Published online Aug 15, 2015. doi: 10.4291/wjgp.v6.i3.43
Peer-review started: January 10, 2015
First decision: March 6, 2015
Revised: March 16, 2015
Accepted: June 1, 2015
Article in press: June 2, 2015
Published online: August 15, 2015
Processing time: 220 Days and 13.4 Hours
Myeloid derived suppressor cells (MDSC) are a heterogeneous population of immune cells that are potent suppressors of immune responses. MDSC emerge in various compartments in the body, such as blood, bone marrow or spleen, especially in conditions of cancer, infections or inflammation. MDSC usually express CD11b, CD33, and low levels of human leukocyte antigen-DR in humans or CD11b and Gr1 (Ly6C/G) in mice, and they can be further divided into granulocytic or monocytic MDSC. The liver is an important organ for MDSC induction and accumulation in hepatic as well as extrahepatic diseases. Different hepatic cells, especially hepatic stellate cells, as well as liver-derived soluble factors, including hepatocyte growth factor and acute phase proteins (SAA, KC), can promote the differentiation of MDSC from myeloid cells. Importantly, hepatic myeloid cells like neutrophils, monocytes and macrophages fulfill essential roles in acute and chronic liver diseases. Recent data from patients with liver diseases and animal models linked MDSC to the pathogenesis of hepatic inflammation, fibrosis and hepatocellular carcinoma (HCC). In settings of acute hepatitis, MDSC can limit immunogenic T cell responses and subsequent tissue injury. In patients with chronic hepatitis C, MDSC increase and may favor viral persistence. Animal models of chronic liver injury, however, have not yet conclusively clarified the involvement of MDSC for hepatic fibrosis. In human HCC and mouse models of liver cancer, MDSC are induced in the tumor environment and suppress anti-tumoral immune responses. Thus, the liver is a primary site of MDSC in vivo, and modulating MDSC functionality might represent a promising novel therapeutic target for liver diseases.
Core tip: Myeloid derived suppressor cells (MDSC) are a heterogeneous population of immune-suppressive cells with important roles during inflammation, infection and cancer. The liver is a primary site for MDSC induction and accumulation, and recent studies linked these cells to the pathogenesis of hepatic inflammation, fibrosis and hepatocellular carcinoma. MDSC can limit tissue injury during acute hepatitis, while they may favor viral persistence in chronic hepatitis. MDSC are also induced during development of liver cancer and suppress anti-tumoral immunity, but their involvement in hepatic fibrosis is less clear. Thus, modulating MDSC functionality might represent a promising novel therapeutic target for liver diseases.
- Citation: Hammerich L, Tacke F. Emerging roles of myeloid derived suppressor cells in hepatic inflammation and fibrosis. World J Gastrointest Pathophysiol 2015; 6(3): 43-50
- URL: https://www.wjgnet.com/2150-5330/full/v6/i3/43.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v6.i3.43
Myeloid-derived suppressor cells (MDSC) are a heterogeneous cell population of myeloid origin originally described in tumor-bearing hosts[1] that are also induced under various inflammatory conditions - including sepsis[2], hepatitis[3,4] and viral infections[5-7]. MDSC regulate immune responses by potently suppressing T cell function[8]; although these T cell suppressive activities have been functionally linked to tumor progression or evasion from immune responses, the exact roles of MDSC appear to be context-dependent and vary between infectious, autoimmune or malignant diseases. MDSC are usually identified as CD11b+ CD33+ HLA-DRlow cells in humans and CD11b+ Gr1+ cells in mice[9]. However, a specific marker for MDSC has not been described so far, which can make identification of these cells difficult as all those surface molecules are shared with other myeloid cell types such as neutrophils, monocytes or myeloid dendritic cells. Therefore, the most reliable feature to distinguish MDSC from other myeloid cells seems to be their suppressive function.
MDSC consist of at least two major subpopulations that are termed monocytic MDSC (mMDSC) and granulocytic MDSC (gMDSC) according to their side scatter (SSC) profile and Gr1 (Ly6C/G) expression in mice[10]. Whereas murine mMDSC have a low SSC profile and are Ly6Chi Ly6G-, gMDSC are Ly6Clo Ly6Ghi and show a higher SSC profile. In humans, CD14 and CD15 have been suggested as markers for mMDSC and gMDSC, respectively, but further investigation is needed to verify this hypothesis[11]. The two subsets seem to differ in their suppressive capacity and functional mechanism(s) depending on the disease studied.
As MDSC are heterogeneous myeloid cells with immune suppressive functions, several mechanisms of T cell suppression have been described. These mechanisms include L-arginine depletion by the enzymes arginase 1 (Arg1) or inducible nitric oxide synthase (iNOS) and generation of reactive oxygen species (ROS)[8,10,12]. Furthermore, MDSC have also been shown to secrete anti-inflammatory cytokines like IL-10[13]. Again, the suppressive mechanisms used by the different subsets as well as the requirement of cell-cell-contacts vs secretion of soluble factors seem to be highly dependent on the underlying pathology (Table 1). A recent study on the development of murine MDSC suggested that the two subsets depend on the expression of distinct anti-apoptotic proteins and that T cell suppressive functions are restricted to the mMDSC subset[14].
| Species | Type of disease | Surface phenotype | Function of MDSC | Mechanism | Ref. |
| Human | Chronic HCV infection | CD11b+ HLA-DRlo CD33+ CD14+ | Inhibition of T cell proliferation and IFNγ production | Arginase1 | [6] |
| Human | HCV-infected hepatocytes | CD11b+/lo HLA-DRlo/- CD33+ CD14+ | Inhibition of T cell cytokine production | ROS Cell-cell-contact | [7] |
| Human | HCC | CD11b+ HLA-DR- CD33+ CD14- | Long-lasting inhibition of effector T cells | [22-30] | |
| Human | HCC | HLA-DRlo/- CD14+ | Inhibition of natural killer cells | Cell-cell-contact NKp30 | [33] |
| Human | HCC | HLA-DRlo/- CD14+ | Induction of Treg and inhibition of effector T cells | Arginase | [29] |
| Mouse | CCl4-mediated fibrosis | CD11b+Ly6G-Ly6ChiF4/80+ | Amelioration of fibrosis through inhibition of HSC | IL-10 production | [13] |
| CD11b+Ly6G+Ly6CloF4/80- | |||||
| Mouse | Th1-mediated inflammation | CD11b+Ly6G-Ly6Chi | Inhibition of T cell proliferation (CD4+ and CD8+) | iNOS cell-cell-contact | [48] |
| CD11b+Ly6G+Ly6Clo | |||||
| Mouse | Sepsis | CD11b+Gr1+ | Inhibition of IL-12 and induction of IL-10 release by macrophages | Cell-cell-contact | [2] |
| Mouse | Immune-mediated hepatitis | CD11b+Ly6GloLy6Chi | Suppression of CD4+ T cell proliferation | iNOS | [46,47] |
| CD11b+Ly6G+Ly6Clo | |||||
| Mouse | ConA-mediated hepatitis | CD11b+Ly6G-Ly6C+ | Protection against liver injury through inhibition of T cells | Arginase | [4] |
| CD11b+Ly6G+Ly6C+(int) | |||||
| Mouse | ConA/LPS-mediated hepatitis | CD11b+Ly6GloLy6Chi | Suppression of CD4+ T cell proliferation and cytokine production | iNOS cell-cell-contact | [3,45] |
| CD11b+Ly6GhiLy6Cint | |||||
| Mouse | CTL-mediated liver injury | CD11b+Gr1+ | Suppression of CTL proliferation and IFNγ production | [21] | |
| Mouse | HBV (transgenics) | CD11b+Gr1+ | Suppression of HBV-specific CTL | Arginase iNOS | [5] |
| Mouse | HCC/primary liver tumors | CD11b+Gr1+ | Suppression of anti-tumor CTL | [35,36,38] | |
| Mouse | Gastrointestinal cancer with liver metastasis | CD11b+Gr1+/int | Inhibition of T cell proliferation and tumor cell lysis | [40] |
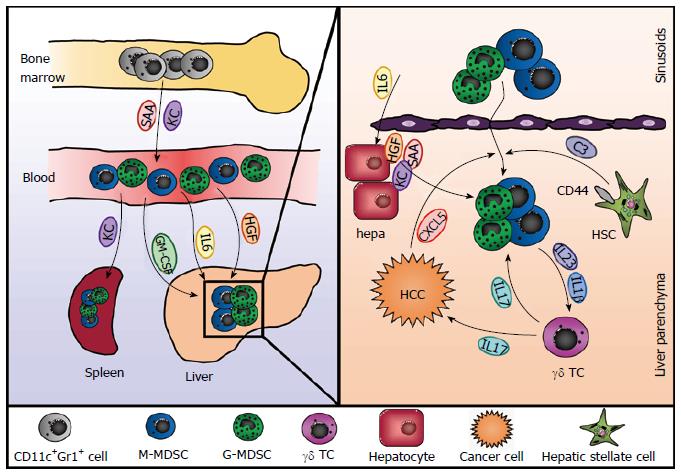
The liver has been shown to be a site of MDSC accumulation, and this seems to apply to hepatic and also to extrahepatic diseases. Different hepatic cell types as well as liver-derived soluble factors have been implicated in the recruitment and differentiation of MDSC under various conditions (Figure 1). In tumor-bearing mice with various types of cancer - including breast, lung and skin cancer - MDSC numbers increased in the liver irrespective of whether the mice had tumor manifestation in the liver, namely hepatic metastasis, or not[15]. Furthermore, adoptively transferred MDSC homed to livers and spleens of tumor-bearing mice in a comparable fashion. Ilkovitch et al[15] could show that this increase in hepatic MDSC is at least in part due to elevated levels of GM-CSF, a hematopoietic growth factor produced by many different types of tumors and associated with splenic accumulation of MDSC.
Additionally, hepatic stellate cells (HSC), a cell type associated with various immune-modulatory functions[16], have been shown to induce MDSC from myeloid cells in mice and men. Primary human HSC were able to induce differentiation of MDSC from PBMC in vitro[17]. This induction was dependent on direct cell-cell contacts as well as on the expression of CD44 by HSC and led to generation of CD14+ HLA-DRlo cells able to suppress T cell responses in an arginase 1-dependent manner. Similarly, murine hepatic stellate cells were proven to induce CD11b+Gr1+ MDSC from bone marrow-derived cells[18,19]. However, this induction seems to be mediated by soluble factors rather than cell-cell contact. Chou et al[18] implicated a critical role for IFNγ signaling in HSC, and an additional study from the same group showed that MDSC induction was mediated by complement component C3 released by HSC[19]. In addition, both studies demonstrated that HSC could also induce MDSC in vivo in the context of islet cell transplantation and therefore contribute to allograft survival.
Furthermore, liver-derived soluble factors can also promote the generation of MDSC (Figure 1). Human mesenchymal stromal cells and an osteosarcoma cell line are able to induce the expansion of CD11b+ CD33+ CD14- MDSC from peripheral blood leukocytes in vitro, an effect that is mediated by hepatocyte growth factor (HGF) and its receptor c-Met[20]. Since the liver usually harbors high levels of HGF this might be an explanation for the high numbers of MDSC present in the liver even under steady state conditions. Indeed, inhibition of the HGF/c-Met pathway in mice led to a significant reduction in hepatic but not splenic MDSC[20]. In the context of polymicrobial sepsis in mice hepatic acute-phase proteins play a critical role for controlling the inflammatory reaction to infection. Both serum amyloid A (SAA) and the chemokine CXCL1/KC work synergistically to mobilize MDSC from the bone marrow and induce their accumulation in the spleen[2]. Mice lacking the production of acute phase proteins due to the deletion of the IL-6 cytokine family receptor gp130 in hepatocytes showed less accumulation of MDSC and increased mortality during sepsis, which could be reversed by adoptive transfer of MDSC or administration of recombinant SAA and KC[2]. Consistently, the ectopic expression of IL-6 in the liver induced accumulation of MDSC in liver and spleen, which protected mice from CD8+ T cell-mediated liver injury[21].
Another factor that may contribute to for the accumulation of MDSC in the liver is activation of inflammasomes, proteolytic complexes activated by pattern recognition receptors (PRR), and resulting in the production of IL-1β and IL-18. In murine cancer models activation of the Nlrp3 inflammasome has been associated with the accumulation of MDSC and suppression of anti-tumor immune responses[22,23]. This may also apply to liver diseases as inflammasome activation is important in a wide range of conditions[24,25]. Chronic human liver diseases are often associated with changes in the intestinal microbiome with the resulting inflammation leading to disruption and enhanced permeability of the intestinal epithelial barrier[26,27]. This enables the translocation of microbial products, which can travel to the liver via the portal vein and activate the inflammasome complex through PRRs. So far, this process has mainly been described for liver macrophages[28], but considering what has been observed for tumor-associated MDSC, inflammasome activation might also induce accumulation of hepatic MDSC.
While the above mentioned data demonstrated that the liver is an important site of MDSC induction for extrahepatic infections and cancer, more recent data implied hepatic MDSC as essential regulators of liver diseases as well. Several studies have concordantly reported that patients with hepatocellular carcinoma (HCC) or chronic hepatitis C virus (HCV) infection show increased frequencies of MDSC in the peripheral blood[6,7,29-32]. Human MDSC in HCC patients are mainly CD14+ HLA-DR-/low and able to inhibit T cell proliferation in an arginase dependent manner[29]. Furthermore, these cells induce a regulatory phenotype in CD4+ T cells and inhibit natural killer (NK) cell function in vitro[29,33]. Likewise, MDSC in the blood of patients with chronic HCV were shown to be CD11b+ HLA-DRlow CD14+ CD33+ and suppress T cells using arginase[6]. In addition, ROS production may contribute to T cell inhibition by MDSC, and HCV-infected hepatocytes were found to promote MDSC differentiation from PBMC[7]. This might represent a mechanism of HCV-mediated immune suppression that leads to persistent infection.
Several studies have addressed the function of MDSC in liver cancer by investigating murine models of HCC. Mice bearing liver tumors show increased numbers of MDSC in liver, spleen, and bone marrow[34-37]. Remarkably, the timing of MDSC accumulation seems to be highly dependent on the tumor model studied. Mice with diethylnitrosamine (DEN) or transgenic myc-overexpression induced liver tumors, in which primary liver cancer develops slowly in the “normal” hepatic microenvironment, showed increased MDSC numbers only during late stages of the disease, while mice with orthotopic or subcutaneous tumors displayed increased MDSC numbers early on[34]. In addition, MDSC from mice with transplantable tumors showed higher suppressive capacity than MDSC from mice with DEN-induced HCC. Several studies showed that treatment with the multi-kinase inhibitor sorafenib[34,35] or an agonistic anti-CD137 antibody[37] decreased frequency of MDSC in mice bearing HCC, thereby contributing to anti-tumoral immunity.
Several soluble factors have been implicated in the recruitment of MDSC during HCC development. Tumor derived GM-CSF and KC mediated the accumulation of MDSC during hepatocarcinogenesis, and neutralization of these molecules reduced hepatic MDSC numbers[34]. Interleukin-17 (IL-17) produced by gamma/delta T cells (γδ T cells) also indirectly mediated MDSC accumulation[38]. Ma et al[38] showed that γδ T cell-derived IL-17 induced secretion of CXCL5 by tumor cells, which then recruited MDSC via engagement of CXCR2. Moreover, IL-17 also acted on the MDSC directly by enhancing their suppressive capacity and MDSC enhanced the production of IL-17 by γδ T cells through release of IL-23 and IL-1β. Similarly, γδ T cell-derived IL-17 has also been shown to recruit MDSC to the liver in HBV-transgenic mice, where they induce CD8 T cell exhaustion and HBV tolerance[5].
In DEN-induced liver carcinogenesis IL-18, is also involved in recruitment of MDSC to the liver. Li et al[39] demonstrated recently that TLR2-deficient mice develop more aggressive HCC than wildtype (wt) mice associated with increased numbers of MDSC in the liver. This was mediated by IL-18 produced by hepatocytes and could be reversed through silencing of IL-18.
Interestingly, MDSC have also been associated with the development of liver metastasis. Mice with different types of intra-abdominal tumors showed a significant accumulation of MDSC in the liver that were able to potently suppress cytotoxic T cells and induce regulatory T cells[40]. Hepatic MDSC also differed from splenic MDSC in these models, expressing higher levels of immune-modulatory cytokines and being primarily of a monocytic phenotype. Similarly to HCC development, hepatic accumulation of MDSC was mediated by tumor-derived KC. This suggests that MDSC promote the development of liver metastases and may provide an explanation why human intra-abdominal cancers metastasize preferentially to the liver[41].
The accumulation of neutrophils, monocytes and macrophages is a hallmark of acute and chronic liver inflammation. For instance, hepatic neutrophils are associated with drug-induced liver injury, alcoholic hepatitis or ischemia-reperfusion injury[42]. Hepatic macrophages are a remarkably heterogeneous population comprising myeloid cells with different origins (e.g., resident Kupffer cells vs infiltrating monocyte-derived macrophages) and distinct properties[43]. Some of these neutrophils and macrophages have a clear immunosuppressive phenotype, prompting research on MDSC in acute and chronic liver injury.
Recently, MDSC have been studied in the context of acute liver inflammation and are usually associated with protective functions in this setting. We and others could show that MDSC accumulate in the liver during Concanavalin A (ConA)-, D-galactosamine (D-gal) and picryl chloride-induced hepatitis[3,4,44-47] and protect the liver from excessive damage. However, there seems to be controversy about which subsets are preferentially involved and which suppressive mechanisms they use. Two independent studies showed that administration of cannabidiol[4] or IL-25[3] increases the number of hepatic CD11b+ Gr1+ cells that ameliorated organ damage upon immune-mediated hepatitis. In this setting, the ratio of gMDSC to mMDSC was about 2:1, and T cell responses were inhibited in an arginase-dependent manner with mMDSC being more suppressive than gMDSC[4]. Consistently, we have shown that inhibiting the suppressive capacity specifically in the mMDSC subset led to severely aggravated hepatitis upon ConA-challenge[44]. Similar observations were also made by another group studying the role of FTY720, a sphingosine-1-phosphate receptor agonist, in recruitment of MDSC to the liver[46,47]. However, the suppressive function of these cells was dependent on iNOS and NO production rather than arginine depletion by Arg1. Furthermore, these studies also provided some insight into how MDSC are recruited to the liver. Similarly to what has been observed in liver cancer, MDSC accumulation was mediated via CXCR2[46,47]. In contrast to the aforementioned studies, Zhu et al[45] showed that, although both MDSC subtypes were recruited, only mMDSC were able to suppress T cell responses and limit liver damage in ConA-mediated hepatitis. This was also observed in acutely inflamed livers of Tgfβ1-/- mice[48], where both subtypes of MDSC accumulated but only mMDSC were capable of suppressing T cells utilizing iNOS.
Overall, the liver provides a unique tolerogenic microenvironment, and several antigen-presenting cells contribute to the suppression of immunogenic T cell responses in the liver[49]. It has become increasingly clear that immune tolerance can also occur during chronic liver diseases. On the one hand, such tolerogenic mechanisms may limit intrahepatic immune responses and subsequent tissue injury, but on the other hand, immune tolerance may restrain eradication of pathogens and favor chronic infections[50]. Only limited data is available on the involvement of MDSC in chronic liver injury and the development of liver fibrosis. A recent study by Suh et al[13] indicates that bone marrow-derived MDSC can ameliorate hepatofibrogenesis through the production of IL-10, which downregulates pro-fibrotic functions of activated HSC. Interestingly, IL-10 production was induced upon contact with activated HSC in vitro, suggesting a mechanism for the beneficial effects observed in patients and mice with hepatic fibrosis treated with infusion of bone marrow cells[51]. On the contrary, liver fibrosis development upon chronic injury was not affected in a mouse model of transgenic overexpression of the transcription factor crem-alpha, which impairs the functionality of hepatic mMDSC[44]. Thus, more data are needed to define the possible role of MDSC in chronic inflammatory settings in the liver, and their involvement may likely vary depending on the etiology of the underlying disease, e.g., autoimmunity, chronic viral hepatitis or metabolic injury.
Given that MDSC are mainly associated with pathogenic functions in human chronic liver diseases such as chronic viral infections or liver cancer development, depletion of these cells and/or inhibition of their development may hold high potential in the treatment of such diseases. It has been shown that MDSC can be differentiated from murine bone-marrow cells and human PBMC in vitro in the presence of GM-CSF and IL-6[52-54]. Thus, these cytokines might be therapeutically targeted to avoid development of MDSC in vivo, but due to the various other functions of these cytokines, systemic inhibition might not be feasible and methods of local inhibition should be explored. In tumor bearing mice depletion of MDSC using a Gr-1 specific antibody has proven to help with eradication of tumors and prevention of recurrence[55,56]. However, a more recent study reported that this antibody failed to completely eliminate hepatic MDSC[57], challenging the feasibility of this approach for liver disease therapy. Since MDSC are considered immature cells, influencing the differentiation of these cells into other myeloid cells that promote rather than inhibit immune responses could be a different therapeutic approach. Retinoic acid and vitamin D3 have both been implicated in the differentiation of MDSC to dendritic cells in vitro and administrations of these agents to tumor-bearing mice or cancer patients resulted in the significant improvement of anti-tumor immune responses[58-61].
In murine models of acute liver inflammation MDSC have been associated with protective rather than pathogenic functions. Therefore, it might be helpful to enhance hepatic MDSC numbers for the treatment of patients with acute inflammation or autoimmunity in the liver. The previously mentioned induction of MDSC from PBMC using GM-CSF and IL-6 would allow for the generation and expansion of autologous MDSC that can then be retransferred to the patient. The fact that adoptively transferred MDSC preferentially home to the liver[15] acts in favor of this approach allowing directed delivery of MDSC to the site of inflammation. However, migration of MDSC and “off-target” T cell suppression cannot be ruled out and should be considered in this setting.
Taken together, MDSC represent promising therapeutic targets in the treatment of liver diseases, but more extensive research is needed before these approaches can be used in clinical settings.
| 1. | Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 981] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 2. | Sander LE, Sackett SD, Dierssen U, Beraza N, Linke RP, Müller M, Blander JM, Tacke F, Trautwein C. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med. 2010;207:1453-1464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 278] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 3. | Sarra M, Cupi ML, Bernardini R, Ronchetti G, Monteleone I, Ranalli M, Franzè E, Rizzo A, Colantoni A, Caprioli F. IL-25 prevents and cures fulminant hepatitis in mice through a myeloid-derived suppressor cell-dependent mechanism. Hepatology. 2013;58:1436-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 4. | Hegde VL, Nagarkatti PS, Nagarkatti M. Role of myeloid-derived suppressor cells in amelioration of experimental autoimmune hepatitis following activation of TRPV1 receptors by cannabidiol. PLoS One. 2011;6:e18281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Kong X, Sun R, Chen Y, Wei H, Tian Z. γδT cells drive myeloid-derived suppressor cell-mediated CD8+ T cell exhaustion in hepatitis B virus-induced immunotolerance. J Immunol. 2014;193:1645-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Cai W, Qin A, Guo P, Yan D, Hu F, Yang Q, Xu M, Fu Y, Zhou J, Tang X. Clinical significance and functional studies of myeloid-derived suppressor cells in chronic hepatitis C patients. J Clin Immunol. 2013;33:798-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Tacke RS, Lee HC, Goh C, Courtney J, Polyak SJ, Rosen HR, Hahn YS. Myeloid suppressor cells induced by hepatitis C virus suppress T-cell responses through the production of reactive oxygen species. Hepatology. 2012;55:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 8. | Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969-2975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 471] [Cited by in RCA: 466] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 9. | Poschke I, Kiessling R. On the armament and appearances of human myeloid-derived suppressor cells. Clin Immunol. 2012;144:250-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233-4244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 913] [Cited by in RCA: 996] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 11. | Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11:802-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 338] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 12. | Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791-5802. [PubMed] |
| 13. | Suh YG, Kim JK, Byun JS, Yi HS, Lee YS, Eun HS, Kim SY, Han KH, Lee KS, Duester G. CD11b(+) Gr1(+) bone marrow cells ameliorate liver fibrosis by producing interleukin-10 in mice. Hepatology. 2012;56:1902-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 14. | Haverkamp JM, Smith AM, Weinlich R, Dillon CP, Qualls JE, Neale G, Koss B, Kim Y, Bronte V, Herold MJ. Myeloid-derived suppressor activity is mediated by monocytic lineages maintained by continuous inhibition of extrinsic and intrinsic death pathways. Immunity. 2014;41:947-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 15. | Ilkovitch D, Lopez DM. The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res. 2009;69:5514-5521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Weiskirchen R, Tacke F. Cellular and molecular functions of hepatic stellate cells in inflammatory responses and liver immunology. Hepatobiliary Surg Nutr. 2014;3:344-363. [PubMed] |
| 17. | Höchst B, Schildberg FA, Sauerborn P, Gäbel YA, Gevensleben H, Goltz D, Heukamp LC, Türler A, Ballmaier M, Gieseke F. Activated human hepatic stellate cells induce myeloid derived suppressor cells from peripheral blood monocytes in a CD44-dependent fashion. J Hepatol. 2013;59:528-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Chou HS, Hsieh CC, Yang HR, Wang L, Arakawa Y, Brown K, Wu Q, Lin F, Peters M, Fung JJ. Hepatic stellate cells regulate immune response by way of induction of myeloid suppressor cells in mice. Hepatology. 2011;53:1007-1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Hsieh CC, Chou HS, Yang HR, Lin F, Bhatt S, Qin J, Wang L, Fung JJ, Qian S, Lu L. The role of complement component 3 (C3) in differentiation of myeloid-derived suppressor cells. Blood. 2013;121:1760-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Yen BL, Yen ML, Hsu PJ, Liu KJ, Wang CJ, Bai CH, Sytwu HK. Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3. Stem Cell Reports. 2013;1:139-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Cheng L, Wang J, Li X, Xing Q, Du P, Su L, Wang S. Interleukin-6 induces Gr-1+CD11b+ myeloid cells to suppress CD8+ T cell-mediated liver injury in mice. PLoS One. 2011;6:e17631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | van Deventer HW, Burgents JE, Wu QP, Woodford RM, Brickey WJ, Allen IC, McElvania-Tekippe E, Serody JS, Ting JP. The inflammasome component NLRP3 impairs antitumor vaccine by enhancing the accumulation of tumor-associated myeloid-derived suppressor cells. Cancer Res. 2010;70:10161-10169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Bruchard M, Mignot G, Derangère V, Chalmin F, Chevriaux A, Végran F, Boireau W, Simon B, Ryffel B, Connat JL. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 634] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 24. | Mehal WZ. The inflammasome in liver injury and non-alcoholic fatty liver disease. Dig Dis. 2014;32:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Bieghs V, Trautwein C. Innate immune signaling and gut-liver interactions in non-alcoholic fatty liver disease. Hepatobiliary Surg Nutr. 2014;3:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 26. | Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 777] [Article Influence: 64.8] [Reference Citation Analysis (2)] |
| 27. | Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1616] [Article Influence: 134.7] [Reference Citation Analysis (43)] |
| 28. | Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol. 2012;57:642-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 414] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 29. | Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 649] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 30. | Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73:2435-2444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 31. | Mundy-Bosse BL, Young GS, Bauer T, Binkley E, Bloomston M, Bill MA, Bekaii-Saab T, Carson WE, Lesinski GB. Distinct myeloid suppressor cell subsets correlate with plasma IL-6 and IL-10 and reduced interferon-alpha signaling in CD4⁺ T cells from patients with GI malignancy. Cancer Immunol Immunother. 2011;60:1269-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 32. | Liu Y, She LH, Wang XY, Zhang GL, Yan Y, Lin CS, Zhao ZX, Gao ZL. Expansion of myeloid-derived suppressor cells from peripheral blood decreases after 4-week antiviral treatment in patients with chronic hepatitis C. Int J Clin Exp Med. 2014;7:998-1004. [PubMed] |
| 33. | Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 533] [Cited by in RCA: 538] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 34. | Kapanadze T, Gamrekelashvili J, Ma C, Chan C, Zhao F, Hewitt S, Zender L, Kapoor V, Felsher DW, Manns MP. Regulation of accumulation and function of myeloid derived suppressor cells in different murine models of hepatocellular carcinoma. J Hepatol. 2013;59:1007-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (8)] |
| 35. | Cao M, Xu Y, Youn JI, Cabrera R, Zhang X, Gabrilovich D, Nelson DR, Liu C. Kinase inhibitor Sorafenib modulates immunosuppressive cell populations in a murine liver cancer model. Lab Invest. 2011;91:598-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 36. | Schneider C, Teufel A, Yevsa T, Staib F, Hohmeyer A, Walenda G, Zimmermann HW, Vucur M, Huss S, Gassler N. Adaptive immunity suppresses formation and progression of diethylnitrosamine-induced liver cancer. Gut. 2012;61:1733-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 37. | Gauttier V, Judor JP, Le Guen V, Cany J, Ferry N, Conchon S. Agonistic anti-CD137 antibody treatment leads to antitumor response in mice with liver cancer. Int J Cancer. 2014;135:2857-2867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Ma S, Cheng Q, Cai Y, Gong H, Wu Y, Yu X, Shi L, Wu D, Dong C, Liu H. IL-17A produced by γδ T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 2014;74:1969-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 39. | Li S, Sun R, Chen Y, Wei H, Tian Z. TLR2 limits development of hepatocellular carcinoma by reducing IL18-mediated immunosuppression. Cancer Res. 2015;75:986-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Connolly MK, Mallen-St Clair J, Bedrosian AS, Malhotra A, Vera V, Ibrahim J, Henning J, Pachter HL, Bar-Sagi D, Frey AB. Distinct populations of metastases-enabling myeloid cells expand in the liver of mice harboring invasive and preinvasive intra-abdominal tumor. J Leukoc Biol. 2010;87:713-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | McMillan DC, McArdle CS. Epidemiology of colorectal liver metastases. Surg Oncol. 2007;16:3-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Moles A, Murphy L, Wilson CL, Chakraborty JB, Fox C, Park EJ, Mann J, Oakley F, Howarth R, Brain J. A TLR2/S100A9/CXCL-2 signaling network is necessary for neutrophil recruitment in acute and chronic liver injury in the mouse. J Hepatol. 2014;60:782-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 43. | Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 849] [Article Influence: 70.8] [Reference Citation Analysis (4)] |
| 44. | Hammerich L, Warzecha KT, Stefkova M, Bartneck M, Ohl K, Gassler N, Luedde T, Trautwein C, Tenbrock K, Tacke F. Cyclic adenosine monophosphate-responsive element modulator alpha overexpression impairs function of hepatic myeloid-derived suppressor cells and aggravates immune-mediated hepatitis in mice. Hepatology. 2015;61:990-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Zhu K, Zhang N, Guo N, Yang J, Wang J, Yang C, Yang C, Zhu L, Xu C, Deng Q. SSC(high)CD11b(high)Ly-6C(high)Ly-6G(low) myeloid cells curtail CD4 T cell response by inducible nitric oxide synthase in murine hepatitis. Int J Biochem Cell Biol. 2014;54:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Liu G, Bi Y, Wang R, Yang H, Zhang Y, Wang X, Liu H, Lu Y, Zhang Z, Chen W. Targeting S1P1 receptor protects against murine immunological hepatic injury through myeloid-derived suppressor cells. J Immunol. 2014;192:3068-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Zhang Y, Bi Y, Yang H, Chen X, Liu H, Lu Y, Zhang Z, Liao J, Yang S, Chu Y. mTOR limits the recruitment of CD11b+Gr1+Ly6Chigh myeloid-derived suppressor cells in protecting against murine immunological hepatic injury. J Leukoc Biol. 2014;95:961-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Cripps JG, Wang J, Maria A, Blumenthal I, Gorham JD. Type 1 T helper cells induce the accumulation of myeloid-derived suppressor cells in the inflamed Tgfb1 knockout mouse liver. Hepatology. 2010;52:1350-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 49. | Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 615] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 50. | Crispe IN. Immune tolerance in liver disease. Hepatology. 2014;60:2109-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 219] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 51. | Thomas JA, Pope C, Wojtacha D, Robson AJ, Gordon-Walker TT, Hartland S, Ramachandran P, Van Deemter M, Hume DA, Iredale JP. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011;53:2003-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 292] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 52. | Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273-2284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 488] [Cited by in RCA: 490] [Article Influence: 30.6] [Reference Citation Analysis (7)] |
| 53. | Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 740] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 54. | Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, Taylor PA, Panoskaltsis-Mortari A, Serody JS, Munn DH. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738-5747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 370] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 55. | Seung LP, Rowley DA, Dubey P, Schreiber H. Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumor rejection. Proc Natl Acad Sci USA. 1995;92:6254-6258. [PubMed] |
| 56. | Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741-1752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 430] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 57. | Ma C, Kapanadze T, Gamrekelashvili J, Manns MP, Korangy F, Greten TF. Anti-Gr-1 antibody depletion fails to eliminate hepatic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;92:1199-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 58. | Young MR, Ihm J, Lozano Y, Wright MA, Prechel MM. Treating tumor-bearing mice with vitamin D3 diminishes tumor-induced myelopoiesis and associated immunosuppression, and reduces tumor metastasis and recurrence. Cancer Immunol Immunother. 1995;41:37-45. [PubMed] |
| 59. | Young MR, Lathers DM. Myeloid progenitor cells mediate immune suppression in patients with head and neck cancers. Int J Immunopharmacol. 1999;21:241-252. [PubMed] |
| 60. | Hengesbach LM, Hoag KA. Physiological concentrations of retinoic acid favor myeloid dendritic cell development over granulocyte development in cultures of bone marrow cells from mice. J Nutr. 2004;134:2653-2659. [PubMed] |
| 61. | Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299-9307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 447] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Gassler N, Morini S S- Editor: Ji FF L- Editor: A E- Editor: Wu HL