INTRODUCTION
The pancreas is a complex organ, containing both endocrine and exocrine components. The endocrine component of the pancreas is composed of the Islets of Langerhans, and comprises a relatively small portion of the pancreas, only about 1%-2% of the organ. The endocrine pancreas is responsible for the production of glucagon and insulin, hormones that regulate glucose homeostasis. The exocrine component comprises the vast majority of the pancreas and is comprised of acinar, ductal, and stellate cells. The acinar cells produce digestive enzymes that are synthesized as inactive zymogens and are secreted through ducts to the duodenum where they are activated. The ductal cells produce and secrete large quantities of bicarbonate (HCO3-) and form a network that serves as a conduit for the delivery of the digestive enzymes into the duodenum. The pancreatic stellate cells synthesize and degrade extracellular matrix proteins.
Pancreatitis is a serious gastrointestinal illness and an important health concern both in the United States and worldwide. Typically, pancreatitis is either classified as acute or chronic. Acute pancreatitis is a necro-inflammatory disease that is characterized by infiltration of the pancreas by inflammatory cells and destruction of the pancreatic exocrine cells. Based on clinical observations in human beings, it is believed that in cases of acute pancreatitis, which resolve, the pancreas regenerates to its full structural and functional capacity after an acute episode. This concept is supported by many studies in experimental animals, which have demonstrated structural and functional repair of the pancreas after experimentally induced pancreatitis[1-5]. In cases of severe acute pancreatitis systemic inflammation develops and can lead to multi-organ failure and death.
Of all gastrointestinal ailments, acute pancreatitis is the single most common cause of hospitalization in the United States. It has been estimated that acute pancreatitis accounts for approximately 2.6 billion dollars in annual inpatient costs[6]. Each year, approximately 220000 people are admitted to United States hospitals because of acute pancreatitis[7]. In up to 20% of these cases there are serious complications with a mortality rate ranging from 10% to 30%[8,9]. Currently, there is no specific pharmacotherapy for acute pancreatitis, underscoring the need for research that aids in the prevention and treatment of this disease.
Although the pathogenesis of acute pancreatitis is not entirely known, it appears that the disease originates in injured acinar cells. Over a hundred years ago, Chiari[10] suggested that acute pancreatitis is caused by inappropriate activation of digestive enzymes, ultimately leading to autodigestion of the pancreas. Although inappropriate activation of trypsinogen is important in causing pancreatic injury early in the disease, the induction and progression of local and systemic inflammation associated with acute pancreatitis does not require trypsinogen activation[11]. In fact, it has been demonstrated that intra-acinar cell activation of the transcriptional activator nuclear factor-κB (NF-κB) occurs simultaneous to, but independent of, trypsinogen activation[11]. NF-κB regulates a wide variety of genes involved in cell survival, cellular replication, immunity, and inflammation. The NF-κB-mediated inflammatory response appears to be responsible for up to half of the pancreatic tissue damage that is associated with acute pancreatitis, as well as the potentially fatal severe systemic inflammatory response[11].
Chronic pancreatitis, like acute pancreatitis, is thought to begin as a necro-inflammatory disease. The exact series of events that ultimately result in chronic pancreatitis are not known. Despite this fact, it is generally thought that chronic pancreatitis has an early stage that is characterized by recurrent attacks of acute pancreatitis, and a late stage associated with pancreatic insufficiency, steatorrhea, diabetes, pancreatic calcification, and fibrosis[12].
ALCOHOLIC PANCREATITIS
Alcohol abuse is commonly associated with pancreatitis. This association has been recognized for well over 100 years, yet to this day how alcohol abuse predisposes the pancreas to disease is not entirely understood[13]. In developing countries, approximately 35% of acute pancreatitis cases[9] and approximately 70% of chronic pancreatitis cases are associated with alcohol abuse[14]. Additionally, individuals diagnosed with chronic pancreatitis are 20-times more likely to develop pancreatic cancer[15], a disease with a dismal prognosis. It is thought that changes that occur in the pancreas during chronic injury are associated with, or predispose the organ to, the initiation of pancreatic neoplasia. This has led to the classification of chronic pancreatitis as a preneoplastic disease.
How alcohol abuse contributes to alcoholic pancreatitis is not fully understood. Although there is a tremendous association between alcohol abuse and pancreatitis, relatively few individuals who abuse alcohol develop alcoholic pancreatitis. This fact indicates that alcoholic pancreatitis is not caused by chronic alcohol abuse alone[16-18]. Instead, it appears that the pancreas is sensitized to injury by alcohol consumption, and external or environmental factors trigger initiation of this disease. A number of factors are believed to be triggers of alcoholic pancreatitis, among these are: Genetic predisposition, high lipid diet, cigarette smoking, and infectious agents[19].
Although alcoholic pancreatitis can remain an acute disease, in many cases this acute disease progresses to alcoholic chronic pancreatitis. Many times this progression from an acute disease to a chronic disease is associated with recurring bouts of acute pancreatitis. Interestingly, it was reported, that progression from acute to chronic pancreatitis is most common in habitual alcohol abusers[20]. This indicates that excessive alcohol consumption is involved in acute pancreatitis progressing to a chronic fibrotic disorder. Because ethanol alone is not capable of causing pancreatitis, the question is, how does ethanol alter the physiology of the pancreas and sensitize the organ to disease?
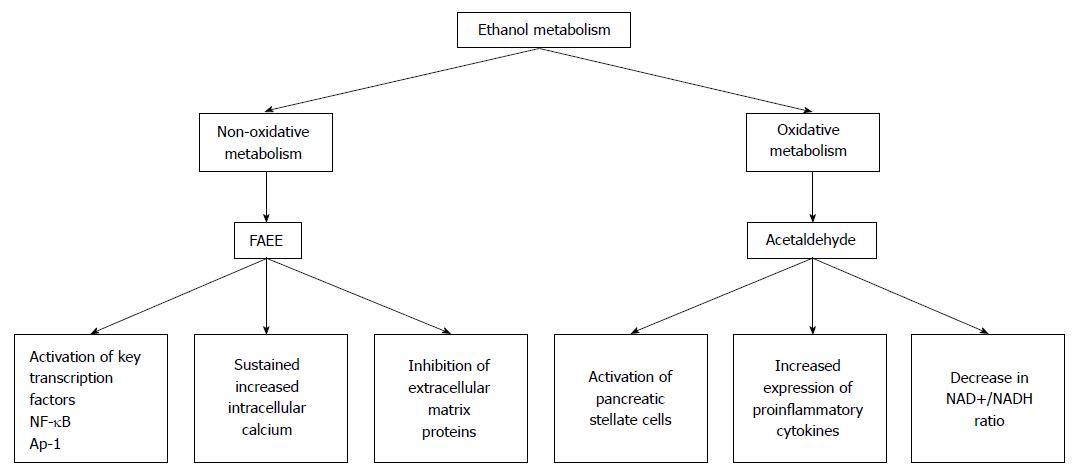
Developmentally, the liver and the pancreas are related[21]. Because of this, it is not terribly surprising that the pancreas can metabolize ethanol. In the pancreas, both nonoxidative and oxidative pathways of ethanol metabolism are functional and have been shown to have a number of deleterious effects on the pancreas (Figure 1).
Figure 1 The by-products of ethanol metabolism cause a number of changes in the pancreas.
In the pancreas, ethanol is metabolized by both nonoxidative and oxidative pathways. The major by-products of the nonoxidative metabolism of ethanol are FAEEs. The major metabolic by-product of the oxidative metabolism of ethanol is acetaldehyde. Metabolism of ethanol by both of these pathways has been shown to cause a number of changes that can predispose the pancreas to acute pancreatitis. FAEE: Fatty acid ethyl ester; NF-κB: Nuclear factor-κB.
Two enzymes, alcohol dehydrogenase (ADH) and cytochrome P450 2E1 (CYP 2E1) catalyze oxidative ethanol metabolism. Ethanol metabolized by both ADH and CYP 2E1 results in the production of reactive oxygen species (ROS) and acetaldehyde. Although the pancreas expresses both ADH and CYP 2E1, the expression of these enzymes is much lower than in the liver. Consequently, the oxidative metabolism of ethanol by the pancreas is also much lower than in the liver[22,23]. In spite of this fact, acetaldehyde, a reactive metabolite of ethanol oxidation, mediates some detrimental effects in pancreatic acinar cells[24].
Nonoxidative ethanol metabolism is accomplished by a diverse group of enzymes known as fatty acid ethyl ester (FAEE) synthases[25]. Ethanol metabolism by these enzymes combines free fatty acids (FA) with ethanol generating FAEEs. In the pancreas fatty acid ester synthase activity is relatively high. Therefore, ethanol metabolism by the nonoxidative pathway is also relatively high[26]. Because ADH and CYP 2E1 activity is relatively low in the pancreases, ethanol metabolism by FAEE synthases and the production of FAEEs likely has an important role in alcohol associated pancreatic dysfunctions and development of alcoholic pancreatitis.
Effects of ethanol on the cellular mobilization of calcium and the inappropriate activation of pancreatic enzymes
It is generally thought that one of the initiating events of acute pancreatitis is the intracellular activation of trypsinogen and other digestive enzymes produced by acinar cells. This inappropriate enzyme activation is mediated by sustained elevation in the concentration of cytoplasmic calcium[27,28].
Intracellular calcium has a critical role in both normal and pathologic actions of acinar cells. The majority of calcium in acinar cells is stored in the endoplasmic reticulum (ER), although there exists an important acidic granular reservoir located in the apical region of the cell. Zymogen granules contain substantial quantities of calcium and constitute a major portion of this acidic reservoir in acinar cells[29].
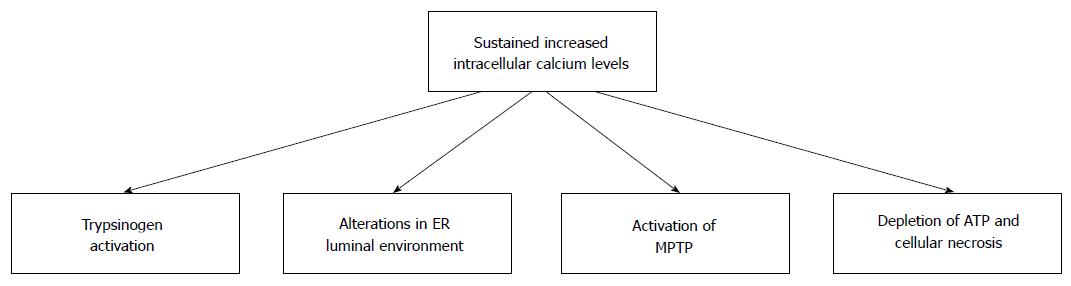
Secretion of zymogens from acinar cells is controlled by the local release of small quantities of calcium from the zymogen-containing granules. In contrast, global sustained release of calcium from intracellular stores sets in action a number of pathologic changes in acinar cells (Figure 2). Thus, intracellular calcium is involved both in the normal and the pathologic processes of acinar cells[30].
Figure 2 Consequences of sustained increased intracellular calcium in pancreatic acinar cells.
Ethanol and its metabolic by-products can cause sustained increases in the level of intracellular calcium. Sustained increases in intracellular calcium results in cellular changes that can damage pancreatic acinar cells. Many of these changes can predispose individuals to the development of alcoholic acute pancreatitis. MPTP: Mitochondrial permeability transition pore; ER: Endoplasmic reticulum.
It has been demonstrated that stimulation of inositol trisphosphate (IP3) type 2 and 3 receptors (IP3Rs) and to a lesser extent ryanodine receptors, located on the ER and zymogen granules results in calcium release[30]. Importantly, in both whole cells and 2-photon permeabilized cells, ethanol and FAEEs induce the sustained release of calcium from intracellular stores by activation of IP3Rs[31]. The critical role of IP3Rs in the pathologic sustained intracellular release of calcium has been demonstrated by studies in which antibodies specific to IP3R2 and 3, pharmacologic inhibition of IP3Rs, and the use of genetically modified mice that lack IP3R2 and 3, attenuate the intensity of calcium release, as well as the extent of trypsinogen activation and tissue necrosis[30-33].
Acinar cells do not contain infinite stores of calcium. In response to increases in cytosolic calcium concentrations, ATP-dependent calcium pumps located on the plasma membrane are activated and eliminate calcium. Therefore, to maintain sustained elevated levels of calcium there must be a mechanism by which acinar cells take up calcium from the extracellular environment.
Located on the basolateral portion of the plasma membrane of acinar cells are calcium-release activated calcium (CRAC) channels. When calcium concentrations in the ER are reduced, a calcium sensing protein (STIM1) located in the ER translocates to these CRAC channels where it interacts with Orai1. The channels are activated and extracellular calcium is taken up from the extracelluar environment. This uptake sustains the elevated levels of intracellular calcium[34]. Importantly, it has been shown that a CRAC channel inhibitor, GSK-7975A, inhibited the calcium entry into acinar cells. Inhibition of calcium entry was able to abrogate trypsin and protease activity, as well as necrosis induced by treatment of acinar cells with FAEEs[32].
Acinar cells are not without some protection from the deleterious effects of sustained elevated calcium levels. Using 2-photon permeabilized acinar cells Gerasimenko et al[33] showed that the actions of ethanol treatment were accentuated in permeabilized cells compared with intact cells. This increased severity could be overcome if physiologic concentrations of calmodulin were included in the extracellular media. The authors speculated that calmodulin was lost from the permeabilized cells and that inclusion of calmodulin in the extracellular media allowed calmodulin to reenter the cells and protect them from the actions of elevated calcium[33]. In support of this contention, the authors demonstrated that pharmacologic inhibition of calmodulin with calmodulin inhibitory peptide resulted in activation of trypsin in permeabilized cells. Conversely, pharmacologic activation of calmodulin with the cell permeable calmodulin activator, CALP-3, substantially abolished the detrimental actions of ethanol in both permeabilized and intact cells[33].
The findings that the addition of calmodulin and inhibition of CRAC channels attenuate the deleterious effects of sustained elevated levels of cytosolic calcium provide novel targets for therapeutic intervention and the treatment of acute pancreatitis[30].
Mitochondrial dysfunction in alcoholic pancreatitis
Mitochondria are intimately involved in the life and death of cells. Mitochondria are responsible for the production of ATP and thus, the energy required to perform all cellular functions. Conversely, mitochondrial damage or dysfunction can lead to cell death by either apoptosis or necrosis. Mitochondria are involved in the activation of the classical apoptosis pathway of cell death in which there is little release of intracellular material and limited activation of the inflammatory response. If mitochondrial dysfunction becomes severe enough, ATP production is reduced or inhibited. In the absence of ATP cell death is necrotic. Cellular necrosis is characterized by disruption of the plasma membrane, release of cellular contents, including activated enzymes, and the activation of the inflammatory response. Of clinical importance, necrotic cell death is associated with more severe pancreatitis[35,36].
In pancreatic acinar cells mitochondria play an important role in maintaining calcium homeostasis. This may be, in part, because of their juxtaposition to sites of calcium release from the ER. It has been shown that peri-apical mitochondria take up cytosolic calcium released during local calcium spikes and respond by increasing ATP production. This ATP is used to drive the sarcoER Ca2+ ATPase pump (SERCA), which restores ER calcium and the plasma membrane Ca2+ pump (PMCA), which restores normal cytosolic calcium levels, thereby terminating the signal and preventing the spread of the signal throughout the cell[37,38]. Unfortunately, this normal physiologic response of mitochondria to increased cytosolic calcium levels can also lead to cell death. If the elevated cytoplasmic calcium concentration is global and sustained this normal cellular compensatory mechanism can be overwhelmed and result in cell death.
As mentioned above, the nonoxidative metabolites of ethanol, FAEEs, can bind IP3Rs on the ER and zymogen granules causing the release of calcium. Excessive mitochondrial calcium can cause permeabilization of the mitochondrial membrane. Mitochondrial membrane permeabilization is a trigger that initiates both apoptotic and necrotic cell death pathways[39]. Permeabilization of the mitochondrial membrane leads to the loss of mitochondrial membrane potential (Δψm) by opening the mitochondrial permeability transition pore (MPTP). Activation of the MPTP allows nonspecific entry of substances with a nuclear mass of less than 15000 Daltons into the inner mitochondrial matrix. This can disrupt the ability of mitochondria to produce ATP and ultimately in necrosis.
FAEEs have also been shown to bind to the inner mitochondrial membrane where they undergo hydrolysis to FA by FAEE hydrolases[40]. This results in the production of locally high concentrations of FA, which can uncouple oxidative phosphorylation and deplete Δψm, thereby inhibiting ATP production[31]. The lack of ATP production exacerbates the effects of the cytosolic calcium because the lack of ATP to drive the ATP-dependent SERCA and PMCA pumps results in the inability of the cell to regain calcium homeostasis.
In cases where the oxidative metabolism of ethanol is diminished the levels of FAEE are increased and can result in tissue damage[41,42]. This is thought to be the explanation for the high level of FAEEs in the pancreas. In support of this, treatment of isolated pancreatic acinar cells with low levels of ethanol and the fatty acid palmitoleic acid caused transient rises in intracellular calcium. Inhibition of the oxidative metabolism of ethanol with 4-methylpyrazol in these cells resulted in the conversion of transient calcium rises to sustained increases in calcium. The sustained elevated levels of calcium resulted in mitochondrial membrane depolarization and cellular necrosis[43]. Inhibition of the FAEE synthase carboxylester lipase with 3-benzyl-6-chloro-2-pyrone (3-BCP) ameliorated the adverse actions of the combined treatment[43]. In vivo studies demonstrated that mice treated with ethanol and palmitoleic acid resulted in increased levels of palmitoleic acid ethyl ester, extensive edema, neutrophil infiltration and acinar cell necrosis. Furthermore, these pathologic changes were accentuated by the inclusion of 4-methylpyrazol treatment. Treatment of these mice with 3-BCP significantly reduced the pathologic effects[43]. Thus, inhibition of fatty acid ethyl synthases reduced the tissue injury associated with ethanol and fatty acid treatment and may be an effective strategy to attenuate the severity of alcohol acute pancreatitis.
Oxidative metabolism of ethanol has also been shown to have deleterious effects on mitochondria[24]. Using isolated mouse acinar cells, as well as, in vivo and ex vivo models of pancreatitis it has been shown that ethanol treatment reduces the Δψm and converts the normal transient decrease in Δψm caused by treatment with physiologic concentrations of cholecystokinin (CCK) to a sustained decrease in Δψm. The sustained decrease in Δψm results in reduced cellular ATP concentrations and necrosis[24]. Using mice deficient in cyclophilin-D, a major component of the MPTP, it was demonstrated that the MPTP plays a major role in the ethanol-mediated sensitivity to mitochondrial depolarization. Further studies revealed that the ethanol-induced effects on Δψm were dependent on the decreased NAD+/NADH ratio associated with the oxidative metabolism of ethanol and its by-product acetaldehyde, and not dependent on calcium.
Metabolism of acetaldehyde is primarily carried out by aldehyde dehydrogenase-2 (ALDH2) a NAD+ requiring enzyme residing on the inner mitochondrial membrane. Thus, metabolism of acetaldehyde by ALDH2 depletes NAD+ and increases the concentration of NADH. Because of this, the authors speculated that the decreased mitochondrial NAD+/NADH ratio and reduced Δψm is a result of the metabolism of acetaldehyde to acetate. This contention is supported by the facts that pharmacologic depletion of NAD+ with FK866 also results in mitochondrial depolarization, and the fact that supplementation with NAD+ ameliorates the effects of ethanol[24].
Interestingly, in mitochondria isolated from the liver, ethanol metabolism induces activation of the MPTP, at least in part, through increased cyclophilin-D activity and the increased association of cyclophilin-D with adenine nucleotide translocator-1 (ANT-1)[44]. This increased cyclophilin activity appears to be linked to sirtuin-3, a NAD+-dependent deacetylase localized to the mitochondrial matrix that is involved in the regulation of cyclophilin-D acetylation[45]. Ethanol oxidation-mediated decrease in the NAD+/NADH ratio leads to decreased sirtuin-3 activity and consequently, hyperacetylation of cyclophilin-D. Hyperacetylation of cyclophilin-D results in increased cyclophilin-D activity, increased binding to ANT-1, and MPTP induction[44]. The role of NAD+ in MPTP in the pancreas makes it tempting to speculate that the ethanol oxidation-mediated induction of the MPTP in pancreatic mitochondria is mediated by a similar NAD+-sirtuin-3-cyclophilin-D mechanism. Thus, NAD+/NADH may be a novel target to ameliorate alcoholic acute pancreatitis.
ER-stress in alcoholic pancreatitis
The primary function of pancreatic acinar cells is to produce digestive enzymes[46]. Synthesis of these proteins requires an extensive ER network. In the ER, newly synthesized proteins undergo posttranslational modification, disulfide bond formation, and chaperone-facilitated protein folding before being transported to the Golgi apparatus. Once in the Golgi these proteins undergo further modification before being transported to zymogen granules or other cellular organelles. Proper protein folding and sorting are critical in preventing inappropriate activation of digestive proenzymes in acinar cells.
Proper protein modification and folding in the ER requires the appropriate levels of intraluminal calcium and ATP, as well as the proper oxidizing environment for disulfide bond formation. Perturbations in these environmental factors, results in the production of misfolded proteins and what is referred to as ER stress. Detection of these misfolded proteins initiates the unfolded protein response. The unfolded protein response is mediated by the activation of three pathways: (1) the inositol-requiring protein-1 pathway (IRE-1); (2) the activating transcription factor-6 pathway; and (3) the RNA-activated protein kinase-like ER kinase (PERK) pathway. In general, activation of the unfolded protein response decreases the production of cellular proteins and increases the expression of proteins involved in protein folding. It is thought that this adaptive response is protective and aids cells in riding themselves of misfolded proteins, the presence of which can be detrimental to cells[47,48].
It has been shown in mice that administration of ethanol by the intragastric feeding model causes ER stress in pancreatic acinar cells[49]. This is characterized by dilation of the ER, alteration of the redox state of the ER, and up regulation of the oxidoreductase, protein disulfide isomerase. It was suggested that the altered redox state was the result of ROS generated in the ER. Additionally, expression of IRE-1 and its downstream effector the spliced form of X-box binding protein-1 (sXBP1) were increased.
The authors speculated that the increased expression of IRE-1 and sXBP1 were critical for the adaptive protective response, and that induction of this pathway protected acinar cells from the detrimental effects of ROS-induced ER stress. To test this hypothesis, the authors examined the effects of ethanol in mice heterozygous for XBP1 (XBP1+/-). Ethanol administration to XBP1+/- mice resulted in a number of ultrastructural changes. These changes included extensive dilation of the ER, a dramatic increase in the number of autophagic vesicles, a substantial decrease in the number of zymogen granules, and the inappropriate localization of zymogen granules throughout the cell. These changes in ultrastructure were also associated with decreased expression of the pancreatic digestive enzyme amylase[49]. Additionally, compared with wild type mice administered ethanol, XBP1+/- mice administered ethanol demonstrated marked increase in PERK, eIF2α phosphorylation, and expression of ATF4[49]; adaptations associated with severe ER stress[50]. Furthermore, approximately 20% of the pancreas in XBP1+/- mice administered ethanol contained pathologic lesions characterized by areas of necrosis, apoptosis, and inflammation[49]. Thus, it appears that ER stress and activation of the unfolded protein response if controlled can be a protective adaptive response. Alternatively, an uncontrolled unfolded protein response can result in cell death and tissue injury[51]. These findings indicate that modulation of ER stress and the unfolded protein response may provide some protection from alcoholic acute pancreatitis.
Pancreatitis and impaired autophagy
Autophagy is an important cellular process by which unneeded or damaged cellular components or organelles are sequestered in autophagic vacuoles and targeted to the lysosomes for degradation. Impairment of this process has been implicated in the pathogenesis of a number of diseases including pancreatitis[52-56]. Ethanol can affect autophagy in a number of organs, including the pancreas[53,57,58].
One histological characteristic of pancreatitis is the accumulation of large vacuoles within acinar cells[59]. It has been demonstrated in preclinical animal models of pancreatitis, as well as in tissue from human beings, that these vacuoles are autophagic vacuoles[55,56]. These vacuoles possess markers of both autophagosomes and lysosomes, and contain undegraded or partially degraded cellular material[56]. The finding that these vacuoles contain undegraded or partially degraded cellular material indicates that the degradation of the material in the autolysosomes, a late event in the autophagic process, is impaired during pancreatitis[56]. Thus, it appears that the inability to complete the autophagic process is responsible for the accumulation of the vacuoles characteristic of pancreatitis.
Cathepsin L and cathepsin B are two important lysosomal hydrolases. Cathepsin L degrades trypsinogen and trypsin, whereas cathepsin B cleaves trypsinogen forming active trypsin. Thus, the activity of these two enzymes has a pivotal role in trypsin activity. Inappropriate trypsin activation is thought to be an early event in the initiation of pancreatitis. How trypsin is inappropriately activated in acinar cells is poorly understood. It has been proposed that cathepsin B is mis-sorted to the zymogen granules, where it cleaves trypsinogen, forming trypsin. How trypsinogen and cathepsin B come in contact has always been a mystery. Recent studies indicate that impairment in the completion of the autophagic process has a role in the co-localization of these two enzymes[56].
Pancreatitis leads to increased levels of cathepsin L and cathepsin B in the zymogen granule fraction. In alcoholic pancreatitis, as well as other forms of acute pancreatitis, the processing and activation of these two enzymes is impaired[56,60]. Importantly, the impairment in cathepsin L activity is more severe than the impairment in cathepsin B activity, especially in the zymogen granules[56]. Additionally, analysis of the autophagosome/autolysosome fraction revealed the presence of zymogen granules. Thus, in these zymogen granule-containing autophagosomes/autolysosomes trypsinogen and cathepsin B come in contact[56]. The imbalance between cathepsin B and cathepsin L activity in these vacuoles would favor the activation of trypsin, and the initiation of pancreatitis. Therefore, lysosomal dysfunction may not only contribute to the accumulation of vacuoles, but may also have an important role in the inappropriate intracellular activation of trypsin and the initiation of pancreatitis.
Ethanol impairs other aspects of autophagy. It has been demonstrated that lysosomal-associated membrane protein-2 (Lamp-2), a lysosomal membrane protein required for the fusion of autophagosomes with lysosomes, is depleted in the pancreata of rats suffering from alcoholic pancreatitis[53,61]. Importantly, analysis of pancreata from human beings revealed that in patients suffering from chronic alcoholic pancreatitis Lamp-2 expression is also decreased[53]. These results indicate that ethanol consumption can inhibit the expression of lysosomal proteins, particularly Lamp-2. Decreased expression of Lamp-2 impairs the fusion of autophagosomes with lysosomes, and autophagic flux. This impairment may be another contributing factor to alcoholic pancreatitis in human beings.
INVOLVEMENT OF PANCREATIC STELLATE CELLS IN ALCOHOLIC PANCREATITIS
The pancreas, like the liver, contains a population of periacinar vitamin A storing cells known as stellate cells[62,63]. These cells, like their hepatic counterparts, synthesize extracellular matrix proteins, as well as matrix metalloproteinases (enzymes that degrade extracellular matrix proteins) and in the healthy organ, function to maintain the architecture of the organ by regulating the deposition and degradation of extracellular matrix components[64]. In response to injury, pancreatic stellate cells transform into highly proliferative myofibroblast-like cells. These “activated” pancreatic stellate cells synthesize large amounts of extracellular matrix proteins, the accumulation of which results in fibrosis. Thus, pancreatic stellate cells are intimately involved in the regulation of both physiologic, as well as pathologic aspects of the pancreas[64,65].
Both rat and human pancreatic stellate cells express ADH[66,67]. Furthermore, ADH activity is up regulated in pancreatic stellate cells of individuals suffering from chronic pancreatitis and pancreatic cancer[67].
The fact that pancreatic stellate cells express ADH indicates that these cells can produce acetaldehyde when exposed to ethanol. Pancreatic stellate cells are activated when exposed to physiologic concentrations of either ethanol or acetaldehyde[66,68]. Ethanol and acetaldehyde not only activate pancreatic stellate cells, but also induce secretion of type-1 collagen and matrix metalloproteinases[66,68,69]. Thus, expression of ADH by pancreatic stellate cells may have an important role in the activation of these cells and development of alcoholic pancreatitis
Treatment of pancreatic stellate cells with ethanol or acetaldehyde also induces the synthesis of cytokines and growth factors involved in their activation[68,70]. These findings have lead to the suggestion that these cytokines and growth factors act on pancreatic stellate cells in an autocrine manner, thereby perpetuating their activation[16]. This autocrine loop may help to explain both the apparent inability of the pancreas to fully recover from injury in the continued presence of ethanol, and the extremely common association between alcohol abuse and chronic pancreatitis[3,14].
Although it is well established that pancreatic stellate cells are primarily responsible for the deposition and degradation of components of the extracellular matrix, acinar cells can also contribute to the deposition of extracellular matrix components. Using isolated rat acinar cells Lugea et al[71] demonstrated that treatment with FAEEs increase the levels of extracellular matrix proteins by inhibiting the acinar cell activity of plasmin and urokinase-type plasminogen activator, proteins that are involved in the degradation of the extracellular matrix components[71]. Thus, it is apparent that ethanol acts by a number of mechanisms to alter the extracellular environment of pancreatic cells.
ROLE OF THE INFLAMMATORY RESPONSE IN ALCOHOLIC PANCREATITIS
Inflammation mediated by cytokines, chemokines, and adhesion molecules is involved in the initiation and progression of pancreatitis[75-77]. In fact, the NF-κB-dependent inflammatory response is responsible for up to half of the pancreatic tissue damage that is associated with acute pancreatitis, as well as the potentially fatal severe systemic inflammatory response[11].
NF-κB and AP-1, important transcriptional activators involved in the inflammatory response have been shown to have prominent roles in pancreatitis[78,79]. Interestingly, it appears that oxidative and nonoxidative metabolites of ethanol have different effects on the expression of these two regulators of the inflammatory response.
Treatment of isolated acini with ethanol or acetaldehyde decreases the activity of these two factors, whereas; treatment with FAEEs increases their activity[78]. The activity of NF-κB is also reduced in the pancreata of animals chronically fed ethanol[17]. This finding led the authors to hypothesize that in vivo attenuation of NF-κB by ethanol reflects a mechanism to protect the pancreas from ethanol-induced damage[17]. Interestingly, administration of CCK, at concentrations that do not normally cause pancreatitis results in pancreatic damage in animals chronically fed ethanol. This damage is associated with increased NF-κB activity, as well as increases in the mRNA levels of a number of proinflammatory cytokines, including: Tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), macrophage inflammatory protein-1 (MIP-1), and monocyte chemotactic protein-1[17]. These findings demonstrate that although attenuation of NF-κB activity by ethanol may reflect a protective adaptation, ethanol also sensitizes the pancreas to damage that is at least partially mediated by the inflammatory response.
Ethanol abuse is one of the primary risk factors associated with chronic pancreatitis. Because of this, the inflammatory response in chronic alcoholic pancreatitis has also been investigated[80]. Using rats pair-fed the Lieber-Decarli diet, Deng et al[80] demonstrated that chronic ethanol administration reduced the number of resident mononuclear cells in the pancreas. They, like others, suggested that this reduction likely reflects a general immunologic suppression in the pancreas of animals chronically fed ethanol[17,78]. Furthermore, the authors suggested this suppression might explain why animals chronically provided ethanol do not develop chronic pancreatitis in the absence of acute pancreatic damage[80].
Similar to previous findings, induction of acute pancreatitis enhanced the inflammatory response in these animals[80]. Repeated episodes of caerulein-induced acute pancreatitis increased the expression of both pro-inflammatory cytokines such as TNF-α, MIP-1α, and RANTES, as well as anti-inflammatory cytokines such as tissue growth factor-β, and IL-10. The increases in cytokine expression were only detected in ethanol-fed rats in which repeated episodes of acute pancreatitis were induced. Additionally, increased activation of pancreatic stellate cells and fibrosis were observed in the animals. These findings led the authors to suggest that ethanol not only sensitizes the pancreas to acute pancreatitis, but also facilitates the progression of acute to chronic pancreatitis following repeated episodes of acute pancreatic injury[80].
CONCLUSION
Unfortunately, there is currently no pharmacotherapy to attenuate the severity of acute pancreatitis in general and alcoholic acute pancreatitis in particular. Despite the fact that ethanol is commonly associated with pancreatitis, it is apparent that ethanol itself is unable to cause pancreatitis and that an additional trigger is required for the initiation of alcoholic acute pancreatitis. Although the actual triggers that initiate alcoholic acute pancreatitis may differ, the inappropriate activation of trypsin and other pancreatic enzymes, as well as the activation of the inflammatory response are key events in its development and progression.
A tremendous amount of work has revealed a number of mechanisms by which ethanol and both its oxidative and nonoxidative metabolites damage pancreatic cells. Greater understanding of the mechanisms by which ethanol alter the normal physiology of pancreatic cells has provided some promising therapeutic targets.
Among the potential targets are the transcriptional activators NF-κB and AP-1. Numerous studies have demonstrated the importance of these factors in the activation of the inflammatory response and alcoholic acute pancreatitis. Thus, attenuating or regulating the activation of these factors should decrease the severity of acute pancreatitis.
Ethanol and its metabolites cause sustained elevation of intracellular calcium, which results in a number of dysfunctions in acinar cells. Modulation of intracellular calcium attenuates many of these dysfunctions. Thus, regulation or modulation of intracellular calcium levels is an attractive strategy for the treatment of acute pancreatitis.
The nonoxidative metabolites of ethanol metabolism FAEEs have been shown to cause a number of pathologic changes in pancreatic cells. It has been demonstrated that inhibiting FAEE synthase attenuates experimental acute pancreatitis. This is also an extremely attractive area to pursue. Regulating or reestablishing the normal NAD+/NADH ratio in acinar cells may also attenuate the severity of alcoholic acute pancreatitis. Lastly, numerous studies have demonstrated that the pancreas responds to ethanol by compensatory mechanisms such as the unfolded protein response and suppression of the inflammatory response. Thus, manipulation of these compensatory mechanisms may be a fruitful strategy for treatment of this disease.
It is clear that ethanol sensitizes the pancreas to injury. By understanding the mechanisms by which ethanol alter the normal physiology of the pancreas, we have uncovered potential targets for therapeutic intervention. Further experimental work and clinical studies are required to determine the utility of these targets in treating alcoholic pancreatitis.