INTRODUCTION
Gastrointestinal (GI) diseases include gastritis[1], gastroesophageal reflux[2,3], dyspepsia[4-6], irritable bowel syndrome[4,7-9] and gastritis associated with cancer[10], inflammation[11], emotional state[12], obstruction[13], Helicobacter pylori infection[14,15] and gastroenteritis[1,16]. If people are susceptible to these diseases and dysfunctions[17], there is a high probability that they will undergo clinical diagnosis for GI disease at least once during their lifetime.
The search for ways to diagnose GI diseases and define normal gastric activity has generated a variety of techniques, some of which are invasive or expose the patient to a high dose of ionizing radiation. Nevertheless, it is important to point out that they have been used to monitor diseases such as diabetic gastroparesis. One of these traditional techniques, scintigraphy, is considered the gold standard for GI tract disease diagnosis[6,18]. However, alternative noninvasive techniques such as the 13C octanoic acid[19,20], superficial electrogastrography[21], ultrasound[22] and biomagnetism are available[12,23-28].
The gold standard technique for evaluation and diagnosis of diseases of the GI system is scintigraphy[28,29]. This is an excellent technique and generates information quickly and almost directly. However, it involves ionizing radiation. Although exposure to ionizing radiation does not have adverse effects if administered in controlled doses, it does limit the frequency with which chronic patients such as those with diabetes concomitant with gastroparesis can be monitored[30].
On the other hand, studies on the anatomy and physiology of the GI tract indicate that more information is required to understand fully this system. In particular, it is known that food is propelled down the esophagus by peristalsis. Furthermore, the GI system consists of accessory organs and the digestive tract that the lumen content of the GI tract passes through. The lumen of the GI tract passes through the mouth, pharynx, esophagus, stomach, small and large intestine, sigmoid colon, rectum and anus. The content of the lumen is transported through the digestive tract by peristaltic activity[31-33]. The GI system is an autonomous system in which the myenteric nervous system controls peristaltic activity. Problems arise when transit time is impeded in any part of the tract by conditions such as diabetes or surgical intervention.
Biomagnetic assessment of the GI system can be conducted using either of two methods. In one, the magnetic field generated by the electrical activity of the myenteric nervous system is measured. This can be performed on any segment of the digestive tract in which peristaltic activity is present. In the second method, a magnetic tracer or marker is introduced into the GI tract and its magnetic field is monitored using an external sensor. In the first method, the signal generated is in the femtotesla range, detection of which requires a magnetometer superconducting quantum interference device and a magnetically shielded room[34]. In the second method, the markers generate magnetic signal variation in the nano- to microtesla range, which can be detected using a magnetometer at room temperature. A digital fluxgate magnetometer or a magnetoresistor can be used to detect the signal and a shielded room is not necessary[34]. Recordings obtained using these techniques are similar or equivalent to GI system logs obtained using scintigraphy, the gold standard.
As far as we are aware, the first GI system assessments were made in the late 1950s and early 1960s[35,36]. However, biomagnetic studies on different parts of the body only began to proliferate after 1970. It is important to note that these early studies involved one of three different biomagnetic sources: (1) a magnetic field generated by the movement of ions; (2) a magnetic field created by the accumulation of ferromagnetic material in some organs of the body; and (3) tracers and magnetic markers[34-42]. Progress continued using these techniques until early 2000.
Herein, we present a detailed description of how to measure the transit time of digesta through the esophagus, stomach and colon, and the frequency of peristalsis using a biomagnetic technique in which signals from magnetic markers or tracers are recorded using a fluxgate magnetometer or a magnetoresistor in an unshielded laboratory.
APPROACH TO MEASURING TRANSIT TIME OF DIGESTA
For all the aforementioned measurements, an approximation must be made to measure the magnetic dipole, which is assumed to be produced by a small magnetic source distant from the observer, who is located near to the magnetic sensor. The induced magnetic field (B) is calculated as follows: B=μ04π[3r(r⋅m)r5−mr3] Where μ0 is the magnetic permeability, r is the distance from source to magnetic sensor, and m is the magnetic moment of the magnetic marker.
It is important to point out that with this biomagnetic modality, it is possible to perform the measurements out of a magnetic shielding room. This particularity is an additional advantage for this technique to be implemented in hospitals with a low cost.
Esophagus
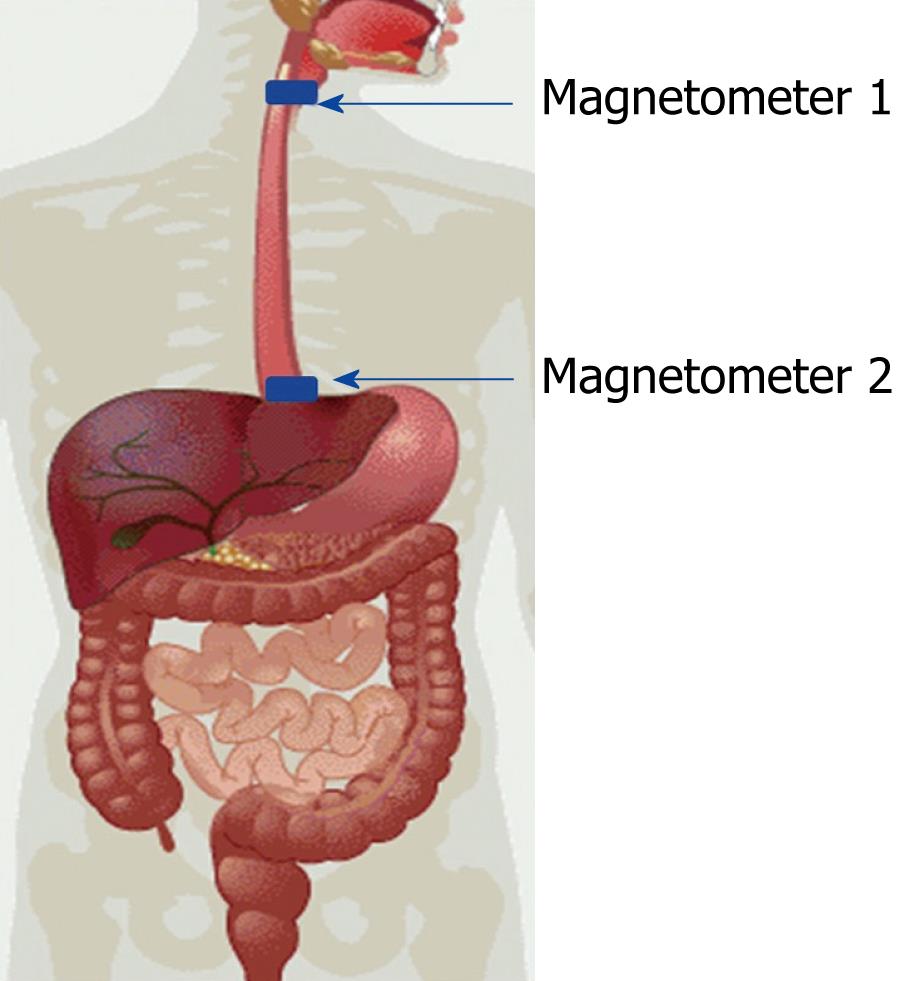
The human esophagus has an average length of 25 cm in adults. The top of the esophagus is connected to the pharynx and the bottom of the esophagus is connected to the stomach at the cardia. Peristalsis begins in the middle of the esophagus, the position at which food arrives when it is propelled from the mouth. To our knowledge, Daghastanli et al[24] (1998) were the first to evaluate esophageal transit time with biomagnetic techniques; they used food containing tracers and magnetic coils that were placed over each end of the esophagus. The standard test is nuclear medicine, so they also correlated measurements using radiotracers and reported that there was a strong correlation between measurements made using radiotracers and magnetic tracers. Later, Córdova-Fraga et al[25] used a cylindrical magnetic marker 3 mm in diameter and 4 mm in length and a pair of fluxgate magnetometers 25 cm apart (just above the ends of the esophagus) (Figure 1). A particularity of these studies was that transit time was measured with the subject in four different positions (0°, 45°, 90° and 85°)[27]. The transit times so measured were consistent with those reported by Daghastanli et al[24], in particular those measured when the subject was at 90° (in the vertical position). Previous work showed that estimates of transit time through the esophagus are accurate when biomagnetism is used with contrast or magnetic markers and sensors at room temperature.
Figure 1 Position of magnetometers used for measuring esophageal transit time.
The advantage of using magnetic markers to measure esophageal transit time is that the signal does not have to be filtered because the high intensity of the magnetic field produced by the magnet results in an excellent signal-to-noise ratio. A typical magnetometer configuration is shown in Figure 1. In this configuration, two signals are evident, each associated with magnetometers located at the extremities of the esophagus and separated by a predetermined distance. As the position from which a signal originates can be calculated, the transit time of luminal contents through the esophagus can be calculated. We demonstrated that esophageal transit times assessed in the upright, Fowler’s and the supine positions (90°, 45° and 0°, respectively) differed significantly (5.2 ± 1.1 s, 6.1 ± 1.5 s and 23.6 ± 9.2 s, respectively; ANOVA with Tukey’s post hoc test).
Stomach
The human stomach is a hollow organ in the form of a J and is located between the esophagus and the small intestine. The former attaches to the stomach at the esophageal sphincter in the cardia and the latter attaches to the stomach at the pylorus. The average volume of the stomach is 150 mL at baseline, reference study in the same experiment, and can expand to a volume of 1.5 L without causing discomfort. From a physiological standpoint, the human stomach is divided into two segments, the proximal (upper) and distal (lower) portions. Anatomically, it has three sections, the bottom, the body and the antrum. When food is ingested and enters the stomach, it is stored at the fundus and is mixed by contractions initiated by action potentials generated in the pacemaker area in the middle of the body. The rings of contraction travel down the stomach to the pyloric sphincter and force some of the luminal contents at the fundus up to the antrum, where it encounters a shock wave that contributes to the reduction in particle size necessary for it to pass into the duodenum. The rate of passage of food from the stomach to the duodenum is a function of two processes: peristalsis, which is directly related to the frequency of contraction generated by the pacemaker area, and gastric emptying, the average time taken for the stomach to empty half of the contents of the lumen. Both processes are altered by diabetes.
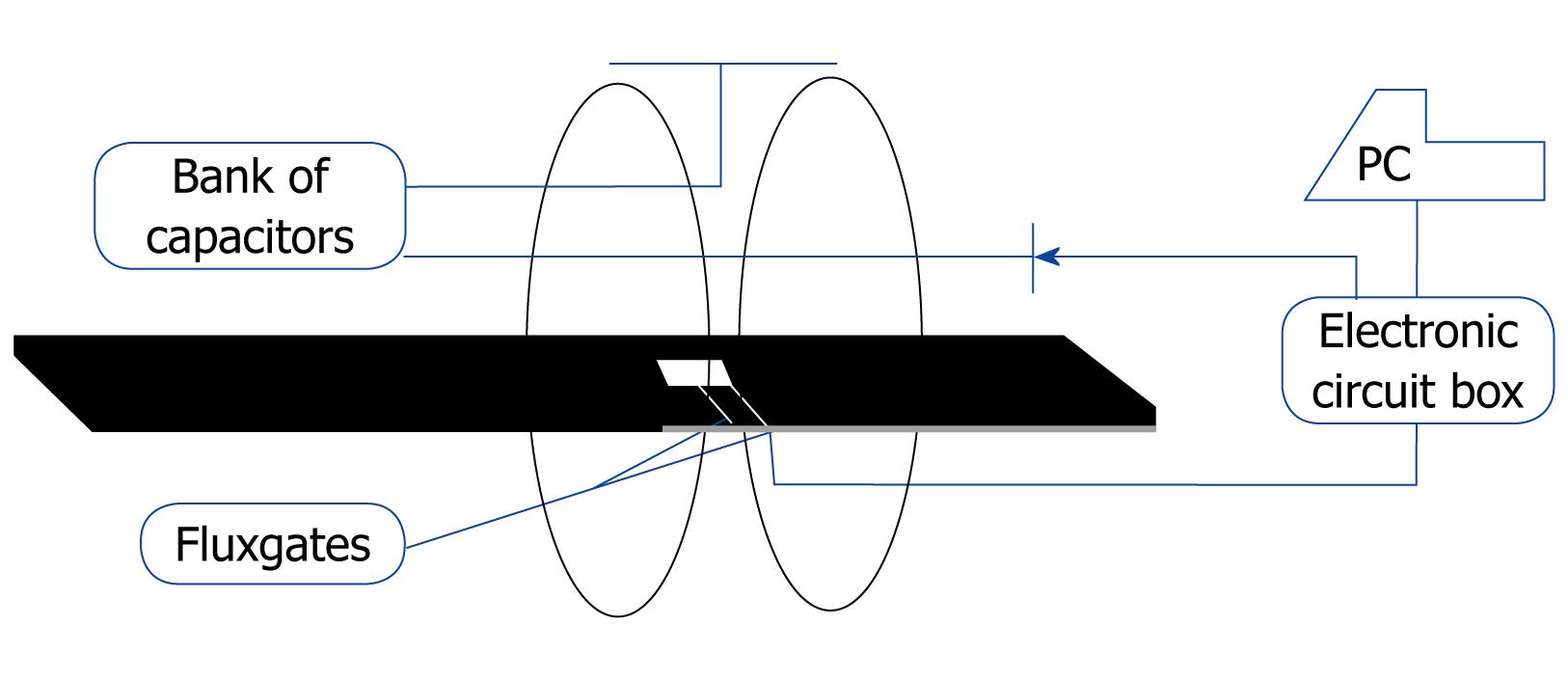
Both processes can be measured using magnetic tracers and a vehicle to deposit them in the gastric segment. This can be achieved by diluting 2 to 5 g of magnetite (Fe3O4) powder in 200 mL of yogurt[42,43] or by incorporating magnetite into a pancake by mixing it with flour[28]. When these preparations are consumed, it is assumed that the magnetite becomes distributed throughout the space occupied by the food in the stomach and that it has a random magnetic alignment. Consequently, there is no net magnetic signal at this stage and the particles must be magnetized to obtain a signal. For this purpose, we recommend using a device consisting of two identical coils assembled as an array of Helmholtz coils (Figure 2). This configuration requires the coils to be on parallel planes that share the same axis and to be separated by a distance equal to their radius. These coils consist of 60 turns of 4-gauge (6 mm diameter) copper wire (6 mm diameter).
Figure 2 Magnetic stimulator prototype.
The coils are supported by two aluminum pulleys. In order to break out the induced electric current, each contains two cuts to eliminate the induction of a field opposite to that produced by the field generated by the coils. Each of the coils contains five 12-turn layers and their diameters are the same. When the two coils are connected in parallel, they have an equivalent resistance of 260 mΩ and an equivalent inductance of 0.93 mH. To generate the magnetic pulse, a bank of capacitors with an equivalent capacitance of 46 mF is connected in parallel.
This bank of capacitors is connected to a 220 V electrical source and the voltage is rectified before it reaches the bank of capacitors, which are then loaded to maximum capacity before closing the circuit (Figure 2) and produce a discharge within 17 μs. This discharge is sufficient to generate peak current in the coils and a magnetic intensity of the order of 32 mT. This generates a magnetic intensity in the tracers of 100 to 300 nT, which can be detected by a fluxgate magnetometer or a magnetoresistor (Figure 2). Several studies have described validation procedures for such systems[26,28,34,40,41].
On the other hand, it is important to point out that the procedure for measuring gastric emptying is standardized by scintigraphy tests, as is that for the measurement of peristalsis, in that they both involve digital signal analysis of spectral density (in our method, the dominant frequency coordinates are determined). On the other hand, magnetic tracers have been diluted in semisolid[26] and solid meals[28] for consumption by healthy subjects and patients. The results of all studies performed by our group were compared with the results of techniques such as scintigraphy and we have carried out reproducibility studies[28].
Magnetic markers are harmless to the human digestive tube as they are inert in gastric pH.
REPRODUCIBILITY AND SENSITIVITY
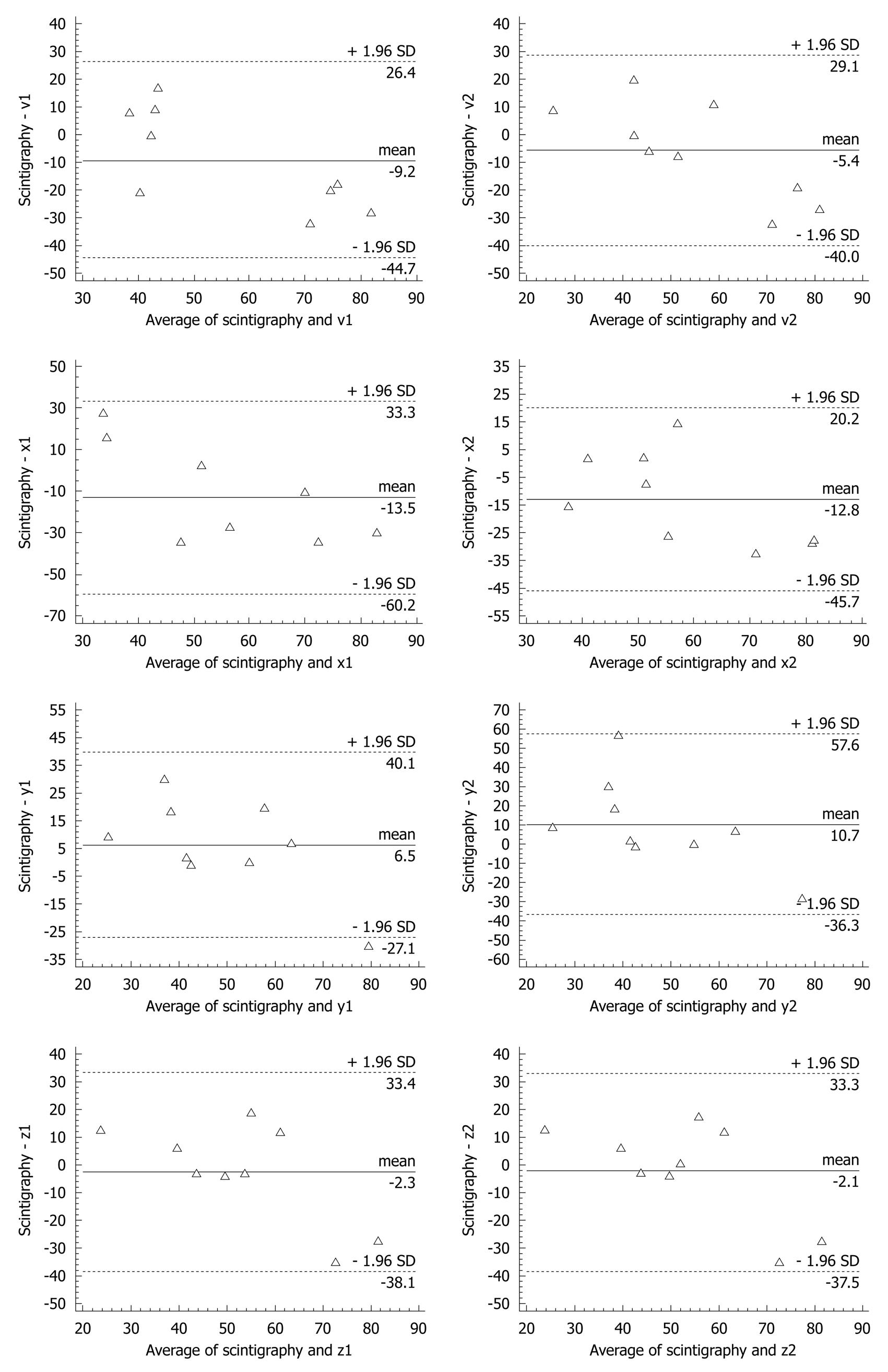
Several studies have been conducted to evaluate magnetogastrography for use in clinical settings. Reproducibility was greater than 85%[26] and a comparison between magnetogastrography and scintigraphy using the Bland-Altman test showed that the best estimate of the sensor axis was BZ and the results of the two methods were similar. Bland-Altman analysis also showed that differences between scintigraphy and magnetogastrography estimates of emptying time were similar for each of the magnetization components (Figure 3). The mean scintigraphy estimate for gastric transit time was (23 ± 12.9) min and the mean ± SD magnetogastrography estimate was (25.8 ± 23) min[28].
Figure 3 Bland Altman analysis for different vectors.
Although this study was performed on young subjects, information on its sensitivity and specificity is lacking. Such data could be obtained by comparing estimates derived using the two techniques for healthy subjects and patients with a specific disease. Therefore, further research is required before this technology can be released for commercial use.
GASTRIC EMPTYING AND PHYSICAL AND EMOTIONAL HEALTH
Previous studies have demonstrated that stress, anxiety and gastric emptying influence the symptoms of functional dyspepsia (FD). Patients with FD have a significantly elevated gastric emptying half time (42.27 ± 7.4) min
compared with healthy volunteers (33.8 ± 8) min, but gastric peristaltic frequency does not differ between these groups (t = −1.60, P = 0.12)[12].
“The biomagnetic technique, in combination with other test, can be used for assessing physiological factors related to emotional state using people, particularly, persons who would be unwilling to undergo invasive or isotopic procedures, for this end. In addition, magnetogastrography shows that patients with FD have a delayed gastric emptying time, which may be associated with this kind of symptoms.” Magnetic tracers are particles of size from 50 to 125 micrometer, such that cannot be absorbed by the microvilli on the villi in the small intestine so they are eliminated by the human body naturally.
Large intestine
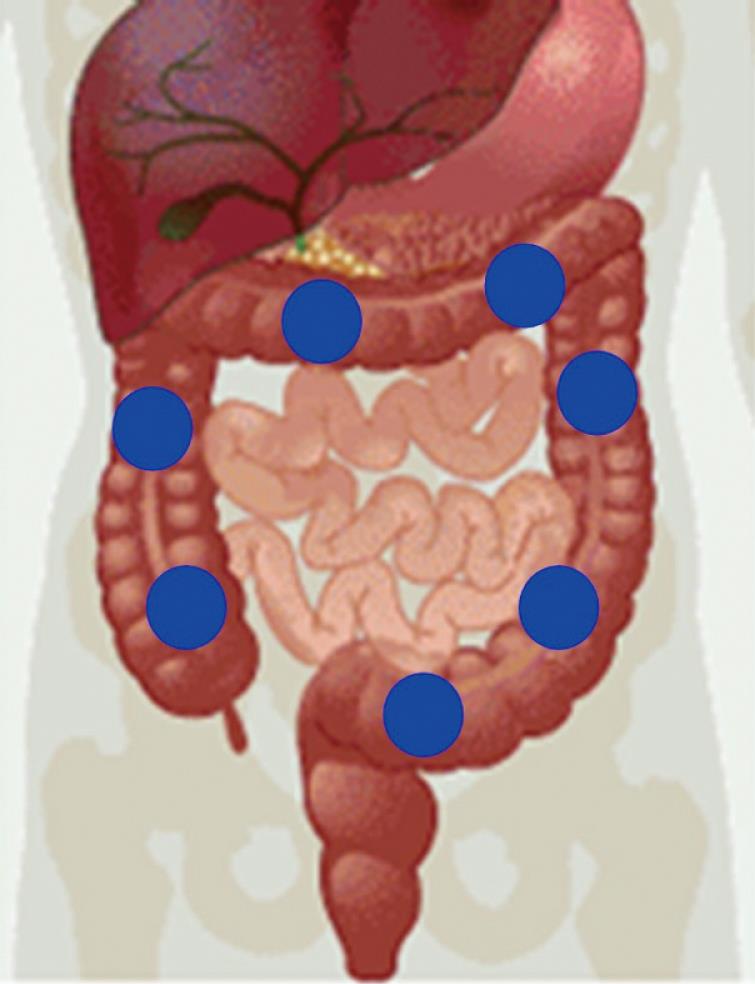
The length of the adult human large intestine is 1.5 m; it consists of the ascending colon, transverse colon, descending colon and sigmoid segment and ends at the rectum. Unlike other segments of the GI tract, the large intestine has three types of motion: mass movement, peristaltic reflex and peristaltic frequency. According to previous work[25,41], digesta take about 13 h to reach the ascending colon. This means that any study on this segment of the GI tract should involve measurements for a minimum of 14 h. We believe that any of the three movements of the large intestine can be measured using a magnetic marker instead of food containing a magnetic tracer. If a healthy person ingests a magnetic marker, it will reach the large intestine, in particular the ascending colon, 13 h later. Using a pair of fluxgate magnetometers in a first-order gradiometer array, it is possible to determine the position of the magnetic marker with a high degree of accuracy (Figure 4). Assuming that it is in the same plane as the umbilical scar, this is assumed as the zero for a virtual Euclide’s plane. Data acquisition is completed within a few minutes, during which the subject remains motionless. After applying a Fourier transform, the data are analyzed using Matlab software.
Figure 4 Magnetic marker positions in the large intestine over time.
If this process is repeated every 2 h, one can estimate transit time through the large intestine and the peristaltic frequency at the position at which the magnetic marker is located at the time of measurement. Such an estimate of point to point is determined in each recording. The above is used in order to estimate the dynamics of this part of the GI tract. For example, we could monitor colon transit time at various phases of the menstrual cycle and determine the effects of stress on colonic transit time.
CONCLUSION
It has been shown that the biomagnetic technique is versatile when used with oral contrast material. It is possible to perform studies using a magnetic sensor that functions at room temperature and a shielded magnetic room is not necessary. It is easy to assess peristalsis in the esophagus, stomach and large intestine with this method. Moreover, it represents an alternative to procedures based on ionizing radiation for determining gastric emptying.
Nevertheless, more studies should be performed on hospital patients to define the best diagnostic protocols for various GI diseases. The age and sex of the patients should be considered in such protocols. The oral contrast medium should also be taken into account when magnetic tracers or markers are used.
Because the biomagnetic technique enables measurement of average gastric emptying time for medical diagnostic purposes and associated emotional states, it could be used to develop a comprehensive health model.
With small modifications, this new biomagnetic technique could be used in hospitals throughout the world for diverse GI applications. Furthermore, it does not involve ionizing radiation, is not invasive and does not cause discomfort, which makes it appropriate for monitoring the effects of therapy in chronic diseases such as gastroparesis, colon constipation and FD[12].
It is still a lab prototype but nevertheless, its individual price of productions is around US$3000.00 and each human evaluation or human study is around US$10.00.
Peer reviewers: Daniel Keszthelyi, Dr., Institute of Maastricht, PO Box 5800, Maastricht 6202 AZ, The Netherlands; Stelios F Assimakopoulos, Dr., Department of Internal Medicine, University Hospital of Patras, Patras 26504, Greece; Angelo Izzo, Professor, Department of Experimental Pharmacology, University of Naples Federico II, via D Montesano 49, 80131 Naples, Italy
S- Editor Wu X L- Editor Roemmele A E- Editor Zheng XM