Published online Jun 22, 2025. doi: 10.4291/wjgp.v16.i2.107994
Revised: April 17, 2025
Accepted: May 18, 2025
Published online: June 22, 2025
Processing time: 78 Days and 13.1 Hours
The rising global prevalence of gastroesophageal reflux disease (GERD) has been closely linked to lifestyle changes driven by globalization. GERD imposes a sub
To develop and validate a GERD Risk Scoring System (GRSS) aimed at identifying high-risk individuals and promoting primary prevention strategies.
A 45-item questionnaire encompassing major lifestyle and demographic risk factors was developed and validated. It was administered to healthy controls and GERD patients. Two regression models—one using continuous variables and another using categorized variables—were used to develop a computational prediction equation and a clinically applicable scoring scale. An independent validation cohort of 355 participants was used to assess model performance in terms of discrimination (C-index), calibration, sensitivity, specificity, internal consistency (Cronbach's alpha), and test-retest reliability (intraclass correlation coefficient, Bland–Altman analysis).
Significant associations were observed between GERD and key lifestyle factors. The derived GRSS equation and scoring scale demonstrated strong discriminative ability, with high sensitivity and specificity. The scoring system exhibited excellent internal consistency (Cronbach’s alpha) and strong test-retest reliability. The C-index indicated excellent predictive accuracy in both derivation and validation cohorts.
GRSS offers a novel and validated approach to GERD risk prediction, combining a robust equation for digital applications and a practical scale for clinical use. Its ability to accurately identify at-risk individuals supports a paradigm shift toward primary prevention, underscoring its significance in addressing the growing burden of GERD at the population level.
Core Tip: There has been a steady rise in the prevalence of gastroesophageal reflux disease (GERD) due to globalization. This study introduces the GERD Risk Scoring System (GRSS), a novel primary prevention tool for assessing GERD susceptibility based on key lifestyle and demographic factors. Using logistic lasso regression, the model demonstrated high predictive accuracy, with strong internal consistency and reliability. GRSS provides both a computational risk equation and a practical scoring scale for clinical use. By enabling early identification of at-risk individuals, GRSS facilitates targeted lifestyle modifications, supporting primary prevention and reducing the long-term burden of GERD-related complications.
- Citation: Subramanian S, Sundararaju U, Rajakumar HK, Coimbatore Sathyabal V, Murugan A, Gnanavel P, Sathishkumar K. Development and validation of a risk prediction model for gastroesophageal reflux disease: Gastroesophageal Reflux Disease Risk Scoring System. World J Gastrointest Pathophysiol 2025; 16(2): 107994
- URL: https://www.wjgnet.com/2150-5330/full/v16/i2/107994.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v16.i2.107994
As defined by the World Gastroenterology Organization, gastroesophageal reflux disease (GERD) is characterized by troublesome symptoms that significantly impact an individual's quality of life or injuries and complications resulting from the retrograde flow of gastric contents into the esophagus, oropharynx, and/or respiratory tract[1].
The Global Burden of Disease Study reported 783.95 million cases of GERD worldwide in 2019[2]. A survey conducted by the Indian Society of Gastroenterology reported that the prevalence of GERD in India ranged from 7.6% to 30%[3]. This high prevalence is a major cause for concern, as GERD leads to complications such as dysphagia, perforation, bleeding, metaplasia, and adenocarcinoma, resulting in significant morbidity and mortality[4].
The American College of Gastroenterology (ACG) recommends a multimodal approach to diagnosing GERD. This starts with a detailed medical history and clinical assessment. Empirical treatment with acid-suppressing medications can serve as a diagnostic test, with symptom improvement indicating GERD. Upper gastrointestinal endoscopy is advised for severe symptoms or to confirm esophageal issues like inflammation, erosions, ulcers, or Barrett's esophagus. Additional diagnostic tools include pondus hydrogenii (pH) monitoring, esophageal manometry, barium swallow X-ray, impedance-pH monitoring, and biopsies during endoscopy[5].
While several symptom-based scoring systems for diagnosing GERD have been developed and validated worldwide, they predominantly rely on self-reported symptoms and do not account for the lifestyle factors that significantly contri
Established lifestyle risk factors associated with the development of GERD include[7-10] poor sleep quality and quantity, elevated body mass index (BMI), older individuals, tobacco use, alcohol consumption, dietary habits, and inadequate fiber content in the diet[11-13].
Our study aims to develop and validate a questionnaire based on risk factors to create a scoring scale to predict the risk of developing GERD. This tool can be used in the general population to identify high-risk populations so that early intervention in the form of lifestyle modifications can be advised to prevent the development of the disease and its complications.
We designed a prospective case-control study following the principles outlined in the Helsinki Declaration. All participants in the study signed written informed consent forms. The participants in this study were recruited from the Outpatient Department of our institution.
The sample size, totaling 321 individuals (107 cases and 214 controls), was calculated using the formula described by Kelsey et al[14]. It is rounded off to the nearest ten (110 cases + 220 controls) for ease of calculation. The detailed sample size calculation can be found in the Supplementary material.
After explaining the study protocol to the participants, we obtained a thorough clinical history and conducted a comprehensive clinical examination. We followed the diagnostic guidelines recommended by the ACG to identify cases of GERD. Patients with typical GERD symptoms underwent endoscopy, and cases were confirmed based on evidence of erosive esophagitis, Los Angeles (LA) grade A or above. The control group subjects were asymptomatic, healthy volun
The participants in both study groups were interviewed, a questionnaire was administered, and data were collected. To assess the test-retest reliability of the GERD Risk Scoring System (GRSS) questionnaire, a subset of 40 study participants was selected to undergo a second interview with the questionnaire at a different time frame. During the time of the interview for data collection, based on the responses received, as a matter of goodwill, we advised the participants about their poor lifestyle and suggested modifications. After 6 months, we randomly selected 25 participants from our study population and telephonically interviewed them with the GRSS. We wanted to see, as a pilot, if there was a significant change in the GRSS scores following the lifestyle modification advice.
The GRSS questionnaire was developed as a novel tool to assess GERD risk based on lifestyle and demographic factors. Our approach involved adapting items from previously validated questionnaires, modifying them to target GERD-specific behaviors, and integrating newly created questions informed by known GERD risk factors identified through literature review. The questionnaire is divided into 6 modules, with 45 questions in total. The first module collects the demographic details of the patient. The other 5 modules concern smoking, alcoholism, sleep quality, diet (quantity of fiber), and stress.
Demographics: Patient identifying particulars are collected along with age, gender, weight, and height. The socioeco
Smoking: The questionnaire investigates whether the participant has a habit of smoking. The type of tobacco product smoked, quantity, and duration of smoking are questioned. Using these data, the participant's lifetime exposure to tobacco products is quantified in the form of pack years.
Alcoholism: The questionnaire investigates whether the participant has a habit of alcohol consumption. The type of alcohol consumed and quantity consumed per day are questioned to quantify the alcohol consumption per sitting in units. Using proven, multicentrically validated questionnaires for assessing alcohol abuse, such as the Alcohol Use Disorders Identification Test and Self-Rating of the Effects of Alcohol questionnaire as templates, we developed questions for the GRSS[16,17]. From these questions, a score ranging from 0 to 22 is assigned to each patient based on the responses obtained.
Sleep: The questionnaire assesses the quality of sleep of the participant. The questions were developed keeping self-rated, validated questionnaires such as the Sleep Quality Scale, Pittsburgh Sleep Quality Index, and Epworth Sleepiness Scale as templates[18-20]. From these questions, a score ranging from 0 to 12.5 is assigned to each patient based on the responses obtained. Higher scores imply a lower quality of sleep.
Diet: The questionnaire investigates whether the participant has adequate dietary fiber consumption in his diet. The questions were developed keeping an 18-item Fiber Screen and food frequency questionnaire as templates[21,22]. The questions were modified for South Indian food culture. From these questions, a score ranging from 1 to 27 is assigned to each patient based on the responses obtained. Additionally, questions were asked about the type of diet consumed, spice preference, and the time elapsed between the consumption of the last meal and bedtime.
Stress: The questionnaire assesses the psychological stress of the participants. The questions were developed keeping validated self-rated questionnaires such as the Perceived Stress Scale–4 and Holmes and Rahe Stress Scale as templates[23,24]. A score ranging from 0 to 10 is assigned to each patient based on the responses obtained. Higher scores imply that the participant faced greater psychological stress. Additionally, questions on the impact of stress on diet and destressing measures (if any) are included.
The final questionnaire is structured as a closed-ended questionnaire. Smoking and alcoholism questions serve as contingency questions, while the rest are either matrix questions, Likert scale questions, or multiple-choice questions. The GRSS features new questions specifically related to GERD development, which were not found in existing questionnaires. The questionnaire can be found in Supplementary material. The questionnaire underwent validation, and a pilot study was conducted to optimize its design. A scoring system was developed for each module, and scores for each module were obtained according to a designed scoring legend. Content validity was established through expert consultation within their respective fields. We also employed confirmatory factor analysis to assess the construct validity and validate the questionnaire used. It is detailed in Supplementary material.
The data from the questionnaire were entered into Microsoft Excel 2020 (Microsoft Corporation, Redmond, United States). Statistical analysis was carried out using Statistical Package for the Social Sciences 26.0 for Windows (IBM, Armonk, NY, United States) and R version 4.3.2 software. Age and BMI were treated as continuous variables, while sleep score, stress score, and diet score were treated as ordinal variables. Smoking was categorized as non-smoker, smoker (< 5 pack years), or smoker (> 5 pack years), and alcoholism was classified into nonalcoholic, light drinker, or heavy drinker categories. Participants' livelihood was categorized as either urban or rural, and socioeconomic status was considered as categorical variables in our analysis. Participants were categorized as cases (GERD+ve) or controls (GERD-ve). Continuous variables were expressed as the mean ± SD or median with a 25-75 percent interquartile range. Categorical variables were expressed as percentages with a 95%CI. We considered P values < 0.05 as significant. To check the normal distribution of the data (normality), we used the Kolmogorov–Smirnov test and Anderson–Darling test. Descriptive analysis was performed to provide an overview of the collected data. Any missing data were excluded from the analysis to ensure the accuracy and reliability of the results.
We developed the GRSS using two distinct approaches: (1) Logistic regression with continuous variables (GRSS scores) for integration into websites and mobile applications, providing a user-friendly risk assessment tool; and (2) Logistic regression with categorical variables (GRSS scores categorized into intervals) was employed to create a tool tailored to be physician-friendly, ensuring ease of calculation and practicality in clinical settings.
Initially, Spearman rank correlation was used to explore associations between component scores and GERD status. Significant variables were then subjected to least absolute shrinkage and selection operator (LASSO) logistic regression analysis, including predictors such as age, BMI, sleep score, diet score, stress score, smoking status, and alcoholism. The ‘glmnet’ package in R was employed, applying L1 regularization (alpha = 1), and stability assessment involved boots
Simultaneously, all factors were converted into categorical variables by dividing them into equally spaced intervals to design the scale. We created a contingency table for each factor and GERD status, conducting bivariate analysis using the two-tailed Fisher's exact test to qualitatively assess the association. The strength of the association between each factor and GERD was determined by Cramer’s V (effect size).
We included significant variables from the bivariate analysis to create a LASSO logistic regression equation where the dependent variable (GERD) depends on the categorized component scores. Categorical coefficients, representing the change in log odds of 'GERD' with the presence of each category for a given predictor, were used to calculate a linear predictor. Subsequently, the probability of 'GERD' was derived from the linear predictor using the logistic function. The logistic regression coefficient β for each variable was utilized to create the scoring scale based on the regression coefficient-based scoring algorithm[25]. This algorithm was found to be superior to the risk ratio-based scoring algorithm. The coefficients were rounded off, and the scoring model was optimized for ease of assessment.
We recruited an additional 355 participants to prospectively evaluate the scoring scale, capturing GRSS scores as continuous variables. This expanded dataset also includes the original set of 330 participants. To illustrate the distribution of patients across different score intervals classified as non-GERD and GERD, we created two bar plots. Additionally, we generated a smooth curve to depict the predicted probability of GERD across various score values. The shaded area around the curve reflects the 95%CI, with dashed lines indicating the upper and lower bounds of this interval.
We used the 'rms' package in R to construct the logistic regression model ('f.score') and performed validation. C-index was employed as a metric to evaluate the model's discrimination ability. The validation process incorporates boots
The 'pROC' package in R was used to construct the receiver operating characteristic (ROC) curve for evaluating the model's ability to discriminate between individuals with and without GERD. Additionally, we calculated the area under the curve (AUC) to quantify the overall predictive accuracy. Sensitivity and specificity were computed to evaluate the model's classification performance.
To evaluate the test-retest reliability of the GRSS, we calculated the intraclass correlation coefficient (ICC) and used Bland–Altman plots to assess the consistency and agreement between the GRSS scores obtained from participants undergoing a second interview at a different time frame. The reliability of the questionnaire was assessed by Cronbach’s alpha value. We used the Wilcoxon signed-rank test to determine if there was a significant change in the GRSS scores post 6 months. The flowchart for the statistical analysis conducted is depicted in Supplementary Figure 1.
We enrolled 330 patients: (1) 110 patients who had GERD; and (2) 220 healthy controls. All the patients enrolled in the study were South Asians. Out of the 110 cases included in the study, 46 participants were classified as LA grade A, 32 participants as LA grade B, 19 participants as LA grade C, and 13 participants as LA grade D.
The dataset exhibited a non-normal distribution, as indicated by the Kolmogorov–Smirnov and Anderson–Darling tests. Spearman rank correlation was used to find the association between continuous variables and GERD. The continuous variables were then converted into categorical variables by creating equally spaced intervals (code definitions are tabulated in Supplementary Table 1). A contingency table was created for each factor (rows) and GERD status (columns). The association between the factors and disease was qualitatively determined by the two-tailed Fisher's exact test. The strength of the association was calculated by effect size (Cramer’s V). The results of the associations are tabulated in Table 1.
| Factor | Continuous variables | Categorical variables | |||
| Spearman ‘rho’ | P value | Fischer’s test | Cramer’s v value | Strength of association | |
| Age | 0.573 | < 0.0011 | < 0.0011 | 0.646 | Strong |
| Body mass index | 0.749 | < 0.0011 | < 0.0011 | 0.745 | Strong |
| Smoking | - | < 0.0011 | 0.476 | Relatively strong | |
| Alcoholism | - | < 0.0011 | 0.434 | Relatively strong | |
| Sleep | 0.710 | < 0.0011 | < 0.0011 | 0.642 | Strong |
| Diet | 0.492 | < 0.0011 | < 0.0011 | 0.639 | Strong |
| Stress | 0.289 | < 0.0011 | < 0.0011 | 0.727 | Strong |
| Community2 | - | 0.129 | 0.0900 | Very weak | |
| Socio-economic status2 | - | 0.932 | 0.0465 | Very weak | |
To assess potential multicollinearity among predictors, variance inflation factor (VIF) values were calculated using a linear model. The VIF values were: (1) Age = 1.45; (2) BMI = 1.90; (3) Sleep = 1.79; (4) Diet = 1.36; (5) Stress = 1.09; (6) Smoking status = 1.04; and (7) Alcohol level = 1.06. All VIF values were well below the commonly accepted threshold of 5, indicating no multicollinearity concerns.
A logistic LASSO regression was applied to continuous variables to formulate an equation, with the optimal regularization parameter (lambda) determined as 0.95 through bootstrapping 10000 datasets, enhancing generalization performance. The coefficients are tabulated in Table 2.
| Factor | Coefficient β |
| Age | 0.01123340 |
| Body mass index | 0.05075936 |
| Smoking | -0.01586711 |
| Alcoholism | -0.03592046 |
| Sleep | 0.09640941 |
| Diet | 0.00588875 |
| Stress | 0.03304126 |
The equation obtained from this model is complex, as shown below, but it can be easily programmed for practical use in computational applications such as websites and mobile applications. GRSS scores = (-1.93) + (0.0112 × age) + (0.051 × BMI) + (0.0964 × sleep score) + (0.00589 × diet score) + (0.033 × stress score) + (-0.0159 × smoker category) + (-0.036 × alcohol category).
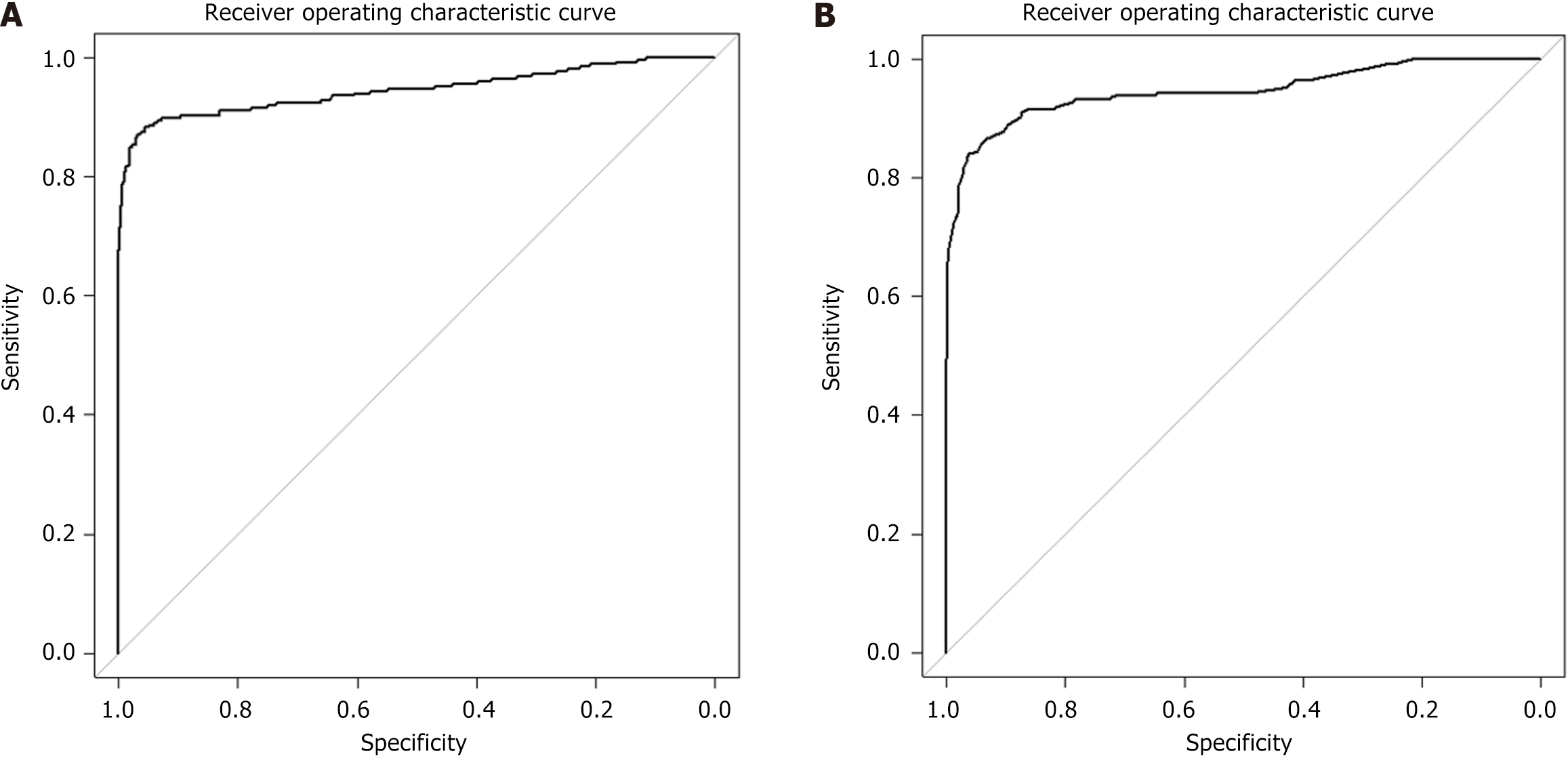
Scores were calculated for both the study participants and an additional cohort of 355 patients. Model validation yielded a C-index of 0.8884 through 100 bootstrap iterations. The ROC curve shown in Figure 1A, demonstrated an AUC of 0.9442, with a sensitivity of 0.8694 and specificity of 0.9659. The optimal cut-off value of 0.511 was determined based on the highest Youden index. Further plots and details of the model are available in the Supplementary material.
The GRSS scores can be converted into the risk of GERD by the following equation: Risk = 1/(1+e3.58 - 8.32 × GRSS scores).
A practical scoring scale for clinical use was developed through logistic lasso regression using categorical variables. An optimal regularization parameter (lambda) of 0.95 was determined by bootstrapping (10000 datasets). The logistic regression coefficient β for each variable was used to create the scoring scale based on the regression coefficient-based scoring algorithm, which proved superior to the risk ratio-based algorithm. Coefficients were rounded and used to assign weights to each interval to create the scoring scale. The scoring scale is shown in Table 3.
| Factor | Parameter | Score |
| Age (years) | Less than 20 | 3 |
| 21-40 | 5 | |
| 41-60 | 8 | |
| 61-80 | 11 | |
| Greater than 81 | 13 | |
| Body mass index | Less than 18 | 3 |
| 18-22.9 | 7 | |
| 23-24.9 | 10 | |
| More than 25 | 13 | |
| Smoking | Non-smoker | 0 |
| Smoker < 5 pack years | 0.2 | |
| Smoker > 5 pack years | 0.4 | |
| Alcohol score | Non-alcoholic | 0 |
| 0–6 | 0·2 | |
| 7–12 | 1.4 | |
| Diet score | < 4 | 2 |
| 4–8 | 1.5 | |
| 8–12 | 1 | |
| > 12 | 0.5 | |
| Stress score | < 4.5 | 1 |
| 4.5–6 | 2 | |
| > 6 | 2.5 | |
| Sleep score | < 4 | 3 |
| 4–8 | 6 | |
| > 8 | 9 |
Scores were calculated for both the study participants and an additional cohort of 355 patients. Model validation yielded a C-index of 0.891475 through 100 bootstrap iterations. The ROC curve shown in Figure 1B, demonstrated an AUC of 0.9457, with a sensitivity of 0.8408 and specificity of 0.9590. The optimal cut-off value of 25.5 was determined based on the highest Youden index. Further plots and details of the model are available in the Supplementary material.
The GRSS scores can be converted into the risk of GERD by the following equation: Risk = 1/(1+e15.57060 - 0.612528 × GRSS scores). In Supplementary Table 2, we provide a translation of scores into corresponding risk percentages.
The reliability of the questionnaire is measured by using Cronbach’s alpha value, which was found to be 0.850 indicating that the internal consistency is good. To assess the test-retest reliability of the GRSS questionnaire, 40 participants completed the questionnaire on two separate occasions. ICC analysis showed an ICC value of 0.836 (P < 0.001), indicating strong consistency between the two administrations. Bland-Altman analysis revealed a minimal mean difference and most data points within the limits of agreement, demonstrating high reliability. The plot is shown in Supplementary Figure 2. Wilcoxon signed-rank test to compare the GRSS scores at baseline and after 6 months indicated a statistically significant difference between the two time points, with a Wilcoxon W value of 252 (P < 0.001).
Our study involved 330 participants, comprising 110 GERD cases and 220 healthy controls, all of whom were of South Asian descent. Spearman rank correlation revealed an association between the risk factors and GERD. Conversion of continuous variables into categorical variables also revealed clear associations between these factors and GERD. Notably, age, BMI, smoking, alcoholism, sleep quality, diet, and stress showed significant correlations with GERD status. However, community and socioeconomic status exhibited weaker associations. After excluding non-significant factors, we took two approaches to building our risk prediction model. The first involved a lasso logistic regression with continuous variables, resulting in a rather complex equation suitable for programming websites and mobile apps. The second approach used categorical variables derived from equally spaced intervals, creating a practical scoring scale for physicians based on coefficients from the lasso logistic regression. Both models demonstrated excellent accuracy in predicting GERD risk when tested on an additional cohort of 355 individuals. Moreover, our questionnaire exhibited good internal consistency with a Cronbach’s alpha value of 0.850. The test-retest reliability of the GRSS assessed by an ICC of 0.836 indicated the stability of GRSS scores over time. We found a significant change in GRSS scores following the lifestyle modification advice provided. These findings suggest that the GRSS could be a valuable tool for assessing GERD risk, considering key demographic and lifestyle factors, and facilitating timely intervention and management.
To develop GRSS, we chose a case-control study design for its efficiency in comparing individuals with and without GERD. This approach facilitated the identification of risk factors and the creation of a GRSS. As there was no suitable existing questionnaire for primary prevention, we developed one in conjunction with the case-control study. Additio
We carried out an extensive literature review on databases such as PubMed/Medline, Scopus, EMBASE, and Google Scholar, focused on studies related to GERD risk prediction. We employed search terms such as "GERD risk prediction", "GERD questionnaire", "Gastroesophageal Reflux Disease prediction tool", and "GERD risk assessment". We found that various questionnaires are available to assess GERD. The GERD-Q is a validated tool designed to gauge the frequency and severity of GERD symptoms, and how these symptoms affect daily life[6]. Similarly, the GERD Symptom Assessment Scale evaluates the severity and frequency of GERD symptoms such as heartburn, regurgitation, chest pain, and swallowing difficulties, while also delving into their repercussions on a patient's quality of life[26]. The Reflux Disease Questionnaire is widely employed to gauge the frequency and severity of heartburn, regurgitation, and epigastric pain, giving a composite score that quantifies GERD symptom severity[27]. The Carlsson-Dent GERD Questionnaire investigates symptom presence and severity, assigning a score indicative of GERD likelihood[28]. The Quality of Life in Reflux and Dyspepsia captures the impact of GERD on a patient's quality of life, encompassing physical and emotional well-being[29]. We also found several studies that aimed to create risk prediction models for assessing Barett's esophagus[30-32]. However, we found a significant gap in studies specifically addressing GERD risk prediction, prompting our central research question: How can we strategically identify individuals at risk of developing GERD. In the realm of primary prevention, how can we tailor preventive measures using a predictive risk factor model-GRSS to curtail the onset of GERD and its related complications.
GERD is linked to various lifestyle factors, with six major ones examined in our study. Older individuals experience higher GERD prevalence due to lax lower esophageal sphincters, as noted by Euseb et al[33]. Increased body weight, reflected by BMI, leads to more adipocytes which secrete adiponectin and leptin, disrupting sphincter function and causing regurgitation of gastric contents[34]. Luminal nitric acid, known for its cytotoxic effects, may impair the epithelial barrier, with high-fiber foods potentially mitigating this risk[35]. Smoking lowers the resting tone of the lower esophageal sphincter, enhances acid reflux, and reduces bicarbonate levels in saliva[10]. Alcohol consumption results in toxic byproducts like acetaldehyde, which disrupt sphincter function and acid secretion, impacting GERD[36]. Sleep disturbances and lying down exacerbate symptoms, particularly if sleep occurs within three hours of eating[37]. Stress increases catecholamine levels, worsening esophageal sphincter action and maintaining inflammatory states that exacerbate GERD symptoms[38].
From the patient's point of view, this innovative tool provided a chance for individuals to detect their vulnerability to GERD at an early stage and facilitated prompt adjustments to their lifestyle. By enabling early identification of risk and emphasizing lifestyle changes, this tool enhanced personal well-being and reduced the risk of complications associated with GERD. From adopting dietary adjustments to healthier sleep patterns and stress management, patients found that this screening tool aligned with their desire for preventive healthcare. The questionnaire was carefully designed with the patient's convenience in mind, ensuring that it was easy to use and did not take more than 8 minutes to complete, further enhancing user-friendliness.
An added advantage of the GRSS questionnaire lies in its component scores, allowing us to pinpoint specific risk factors for each individual. This precision enables us to recommend tailored lifestyle modifications to address the particular risk factor identified. By offering personalized advice, the GRSS allows individuals to make changes that directly mitigate their unique risks. This not only enhances the effectiveness of preventive measures but also ensures a more individualized and patient-centric approach to managing GERD risk. The risk factors selected in our study are primarily associated with lifestyle habits. Although we have developed the GRSS as a tool for assessing the risk of GERD development, our study did not assess whether improvements in lifestyle leading to a decrease in GRSS scores led to a reduced risk of GERD. This suggests a potential direction for future research, which could involve an intervention study to assess its clinical utility.
GRSS includes non-integer values, which may appear unconventional in clinical scoring tools. While integer scores are often preferred for their simplicity, we chose to retain non-integer scores because they better reflect clinically meaningful risk thresholds. For instance, in the smoking module, we initially considered higher pack-year thresholds (< 50 pack years and > 50 pack years) yielding integer based scores. However, this approach proved suboptimal for clinical utility. Most young individuals in our primary prevention target group do not yet reach such high cumulative exposures. As a result, the scoring system would fail to capture early, modifiable risk in this crucial population. By lowering the threshold to < 5 pack years and > 5 pack years, we ensured that the tool remains sensitive to early smoking exposure. This adjustment resulted in non-integer scores but it preserves the lead time advantage essential to any effective screening or preventive measure.
Beyond its utility as a research instrument, the GRSS holds immense promise in clinical settings. Its user-friendly design facilitates regular and accurate GERD risk assessment by patients or healthcare workers. Clinicians should maintain a high index of suspicion for GERD in patients with higher GRSS scores and initiate appropriate interventions promptly to prevent the development of complications. While the development of the GRSS represents a significant leap forward, we acknowledge certain limitations and areas for future refinement. These include the need for further validation of the tool in multicentric studies to establish the reliability of this scoring system. The GRSS was developed and validated in a South Asian setting. Consequently, the generalizability of the questionnaire to different populations may be affected, highlighting the need for regional modifications in the questionnaire by further studies. GERD is influenced by 19 risk factors; our questionnaire focuses on the six major risk factors. While this strategic selection streamlines the questionnaire's practicality, it also limits the complete evaluation of all the risk factors for GERD. Additionally, self-reported data introduces the possibility of recall bias, which is an inherent limitation in questionnaire-based studies assessing lifestyle risk factors. However, such tools remain essential for early screening and public health utility due to their practicality and scalability. To mitigate recall bias, the GRSS questionnaire was carefully designed with objective, specific, and behaviorally anchored questions to facilitate more accurate recall and reporting. Another limitation is that, in our pilot, we assessed if there was a change in GRSS scores following unofficial lifestyle modification advice but could not assess compliance. Future research should address this aspect. We are committed to addressing these issues and continually enhancing the GRSS to meet the evolving needs of both researchers and clinicians. A visual illustration of the clinical implication of GRSS is shown in Figure 2.
In conclusion, we have developed a new novel questionnaire–GRSS that may predict the susceptibility of an individual to developing GERD in the future. Based on the GRSS scores, lifestyle modifications can be advised to the individual. GRSS proves itself on two fronts—first, with a sophisticated equation suitable for computational applications, and second, with a user-friendly scoring scale for clinicians. Both approaches showed remarkable precision in predicting GERD risk which highlighted the GRSS's potential for early intervention in clinical settings.
The substantial prevalence of GERD within our South Asian study group emphasizes the broader public health implications of GRSS. This approach focuses on primary prevention rather than the traditional secondary prevention tools such as screening, which typically identify individuals after GERD has already developed. Our method allows us to use the tool across the entire population, categorizing people into low-risk, medium-risk, and high-risk groups. Those in the low-risk and medium-risk categories are advised on lifestyle changes to minimize the risk of GERD, while high-risk individuals undergo additional screening to check for the disease. This not only optimizes preventive strategies but also ensures more targeted and efficient healthcare interventions based on individual risk levels, marking a significant step forward in promoting overall health and well-being. While our study has inherent limitations, it serves as a stepping-stone for future research in the field of GERD.
We thank all the study participants who consented to be a part of the study. We extend our gratitude to the Department of General Surgery, Government Medical College, Omandurar for inculcating clinical acumen, providing the study population, and permitting us access to the upper gastrointestinal endoscopy reports of the patients. We are extremely grateful to the faculty and other support staff of Government Medical College, Omandurar who have supported us throughout the study period.
| 1. | World Gastroenterology Organisation. World Gastroenterology Organisation Global Guidelines – GERD - Global Perspective on Gastroesophageal Reflux Disease. Available from: https://www.worldgastroenterology.org/guidelines/gastroesophageal-reflux-disease/gastroesophageal-reflux-disease-english. |
| 2. | Zhang D, Liu S, Li Z, Wang R. Global, regional and national burden of gastroesophageal reflux disease, 1990-2019: update from the GBD 2019 study. Ann Med. 2022;54:1372-1384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 3. | Bhatia SJ, Makharia GK, Abraham P, Bhat N, Kumar A, Reddy DN, Ghoshal UC, Ahuja V, Rao GV, Devadas K, Dutta AK, Jain A, Kedia S, Dama R, Kalapala R, Alvares JF, Dadhich S, Dixit VK, Goenka MK, Goswami BD, Issar SK, Leelakrishnan V, Mallath MK, Mathew P, Mathew P, Nandwani S, Pai CG, Peter L, Prasad AVS, Singh D, Sodhi JS, Sud R, Venkataraman J, Midha V, Bapaye A, Dutta U, Jain AK, Kochhar R, Puri AS, Singh SP, Shimpi L, Sood A, Wadhwa RT. Indian consensus on gastroesophageal reflux disease in adults: A position statement of the Indian Society of Gastroenterology. Indian J Gastroenterol. 2019;38:411-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 4. | Richter JE, Rubenstein JH. Presentation and Epidemiology of Gastroesophageal Reflux Disease. Gastroenterology. 2018;154:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 413] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 5. | Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am J Gastroenterol. 2022;117:27-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 748] [Cited by in RCA: 578] [Article Influence: 144.5] [Reference Citation Analysis (1)] |
| 6. | Jones R, Junghard O, Dent J, Vakil N, Halling K, Wernersson B, Lind T. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 524] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 7. | Dickman R, Green C, Fass SS, Quan SF, Dekel R, Risner-Adler S, Fass R. Relationships between sleep quality and pH monitoring findings in persons with gastroesophageal reflux disease. J Clin Sleep Med. 2007;3:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Chang P, Friedenberg F. Obesity and GERD. Gastroenterol Clin North Am. 2014;43:161-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 9. | Moshkowitz M, Horowitz N, Halpern Z, Santo E. Gastroesophageal reflux disease symptoms: prevalence, sociodemographics and treatment patterns in the adult Israeli population. World J Gastroenterol. 2011;17:1332-1335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Pandolfino JE, Kahrilas PJ. Smoking and gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol. 2000;12:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Pan J, Cen L, Chen W, Yu C, Li Y, Shen Z. Alcohol Consumption and the Risk of Gastroesophageal Reflux Disease: A Systematic Review and Meta-analysis. Alcohol Alcohol. 2019;54:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Taraszewska A. Risk factors for gastroesophageal reflux disease symptoms related to lifestyle and diet. Rocz Panstw Zakl Hig. 2021;72:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Morozov S, Isakov V, Konovalova M. Fiber-enriched diet helps to control symptoms and improves esophageal motility in patients with non-erosive gastroesophageal reflux disease. World J Gastroenterol. 2018;24:2291-2299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (6)] |
| 14. | Kelsey JL, Thompson WD, Evans AS. Methods in observational epidemiology. New York: Oxford University Press, 1986. |
| 15. | Wani RT. Socioeconomic status scales-modified Kuppuswamy and Udai Pareekh's scale updated for 2019. J Family Med Prim Care. 2019;8:1846-1849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 231] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 16. | Alcohol use disorders identification test (AUDIT). Available from: https://auditscreen.org/. |
| 17. | Schuckit MA, Smith TL, Danko GP, Pierson J, Hesselbrock V, Bucholz KK, Kramer J, Kuperman S, Dietiker C, Brandon R, Chan G. The ability of the Self-Rating of the Effects of Alcohol (SRE) Scale to predict alcohol-related outcomes five years later. J Stud Alcohol Drugs. 2007;68:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Snyder E, Cai B, DeMuro C, Morrison MF, Ball W. A New Single-Item Sleep Quality Scale: Results of Psychometric Evaluation in Patients With Chronic Primary Insomnia and Depression. J Clin Sleep Med. 2018;14:1849-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 279] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 19. | Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17520] [Cited by in RCA: 23494] [Article Influence: 635.0] [Reference Citation Analysis (0)] |
| 20. | Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9909] [Cited by in RCA: 11611] [Article Influence: 331.7] [Reference Citation Analysis (0)] |
| 21. | Rijnaarts I, de Roos N, Zoetendal EG, de Wit N, Witteman BJM. Development and validation of the FiberScreen: A short questionnaire to screen fibre intake in adults. J Hum Nutr Diet. 2021;34:969-980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Neyrinck AM, Nazare JA, Rodriguez J, Jottard R, Dib S, Sothier M, Berghe LVD, Alligier M, Alexiou H, Maquet V, Vinoy S, Bischoff SC, Walter J, Laville M, Delzenne NM. Development of a Repertoire and a Food Frequency Questionnaire for Estimating Dietary Fiber Intake Considering Prebiotics: Input from the FiberTAG Project. Nutrients. 2020;12:2824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16831] [Cited by in RCA: 18407] [Article Influence: 428.1] [Reference Citation Analysis (0)] |
| 24. | Noone PA. The Holmes-Rahe Stress Inventory. Occup Med (Lond). 2017;67:581-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Mehta HB, Mehta V, Girman CJ, Adhikari D, Johnson ML. Regression coefficient-based scoring system should be used to assign weights to the risk index. J Clin Epidemiol. 2016;79:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 26. | Rothman M, Farup C, Stewart W, Helbers L, Zeldis J. Symptoms associated with gastroesophageal reflux disease: development of a questionnaire for use in clinical trials. Dig Dis Sci. 2001;46:1540-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Shaw M, Dent J, Beebe T, Junghard O, Wiklund I, Lind T, Johnsson F. The Reflux Disease Questionnaire: a measure for assessment of treatment response in clinical trials. Health Qual Life Outcomes. 2008;6:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Contreras-Omaña R, Sánchez-Reyes O, Ángeles-Granados E. Comparison of the Carlsson-Dent and GERD-Q questionnaires for gastroesophageal reflux disease symptom detection in a general population. Rev Gastroenterol Mex. 2017;82:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Wiklund IK, Junghard O, Grace E, Talley NJ, Kamm M, Veldhuyzen van Zanten S, Paré P, Chiba N, Leddin DS, Bigard MA, Colin R, Schoenfeld P. Quality of Life in Reflux and Dyspepsia patients. Psychometric documentation of a new disease-specific questionnaire (QOLRAD). Eur J Surg Suppl. 1998;41-49. [PubMed] |
| 30. | Rosenfeld A, Graham DG, Jevons S, Ariza J, Hagan D, Wilson A, Lovat SJ; BEST2 study group, Sami SS, Ahmad OF, Novelli M, Rodriguez Justo M, Winstanley A, Heifetz EM, Ben-Zecharia M, Noiman U, Fitzgerald RC, Sasieni P, Lovat LB. Development and validation of a risk prediction model to diagnose Barrett's oesophagus (MARK-BE): a case-control machine learning approach. Lancet Digit Health. 2020;2:E37-E48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Thrift AP, Kendall BJ, Pandeya N, Vaughan TL, Whiteman DC; Study of Digestive Health. A clinical risk prediction model for Barrett esophagus. Cancer Prev Res (Phila). 2012;5:1115-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Ireland CJ, Fielder AL, Thompson SK, Laws TA, Watson DI, Esterman A. Development of a risk prediction model for Barrett's esophagus in an Australian population. Dis Esophagus. 2017;30:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Eusebi LH, Ratnakumaran R, Yuan Y, Solaymani-Dodaran M, Bazzoli F, Ford AC. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67:430-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 480] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 34. | Vaishnav B, Bamanikar A, Maske P, Reddy A, Dasgupta S. Gastroesophageal Reflux Disease and its Association with Body Mass Index: Clinical and Endoscopic Study. J Clin Diagn Res. 2017;11:OC01-OC04. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Iijima K, Shimosegawa T. Involvement of luminal nitric oxide in the pathogenesis of the gastroesophageal reflux disease spectrum. J Gastroenterol Hepatol. 2014;29:898-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Chen SH, Wang JW, Li YM. Is alcohol consumption associated with gastroesophageal reflux disease? J Zhejiang Univ Sci B. 2010;11:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Jung HK, Choung RS, Talley NJ. Gastroesophageal reflux disease and sleep disorders: evidence for a causal link and therapeutic implications. J Neurogastroenterol Motil. 2010;16:22-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Song EM, Jung HK, Jung JM. The association between reflux esophagitis and psychosocial stress. Dig Dis Sci. 2013;58:471-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/