Published online Nov 15, 2015. doi: 10.4291/wjgp.v6.i4.235
Peer-review started: June 5, 2015
First decision: July 6, 2015
Revised: September 9, 2015
Accepted: October 16, 2015
Article in press: October 19, 2015
Published online: November 15, 2015
Processing time: 167 Days and 15.8 Hours
AIM: To investigate the survival impact of common pharmaceuticals, which target stromal interactions, following a pancreaticoduodenectomy for pancreatic ductal adenocarcinoma.
METHODS: Data was collected retrospectively for 164 patients who underwent a pancreaticoduodenectomy for pancreatic ductal adenocarcinoma (PDAC). Survival analysis was performed on patients receiving the following medications: angiotensin-converting enzyme inhibitors (ACEI)/angiotensin II receptor blockers (ARB), calcium channel blockers (CCB), aspirin, and statins. Statistical analysis included Kaplan-meier survival estimates and cox multivariate regression; the latter of which allowed for any differences in a range of prognostic indicators between groups. Medications showing a significant survival benefit were investigated in combination with other medications to evaluate synergistic effects.
RESULTS: No survival benefit was observed with respect to ACEI/ARB (n = 41), aspirin or statins on individual drug analysis (n = 39). However, the entire CCB group (n = 26) showed a significant survival benefit on multivariate cox regression; hazard ratio (HR) of 0.475 (CI = 0.250-0.902, P = 0.023). Further analysis revealed that this was influenced by a group of patients who were taking aspirin in combination with CCB; median survival was significantly higher in the CCB + aspirin group (n = 15) compared with the group taking neither drug (n = 98); 1414 d vs 601 d (P = 0.029, log-rank test). Multivariate cox regression revealed neither aspirin nor CCB had a statistically significant impact on survival when given alone, however in combination the survival benefit was significant; HR = 0.332 (CI = 0.126-0.870, P = 0.025). None of the other medications showed a survival benefit in any combination.
CONCLUSION: Aspirin + CCB in combination appears to increase survival in patients with PDAC, highlighting the potential clinical use of combination therapy to target stromal interactions in pancreatic cancer.
Core tip: Stromal interactions play a large part in the dismal prognosis of pancreatic cancer. Recent laboratory studies have examined the potential use of common pharmaceuticals, such as calcium channel blocker (CCB), aspirin, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers and statins, in inhibiting these protumorigenic stromal interactions. We retrospectively collected data from 164 patients whom underwent a pancreaticoduodenectomy to remove a pancreatic ductal adenocarcinoma, to see if the potential benefits of these drugs translated into increased survival. Our finding that those taking a combination of aspirin and CCB survived over twice as long as those on neither drug, highlights the potential of novel drug combinations to increase survival in pancreatic cancer.
- Citation: Tingle SJ, Moir JA, White SA. Role of anti-stromal polypharmacy in increasing survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. World J Gastrointest Pathophysiol 2015; 6(4): 235-242
- URL: https://www.wjgnet.com/2150-5330/full/v6/i4/235.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v6.i4.235
Pancreatic cancer is one of the most aggressive malignancies with a dismal prognosis. In the United Kingdom it is the 5th most common cause of cancer death, with 1- and 5-year survival rates of 20.8% and 3.3% respectively[1]. The most common type of pancreatic cancer is pancreatic ductal adenocarcinoma (PDAC), making up 95% of cases. Given this poor prognosis, with little improvement over the last 40 years, novel options for therapeutic targets are being investigated, in both the palliative setting to improve survival and the post-resection setting to reduce recurrence rates. One such target is the complex interaction between pancreatic cancer and the surrounding tissue, which is termed stroma.
The stroma is the local microenvironment which surrounds the tumour and is made up of a variety of cellular (vascular, inflammatory and neural cells) and non-cellular components. Most of these are present in the normal pancreas and aid in regulating normal pancreatic function. In the presence of a pancreatic tumour stromal cells become activated, resulting in a desmoplastic reaction that increases tumour proliferation, chemotherapy resistance and metastasis[2-5]. PDAC has the most significant interactions with surrounding stroma out of all solid organ epithelial cancers, which may partly explain the aggressive nature of the disease, and as such is currently a hot topic in pancreatic cancer research.
Patients who receive surgery benefit from improved outcomes, but surgical resection is only an option in around 20% of patients[6]. Previous studies have shown that despite the curative intent of surgery, the majority of patients experience recurrence[7]. This is largely due to incomplete R1 resection. However, the activated stroma which is left behind in the remnant pancreas, even in theoretically complete R0 resections, may have a role in creating a protumorigenic environment and encouraging recurrence of disease.
Various scientific studies have demonstrated that commonly used pharmaceutical agents may influence the protumorigenic cancer-stroma relationship. Calcium channel blockers (CCB)[8], aspirin[9], statins[10,11], angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB)[12,13] demonstrate inhibitory effects on stromal interactions, manifesting as reduced growth and/or metastasis of PDAC cells in a mixture of in-vitro and animal studies. This effect is enhanced in combination with gemcitabine (the current first line chemotherapeutic agent in pancreatic cancer), suggesting that these medications may work by improving chemo penetrance[9,14].
ACEI and ARBs, which affect stromal interactions via the local renin-angiotensin system (RAS), have been shown to improve survival[15]. Furthermore aspirin[16] and statins[17] have been shown to reduce the risk of pancreatic cancer development, suggesting an inhibitory effect on carcinogenesis. The anticancer potential of these drugs has been examined in a whole range of other cancer types[18-21].
This study aims to investigate whether the aforementioned laboratory findings translate into a significant clinical survival benefit in the post-resection setting, and to observe if any of these medications could act in combination to give a synergistically beneficial effect on survival.
All patients included in the study had a histologically confirmed PDAC removed from the head of the pancreas by Whipple’s pancreaticoduodenectomy between December 2004 and March 2013. Data was retrospectively collected from hand held and electronic patient notes. This included whether they were taking ACEI/ARB (which were grouped as they both affect the local RAS), CCB, aspirin or statins as regular medications upon discharge after their operation.
Any drug which offered a significant benefit in survival was then investigated in combination with the other drugs to determine if any synergistic benefits were present.
Kaplan-Meier was used to calculate estimated median overall survival, which was measured in days after surgery, and the log-rank test was applied to compare groups. As some of the patients were still alive at the end of the study, censoring was applied, allowing these patients to be included in the analysis. χ2 test was used to compare categorical variables. A P < 0.05 was considered significant.
Cox regression was used to exclude possible cofounding factors, and estimate the hazard ratios for various drug groups, adjusting for prognostic indicators. Prognostic indicators included sex, age (< 60 or ≥ 60 years), blood pressure status (hypertensive or normotensive), pre-operative body mass index (< 18.5, 18.5-25, > 25), post-operative adjuvant chemotherapy, CA19-9 level at diagnosis (< 47, 47-1000, > 1000), American Society of Anesthesiologists (ASA) grade (1-2 or 3-4), resection margin status and TNM staging.
SPSS was used for all of the statistical analysis.
In total, 195 patients had a Whipple’s pancreaticoduodenectomy to remove a PDAC at the Newcastle Freeman Hospital between December 2004 and March 2013. Of these data could be collected for 164 patients with a median follow up time of 23.9 mo.
Drugs were initially looked at on an individual basis, creating four groups; ACEI/ARB (n = 30/11 = 41), CCB (n = 26), aspirin (n = 55), and statins (n = 39). Median daily dose of the various drugs were as follows; aspirin 75 mg, CCB 10 mg (range: 5-180 mg), statin 40 mg (5-40 mg), ACEI 10 mg (1.25-40 mg) and ARBs 60 mg (4-300 mg). Information on adjuvant chemotherapy could be collected for 153 patients. In total 110 (71.9%) received post-operative adjuvant chemotherapy. Of these 53 (48.2%) received 5FU treatment in the MAYO regime, 53 received Gemcitabine (48.2%), and the remaining 4 (3.6%) received other chemotherapeutic agents. Of the 53 patients taking Gemcitibine, 4 were also receiving Capecitabine and 2 were also receiving Carboplatin. None of the patients received radiotherapy.
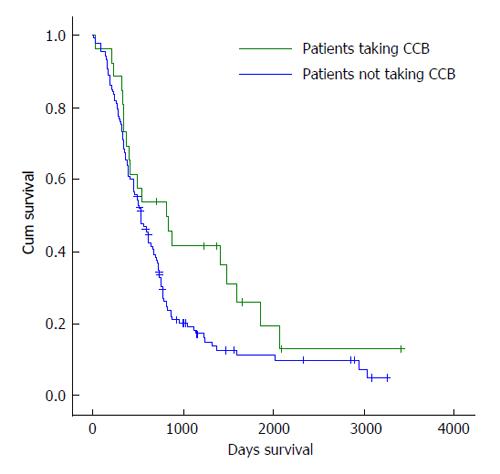
Initial analysis compared median survival of every patient taking a particular drug, with those not taking that drug (Table 1). This initial analysis did not investigate whether the drug was being taken in combination with any of the other medications. None of the medications showed a statistically significant impact on survival when a Log rank test was applied. The only drug which showed an increase in median survival was CCB, (Figure 1) with those taking the drug having a median survival of 815 d compared with 528 d in those not taking the drug (P = 0.061). At this stage, the CCB group included every person taking CCB, some of which were also taking other medications such as aspirin, statins or ACEI/ARBs in various combinations.
| Drug name | Number taking the drug out of 164 patients | Median survival estimate for those taking the drug (d) | Median survival for those not taking the drug (d) | P value (log rank test) |
| ACEI/ARB | 41 | 539 | 611 | 0.652 |
| CCB | 26 | 815 | 528 | 0.061 |
| Aspirin | 55 | 504 | 546 | 0.846 |
| Statins | 39 | 504 | 577 | 0.368 |
When multivariate analysis was applied, being in the CCB group was an independent predictor of improved survival with a hazard ratio of 0.475 (P = 0.023) as can be seen in Table 2. All of the other drugs resulted in worsened survival, but this was not statistically significant.
| Drug name | Number taking the drug out of 164 patients | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P-value | HR (95%CI) | P-value | ||
| ACEI/ARB | 41 | 1.094 (0.741-1.614) | 0.653 | 1.129 (0.617-2.065) | 0.693 |
| CCB | 26 | 0.635 (0.393-1.025) | 0.063 | 0.475 (0.250-0.902) | 0.023 |
| Aspirin | 55 | 1.036 (0.726-1.479) | 0.846 | 1.041 (0.651-1.667) | 0.865 |
| Statins | 39 | 1.200 (0.806-1.787) | 0.369 | 1.055 (0.614-1.814) | 0.845 |
After observing a statistically significant benefit in the entire CCB group, this drug was analysed in combination with the other drugs in the study, as seen at the top of Table 3. Both Kaplain-Meier median survival estimates and multivariate cox regression showed that there was no significant survival benefit in people taking either statins or ACEI/ARBs along with CCB (Table 3). However, the CCB + aspirin group (n = 15) had a significantly improved median survival; 1414 d compared to 528 d in those not on this drug combination (P = 0.012 Log rank test). This benefit was confirmed in the multivariate cox regression analysis; being in the CCB + aspirin group gave a HR of 0.300 (CI = 0.122-0.735, P = 0.008). Further analysis later revealed that this CCB + aspirin group was solely responsible for the increase in median survival seen in the initial entire CCB group (Figure 2). No other combination of ACEI/ARB, statins or aspirin showed a significant improvement in survival as seen in Table 3.
| Drug combination | Number of people on | Kaplan-Meier estimated median survival | Multivariate cox regression | |||
| combination out of 164 | For those on the drug combination | For those not on drug combination | P-value (log rank) | HR (95%CI) | P-value | |
| CCB + aspirin | 15 | 1414 | 528 | 0.012 | 0.300 (0.122-0.735) | 0.008 |
| CCB + statin | 12 | 544 | 539 | 0.284 | 0.413 (0.155-1.101) | 0.077 |
| CCB + ACEI/ARB | 12 | 485 | 541 | 0.450 | 0.512 (0.194-1.348) | 0.175 |
| Aspirin + statin | 27 | 504 | 546 | 0.697 | 0.969 (0.509-1.844) | 0.924 |
| Aspirin + ACEI/ARB | 22 | 485 | 569 | 0.923 | 0.948 (0.438-2.054) | 0.893 |
| Statin + ACEI/ARB | 21 | 368 | 577 | 0.426 | 1.126 (0.533-2.379) | 0.756 |
Further statistical analysis of patients taking CCB and/or aspirin was then performed. This divided the 164 patients into four groups; those taking CCB + aspirin in combination (n = 15), those taking aspirin without CCB (n = 40), those taking CCB without aspirin (n = 11), and those taking neither drug which acted as the control group (n = 98). χ2 tests were then used to compare differences in the various prognostic indicators between these drug groups (Table 4). None of these prognostic indicators showed statistically significant differences between groups, except blood pressure status, ASA grade, and resection value; those taking CCB and/or aspirin were more likely to suffer from hypertension (P = 0.000), more likely to have a higher ASA grade (P = 0.008), and more likely to have a successful surgical resection (P = 0.020).
| Characteristics | No CCB/aspirin, n (%) | CCB and aspirin, n (%) | Aspirin without CCB, n (%) | CCB without Aspirin, n (%) | P value |
| n = 98 | n = 15 | n = 40 | n = 11 | ||
| Sex | |||||
| Male | 56 (57.1) | 5 (33.3) | 25 (62.5) | 8 (72.7) | 0.169 |
| Age (yr) | |||||
| < 60 | 39 (39.8) | 5 (33.3) | 8 (20.0) | 3 (27.3) | 0.157 |
| ≥ 60 | 59 (60.2) | 10 (66.7) | 32 (80.0) | 8 (72.7) | |
| Blood pressure status | |||||
| Hypertensive | 27 (27.6) | 13 (86.7) | 22 (55.0) | 11 (100.0) | 0.000 |
| Non-hypertensive | 71 (72.4) | 2 (13.3) | 18 (45.0) | 0 (0.0) | |
| BMI | |||||
| < 18.5 | 2 (2.0) | 0 (0) | 1 (2.5) | 0 (0.0) | 0.307 |
| 18.5-25 | 52 (53.1) | 5 (33.3) | 24 (60.0) | 3 (27.3) | |
| > 25 | 41 (41.8) | 9 (60.0) | 14 (35.0) | 8 (72.7) | |
| Adjuvant chemotherapy | |||||
| Received post-op | 69 (70.4) | 9 (60.0) | 23 (57.5) | 9 (81.8) | 0.333 |
| Not received | 24 (24.5) | 5 (33.3) | 13 (32.5) | 1 (9.1) | |
| CA19-9 | |||||
| < 47 | 27 (27.6) | 3 (20.0) | 9 (22.5) | 1 (9.1) | 0.437 |
| 47-1000 | 51 (52.0) | 7 (46.7) | 20 (50.0) | 7 (63.6) | |
| > 1000 | 8 (8.2) | 3 (20.0) | 6 (15.0) | 3 (27.3) | |
| ASA grade | |||||
| 1-2 | 81 (82.7) | 12 (80.0) | 22 (55.0) | 8 (72.7) | 0.008 |
| 3-4 | 17 (17.3) | 3 (20.0) | 18 (45.0) | 3 (27.3) | |
| Resection value | |||||
| R0 | 14 (14.3) | 6 (40.0) | 9 (22.5) | 5 (45.5) | 0.020 |
| R1 | 83 (84.7) | 9 (60.0) | 31 (77.5) | 6 (54.5) | |
| T status | |||||
| T1-2 | 3 (3.1) | 2 (13.3) | 1 (2.5) | 0 (0.0) | 0.199 |
| T3-4 | 95 (96.9) | 13 (86.7) | 39 (97.5) | 11 (100.0) | |
| N status | |||||
| N0 | 7 (7.1) | 3 (20.0) | 5 (12.5) | 0 (0.0) | 0.242 |
| N1 | 90 (91.8) | 12 (80) | 35 (87.5) | 11 (100.0) |
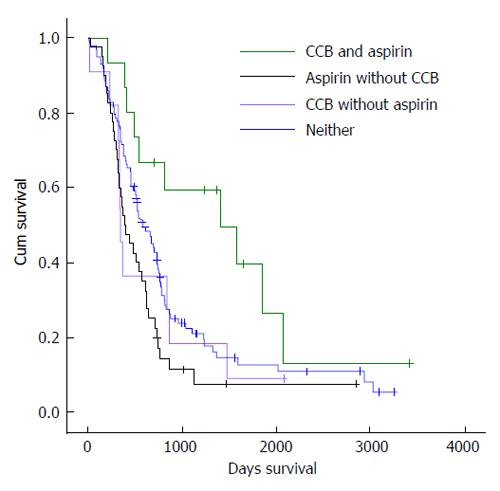
Kaplan-Meier estimated median survival was 601 d in those taking neither drug (Table 5). At 1414 d, combination of CCB + aspirin made a statistically significant improvement in median survival (P = 0.029 log rank test). Taking either drug alone led to a decrease in median survival time; median survival in the aspirin without CCB group was 392 d (P = 0.032), and was 343 d in the CCB without aspirin group (P = 0.563). Differences in survival between groups can be seen in Figure 2.
| Drug group | Number of people | Estimated median | P-value (log rank) | Multivariate cox regression | |
| in group | survival (d) | compared to control | HR (95%CI) | P-value | |
| Control (no CCB/aspirin) | 98 | 601 | - | 1 | - |
| CCB + aspirin | 15 | 1414 | 0.029 | 0.332 (0.126-0.870) | 0.025 |
| Aspirin without CCB | 40 | 392 | 0.032 | 1.658 (0.968-2.840) | 0.066 |
| CCB without Aspirin | 11 | 343 | 0.563 | 1.039 (0.416-2.595) | 0.935 |
The previously observed benefit of taking CCB + aspirin remained statistically significant when multivariate cox regression was used; this allowed for any differences in prognostic indicators, including resection status, and compared the CCB + aspirin group with those taking neither drug, to find a hazard ratio of 0.332 (CI = 0.126-0.870, P = 0.025). Taking either of the drugs in isolation made no statistically significant impact on survival when multivariate cox regression was applied.
This study interestingly demonstrates a greater than twofold increase in post-operative median survival in patients who take a combination of CCB and aspirin, as compared to those taking neither drug. The estimated median survival in patients taking neither drug was comparable to that in similar studies[22-24]. These observations remained significant when allowing for a range of prognostic indicators using multivariate cox regression. In contrast taking any of these medications in isolation or other combinations did not impact on survival. One may therefore postulate that aspirin and CCB’s may act in synergy to inhibit cancer-stromal interactions and thus improve survival.
It has been suggested that the dense desmoplastic reaction that surrounds tumours may account for up to 90% of tumour volume[25]. This represents an intriguing concept in tumour staging, as whilst one may theoretically achieve a tumour-free R0 resection margin, large amounts of activated tumour stroma may be left behind and act as a catalyst for recurrent disease. Therefore, in the context of this study’s findings, it may be that aspirin and CCB act in combination to inhibit any subsequent protumorigenic activity, thus reducing/slowing recurrence and improving survival.
A vast array of different signalling pathways exist which are involved in the development and progression of cancer. The benefits of inhibiting multiple pathways, or multiple points on a single pathway, via combination drug therapy is supported by clinical data showing the synergistic effects of combining anti-cancer therapies leading to improved outcomes compared to the sum of each individual drug’s benefits[26]. To the author’s knowledge, this is the first study looking at a combination of CCB and aspirin as a therapeutic option in pancreatic cancer. As a result, the mechanisms of action are poorly understood. However, we can consider some of the laboratory work which prompted this study, to appreciate some of the potential underlying mechanisms.
Aspirin’s role as an anti-inflammatory, anti-platelet drug is well established through its inhibitory action on the inflammatory enzyme cyclooxygenase-1 (COX-1), and is known to have a key role in reducing the risk of cancer development in a variety of malignancies, including pancreatic cancer[16,27,28]. The mechanism of effect is likely due to the inhibition of stromal-interactions which interfere with local inflammation. This is particularly pertinent in PDAC given the significant inflammatory environment observed, with a weak and fragile extra-cellular matrix promoting cancer development[29]. The fact chronic pancreatitis is a key risk factor in PDAC supports this.
There are various pathways aspirin exerts an influence upon in this setting. Incorrect regulation of the transcription factor nuclear factor kappa B (NF-κB) can lead to excess local inflammation and a positive feedback loop amplifying the activity of the local RAS to oncogenic levels[30]. NF-κB is frequently activated in pancreatic cancer which suggests a link between local inflammation and progression of pancreatic cancer[31]. Aspirin’s inhibitory effect on inflammation and NF-κB have been demonstrated in laboratory studies[31,32], and a resulting decrease in the progression and development of PDAC has been observed in mouse models[33].
Another molecule involved in inflammation is COX-2, an inflammatory enzyme which is also often raised in pancreatic cancer; the inhibition of which leads to decreased carcinogenesis[34]. Although aspirin has a greater effect on COX-1, it may have a role in inhibiting COX-2 in pancreatic cancer. The immune system also plays a role in inflammation, and immune inflammatory cells are one of the cellular components of pancreatic stroma. One such immune cell is the FOXP3 regulatory T cell, which aspirin has been shown to inhibit in the context of pancreatic stroma[9].
CCBs have also shown promise in the laboratory, with an earlier study showing that CCBs can inhibit growth and decrease the doubling time of pancreatic cancer cells[35]. Furthermore the stroma is known to represent a barrier to chemotherapy, and CCBs may have a role in improving chemo penetrance in a range of cancer types, including pancreatic[36]. CCBs have been shown to increase the effectiveness of chemotherapy on a resistant pancreatic adenocarcinoma via its effect on P-glycoprotein, which is also known as multidrug resistance protein[37].
Another possible mechanism of action involves cholecystokinin (CCK), an intracellular peptide hormone which has various roles in control of the pancreas[38]. It is known that high levels of CCK can cause both formation and progression of pancreatic cancer[39]. CCBs have the ability to limit the effects of CCK on pancreatic cells and lead to decreased carcinogenesis[40] and metastasis[41]. Alternatively CCBs have been shown to inhibit the proliferation of pancreatic cancer through the blockade of IK calcium-activated potassium channels[8].
This study is limited by the small sample size of patients taking aspirin and CCB in combination. It is also limited by the fact we looked at regular medications being taken on discharge from hospital, which did not allow any analysis into the effect of altering the duration of administration of these medications.
The retrospective nature of this work brings an inherent selection bias however this was countered through multivariate analysis including a range of prognostic indicators. The one key difference between groups related to the resection margin status, where those taking aspirin and CCB in combination were more likely to have an R0 resection. However, when allowed for using multivariate analysis, the benefits of combining aspirin and CCB still remained statistically significant. One may potentially hypothesise that the anti-stromal effects of taking this combination of medications pre-operatively led to a less locally advanced tumour and therefore a higher chance of full resection.
It could be argued that it is simply the CCB which are having an effect on survival, as seen in our initial individual drug analysis, and that aspirin was only found as a coincidence as we were looking at combinations in the already beneficial CCB group. However, our statistics would suggest that the only reason that CCB showed a benefit on individual drug analysis was the presence of 15 people within the group who were also taking aspirin. Indeed, without those 15 patients, CCB in isolation showed no benefit.
In conclusion, this novel retrospective study has shown that the potential anti-stromal benefits of CCB and aspirin demonstrated by previous laboratory studies do translate into survival benefits in patients with pancreatic ductal adenocarcinoma. Laboratory studies would be useful to determine the mechanism of action of the synergistic effect observed. Further clinical studies with larger patient groups, as well as randomised prospective studies, will help to determine the true anti-cancer potential of these drugs. This study builds on previous laboratory research and represents an exciting new range of potential therapeutics for pancreatic cancer, especially given the cheap, accessible and safe nature of these drugs.
The complex interaction between pancreatic ductal adenocarcinoma and its surrounding tissue microenvironment (termed stroma) plays a large part in the dismal prognosis of pancreatic cancer. Recent laboratory studies have examined the potential use of common pharmaceuticals, such as calcium channel blockers (CCB), aspirin, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers and statins, in inhibiting these protumorigenic stromal interactions. Further clinical research is required to look at the effects of these drugs on mortality.
Studies looking at whether the potential benefits suggested by laboratory research translates into increased survival in clinical research is a current hotspot in this field. There has also been growing interest into the effect of combining therapies to get a synergistic effect; an area which this study explores.
This study built on previous laboratory research to show survival benefits in the clinical setting. The authors demonstrated a statistically significant improvement in Kaplan-Meier estimated median survival in patients taking a combination of aspirin and CCB, a combination which has not been studied in this setting before. The twofold increase in estimated median survival seen in the aspirin + CCB group was confirmed by multivariate cox regression which found the increase in survival to remain significant when a range of prognostic indicators was allowed for.
If the findings of this study are confirmed by further research, patients with pancreatic cancer could expect improvements in life expectancy, with the simple addition of extremely cheap, well tolerated, and readily available medications.
The stroma is the local microenvironment which surrounds the tumour and is made up of a variety of cellular (vascular, inflammatory and neural cells) and non-cellular components. Most of these are present in the normal pancreas and aid in regulating normal pancreatic function. In the presence of a pancreatic tumour stromal cells become activated, resulting in increased tumour proliferation, chemotherapy resistance and metastasis.
This is a novel look at a very interesting topic. In the clinical finding presented in this manuscript, the authors showed that combination CCB and aspirin can increase survival in patients with pancreatic cancer pancreatic ductal adenocarcinoma following pancreaticoduodenectomy although in a small number of patients. A potential mechanism related to targeting stromal interactions in pancreatic cancer was proposed.
| 1. | Cancer Research UK. Pancreatic cancer key stats. Cancer Research UK. 2014;. |
| 2. | Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 592] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 3. | Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1527] [Cited by in RCA: 1540] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 4. | Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1526] [Cited by in RCA: 1616] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 5. | Vonlaufen A, Phillips PA, Xu Z, Goldstein D, Pirola RC, Wilson JS, Apte MV. Pancreatic stellate cells and pancreatic cancer cells: an unholy alliance. Cancer Res. 2008;68:7707-7710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2230] [Article Influence: 139.4] [Reference Citation Analysis (2)] |
| 7. | Barugola G, Falconi M, Bettini R, Boninsegna L, Casarotto A, Salvia R, Bassi C, Pederzoli P. The determinant factors of recurrence following resection for ductal pancreatic cancer. JOP. 2007;8:132-140. [PubMed] |
| 8. | Jäger H, Dreker T, Buck A, Giehl K, Gress T, Grissmer S. Blockage of intermediate-conductance Ca2+-activated K+ channels inhibit human pancreatic cancer cell growth in vitro. Mol Pharmacol. 2004;65:630-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Plassmeier L, Knoop R, Waldmann J, Kesselring R, Buchholz M, Fichtner-Feigl S, Bartsch DK, Fendrich V. Aspirin prolongs survival and reduces the number of Foxp3+ regulatory T cells in a genetically engineered mouse model of pancreatic cancer. Langenbecks Arch Surg. 2013;398:989-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Ishikawa S, Nagai Y, Masuda T, Koga Y, Nakamura T, Imamura Y, Takamori H, Hirota M, Funakosi A, Fukushima M. The role of oxysterol binding protein-related protein 5 in pancreatic cancer. Cancer Sci. 2010;101:898-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Kusama T, Mukai M, Iwasaki T, Tatsuta M, Matsumoto Y, Akedo H, Inoue M, Nakamura H. 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors reduce human pancreatic cancer cell invasion and metastasis. Gastroenterology. 2002;122:308-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 169] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Arafat HA, Gong Q, Chipitsyna G, Rizvi A, Saa CT, Yeo CJ. Antihypertensives as novel antineoplastics: angiotensin-I-converting enzyme inhibitors and angiotensin II type 1 receptor blockers in pancreatic ductal adenocarcinoma. J Am Coll Surg. 2007;204:996-1005; discussion 1005-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Masamune A, Hamada S, Kikuta K, Takikawa T, Miura S, Nakano E, Shimosegawa T. The angiotensin II type I receptor blocker olmesartan inhibits the growth of pancreatic cancer by targeting stellate cell activities in mice. Scand J Gastroenterol. 2013;48:602-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Bocci G, Fioravanti A, Orlandi P, Bernardini N, Collecchi P, Del Tacca M, Danesi R. Fluvastatin synergistically enhances the antiproliferative effect of gemcitabine in human pancreatic cancer MIAPaCa-2 cells. Br J Cancer. 2005;93:319-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Nakai Y, Isayama H, Ijichi H, Sasaki T, Sasahira N, Hirano K, Kogure H, Kawakubo K, Yagioka H, Yashima Y. Inhibition of renin-angiotensin system affects prognosis of advanced pancreatic cancer receiving gemcitabine. Br J Cancer. 2010;103:1644-1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 16. | Tan XL, Reid Lombardo KM, Bamlet WR, Oberg AL, Robinson DP, Anderson KE, Petersen GM. Aspirin, nonsteroidal anti-inflammatory drugs, acetaminophen, and pancreatic cancer risk: a clinic-based case-control study. Cancer Prev Res (Phila). 2011;4:1835-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Khurana V, Sheth A, Caldito G, Barkin JS. Statins reduce the risk of pancreatic cancer in humans: a case-control study of half a million veterans. Pancreas. 2007;34:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Corcos L, Le Jossic-Corcos C. Statins: perspectives in cancer therapeutics. Dig Liver Dis. 2013;45:795-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Cuzick J, Thorat MA, Bosetti C, Brown PH, Burn J, Cook NR, Ford LG, Jacobs EJ, Jankowski JA, La Vecchia C. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol. 2015;26:47-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 263] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 20. | Chiang YY, Chen KB, Tsai TH, Tsai WC. Lowered cancer risk with ACE inhibitors/ARBs: a population-based cohort study. J Clin Hypertens (Greenwich). 2014;16:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Holmes MD, Chen WY. Hiding in plain view: the potential for commonly used drugs to reduce breast cancer mortality. Breast Cancer Res. 2012;14:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199-1210; discussion 1210-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1140] [Article Influence: 57.0] [Reference Citation Analysis (1)] |
| 23. | Eeson G, Chang N, McGahan CE, Khurshed F, Buczkowski AK, Scudamore CH, Warnock GL, Chung SW. Determination of factors predictive of outcome for patients undergoing a pancreaticoduodenectomy of pancreatic head ductal adenocarcinomas. HPB (Oxford). 2012;14:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Lewis R, Drebin JA, Callery MP, Fraker D, Kent TS, Gates J, Vollmer CM. A contemporary analysis of survival for resected pancreatic ductal adenocarcinoma. HPB (Oxford). 2013;15:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 642] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 26. | Alexis F. Nano-polypharmacy to treat tumors: coencapsulation of drug combinations using nanoparticle technology. Mol Ther. 2014;22:1239-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006;6:130-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 419] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 28. | Ye X, Fu J, Yang Y, Gao Y, Liu L, Chen S. Frequency-risk and duration-risk relationships between aspirin use and gastric cancer: a systematic review and meta-analysis. PLoS One. 2013;8:e71522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195-4200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 2936] [Article Influence: 195.7] [Reference Citation Analysis (0)] |
| 30. | Daniluk J, Liu Y, Deng D, Chu J, Huang H, Gaiser S, Cruz-Monserrate Z, Wang H, Ji B, Logsdon CD. An NF-κB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J Clin Invest. 2012;122:1519-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 31. | Sclabas GM, Uwagawa T, Schmidt C, Hess KR, Evans DB, Abbruzzese JL, Chiao PJ. Nuclear factor kappa B activation is a potential target for preventing pancreatic carcinoma by aspirin. Cancer. 2005;103:2485-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Yue W, Yang CS, DiPaola RS, Tan XL. Repurposing of metformin and aspirin by targeting AMPK-mTOR and inflammation for pancreatic cancer prevention and treatment. Cancer Prev Res (Phila). 2014;7:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 33. | Fendrich V, Chen NM, Neef M, Waldmann J, Buchholz M, Feldmann G, Slater EP, Maitra A, Bartsch DK. The angiotensin-I-converting enzyme inhibitor enalapril and aspirin delay progression of pancreatic intraepithelial neoplasia and cancer formation in a genetically engineered mouse model of pancreatic cancer. Gut. 2010;59:630-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Schuller HM, Zhang L, Weddle DL, Castonguay A, Walker K, Miller MS. The cyclooxygenase inhibitor ibuprofen and the FLAP inhibitor MK886 inhibit pancreatic carcinogenesis induced in hamsters by transplacental exposure to ethanol and the tobacco carcinogen NNK. J Cancer Res Clin Oncol. 2002;128:525-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Sato K, Ishizuka J, Cooper CW, Chung DH, Tsuchiya T, Uchida T, Rajaraman S, Townsend CM, Thompson JC. Inhibitory effect of calcium channel blockers on growth of pancreatic cancer cells. Pancreas. 1994;9:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Ford JM, Hait WN. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev. 1990;42:155-199. [PubMed] |
| 37. | Liu B, Staren ED, Iwamura T, Appert HE, Howard JM. Mechanisms of taxotere-related drug resistance in pancreatic carcinoma. J Surg Res. 2001;99:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Guo YS, Townsend CM. Roles of gastrointestinal hormones in pancreatic cancer. J Hepatobiliary Pancreat Surg. 2000;7:276-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Smith JP, Solomon TE. Cholecystokinin and pancreatic cancer: the chicken or the egg? Am J Physiol Gastrointest Liver Physiol. 2014;306:G91-G101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Nakaizumi A, Uehara H, Baba M, Iishi H, Tatsuta M. Inhibition by verapamil of cholecystokinin-enhancement of pancreatic carcinogenesis induced by azaserine in Wistar rats. Cancer Lett. 1996;105:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Matters GL, Cooper TK, McGovern CO, Gilius EL, Liao J, Barth BM, Kester M, Smith JP. Cholecystokinin mediates progression and metastasis of pancreatic cancer associated with dietary fat. Dig Dis Sci. 2014;59:1180-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
P- Reviewer: Lu Z, Reddy SS S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/