polymorphic EPIYA motifs at the C-terminal of CagA are involved in pathogenesis of H. pylori-related gastroduodenal diseases[73]. CagA containing EPIYA motifs can activate the STAT3 pathway and promote cell migration[74]. The EPIYA motifs are distinguished by different amino acid sequences surrounding the EPIYA motif, and an increased number of CagA EPIYA-C sites confers a heightened risk for GC developing[75,76]. The sequences from Western and East Asian strains contain EPIYA-C and -D, respectively, and the strains with two segment C have more chances to develop GC than those with one[73]. The significantly higher prevalence of East Asian CagA in patients from Japan with H. pylori infection may be involved in the pathogenesis of GC[77].
Polymorphisms among the vacA alleles of H. pylori strains contribute to various levels of cytotoxicity, while variations in various regions can influence activity of VacA, including vacuolating activity[78,79]. It has been confirmed that vacuolating activity is highest in s1/m1 strains, vacA s1/m1 strains are closely relevant with GC in western countries[80,81]. Nevertheless, this situation is not universal worldwide, for example, s1/m1 strains in districts of Asia is irrelevant to clinical outcome[82,83].
Additionally, investigation of the prevalence of oipA and iceA1/iceA2 positive strains among patients suffering from GC or gastritis results in that the frequency of iceA1 allele in patients with GC is significantly higher than those with gastritis[72]. However, there is no significant difference in prevalence of oipA and iceA2 genes among the two groups of patients (P > 0.05), suggesting the iceA1 gene might take a role in pathogenesis of H. pylori-induced GC[72]. Studies also indicated that certain genetic types of H. pylori hopQ were closely related to GC[84].
Polymorphisms in the genes encoding innate immune factors are involved in pathogenesis of H. pylori-related diseases, and the polymorphisms of cytokine genes cause inter-individual variation in cytokine responses which contributes to diversity of clinical outcome[85].
As reported, the risk of GC in many populations was affected by the polymorphism of the genes encoding IL-1β, TNFα, IL-8, IL-17 and IL-10 or their receptor antagonist[25]. An elevated risk of GC was observed in IL-8-251 AA or IL-10-1082 G genotype carriers with H. pylori infection[86]. IL-17 A/F plays critical function in inflammation and probably in cancer. Studies concluded that polymorphism of IL-17F was involved in susceptibility to GC[87].
Current evidences support that TLRs are play roles in both recognition of H. pylori and gastric carcinogenesis, and polymorphisms in genes involved in the TLR signaling pathways modulate the risk of GC[88].
Additionally, peroxisome proliferator-activated receptors may play roles in H. pylori-related gastric carcinogenesis[89]. The G/G variant rs2076167 is relevant to increased risk of GC in an animal model. The association between G/G variants of rs2016167 and GC is close among those consuming higher salt diet[89]. The insertion/deletion polymorphism of the angiotensin I-converting enzyme gene was recently proved to be linked to the pathogenesis and progression of human cancers[90]. As demonstrated, both bacterial and host gene polymorphisms affect oxidative stress and DNA damage, as thought to be a key mechanism in gastric carcinogenesis. The interaction of bacterial and host gene polymorphisms may become an explanation for why GC only occurs in a small proportion of H. pylori-infected individuals[91].
There are multiple ways by which H. pylori manipulates the host to lower the threshold for carcinogenesis, gastric microbiota, high-salt diet, smoking habit, low iron levels and use of proton pump inhibitors (PPIs) may enhance risk of H. pylori-associated carcinogenesis[92].
Alterations of microbiota inhabiting human digestive tract can favor carcinogenesis[93]. Conventional wisdom espoused the dogma that pH values < 4 were able to sterilize the stomach, but since the discovery of H. pylori[94], a complex community of noncultivatable inhabitants have been uncovered in the stomach[95]. The interaction of gastric microbiota with H. pylori likely affects gastric immunobiology and the outcome of infection[95]. Data indicate that the microbial density in the normal stomach is low (101-103 CFU/g)[94], and the low bacterial densities within this portion of gastrointestinal tract is attributed to rapid peristalsis, low pH and/or high bile concentration[96]. The parietal cell loss caused by H. pylori infection leads to hypochlorhydria or even achlorhydria, thereby increase the risk of bacterial overgrowth and detrimental infection[97]. Alteration of gastric microbiota may promote the development of GC by up-regulating production of N-nitroso compounds[93].
Studies demonstrated that virulence factors like cagA and smoking might have synergistic effect in carcinogenesis of GC, and cagA genotype of H. pylori strains was closely related to active-smoking in population with H. pylori infection as shown in Table 1[98].
| N cagA neg | N cagA pos | OR | 95%CI | OR1 | 95%CI | |
| Smoking status at endoscope | ||||||
| Non active smokers | 34 | 31 | 1.00 | 1.00 | ||
| Active smokers | 8 | 23 | 3.15 | 1.23-8.07 | 4.52 | 1.28-15.98 |
| Smoking status | ||||||
| Never | 22 | 23 | 1.00 | 1.00 | ||
| Former smoker | 12 | 8 | 0.64 | 0.22-1.86 | 0.36 | 0.09-1.53 |
| Current smoker | 8 | 23 | 2.75 | 1.02-7.43 | 3.24 | 0.84-12.47 |
| P value for linear trend | 0.067 | 0.123 |
As evidenced, CagA expression is significantly upregulated when certain H. pylori strains are cultured in a medium of high salt concentrations. Through sequence analysis and site-directed mutagenesis, it was determined that salt-responsive H. pylori strains were more likely to contain two copies of TAATGA motif within the cagA gene promoter, while the strains containing only a single copy of this motif were less likely to possess properties of salt-responsive CagA expression[92,99]. However, another study showed that the severity of gastritis in H. pylori infected population might be unassociated with high-salt diet[100].
The iron level in the host has also been proved to manipulate the virulence potential of H. pylori. The bacteria harvested from gerbils with low iron levels were found to assemble more T4SS pili per bacterium, translocate increased amounts of CagA, and augment more IL-8 secretion compared to those isolated from gerbils with normal iron levels[101,102]. Furthermore, the H. pylori strains isolated from patients with low ferritin levels induce significantly higher levels of IL-8 compared to the strains from patients with the highest ferritin levels, suggesting that iron deficiency in the host might enhance the bacterial virulence and the risk for carcinogenesis of gastric tissues[101,102].
It has been evidenced that long-term use of proton PPIs might aggravate corpus atrophic gastritis in H. pylori-infected patients[97]. The worsening atrophic gastritis contributes to development of gastric carcinoma, particularly owing increasing production of potentially carcinogenic N-nitroso compounds by the bacteria overgrowing under conditions of hypochlorhydria[97]. Hypergastrinemia induced by PPI administration might also promote the development of GC[97].
Di (2-ethylhexyl) phthalate (DEHP), as an essential additive in plastic manufacturing, has been used as plasticizer for many products including plastic food packaging[103]. Recent studies confirmed that DEHP was a teratogenic compound closely related to carcinogenesis[103]. DEHP may enhance H. pylori cytotoxicity, induce gastric epithelial cell apoptosis, disrupt the gastric mucosa integrity and promote pathogenesis of gastric carcinogenesis[103].
The genomes of H. pylori are highly diverse, multiple virulence factors take effects on host epithelium in various manners, including direct action and indirect action like eliciting immune response. Genetic polymorphism of host, dietary factors, smoking, gastric microbiota and long-term consuming of PPIs influence the progression of H. pylori-relate gastric lesion. The pathogenesis of H. pylori-associated GC is a multi-factorial and multi-step process, and its development depends on a combination of host, bacterial and environmental factors as shown in Figure 2. It is important to further reveal the carcinogenesis of H. pylori-related GC in order to develop more effective treatments for this common but deadly malignancy.
| 1. | Goh LY, Leow AH, Goh KL. Observations on the epidemiology of gastrointestinal and liver cancers in the Asia-Pacific region. J Dig Dis. 2014;15:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 2. | Linz B, Balloux F, Moodley Y, Manica A, Liu H, Roumagnac P, Falush D, Stamer C, Prugnolle F, van der Merwe SW. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445:915-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 720] [Cited by in RCA: 645] [Article Influence: 33.9] [Reference Citation Analysis (3)] |
| 3. | Mishra S. Is Helicobacter pylori good or bad? Eur J Clin Microbiol Infect Dis. 2013;32:301-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Watari J, Chen N, Amenta PS, Fukui H, Oshima T, Tomita T, Miwa H, Lim KJ, Das KM. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol. 2014;20:5461-5473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 202] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (6)] |
| 5. | Xia HH, Talley NJ. Apoptosis in gastric epithelium induced by Helicobacter pylori infection: implications in gastric carcinogenesis. Am J Gastroenterol. 2001;96:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Wen S, Moss SF. Helicobacter pylori virulence factors in gastric carcinogenesis. Cancer Lett. 2009;282:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Tegtmeyer N, Wessler S, Backert S. Role of the cag-pathogenicity island encoded type IV secretion system in Helicobacter pylori pathogenesis. FEBS J. 2011;278:1190-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 196] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 8. | Murata-Kamiya N, Kikuchi K, Hayashi T, Higashi H, Hatakeyama M. Helicobacter pylori exploits host membrane phosphatidylserine for delivery, localization, and pathophysiological action of the CagA oncoprotein. Cell Host Microbe. 2010;7:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Fischer W. Assembly and molecular mode of action of the Helicobacter pylori Cag type IV secretion apparatus. FEBS J. 2011;278:1203-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Gopal GJ, Pal J, Kumar A, Mukhopadhyay G. C-terminal domain of CagX is responsible for its interaction with CagT protein of Helicobacter pylori type IV secretion system. Biochem Biophys Res Commun. 2015;456:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Hayashi T, Senda M, Morohashi H, Higashi H, Horio M, Kashiba Y, Nagase L, Sasaya D, Shimizu T, Venugopalan N. Tertiary structure-function analysis reveals the pathogenic signaling potentiation mechanism of Helicobacter pylori oncogenic effector CagA. Cell Host Microbe. 2012;12:20-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Kim SS, Ruiz VE, Carroll JD, Moss SF. Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer Lett. 2011;305:228-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | Kalaf EA, Al-Khafaji ZM, Yassen NY, Al-Abbudi FA, Sadwen SN. Study of the cytoxin-associated gene a (CagA gene) in Helicobacter pylori using gastric biopsies of Iraqi patients. Saudi J Gastroenterol. 2013;19:69-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Stein M, Bagnoli F, Halenbeck R, Rappuoli R, Fantl WJ, Covacci A. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol Microbiol. 2002;43:971-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 353] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 15. | Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 790] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 16. | Bourzac KM, Guillemin K. Helicobacter pylori-host cell interactions mediated by type IV secretion. Cell Microbiol. 2005;7:911-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Alzahrani S, Lina TT, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE. Effect of Helicobacter pylori on gastric epithelial cells. World J Gastroenterol. 2014;20:12767-12780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (6)] |
| 18. | Backert S, Clyne M. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2011;16 Suppl 1:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 590] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 20. | Tanaka H, Yoshida M, Nishiumi S, Ohnishi N, Kobayashi K, Yamamoto K, Fujita T, Hatakeyama M, Azuma T. The CagA protein of Helicobacter pylori suppresses the functions of dendritic cell in mice. Arch Biochem Biophys. 2010;498:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | McCracken KW, Catá EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, Tsai YH, Mayhew CN, Spence JR, Zavros Y. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516:400-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 649] [Cited by in RCA: 739] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 22. | Palframan SL, Kwok T, Gabriel K. Vacuolating cytotoxin A (VacA), a key toxin for Helicobacter pylori pathogenesis. Front Cell Infect Microbiol. 2012;2:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (2)] |
| 23. | Manente L, Perna A, Buommino E, Altucci L, Lucariello A, Citro G, Baldi A, Iaquinto G, Tufano MA, De Luca A. The Helicobacter pylori’s protein VacA has direct effects on the regulation of cell cycle and apoptosis in gastric epithelial cells. J Cell Physiol. 2008;214:582-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Papini E, Satin B, Norais N, de Bernard M, Telford JL, Rappuoli R, Montecucco C. Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J Clin Invest. 1998;102:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 185] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 398] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 26. | Allen LA, Schlesinger LS, Kang B. Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J Exp Med. 2000;191:115-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 168] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Torres VJ, VanCompernolle SE, Sundrud MS, Unutmaz D, Cover TL. Helicobacter pylori vacuolating cytotoxin inhibits activation-induced proliferation of human T and B lymphocyte subsets. J Immunol. 2007;179:5433-5440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, Incecik ET, Berg DE, Covacci A, Engstrand L, Borén T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 849] [Article Influence: 30.3] [Reference Citation Analysis (1)] |
| 29. | Mahdavi J, Sondén B, Hurtig M, Olfat FO, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson KA. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 673] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 30. | Lu H, Yamaoka Y, Graham DY. Helicobacter pylori virulence factors: facts and fantasies. Curr Opin Gastroenterol. 2005;21:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Ishijima N, Suzuki M, Ashida H, Ichikawa Y, Kanegae Y, Saito I, Borén T, Haas R, Sasakawa C, Mimuro H. BabA-mediated adherence is a potentiator of the Helicobacter pylori type IV secretion system activity. J Biol Chem. 2011;286:25256-25264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Toller IM, Neelsen KJ, Steger M, Hartung ML, Hottiger MO, Stucki M, Kalali B, Gerhard M, Sartori AA, Lopes M. Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proc Natl Acad Sci USA. 2011;108:14944-14949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 264] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 33. | Testerman TL, Morris J. Beyond the stomach: an updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World J Gastroenterol. 2014;20:12781-12808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 188] [Cited by in RCA: 219] [Article Influence: 18.3] [Reference Citation Analysis (3)] |
| 34. | Unemo M, Aspholm-Hurtig M, Ilver D, Bergström J, Borén T, Danielsson D, Teneberg S. The sialic acid binding SabA adhesin of Helicobacter pylori is essential for nonopsonic activation of human neutrophils. J Biol Chem. 2005;280:15390-15397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Yamaoka Y. Increasing evidence of the role of Helicobacter pylori SabA in the pathogenesis of gastroduodenal disease. J Infect Dev Ctries. 2008;2:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Yamaoka Y, Kwon DH, Graham DY. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc Natl Acad Sci USA. 2000;97:7533-7538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 320] [Article Influence: 12.3] [Reference Citation Analysis (2)] |
| 37. | Franco AT, Johnston E, Krishna U, Yamaoka Y, Israel DA, Nagy TA, Wroblewski LE, Piazuelo MB, Correa P, Peek RM. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68:379-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 235] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 38. | Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 500] [Cited by in RCA: 469] [Article Influence: 29.3] [Reference Citation Analysis (2)] |
| 39. | Sugimoto M, Ohno T, Graham DY, Yamaoka Y. Gastric mucosal interleukin-17 and -18 mRNA expression in Helicobacter pylori-induced Mongolian gerbils. Cancer Sci. 2009;100:2152-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Backert S, Clyne M, Tegtmeyer N. Molecular mechanisms of gastric epithelial cell adhesion and injection of CagA by Helicobacter pylori. Cell Commun Signal. 2011;9:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 41. | Rimbara E, Mori S, Kim H, Shibayama K. Role of γ-glutamyltranspeptidase in the pathogenesis of Helicobacter pylori infection. Microbiol Immunol. 2013;57:665-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Olofsson A, Vallström A, Petzold K, Tegtmeyer N, Schleucher J, Carlsson S, Haas R, Backert S, Wai SN, Gröbner G. Biochemical and functional characterization of Helicobacter pylori vesicles. Mol Microbiol. 2010;77:1539-1555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 43. | Gong M, Ling SS, Lui SY, Yeoh KG, Ho B. Helicobacter pylori gamma-glutamyl transpeptidase is a pathogenic factor in the development of peptic ulcer disease. Gastroenterology. 2010;139:564-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 44. | Kim IJ, Blanke SR. Remodeling the host environment: modulation of the gastric epithelium by the Helicobacter pylori vacuolating toxin (VacA). Front Cell Infect Microbiol. 2012;2:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Wroblewski LE, Peek RM. “Targeted disruption of the epithelial-barrier by Helicobacter pylori”. Cell Commun Signal. 2011;9:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Fischer W, Prassl S, Haas R. Virulence mechanisms and persistence strategies of the human gastric pathogen Helicobacter pylori. Curr Top Microbiol Immunol. 2009;337:129-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 47. | Murata-Kamiya N, Kurashima Y, Teishikata Y, Yamahashi Y, Saito Y, Higashi H, Aburatani H, Akiyama T, Peek RM, Azuma T. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617-4626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 378] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 48. | Saadat I, Higashi H, Obuse C, Umeda M, Murata-Kamiya N, Saito Y, Lu H, Ohnishi N, Azuma T, Suzuki A. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447:330-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 403] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 49. | Wroblewski LE, Piazuelo MB, Chaturvedi R, Schumacher M, Aihara E, Feng R, Noto JM, Delgado A, Israel DA, Zavros Y. Helicobacter pylori targets cancer-associated apical-junctional constituents in gastroids and gastric epithelial cells. Gut. 2015;64:720-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 50. | Marcus EA, Vagin O, Tokhtaeva E, Sachs G, Scott DR. Helicobacter pylori impedes acid-induced tightening of gastric epithelial junctions. Am J Physiol Gastrointest Liver Physiol. 2013;305:G731-G739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Osman MA, Bloom GS, Tagoe EA. Helicobacter pylori-induced alteration of epithelial cell signaling and polarity: a possible mechanism of gastric carcinoma etiology and disparity. Cytoskeleton (Hoboken). 2013;70:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Lu H, Murata-Kamiya N, Saito Y, Hatakeyama M. Role of partitioning-defective 1/microtubule affinity-regulating kinases in the morphogenetic activity of Helicobacter pylori CagA. J Biol Chem. 2009;284:23024-23036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | Deen NS, Huang SJ, Gong L, Kwok T, Devenish RJ. The impact of autophagic processes on the intracellular fate of Helicobacter pylori: more tricks from an enigmatic pathogen? Autophagy. 2013;9:639-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Greenfield LK, Jones NL. Modulation of autophagy by Helicobacter pylori and its role in gastric carcinogenesis. Trends Microbiol. 2013;21:602-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 55. | Saberi S, Douraghi M, Azadmanesh K, Shokrgozar MA, Zeraati H, Hosseini ME, Mohagheghi MA, Parsaeian M, Mohammadi M. A potential association between Helicobacter pylori CagA EPIYA and multimerization motifs with cytokeratin 18 cleavage rate during early apoptosis. Helicobacter. 2012;17:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu Rev Biochem. 2004;73:87-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 57. | Ashktorab H, Dashwood RH, Dashwood MM, Zaidi SI, Hewitt SM, Green WR, Lee EL, Daremipouran M, Nouraie M, Malekzadeh R. H. pylori-induced apoptosis in human gastric cancer cells mediated via the release of apoptosis-inducing factor from mitochondria. Helicobacter. 2008;13:506-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Iwai H, Kim M, Yoshikawa Y, Ashida H, Ogawa M, Fujita Y, Muller D, Kirikae T, Jackson PK, Kotani S. A bacterial effector targets Mad2L2, an APC inhibitor, to modulate host cell cycling. Cell. 2007;130:611-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 59. | Fan X, Crowe SE, Behar S, Gunasena H, Ye G, Haeberle H, Van Houten N, Gourley WK, Ernst PB, Reyes VE. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J Exp Med. 1998;187:1659-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 147] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 60. | Fan X, Gunasena H, Cheng Z, Espejo R, Crowe SE, Ernst PB, Reyes VE. Helicobacter pylori urease binds to class II MHC on gastric epithelial cells and induces their apoptosis. J Immunol. 2000;165:1918-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 61. | Lin WC, Tsai HF, Liao HJ, Tang CH, Wu YY, Hsu PI, Cheng AL, Hsu PN. Helicobacter pylori sensitizes TNF-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human gastric epithelial cells through regulation of FLIP. Cell Death Dis. 2014;5:e1109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Beswick EJ, Pinchuk IV, Minch K, Suarez G, Sierra JC, Yamaoka Y, Reyes VE. The Helicobacter pylori urease B subunit binds to CD74 on gastric epithelial cells and induces NF-kappaB activation and interleukin-8 production. Infect Immun. 2006;74:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 63. | Tanahashi T, Kita M, Kodama T, Yamaoka Y, Sawai N, Ohno T, Mitsufuji S, Wei YP, Kashima K, Imanishi J. Cytokine expression and production by purified Helicobacter pylori urease in human gastric epithelial cells. Infect Immun. 2000;68:664-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 64. | Lu H, Wu JY, Kudo T, Ohno T, Graham DY, Yamaoka Y. Regulation of interleukin-6 promoter activation in gastric epithelial cells infected with Helicobacter pylori. Mol Biol Cell. 2005;16:4954-4966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Pinchuk IV, Morris KT, Nofchissey RA, Earley RB, Wu JY, Ma TY, Beswick EJ. Stromal cells induce Th17 during Helicobacter pylori infection and in the gastric tumor microenvironment. PLoS One. 2013;8:e53798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 66. | Kabir S. The role of interleukin-17 in the Helicobacter pylori induced infection and immunity. Helicobacter. 2011;16:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 67. | Paoluzi OA, Blanco del VG, Caruso R, Monteleone I, Monteleone G, Pallone F. Impairment of ghrelin synthesis in Helicobacter pylori-colonized stomach: new clues for the pathogenesis of H. pylori-related gastric inflammation. World J Gastroenterol. 2014;20:639-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Ohnishi N, Yuasa H, Tanaka S, Sawa H, Miura M, Matsui A, Higashi H, Musashi M, Iwabuchi K, Suzuki M. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci USA. 2008;105:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 472] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 69. | Wang F, Wu X, Liu Z, Bu G, Li X, Qu N, Peng J, Xu C, Shen S, Yuan Y. Association between Virulence Factors and TRAF1/4-1BB/Bcl-xL Expression in Gastric Mucosa Infected with Helicobacter pylori. Gastroenterol Res Pract. 2015;2015:648479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Wang TR, Peng JC, Qiao YQ, Zhu MM, Zhao D, Shen J, Ran ZH. Helicobacter pylori regulates TLR4 and TLR9 during gastric carcinogenesis. Int J Clin Exp Pathol. 2014;7:6950-6955. [PubMed] |
| 71. | Hitkova I, Yuan G, Anderl F, Gerhard M, Kirchner T, Reu S, Röcken C, Schäfer C, Schmid RM, Vogelmann R. Caveolin-1 protects B6129 mice against Helicobacter pylori gastritis. PLoS Pathog. 2013;9:e1003251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Aghdam SM, Sardari Z, Safaralizadeh R, Bonyadi M, Abdolmohammadi R, Moghadam MS, Khalilnezhad A. Investigation of association between oipA and iceA1/iceA2 genotypes of Helicobacter pylori and gastric cancer in Iran. Asian Pac J Cancer Prev. 2014;15:8295-8299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Xia Y, Yamaoka Y, Zhu Q, Matha I, Gao X. A comprehensive sequence and disease correlation analyses for the C-terminal region of CagA protein of Helicobacter pylori. PLoS One. 2009;4:e7736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 74. | Lee IO, Kim JH, Choi YJ, Pillinger MH, Kim SY, Blaser MJ, Lee YC. Helicobacter pylori CagA phosphorylation status determines the gp130-activated SHP2/ERK and JAK/STAT signal transduction pathways in gastric epithelial cells. J Biol Chem. 2010;285:16042-16050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 75. | Naito M, Yamazaki T, Tsutsumi R, Higashi H, Onoe K, Yamazaki S, Azuma T, Hatakeyama M. Influence of EPIYA-repeat polymorphism on the phosphorylation-dependent biological activity of Helicobacter pylori CagA. Gastroenterology. 2006;130:1181-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 76. | Ferreira RM, Machado JC, Leite M, Carneiro F, Figueiredo C. The number of Helicobacter pylori CagA EPIYA C tyrosine phosphorylation motifs influences the pattern of gastritis and the development of gastric carcinoma. Histopathology. 2012;60:992-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 77. | Fujiya K, Nagata N, Uchida T, Kobayakawa M, Asayama N, Akiyama J, Shimbo T, Igari T, Banerjee R, Nageshwar Reddy D. Different gastric mucosa and CagA status of patients in India and Japan infected with Helicobacter pylori. Dig Dis Sci. 2014;59:631-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 78. | Rhead JL, Letley DP, Mohammadi M, Hussein N, Mohagheghi MA, Eshagh Hosseini M, Atherton JC. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133:926-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 310] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 79. | Ji X, Fernandez T, Burroni D, Pagliaccia C, Atherton JC, Reyrat JM, Rappuoli R, Telford JL. Cell specificity of Helicobacter pylori cytotoxin is determined by a short region in the polymorphic midregion. Infect Immun. 2000;68:3754-3757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 80. | Atherton JC, Cao P, Peek RM, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771-17777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1078] [Cited by in RCA: 1112] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 81. | Miehlke S, Kirsch C, Agha-Amiri K, Günther T, Lehn N, Malfertheiner P, Stolte M, Ehninger G, Bayerdörffer E. The Helicobacter pylori vacA s1, m1 genotype and cagA is associated with gastric carcinoma in Germany. Int J Cancer. 2000;87:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 82. | Basso D, Zambon CF, Letley DP, Stranges A, Marchet A, Rhead JL, Schiavon S, Guariso G, Ceroti M, Nitti D. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008;135:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 295] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 83. | Ogiwara H, Graham DY, Yamaoka Y. vacA i-region subtyping. Gastroenterology. 2008;134:1267; author reply 1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 84. | Talebi Bezmin Abadi A, Mohabbati Mobarez A. High Prevalence of Helicobacter pylori hopQ II Genotype Isolated from Iranian Patients with Gastroduodenal Disorders. J Pathog. 2014;2014:842469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Oluwasola AO. Genetic determinants and clinico-pathological outcomes of helicobacter pylori infection. Ann Ib Postgrad Med. 2014;12:22-30. [PubMed] |
| 86. | Lu W, Pan K, Zhang L, Lin D, Miao X, You W. Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor {alpha} and risk of gastric cancer in a Chinese population. Carcinogenesis. 2005;26:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 173] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 87. | Wu X, Zeng Z, Chen B, Yu J, Xue L, Hao Y, Chen M, Sung JJ, Hu P. Association between polymorphisms in interleukin-17A and interleukin-17F genes and risks of gastric cancer. Int J Cancer. 2010;127:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 88. | Castaño-Rodríguez N, Kaakoush NO, Mitchell HM. Pattern-recognition receptors and gastric cancer. Front Immunol. 2014;5:336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 89. | Jeon C, Chang SC, Mu L, Zhao J, Rao JY, Lu QY, Zhang ZF. Genetic variants of peroxisome proliferator-activated receptor δ are associated with gastric cancer. Dig Dis Sci. 2013;58:2881-2886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Ebert MP, Lendeckel U, Westphal S, Dierkes J, Glas J, Folwaczny C, Roessner A, Stolte M, Malfertheiner P, Röcken C. The angiotensin I-converting enzyme gene insertion/deletion polymorphism is linked to early gastric cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2987-2989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 91. | Izzotti A, De Flora S, Cartiglia C, Are BM, Longobardi M, Camoirano A, Mura I, Dore MP, Scanu AM, Rocca PC. Interplay between Helicobacter pylori and host gene polymorphisms in inducing oxidative DNA damage in the gastric mucosa. Carcinogenesis. 2007;28:892-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 92. | Wroblewski LE, Peek RM. Helicobacter pylori in gastric carcinogenesis: mechanisms. Gastroenterol Clin North Am. 2013;42:285-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 93. | Wang LL, Yu XJ, Zhan SH, Jia SJ, Tian ZB, Dong QJ. Participation of microbiota in the development of gastric cancer. World J Gastroenterol. 2014;20:4948-4952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 94. | Sheh A, Fox JG. The role of the gastrointestinal microbiome in Helicobacter pylori pathogenesis. Gut Microbes. 2013;4:505-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 95. | Brawner KM, Morrow CD, Smith PD. Gastric microbiome and gastric cancer. Cancer J. 2014;20:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 96. | Manson JM, Rauch M, Gilmore MS. The commensal microbiology of the gastrointestinal tract. Adv Exp Med Biol. 2008;635:15-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 97. | Hagiwara T, Mukaisho K, Nakayama T, Hattori T, Sugihara H. Proton pump inhibitors and helicobacter pylori-associated pathogenesis. Asian Pac J Cancer Prev. 2015;16:1315-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 98. | Santibáñez M, Aguirre E, Belda S, Aragones N, Saez J, Rodríguez JC, Galiana A, Sola-Vera J, Ruiz-García M, Paz-Zulueta M. Relationship between tobacco, cagA and vacA i1 virulence factors and bacterial load in patients infected by Helicobacter pylori. PLoS One. 2015;10:e0120444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 99. | Loh JT, Torres VJ, Cover TL. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007;67:4709-4715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 100. | Lee JY, Kim N, Nam RH, Choi YJ, Seo JH, Lee HS, Oh JC, Lee DH. No Correlation of Inflammation With Colonization of Helicobacter pylori in the Stomach of Mice Fed High-salt Diet. J Cancer Prev. 2014;19:144-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 101. | Noto JM, Gaddy JA, Lee JY, Piazuelo MB, Friedman DB, Colvin DC, Romero-Gallo J, Suarez G, Loh J, Slaughter JC. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest. 2013;123:479-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 102. | Loh JT, Friedman DB, Piazuelo MB, Bravo LE, Wilson KT, Peek RM, Correa P, Cover TL. Analysis of Helicobacter pylori cagA promoter elements required for salt-induced upregulation of CagA expression. Infect Immun. 2012;80:3094-3106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 103. | Lin CH, Wu CY, Kou HS, Chen CY, Huang MC, Hu HM, Wu MC, Lu CY, Wu DC, Wu MT. Effect of Di(2-ethylhexyl)phthalate on Helicobacter pylori-Induced Apoptosis in AGS Cells. Gastroenterol Res Pract. 2013;2013:924769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Adachi Y S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK