Published online Nov 15, 2014. doi: 10.4291/wjgp.v5.i4.400
Revised: March 30, 2014
Accepted: July 17, 2014
Published online: November 15, 2014
Processing time: 298 Days and 5.3 Hours
The purpose of this paper is to review current information about the role of inflammation caused by Helicobacter pylori (H. pylori) infection in neurological diseases such as Parkinson’s disease, Alzheimer’s disease, Guillain-Barré syndrome, multiple sclerosis, and other inflammatory diseases including ischemic stroke. Infection with H. pylori usually persists throughout life, resulting in a chronic inflammatory response with local secretion of numerous inflammatory mediators including chemokines [interleukin (IL)-8, macrophage chemotactic protein (MCP)-1, growth-regulated oncogene (GRO)-α] and cytokines [IL-1β, tumor necrosis factor (TNF)-α, IL-6, IL-12, interferon (IFN)-γ], which can pass into the circulation and have a systemic effect. The persistence of detectable systemic and local concentrations of inflammatory mediators is likely to alter the outcome of neurological diseases. These proinflammatory factors can induce brain inflammation and the death of neurons and could eventually be associated to Parkinson’s disease and also may be involved in the development of Alzheimer’s disease. However, most neurological diseases are the result of a combination of multiple factors, but the systemic inflammatory response is a common component and determinant in the onset, evolution, and outcome of diseases. However, more studies are needed to allow understanding of the effects and mechanisms by which the inflammatory response generated by H. pylori infection affects neurological diseases.
Core tip: Neurological diseases such as Parkinson, Alzheimer, Guillain-Barré syndrome, multiple sclerosis, and ischemic stroke are the result of a combination of multiple factors, but the chronic and systemic inflammatory response to Helicobacter pylori could be a common component and determinant in the onset, evolution, and outcome of these diseases.
-
Citation: Álvarez-Arellano L, Maldonado-Bernal C.
Helicobacter pylori and neurological diseases: Married by the laws of inflammation. World J Gastrointest Pathophysiol 2014; 5(4): 400-404 - URL: https://www.wjgnet.com/2150-5330/full/v5/i4/400.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v5.i4.400
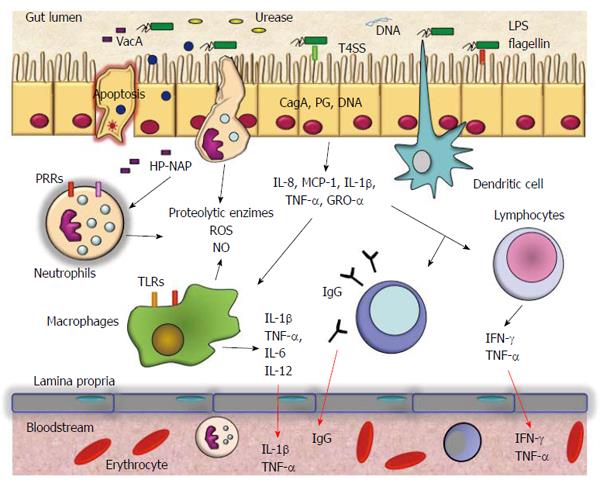
Helicobacter pylori (H. pylori) is a Gram-negative bacterium that chronically infects more than 50% of the human population[1]. It is well known that infection with the bacterium increases the risk of gastric diseases including peptic ulcers and gastric cancer[2,3]. H. pylori is able to infect and live persistently in the human stomach and elicits severe acute and chronic inflammatory responses, which may be of variable magnitude depending on the host’s genetic makeup and lifestyle[4]. Differing inflammatory responses among hosts may help to explain the different outcomes of infection with H. pylori[5]. H. pylori induces infiltration to the gastric mucosa of neutrophils, macrophages, dendritic cells, T and B cells, and stimulates secretion of interleukin (IL)-8, tumor necrosis factor (TNF)-α, IL-6, IL-1β, IL-12, IL-10, and interferon (IFN)-γ[5]. This persistent response causes significant changes in the physiology of the stomach by direct damage to the cells or by regulating cell proliferation and apoptosis (Figure 1). Neutrophils and macrophages release reactive oxygen and nitrogen species, which may induce irreversible changes in the gene expression of cells of the gastric mucosa. The levels of these chemical species decrease when H. pylori infection is eradicated[6]. The secretion of inflammatory mediators is likely to have serious biological implications at the local and systemic level. For example, IL-8 is a chemokine induced early by H. pylori infection. It is a chemoattractant of neutrophils and mediates responses to bacterial infection and to autoimmune disease[7]. IL-6 activates target genes involved in cellular differentiation, survival, apoptosis, and proliferation[8]. IL-6 may perpetuate inflammation by inducing antiapoptotic signals mediated by signal transducer and activator of transcription 3. IL-1β signaling is absolutely necessary for the efficient control of H. pylori infection. IL-1R(-/-) mice failed to developed protective immunity against Helicobacter-associated gastritis and gastric preneoplasia as a result of their inability to generate Helicobacter-specific T helper (Th)1 and Th17 responses[9].
IL-10 is an anti-inflammatory cytokine that inhibits the production of proinflammatory cytokines by inhibition of Th1 lymphocytes and stimulation of B cells and Th2 lymphocytes, and consequently it downregulates the inflammatory response[10]. This cytokine is very important for the maintenance of a balanced response in gastric inflammation. In contrast, IFN-γ mediates responses to bacterial infection and autoimmune disease. It is upregulated in the gastric mucosa by chronic Helicobacter infection[11]. This cytokine is important in the production of gastric acid and its levels are correlated with the damage found in gastritis.
H. pylori infection has been associated with the development and progression of neurological diseases, principally by inducing systemic inflammation, molecular mimicry, and interference with the absorption of drugs. In this review, we summarize the most important research on this issue.
Parkinson’s disease is the second most common neurodegenerative disease worldwide. It is characterized by the accumulation of cytoplasmic proteins, including α-synuclein, which leads to the progressive loss of dopaminergic neurons. The loss of dopaminergic neurons causes the resting tremors, rigidity and bradykinesia that are characteristic symptoms of the disease[12]. H. pylori infection may increase the risk of Parkinson’s disease[13]. The administration of L-3,4-dihydroxyphenylalanine (L-dopa), a precursor of dopamine, is used as a treatment for Parkinson’s disease. H. pylori infection may affect the bioavailability of L-dopa by disrupting the duodenal mucosa, which is the site of primary absorption of L-dopa[14,15]. Recent studies suggested that H. pylori eradication in patients with Parkinson’s disease might improve the bioavailability of L-dopa and reduce motor fluctuations[15-18].
Parkinsonism is a neurological syndrome that shares symptoms found in Parkinson’s disease. It has been suggested that a peripheral immune response characterized by the presence of proinflammatory cytokines such as IL-8, IL-1β, and TNF-α in the bloodstream induces a disruption of the blood-brain barrier and promotes microglia-mediated inflammation and neurotoxicity[19-21]. Several studies have established that that proinflammatory factors associated with chronic gastrointestinal disease can induce brain inflammation and the death of dopaminergic neurons and could eventually be responsible for parkinsonism[22-24]. Dobbs et al[25] proposed that H. pylori infection predisposes to autoimmunity that results in neuronal damage leading to eventual parkinsonism. This was based on the observation of an age-associated increase in the levels of antibodies against H. pylori in Parkinson’s patients, but this association is not clear, and other investigations are required to clarify it.
Alzheimer’s disease is a progressive neurodegenerative disease characterized by both synaptic loss and neuronal death as a result of extracellular and intracellular accumulation of β-amyloid deposits and neurofibrillary tangles in brain regions important for memory and cognitive processes[26]. The inflammatory response plays an important role in the pathophysiology of Alzheimer’s disease. Higher levels of H. pylori-specific IgG antibody, IL-8 and TNF-α have been found in cerebrospinal fluid (CSF) of cognitively impaired Alzheimer’s disease patients infected with H. pylori[27,28], and it has been proposed that the inflammatory response induced by H. pylori may be involved in the development of Alzheimer’s disease. This is consistent with some studies that observed an improvement in parameters of cognitive and functional status and the survival rate of Alzheimer’s disease patients after eradication of H. pylori[29,30]. However, Alzheimer’s disease was independent of H. pylori status in a Japanese population[31].
H. pylori-induced chronic atrophic gastritis causes a decrease in serum vitamin B concentration, thereby increasing the concentration of homocysteine[26]. It has been shown that the serum homocysteine concentration correlates with the severity of dementia. Homocysteine-induced oxidative damage has been described in the brain of subjects with mild cognitive impairment, suggesting that oxidative damage may be one of the earliest events in the onset and progression of Alzheimer’s disease[30].
Guillain-Barré syndrome is an acute inflammatory autoimmune neuropathy, presenting as a progressive motor weakness usually beginning in the legs, and can be triggered by a preceding bacterial or viral infection. Molecular mimicry of host structures by antigens present in the gastrointestinal pathogens Campylobacter jejuni and H. pylori are thought to be connected with the development of the autoimmune sequelae observed in Guillain-Barré syndrome[32,33]. In a case-control study, it was found that serum anti-H. pylori IgG of patients was significantly higher than that of controls, that CSF anti-H. pylori IgG was positive in 80% of patients and in 20% of controls and that the CSF IgG titer was also significantly higher in patients than controls[34]. Furthermore, specific IgG antibodies to vacuolating cytotoxin A (VacA) of H. pylori have been detected in the CSF of patients with Guillain-Barré syndrome. The sequence homology found between VacA and human ATPase A subunit suggests that antibodies to VacA bind to ion channels in Schwann cells, resulting in demyelination of motor neurons in these patients[35]. Moreover, high levels of serum anti-H. pylori IgG antibodies closely correlate with a more advanced clinical status, and elevation of anti-H. pylori-specific IgG antibodies is associated with involvement of the proximal parts of peripheral nerves in patients with acute inflammatory demyelinating polyradiculoneuropathy, the most commonly observed subtype of Guillain-Barré syndrome[32]. Most studies have included only a small sample, so more research is needed to confirm the association between H. pylori infection and Guillain-Barré syndrome.
Multiple sclerosis is the most common inflammatory demyelinating disease of the central nervous system. The association between H. pylori infection and multiple sclerosis is a controversial issue and there are few studies that address the problem. H. pylori infection is significantly less frequent in patients with conventional multiple sclerosis than in healthy controls or patients with opticospinal multiple sclerosis[36]. However, H. pylori infection seems to be one of the risk factors for the development of anti-aquaporin 4 (AQP4) antibody-positive multiple sclerosis, and the eradication of H. pylori may be a possible adjunct therapy[37]. Neuromyelitis optica is an inflammatory disease selectively affecting the optic nerves and spinal cord. Chronic persistent infection may contribute to the development of neuromyelitis optica through molecular mimicry between human AQP4 and bacterial AQP. In addition, H. pylori neutrophil-activating protein (HP-NAP) contributes to the pathology by inducing migration and activation of neutrophils[37].
The pathophysiologic mechanism for the majority of ischemic strokes is occlusion of carotid or cerebral vessels. Infection with H. pylori as a risk factor for stroke is still an unresolved issue because of conflicting results. However, a recent meta-analysis showed that chronic infection with H. pylori and the presence of CagA-positive strains are statistically significant risk factors for ischemic stroke, especially for noncardioembolic ischemic stroke[38,39]. Similarly, CagA-positive strains of H. pylori are significantly associated with atherosclerotic stroke in patients with an active infection[34].
The mechanisms of the high risk for ischemic stroke conferred by chronic H. pylori infection are still not understood. It has been hypothesized that H. pylori activates platelets and affects coagulation, and it has been shown that six months after eradication of H. pylori infection, the plasma levels of total cholesterol, low-density lipoprotein-cholesterol, fibrinogen, and IL-8 were significantly lower than those in H. pylori-positive stroke patients and controls[40].
Most neurological diseases are the result of a combination of multiple factors, but the systemic inflammatory response and the production of autoantibodies are common components and determinants in the onset, evolution, and outcome of these diseases. Future studies need to focus on determining the molecular mechanisms by which inflammatory mediators induced by H. pylori act on the brain, tipping the balance toward a pathological condition.
| 1. | Montecucco C, Rappuoli R. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat Rev Mol Cell Biol. 2001;2:457-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 374] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 2. | Labenz J, Börsch G. Evidence for the essential role of Helicobacter pylori in gastric ulcer disease. Gut. 1994;35:19-22. [PubMed] |
| 3. | Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177-240. [PubMed] |
| 4. | Hamajima N, Goto Y, Nishio K, Tanaka D, Kawai S, Sakakibara H, Kondo T. Helicobacter pylori eradication as a preventive tool against gastric cancer. Asian Pac J Cancer Prev. 2004;5:246-252. [PubMed] |
| 5. | Peek RM, Fiske C, Wilson KT. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol Rev. 2010;90:831-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Ando T, Goto Y, Maeda O, Watanabe O, Ishiguro K, Goto H. Causal role of Helicobacter pylori infection in gastric cancer. World J Gastroenterol. 2006;12:181-186. [PubMed] |
| 7. | Matsuo Y, Ochi N, Sawai H, Yasuda A, Takahashi H, Funahashi H, Takeyama H, Tong Z, Guha S. CXCL8/IL-8 and CXCL12/SDF-1alpha co-operatively promote invasiveness and angiogenesis in pancreatic cancer. Int J Cancer. 2009;124:853-861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 245] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Lin MT, Lin BR, Chang CC, Chu CY, Su HJ, Chen ST, Jeng YM, Kuo ML. IL-6 induces AGS gastric cancer cell invasion via activation of the c-Src/RhoA/ROCK signaling pathway. Int J Cancer. 2007;120:2600-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Hitzler I, Sayi A, Kohler E, Engler DB, Koch KN, Hardt WD, Müller A. Caspase-1 has both proinflammatory and regulatory properties in Helicobacter infections, which are differentially mediated by its substrates IL-1β and IL-18. J Immunol. 2012;188:3594-3602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Xue H, Lin B, An J, Zhu Y, Huang G. Interleukin-10-819 promoter polymorphism in association with gastric cancer risk. BMC Cancer. 2012;12:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Lindgren Å, Yun CH, Sjöling Å, Berggren C, Sun JB, Jonsson E, Holmgren J, Svennerholm AM, Lundin SB. Impaired IFN-γ production after stimulation with bacterial components by natural killer cells from gastric cancer patients. Exp Cell Res. 2011;317:849-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33-39. [PubMed] |
| 13. | Nielsen HH, Qiu J, Friis S, Wermuth L, Ritz B. Treatment for Helicobacter pylori infection and risk of Parkinson’s disease in Denmark. Eur J Neurol. 2012;19:864-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Pierantozzi M, Pietroiusti A, Sancesario G, Lunardi G, Fedele E, Giacomini P, Frasca S, Galante A, Marciani MG, Stanzione P. Reduced L-dopa absorption and increased clinical fluctuations in Helicobacter pylori-infected Parkinson’s disease patients. Neurol Sci. 2001;22:89-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Fasano A, Bove F, Gabrielli M, Petracca M, Zocco MA, Ragazzoni E, Barbaro F, Piano C, Fortuna S, Tortora A. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov Disord. 2013;28:1241-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 263] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 16. | Lee WY, Yoon WT, Shin HY, Jeon SH, Rhee PL. Helicobacter pylori infection and motor fluctuations in patients with Parkinson’s disease. Mov Disord. 2008;23:1696-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Lyte M. Microbial endocrinology as a basis for improved L-DOPA bioavailability in Parkinson’s patients treated for Helicobacter pylori. Med Hypotheses. 2010;74:895-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Rahne KE, Tagesson C, Nyholm D. Motor fluctuations and Helicobacter pylori in Parkinson’s disease. J Neurol. 2013;260:2974-2980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Candelario-Jalil E, Taheri S, Yang Y, Sood R, Grossetete M, Estrada EY, Fiebich BL, Rosenberg GA. Cyclooxygenase inhibition limits blood-brain barrier disruption following intracerebral injection of tumor necrosis factor-alpha in the rat. J Pharmacol Exp Ther. 2007;323:488-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3304] [Cited by in RCA: 3211] [Article Influence: 169.0] [Reference Citation Analysis (0)] |
| 21. | Lo YC, Shih YT, Wu DC, Lee YC. In vitro effects of Helicobacter pylori-induced infection in gastric epithelial AGS cells on microglia-mediated toxicity in neuroblastoma SH-SY5Y cells. Inflamm Res. 2009;58:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Dobbs RJ, Charlett A, Purkiss AG, Dobbs SM, Weller C, Peterson DW. Association of circulating TNF-alpha and IL-6 with ageing and parkinsonism. Acta Neurol Scand. 1999;100:34-41. [PubMed] |
| 23. | Bardou I, Kaercher RM, Brothers HM, Hopp SC, Royer S, Wenk GL. Age and duration of inflammatory environment differentially affect the neuroimmune response and catecholaminergic neurons in the midbrain and brainstem. Neurobiol Aging. 2014;35:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Villarán RF, Espinosa-Oliva AM, Sarmiento M, De Pablos RM, Argüelles S, Delgado-Cortés MJ, Sobrino V, Van Rooijen N, Venero JL, Herrera AJ. Ulcerative colitis exacerbates lipopolysaccharide-induced damage to the nigral dopaminergic system: potential risk factor in Parkinson`s disease. J Neurochem. 2010;114:1687-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 25. | Dobbs SM, Dobbs RJ, Weller C, Charlett A. Link between Helicobacter pylori infection and idiopathic parkinsonism. Med Hypotheses. 2000;55:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Selkoe DJ. Deciphering the genesis and fate of amyloid beta-protein yields novel therapies for Alzheimer disease. J Clin Invest. 2002;110:1375-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Roubaud-Baudron C, Krolak-Salmon P, Quadrio I, Mégraud F, Salles N. Impact of chronic Helicobacter pylori infection on Alzheimer’s disease: preliminary results. Neurobiol Aging. 2012;33:1009.e11-1009.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Kountouras J, Boziki M, Gavalas E, Zavos C, Deretzi G, Grigoriadis N, Tsolaki M, Chatzopoulos D, Katsinelos P, Tzilves D. Increased cerebrospinal fluid Helicobacter pylori antibody in Alzheimer’s disease. Int J Neurosci. 2009;119:765-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Kountouras J, Boziki M, Gavalas E, Zavos C, Grigoriadis N, Deretzi G, Tzilves D, Katsinelos P, Tsolaki M, Chatzopoulos D. Eradication of Helicobacter pylori may be beneficial in the management of Alzheimer’s disease. J Neurol. 2009;256:758-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Kountouras J, Boziki M, Gavalas E, Zavos C, Deretzi G, Chatzigeorgiou S, Katsinelos P, Grigoriadis N, Giartza-Taxidou E, Venizelos I. Five-year survival after Helicobacter pylori eradication in Alzheimer disease patients. Cogn Behav Neurol. 2010;23:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Shiota S, Murakami K, Yoshiiwa A, Yamamoto K, Ohno S, Kuroda A, Mizukami K, Hanada K, Okimoto T, Kodama M. The relationship between Helicobacter pylori infection and Alzheimer’s disease in Japan. J Neurol. 2011;258:1460-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Kountouras J, Deretzi G, Zavos C, Karatzoglou P, Touloumis L, Nicolaides T, Chatzopoulos D, Venizelos I. Association between Helicobacter pylori infection and acute inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 2005;12:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Moran AP, Prendergast MM. Molecular mimicry in Campylobacter jejuni and Helicobacter pylori lipopolysaccharides: contribution of gastrointestinal infections to autoimmunity. J Autoimmun. 2001;16:241-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | De Bastiani R, Gabrielli M, Ubaldi E, Benedetto E, Sanna G, Cottone C, Candelli M, Zocco MA, Saulnier N, Santoliquido A. High prevalence of Cag-A positive H. pylori strains in ischemic stroke: a primary care multicenter study. Helicobacter. 2008;13:274-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Chiba S, Sugiyama T, Yonekura K, Tanaka S, Matsumoto H, Fujii N, Ebisu S, Sekiguchi K. An antibody to VacA of Helicobacter pylori in cerebrospinal fluid from patients with Guillain-Barre syndrome. J Neurol Neurosurg Psychiatry. 2002;73:76-78. [PubMed] |
| 36. | Li W, Minohara M, Su JJ, Matsuoka T, Osoegawa M, Ishizu T, Kira J. Helicobacter pylori infection is a potential protective factor against conventional multiple sclerosis in the Japanese population. J Neuroimmunol. 2007;184:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Li W, Minohara M, Piao H, Matsushita T, Masaki K, Matsuoka T, Isobe N, Su JJ, Ohyagi Y, Kira J. Association of anti-Helicobacter pylori neutrophil-activating protein antibody response with anti-aquaporin-4 autoimmunity in Japanese patients with multiple sclerosis and neuromyelitis optica. Mult Scler. 2009;15:1411-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Wang ZW, Li Y, Huang LY, Guan QK, Xu da W, Zhou WK, Zhang XZ. Helicobacter pylori infection contributes to high risk of ischemic stroke: evidence from a meta-analysis. J Neurol. 2012;259:2527-2537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Zhang S, Guo Y, Ma Y, Teng Y. Relationship between the cytotoxin-associated gene-A status of H pylori strains and cerebral infarction in European Caucasians and Chinese Han: a meta-analysis. World J Gastroenterol. 2008;14:1286-1292. [PubMed] |
| 40. | Majka J, Róg T, Konturek PC, Konturek SJ, Bielański W, Kowalsky M, Szczudlik A. Influence of chronic Helicobacter pylori infection on ischemic cerebral stroke risk factors. Med Sci Monit. 2002;8:CR675-CR684. [PubMed] |
P- Reviewer: Kamer E, Zhao JB S- Editor: Wen LL L- Editor: A E- Editor: Wang CH