Published online Feb 15, 2016. doi: 10.4291/wjgp.v7.i1.181
Peer-review started: May 29, 2015
First decision: June 19, 2015
Revised: August 19, 2015
Accepted: November 30, 2015
Article in press: December 3, 2015
Published online: February 15, 2016
Processing time: 249 Days and 0.4 Hours
AIM: To evaluate the frequency of Helicobacter pylori (H. pylori) reinfection in peptic ulcer patients during 9 years after H. pylori eradication.
METHODS: We invited 117 peptic ulcer patients in whom eradication of H. pylori was confirmed 1 year after eradication treatment both by histology and by rapid urease test. In total, 57 patients were available for the study procedures: 34 (59.6%) male, 23 (40.4%) female; mean age 52.3 ± 13.0 years. There were 45 (78.9%) patients with duodenal ulcer and 12 (21.1%) with gastric ulcer. H. pylori was diagnosed by a rapid urease test and histology if endoscopy was performed. If endoscopy was refused, H. pylori was diagnosed by the C14-urea breath test and serology. H. pylori was established if at least one of the tests was positive.
RESULTS: The mean follow-up was 8.9 ± 1.0 years (range, 6-12). H. pylori was established in 15 patients. In 2 H. pylori-negative patients, H. pylori was established during the follow-up period and eradicated. Therefore, we consider that reinfection occurred in 17 patients. In the per protocol analysis, reinfection was established in 17 of 57 (29.8%; 95%CI: 19.2-42.2) patients during the follow-up period. The annual rate of infection was 3.36%. If all non-responders were considered H. pylori-negative, reinfection would be 14.5% (17/117), the annual rate being 1.63%. The mean age of patients with reinfection was 51.8 ± 14.0 years, and without reinfection was 52.5 ± 13.0 years, P > 0.05; the mean body mass index of patients with reinfection was 27.2 ± 4.1 kg/m2, and without reinfection was 25.7 ± 4.2 kg/m2, P > 0.05. There were no differences in the reinfection rates according the location of the peptic ulcer, the eradication regimen used, and smoking status.
CONCLUSION: The reinfection rate of H. pylori is relatively high in Lithuania and probably related to the high prevalence of H. pylori, what may reflect differences in the socioeconomic status between Western and Eastern European countries.
Core tip: The reinfection rate of Helicobacter pylori (H. pylori) varies according to geographical area. In regions with higher socioeconomic status and lower prevalence of H. pylori it is only 1.68% of cases. In developing areas, the reinfection rate could be much higher. Lithuania, as well as other Eastern and Central European countries, is in transition, and the prevalence of H. pylori is not as low as in Western regions, but not as high as in developing countries. According to our study, the H. pylori reinfection rate in Lithuania is relatively high (the annual rate being 3.36%), probably because of the high prevalence of H. pylori. This could indirectly reflect differences in the socioeconomic status between Western and Eastern European countries.
- Citation: Jonaitis L, Kiudelis G, Slepavicius P, Kupcinskas L. High rate of Helicobacter pylori reinfection in Lithuanian peptic ulcer patients. World J Gastrointest Pathophysiol 2016; 7(1): 181-185
- URL: https://www.wjgnet.com/2150-5330/full/v7/i1/181.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v7.i1.181
It is well established that Helicobacter pylori (H. pylori) infection is the main cause of chronic gastritis and peptic ulcer disease, and a definite risk factor for gastric cancer[1-4]. Consensuses from different parts of the world strongly recommend eradication of H. pylori to cure peptic ulcer disease and MALT lymphoma, and to decrease the risk of gastric cancer[5-7]. H. pylori eradication is recommended to alleviate the burden of functional dyspepsia and some other digestive and extragastric pathologies[8-13]. Current treatment modalities allow eradication of the H. pylori bacterium in up to 90% of cases (less if there is clarithromycin resistance). Nevertheless, in some cases, recrudescence or reinfection of H. pylori may occur. Reinfection is considered when H. pylori is found after confirmed H. pylori eradication. The confirmation of eradication must be performed not earlier than 6 mo after eradication treatment. It has been reported that in highly developed countries reinfection is rare and may account no more than 1.68% of cases[14]. In contrast, in developing areas the reinfection rate could be much higher and has been reported to reach 9.63%[14]. Central and Eastern European countries are areas of medium to high H. pylori prevalence. The reinfection rates could be expected to be in between the rates indicated above[8-32]. H. pylori-related diseases are common in these countries and H. pylori eradication treatment is widely applied. There are little data from Eastern and Central Europe about the reinfection of H. pylori. In the Maastricht consensus, the recommendation to regularly investigate the regional epidemiological status of H. pylori has been proposed, indicating that the data for prevalence, eradication rates, antibacterial resistance, and reinfection rates are important[13].
Therefore, we carried out a long-term follow-up study to evaluate the frequency of H. pylori reinfection in peptic ulcer patients in Lithuania after confirmation of H. pylori eradication.
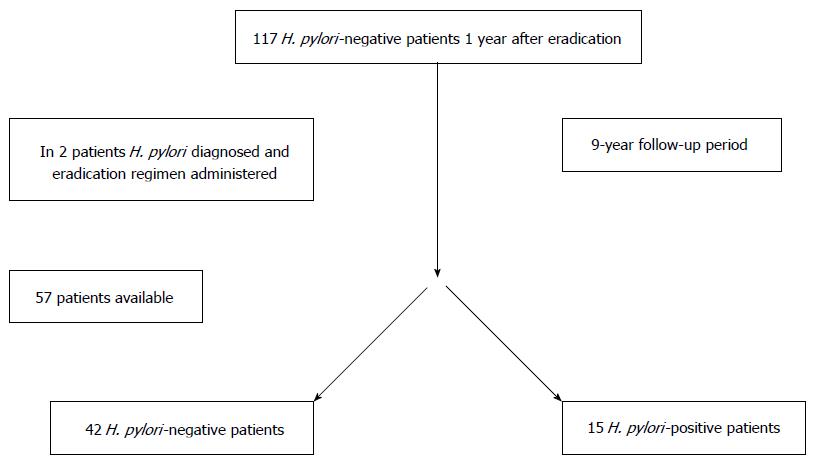
We included peptic ulcer patients from our previous 1-year follow-up studies[15,20]; 117 patients, who were H. pylori-negative 1 year after eradication treatment (therefore, considered to have true eradication), were invited to participate in the study by mail or telephone. Fifty seven patients responded and were available for the study procedures. Written informed consent from all participants and approval of the Kaunas Regional Biomedical Research Ethics Committee was obtained. During the visits, demographic and clinical data were obtained. The flowchart of the study is presented in Figure 1. The previous 1-wk eradication regimens of these patients contained omeprazole, amoxicillin, and clarithromycin [applied to 33 (58%) patients]; omeprazole, amoxicillin and metronidazole [applied to 12 (21%) patients]; omeprazole, clarithromycin and metronidazole [applied to 12 (21%) patients].
According to the protocol of previous studies[15,20], the final H. pylori status was determined after 12 mo following eradication therapy by a rapid urease test (RUT) and histology. Patients were considered as H. pylori-negative if both tests were negative.
The mean follow-up period was 8.9 ± 1.0 years (range, 6-12) after the confirmation of the negative H. pylori status. At this time H. pylori was tested by RUT and histology if patients agreed to undergo endoscopy. If endoscopy was refused, H. pylori was tested by the 14C-urea breath test (UBT) “Heliprobe”[18,24-26] and serology (the quantitative test “SureScreen Diagnostics Ltd” which is CE Marked, Food and Drug Administration Approved[17]). H. pylori-positivity was established, if at least one of the tests was positive.
Forty three patients were tested by RUT and histology; 14 patients (those who refused endoscopy) were tested by UBT and by additional serology.
The data were analyzed and compared using the χ2 or Student t test. Values of P < 0.05 were considered significant.
The 57 patients consisted of 34 (59.6%) males and 23 (40.4%) females, with a mean age of 52.3 ± 13.0 years. There were 45 (78.9%) patients with a duodenal ulcer and 12 (21.1%) with a gastric ulcer.
Endoscopy was performed in 43 (75.4%) patients. H. pylori was established in 15 patients, and 42 patients were H. pylori-negative. In 2 H. pylori-negative patients H. pylori had been established by RUT during the follow-up period (both had peptic ulcer relapse), and successful eradication treatment had been administered. Therefore, we may count 17 patients with H. pylori reinfection during follow-up.
In the per protocol analysis, reinfection was established in 17 (29.8%; 95%CI: 19.2-42.2) of 57 patients, the annual rate being 3.36%. If we consider that all non-responders were H. pylori-negative (the most optimistic analysis), the reinfection rate would be 14.5% (17/117), the annual rate being 1.63%.
The mean age was 51.8 ± 14.0 years in patients with reinfection, and 52.5 ± 13.0 years in patients without reinfection (P > 0.05); the mean body mass index in the 2 groups was 27.2 ± 4.1 kg/m2 and 25.7 ± 4.2 kg/m2, respectively (P > 0.05). A comparison of characteristics of patients with and without reinfection is presented in Table 1.
| Reinfection | No reinfection | P-value | |
| n = 17 | n = 40 | ||
| Male | 12 (70.6) | 22 (55) | > 0.05 |
| Smokers | 7 (41.2) | 19 (47.5) | > 0.05 |
| Duodenal ulcer | 13 (76.5) | 32 (80) | > 0.05 |
| Primary eradication regimen - 7 d triple therapy: | |||
| Omeprazole, clarithromycin, amoxicillin | 9 (52.9) | 24 (60) | > 0.05 |
| Omeprazole, metronidazole, amoxicillin | 4 (33.3) | 8 (20) | > 0.05 |
| Omeprazole, clarithromycin, metronidazole | 4 (33.3) | 8 (20) | > 0.05 |
The reinfection rate of H. pylori could be mostly dependent on the prevalence of H. pylori in the specific region. It could be considered as an indirect indicator of the socioeconomic status of the region. Yan et al[14] analyzed the correlation between H. pylori recurrence rate and socioeconomic development [as represented by Human Development Index (HDI)], using data from 77 studies, which were considered reliable. Countries with very high HDI had a mean annual rate of 1.68%, which was significantly lower than that of high HDI countries at 6.05%, medium HDI countries at 7.04%, and low HDI countries at 9.63% (global annual rate being 2.82%)[14]. Lithuania is placed in the very high HDI category.
The studies indicate that low socioeconomic status was one of the major risk factors for a high prevalence of H. pylori infection[16,23]. Eastern and Central European countries are in a transitional area, where the prevalence of H. pylori is not as low as in Western regions, but not as high as in the developing countries[27]. There are no large epidemiological studies on the prevalence of H. pylori in this region, but there are data on the prevalence of H. pylori in specific groups of patients. The prevalence of H. pylori in middle-aged outpatients was 69%[21]. The prevalence of H. pylori in 22-year-old medical students established by serology was 30.4% and has decreased substantially during last 17 years[22].
In our study, in the per protocol analysis, H. pylori reinfection was established in 29.8% (17/57) (95%CI: 19.2-42.2) of patients during our 9-year follow-up, the annual rate being 3.36%. We also calculated the reinfection rates on the assumption that all non-responders were H. pylori-negatives: In this case reinfection would be 14.5% (17/117), the annual rate being 1.63%. In reality, the reinfection rate is probably in between these numbers, and could be considered a relatively high H. pylori reinfection rate. This may indicate that in Lithuania the decrease in the prevalence of H. pylori infection[21,22] is not as fast as was supposed, probably related to rather slow development of the socioeconomic status of the country, or the H. pylori prevalence is decreasing much slower than the speed of socioeconomic development. It is logical to believe that similar results could be found in neighboring countries.
The advantage of our study is that we established true reinfection, as all our patients were H. pylori-negative 1-year after the eradication regimen. We would like to stress that our study is the first to report on the reinfection rates of adult peptic ulcer patients from Central and Eastern Europe. In contrast to many South European countries, where the prevalence of antimicrobial drug resistance is significant, H. pylori susceptibility to the standard antibiotics in Lithuania remains high[19]. Therefore, high reinfection rates could be the most important issue in the management of H. pylori infection in the country.
There are some limitations of the study. The low number of responses (57 out of 117 patients) to follow-up investigations allowed us to speculate with the most pessimistic and most optimistic numbers, not being able exactly to determine the rates of H. pylori reinfection. Besides, we did not have epidemiological data of our patients, thus we could not examine the reasons for reinfection. Our investigated demographic and clinical characteristics were not predictive of reinfection.
In conclusion, the reinfection rate of H. pylori in a cohort of peptic ulcer patients in Lithuania was relatively high, and this may be related to the relatively high prevalence of H. pylori infection in the country, suggesting that socioeconomic differences between Western and Eastern European countries are probably still marked.
It is strongly recommended to eradicate Helicobacter pylori (H. pylori) in order to cure peptic ulcer disease and MALT lymphoma, to decrease risk of gastric cancer, and to alleviate the burden of functional dyspepsia and some other digestive and extragastric pathologies. At present, the eradication of H. pylori could be achieved in up to 90% of cases. Nevertheless, recrudescence or reinfection may occur over time.
In the Maastricht consensus, the recommendation to regularly investigate the regional epidemiological status of H. pylori has been proposed, indicating that data of prevalence, eradication rates, antibacterial resistance, and reinfection rates are important. The reinfection of H. pylori has been reported to be not infrequent, especially in areas of high H. pylori prevalence. More studies are necessary to establish the rate of reinfection in different parts of the world. Factors which may contribute to the occurrence of H. pylori reinfection have to be elucidated.
There are few available data from Eastern and Central Europe on reinfection rates of H. pylori. This article presents a long-term follow-up study, which evaluates the frequency of H. pylori reinfection in Lithuanian peptic ulcer patients after confirmation of H. pylori eradication. These are probably the first data from Eastern-Central Europe regarding reinfection of H. pylori in peptic ulcer patients.
The results of the study add important scientific information on H. pylori reinfection rates in Central and Eastern Europe. This is important knowledge reflecting the current Maastricht consensus. It also encourages us to rethink the present epidemiological situation regarding the prevalence of H. pylori in Lithuania and the whole region. The reinfection rate of H. pylori in the cohort of peptic ulcer patients in Lithuania may be related to the relatively high prevalence of H. pylori infection in this region, suggesting that socioeconomic differences between Western and Eastern European countries are probably still marked.
Reinfection of H. pylori is considered when a new strain of H. pylori is found after confirmed eradication. The confirmation of eradication must be performed not earlier than 6 mo after eradication treatment. Recrudescence of H. pylori is considered as a recurrence of the previous infection by the same H. pylori strain.
The subject of the present manuscript is interesting and important, as there are not many studies on the annual rate of H. pylori reinfection in this geographic area. However, some additions and clarifications should be performed to improve the manuscript.
| 1. | Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136:1863-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 503] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 2. | Fuccio L, Zagari RM, Eusebi LH, Laterza L, Cennamo V, Ceroni L, Grilli D, Bazzoli F. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 240] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 3. | Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology. 1996;110:1244-1252. [PubMed] |
| 4. | McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597-1604. [PubMed] |
| 5. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [PubMed] |
| 6. | Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 837] [Article Influence: 44.1] [Reference Citation Analysis (3)] |
| 7. | Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587-1600. [PubMed] |
| 8. | Raghunath A, Hungin AP, Wooff D, Childs S. Prevalence of Helicobacter pylori in patients with gastro-oesophageal reflux disease: systematic review. BMJ. 2003;326:737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 233] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Laine L, Sugg J. Effect of Helicobacter pylori eradication on development of erosive esophagitis and gastroesophageal reflux disease symptoms: a post hoc analysis of eight double blind prospective studies. Am J Gastroenterol. 2002;97:2992-2997. [PubMed] |
| 10. | Schwizer W, Thumshirn M, Dent J, Guldenschuh I, Menne D, Cathomas G, Fried M. Helicobacter pylori and symptomatic relapse of gastro-oesophageal reflux disease: a randomised controlled trial. Lancet. 2001;357:1738-1742. [PubMed] |
| 11. | Manes G, Mosca S, Laccetti M, Lioniello M, Balzano A. Helicobacter pylori infection, pattern of gastritis, and symptoms in erosive and nonerosive gastroesophageal reflux disease. Scand J Gastroenterol. 1999;34:658-662. [PubMed] |
| 12. | Malfertheiner P, MOssner J, Fischbach W, Layer P, Leodolter A, Stolte M, Demleitner K, Fuchs W. Helicobacter pylori eradication is beneficial in the treatment of functional dyspepsia. Aliment Pharmacol Ther. 2003;18:615-625. [PubMed] |
| 13. | Bruley Des Varannes S, Fléjou JF, Colin R, Zaïm M, Meunier A, Bidaut-Mazel C. There are some benefits for eradicating Helicobacter pylori in patients with non-ulcer dyspepsia. Aliment Pharmacol Ther. 2001;15:1177-1185. [PubMed] |
| 14. | Yan TL, Hu QD, Zhang Q, Li YM, Liang TB. National rates of Helicobacter pylori recurrence are significantly and inversely correlated with human development index. Aliment Pharmacol Ther. 2013;37:963-968. [PubMed] |
| 15. | Kupcinskas L, Jonaitis L, Kiudelis G. A 1 year follow-up study of the consequences of Helicobacter pylori eradication in duodenal ulcer patients: unchanged frequency of erosive oesophagitis and decreased prevalence of non-erosive gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol. 2004;16:369-374. [PubMed] |
| 16. | Calvet X, Ramírez Lázaro MJ, Lehours P, Mégraud F. Diagnosis and epidemiology of Helicobacter pylori infection. Helicobacter. 2013;18 Suppl 1:5-11. [PubMed] |
| 17. | Available from: https: //www.surescreen.com/diagnostics/picture.php?prodid=H. pyloriT. |
| 18. | Jonaitis LV, Kiudelis G, Kupcinskas L. Evaluation of a novel 14C-urea breath test “Heliprobe” in diagnosis of Helicobacter pylori infection. Medicina (Kaunas). 2007;43:32-35. [PubMed] |
| 19. | Kupcinskas L, Rasmussen L, Jonaitis L, Kiudelis G, Jørgensen M, Urbonaviciene N, Tamosiunas V, Kupcinskas J, Miciuleviciene J, Kadusevicius E. Evolution of Helicobacter pylori susceptibility to antibiotics during a 10-year period in Lithuania. APMIS. 2013;121:431-436. [PubMed] |
| 20. | Jonaitis L, Kiudelis G, Kupcinskas L. Gastroesophageal reflux disease after Helicobacter pylori eradication in gastric ulcer patients: a one-year follow-up study. Medicina (Kaunas). 2008;44:211-215. [PubMed] |
| 21. | Jonaitis L, Kiudelis G, Kupcinskas L, Kupcinskas J. Prevalence of Helicobacter pylori among outpatient middle-aged patients in Lithuania and its relation to dyspeptic symptoms. Helicobacter. 2013;18:104. |
| 22. | Jonaitis L, Kiudelis G, Kupcinskas L. Prevalence of Helicobacter pylori among medical students in Lithuania decreased during last 17 years. Helicobacter. 2012;17:88. |
| 23. | Silva FM, Navarro-Rodriguez T, Barbuti RC, Mattar R, Hashimoto CL, Eisig JN. Helicobacter pylori reinfection in Brazilian patients with peptic ulcer disease: a 5-year follow-up. Helicobacter. 2010;15:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Pathak CM, Kaur B, Khanduja KL. 14C-urea breath test is safe for pediatric patients. Nucl Med Commun. 2010;31:830-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Bentur Y, Matsui D, Koren G. Safety of 14C-UBT for diagnosis of Helicobacter pylori infection in pregnancy. Can Fam Physician. 2009;55:479-480. [PubMed] |
| 26. | Ozdemir E, Karabacak NI, Degertekin B, Cirak M, Dursun A, Engin D, Unal S, Unlü M. Could the simplified (14)C urea breath test be a new standard in noninvasive diagnosis of Helicobacter pylori infection? Ann Nucl Med. 2008;22:611-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Oona M, Rägo T, Maaroos HI. Long-term recurrence rate after treatment of Helicobacter pylori infection in children and adolescents in Estonia. Scand J Gastroenterol. 2004;39:1186-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | McMahon BJ, Bruce MG, Hennessy TW, Bruden DL, Sacco F, Peters H, Hurlburt DA, Morris JM, Reasonover AL, Dailide G. Reinfection after successful eradication of Helicobacter pylori: a 2-year prospective study in Alaska Natives. Aliment Pharmacol Ther. 2006;23:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Kim SY, Hyun JJ, Jung SW, Koo JS, Yim HJ, Lee SW. Helicobacter pylori recurrence after first- and second-line eradication therapy in Korea: the problem of recrudescence or reinfection. Helicobacter. 2014;19:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Kim MS, Kim N, Kim SE, Jo HJ, Shin CM, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH. Long-term follow-up Helicobacter pylori reinfection rate and its associated factors in Korea. Helicobacter. 2013;18:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Ryu KH, Yi SY, Na YJ, Baik SJ, Yoon SJ, Jung HS, Song HJ. Reinfection rate and endoscopic changes after successful eradication of Helicobacter pylori. World J Gastroenterol. 2010;16:251-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Zhang YY, Xia HH, Zhuang ZH, Zhong J. Review article: ‘true’ re-infection of Helicobacter pylori after successful eradication--worldwide annual rates, risk factors and clinical implications. Aliment Pharmacol Ther. 2009;29:145-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Boyanova L, Biernat MM S- Editor: Yu J L- Editor: Cant MR E- Editor: Jiao XK