Published online Jun 22, 2025. doi: 10.4291/wjgp.v16.i2.107265
Revised: April 13, 2025
Accepted: May 18, 2025
Published online: June 22, 2025
Processing time: 92 Days and 11.3 Hours
Pancreatic neuroendocrine tumors (pNETs) are rare, presenting significant cha
Core Tip: Pancreatic neuroendocrine tumors (pNETs) are rare neoplasms with variable clinical and pathobiological characte
- Citation: Rikhraj N, Fernandez CJ, Ganakumar V, Pappachan JM. Pancreatic neuroendocrine tumors: A case-based evidence review. World J Gastrointest Pathophysiol 2025; 16(2): 107265
- URL: https://www.wjgnet.com/2150-5330/full/v16/i2/107265.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v16.i2.107265
Neuroendocrine neoplasms (NENs) refer to neoplasms originating from the enterochromaffin cells of the embryonic gut exhibiting both neural and endocrine differentiation. While the majority of NENs are well-differentiated, less aggressive, but potentially malignant NETs (neuroendocrine tumors), the remaining are poorly differentiated and aggressive NECs (neuroendocrine carcinomas). As per the latest classification, NENs can be divided into gastroenteropancreatic NENs (GEP-NENs) and lung NENs[1]. GEP-NENs constitute 55%-70% of all NENs, 12%-20% of which are formed by pancreatic NENs (pNENs)[2].
The pNENs account for only 1%-2% of all pancreatic neoplasms, and pancreatic NETs (pNETs) account for nearly 90% of all pNENs[3]. The pNENs are mostly diagnosed in the sixth decade of life, and they exhibit a minimal male preponderance. Nearly 70% of the pNENs are non-functioning, whereas 30% are functioning, secreting hormones or peptides associated with the clinical syndrome[4]. Those tumors with detectable peptides on biopsy specimens but that do not exhibit features of hormone hypersecretion syndrome are still considered non-functioning. While most pNENs are sporadic, nearly 10% are associated with hereditary syndromes, including Multiple Endocrine Neoplasia type 1 or type 4 (MEN1 or MEN4), von Hippel-Lindau syndrome, neurofibromatosis type 1 and tuberous sclerosis. The hereditary pNENs are often multifocal, well-differentiated, functioning, possibly secreting multiple peptide hormones simultaneously, with a young age of onset, and are associated with ectopic production of hormones and accompanied by other endocrine disorders or malignancies. Though pNENs are less aggressive with better 5-year survival rates compared to pancreatic adenocarcinomas, they are associated with nearly 23.1% recurrence rates within 8.1 years, even after curative resection[5].
A population-based study using surveillance epidemiology and end results (SEER) 18 registry data showed that in comparison to 2000-2008, the annual incidence of pNETs has increased from 0.27 per 100000 to 1.00 per 100000 in the study period 2009-2016[6]. This increase in annual incidence is accompanied by the early detection of particularly the asymptomatic non-functioning NETs in their early stages, thereby resulting in an improvement in median overall survival from 46 months to 85 months. Moreover, the improved median overall survival was consistent over different stages of the disease - localized disease at 83%, regional disease at 67%, and metastatic disease at 28%[6].
The overall incidence of GEP-NECs is rare, but several studies report increasing incidence particularly across North America, Asia and Europe[7,8]. The SEER 18 registry reported an incidence rate of 9.36 per 100000, nearly 90% of which are lung NECs (8.36 per 100000, 95% small cell NECs and 5% non-small cell NECs), whereas pNECs constitute only 0.07 per 100000 (small cell NECs nearly 50%)[9]. The pNECs have one of the shortest median survivals at 5.7 months [multivariate hazard ratio (HR): 1.10; 95% confidence interval (CI): 1.03-1.18, P < 0.001]. This could be attributed to the advanced stage at diagnosis (75.6% of the pNEC patients presenting with distant metastasis) in comparison to other GEP-NEC subtypes[9].
Due to this cumulative disease burden, studying the pathophysiology, clinical presentation, and response to treatment of pNETs and pNECs make it interesting and relevant.
The pNETs are derived from primitive endoderm, which differentiate into several gastrointestinal (GI) epithelial lineages, inclusive of enterochromaffin cells. These enterochromaffin cells are categorised based on morphology, location and expression of peptide hormones. The proliferation of these cells is dependent on various transcription factors, for example, Neurogenin 3 in the pancreas, which delineates the Notch signalling pathway responsible for secretory and absorptive cell lineages[10].
Germline mutations in tumour suppressor genes MEN1, VHL, NF1, and TSC1/2 account for 10% of familial syndrome-derived pNETs. The majority of pNETs are sporadic, resulting from somatic cell mutations for proteins involved in chromatin modelling, for example, MEN1, DAXX and ATRX. Encoding protein mutations in the mTOR pathway have shown a correlation to the formation of pNETs[11]. Alongside the presence of serum biomarkers, these have vital implications for targeted therapy. NECs can present with specific genetic alterations, for example, TP53 and RB1 in small cell NECs and a variable genetic profile in large cell NECs[12].
Dependent on secretory products and hypersecretion of specified hormones, the pNET are called by different names. Insulinoma is the most common functional pNET (30%-40%), with an annual incidence of 1-32 cases per million population[13-17]. Functional expression means symptomatic management can be achieved effectively with surgical excision. In the case of insulinoma, tumor origin within β-cells of Islets of Langerhans means parenchymal sparing pancreatectomy can relieve symptoms of the classical Whipple’s triad of hypoglycaemia (plasma glucose < 4 mmol/L), neuroglycopenic clinical signs and resolution after administration of glucose[18].
Functionality is an important predictive factor in the presentation of pNENs[19]. Patients with nonfunctioning pNENs have larger, poorly differentiated tumors with hepatic and lymph node involvement associated with reduced survival outcomes compared to those with functioning pNENs[20]. Tumor grade is another important prognostic factor that can indicate cellular morphology according to classes of differentiation[21]. According to the World Health Organization, the pNENs are broadly classified into well-differentiated pNETs (three subtypes: G1 pNETs, G2 pNETs and G3 pNETs) and poorly differentiated pNECs (two subtypes: Small cell and large cell types and are high-grade by definition)[22]. These classifications are summarized in Table 1.
| Terminology | WHO grade | Differentiation | Mitotic rate | Ki-67 index |
| NET | G1 | Well-differentiated NET | < 2/2 mm² | < 3% |
| G2 | 2-20/2 mm² | 3%-20% | ||
| G3 | > 20%/2 mm² | > 20% | ||
| Small cell NEC | High grade | Poorly differentiated NEC | > 20/2 mm² | > 70% |
| Large cell NEC | High grade |
Generally, serum biomarkers can be used to identify the presence of NENs with the presentation of proteins in the secretory granules, synaptic-like vesicles, or cytoplasm of neuroendocrine cells. These have low specificity as these biomarkers can also be elevated in inflammatory conditions like inflammatory bowel disease, pancreatitis, gastritis, proton pump inhibitor and steroid treatment, liver failure, and other neoplasms[12]. Specific pancreatic neuroendocrine markers[23] can be used to distinguish functional vs non-functional pNETs, as summarised in Table 2.
| Circulating biomarkers | ||
| Non-specific biomarkers | Specific to functional pNEN | Specific to nonfunctional pNEN |
| Chromogranin A | Insulin | Pancreatic polypeptide |
| Pancreastatin | Glucagon | Human chorionic gonadotropin |
| Chromogranin B | Gastrin | Neurotensin |
| Neuron-specific enolase | Somatostatin | Ghrelin |
| Alpha fetoprotein | Vasoactive intestinal peptide | Calcitonin |
| Growth hormone or GHRH | ||
| Adrenocorticotropic hormone | ||
| Immunohistochemical biomarkers | ||
| Differentiation markers | Site of origin markers | Prognostic markers |
| Chromogranin A | NESP55 | ATRX/DAXX |
| Synaptophysin | PGR | SSTR2a |
| INSM1 | PDX1 | PD-L1 |
| ARX | ||
| ISL1 | ||
| Molecular biomarkers | ||
| Circulating tumor cells (CTCs) | ||
| Circulating cell-free DNA (cfDNA) | ||
| Circulating microRNAs (miRNAs) | ||
| Circulating transcripts (NETest score) | ||
| G protein-coupled receptor-associated sorting protein 1 (GPRASP1) | ||
| Delta-like protein 3 (DLL3) | ||
| Tumor-associated macrophages (TAMs) | ||
| Glucose transporter 1 (GLUT1) | ||
The diagnosis of NEN is established by immunohistochemical staining showing positivity for markers of neuroendocrine differentiation, including INSM1 (highly sensitive and specific), synaptophysin (highly sensitive but not specific), and chromogranin A (less sensitive than synaptophysin but highly specific)[21]. This should be followed by a stain for transcription factors to know the site of origin: For example, pNENs may express PDX1 (a transcription factor typically expressed by the β cells), ARX (transcription factor typically expressed by the α cells), and ISL1 and stain for the peptide hormones[24].
The typical (indolent) insulinomas are epigenetically similar to β cells: PDX1 positive and ARX negative[25]. They are small (below 2 cm), have a favourable prognosis after resection, and are characterized by YY1 mutations in nearly 30% of cases. On the other hand, the rare aggressive insulinomas are ARX positive, larger (3.5-9.0 cm), have higher metastatic rates, and are characterized by genetic alternations seen characteristically in non-functioning pNENs, including ATRX/DAXX mutations, alternative lengthening of telomeres or CDKN2A deletions[25]. Hence, assessment of ATRX/DAXX mutations and alternative lengthening of telomeres are recommended in pNENs.
The origin, differentiation and tumorigenic mechanisms of the aggressive insulinomas are more closely related to non-functioning pNENs[25]. It is possible that they existed as non-functioning pNENs for a while before exhibiting the functional behaviour (recurrent hypoglycaemia). These tumours might have been producing insulin, though at an asymptomatic level. It is hypothesized that the tumorigenesis of aggressive AXR-positive insulinomas might have happened from a non-functioning β-cell tumor acquiring α-cell characteristics after ATRX/DAXX mutations and the presence of alternative lengthening of telomeres, or from a non-functioning α cell/intermediate tumor acquiring β cell characteristic of insulin secretion[25].
Imaging plays an important role in the diagnosis, follow-up and assessment of response to treatment of pNETs. High-resolution computed tomography (CT) of the thorax, abdomen, and pelvis is the first line in providing radiological imaging of the location and size of the primary tumor, nodal disease, and sites of metastasis. Intravenous contrast, particularly in the late arterial phase, can prove useful for detecting NENs in the pancreas and liver[26]. Magnetic resonance imaging (MRI) has increased specificity and sensitivity compared to CT (100% specificity for pancreas and 98% for liver), and diffusion-weighted (T1/T2) imaging can aid in small hepatic metastasis identification, along with greater detail into tumor margins[19]. Positron emission tomography (PET) with F-fluorodeoxyglucose can show uptake in combination with CT/MRI.
Due to the expression of biomarkers, nuclear imaging is useful in providing functional response information of pNETs. Somatostatin Receptor (SSR) imaging is most commonly used in combination with single-photon emission computed tomography (SPECT)/CT or PET/CT and includes the use of octreotide, 111In-pentereotide or Gallium-DOTA tracer[13]. Receptor positivity has important applications for the use of somatostatin analogues or targeted therapy and can measure treatment response/remission.
Following physical examination, biopsy and imaging for diagnosis, pNETs are staged, as per TNM and AJCC classifications[27], as recorded in Table 3.
| AJCC | TNM | Description |
| I | T1 N0 M0 | Tumor < 2 cm, local to pancreas. No nodal disease/metastasis |
| II | T2 N0 M0 | 2 cm < Tumor < 4 cm, local to pancreas. No nodal disease/metastasis |
| T3 N0 M0 | Tumor > 4 cm, extended to duodenum/common bile duct. No nodal disease or metastasis | |
| III | T1-3/4 N1 M0 | Tumor extended to adjacent organs (stomach, spleen, colon/adrenals) or spread haematogenously (T4). It may present with lymph node spread (N1) but no distant metastasis |
| IV | Tx Nx M1 | Any size (Tx), any nodal spread (Nx), but distant metastasis (M1) |
SEER stages of localized, regional and distant disease can be used to categorize pNETs based on 5-year relative survival rates. Localized tumors to the pancreas report the best prognoses of up to 95%, followed by regional (adjacent organ/Lymph nodal spread; 72%) and distant metastasis to lungs, liver and bone at 23%. Overall, a 53% 5-year survival rate is reported across all stages, meaning that effective diagnosis and treatment can achieve stable disease[28].
Treatment of pNETs falls under two main aims: Curative intent and reduced disease burden with symptomatic manage
Surgery remains the only treatment modality with curative intent. Indications include small pNETs (< 2 cm), sympto
Where surgery (open/Laparoscopic/robotic) is not indicated as in poor general health, high American Society of Anesthesiologists/Eastern Cooperative Oncology Group performance score, extrahepatic metastases or pNEC, local measures can be trialled such as ablation using radiofrequency, microwave, laser or percutaneous or endoscopic cryotherapy[19]. Indications include hepatic metastases < 5 cm and ablation margins > 1 cm. Transarterial embolization can afford debulking via chemo/selective internal radiotherapy with Yttrium-90 isotope. Pancreatitis is the most common reported risk post-local measures. The consensus is that surgery is performed with curative intent for G1/2 tumors even with nodal/hepatic metastasis, coupled with hepatectomy or staged liver resection, followed by liver transplantation if necessary[19]. The role of whether curative intent can be achieved via surgery for G3 tumors over debulking is still debated in the literature.
Medical measures are indicated for symptomatic, functional pNETs or unresectable or incompletely resected tumors to suppress post-operative functionality. Recurrence can be managed symptomatically. In the case of hypoglycaemia secondary to insulinoma, this includes dietary adjustments of frequent small meals, followed by diazoxide (sulphony
For locally advanced, unresectable or metastatic tumors that do not respond to SSA, molecular-targeted therapies can be trialled. Particularly for insulinomas, everolimus acts as an mTOR (mammalian target of rapamycin) pathway inhibitor, and sunitinib as a VEGF/PDGF (vascular endothelium growth factor/platelet-derived growth factor) inhibitor under the broader category of tyrosine kinase pathway inhibitors[13,19]. These can be used individually or in combina
Recent research is showing the promise of peptide receptor radionuclide therapy (PRRT) for the management of functioning metastatic insulinomas with refractory hypoglycaemia. This treatment modality couples the use of SSA therapy, labelled with a radioactive isotope (Luteium-177-DOTA-Tyr3-octreotate) or Indium-111 octreotide, to act as targeted systemic radiation. The choice of this 177 Lu-DOTATE isotope is preferred due to the emission of concomitant beta and gamma-rays, reduced renal and haematological toxicity and modification of C-terminal threoninol on octreotide to threonine to increase affinity to SSRT2[31]. Positive results include improved quality of life secondary to better glycaemic control (70.6%) and stable disease (23.5%)[32]. This is further supported by the fact that 81% of patients having a reduced hypoglycaemic score, 58% requiring reduced antihypoglycaemic medication and median overall survival and progression-free survival of 19.7 months (95%CI: 6.5-32.9 months) and 11.7 months (95%CI: 4.9-18.5 months), respectively, post-PRRT[29].
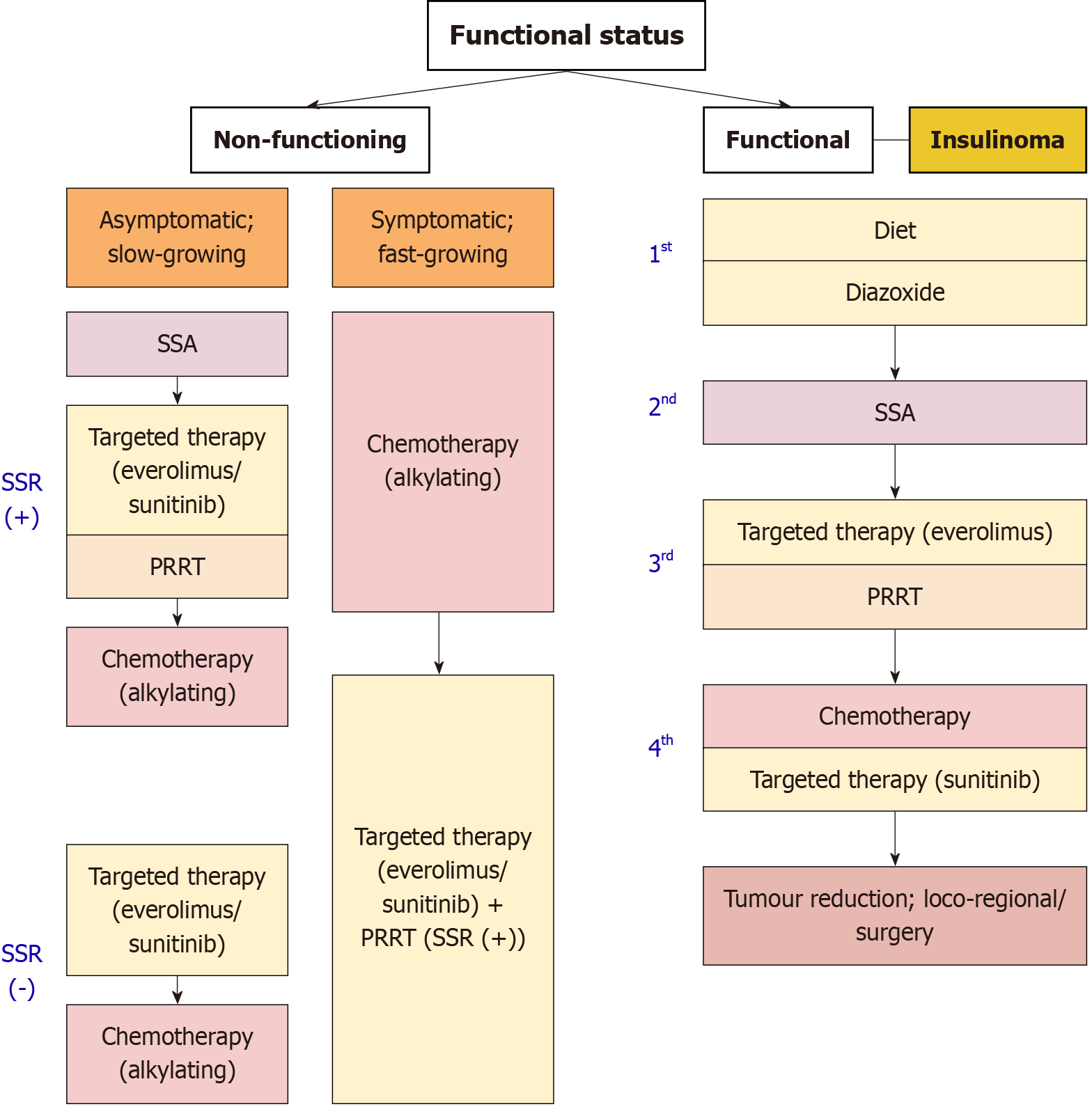
As per current accepted European neuroendocrine tumor society (ENETS) guidelines[33], an algorithm for the management of pNETs is summarized in Figure 1, according to functional status.
Based on the latest available guidance, the treatment strategy for non-functioning pNETs can be summarized for ease of understanding[34]. Patients with locally advanced NF-Pan-NET (stage T3 and T4) can be resected safely with low mortality and acceptable morbidity risk in expert centres. Radical local resection (R0) including portal-venous resection could be considered in selected cases. To help estimate risk of recurrence post-local measures, and guide follow-up schedules, nomograms after resection are recommended. In locally advanced or oligometastatic cases, preoperative treatment with PRRT can be beneficial in reducing tumor bulk.
For slow-growing, advanced G1-G2 non-functioning pNETs that are SSR positive, SSA is the recommended upfront treatment. In progressive G1-G2 non-functioning pNETs with SSR positivity, targeted therapy with everolimus and sunitinib are recommended. These agents should be considered in G3 progressive disease as well. PRRT may be considered second-line in non-functioning pNETs that are SSR positive. Finally, for metastatic disease, systemic chemotherapy (temozolomide in combination with capecitabine or streptozotocin + 5-FU) may be considered for patients with progressive/metastatic or symptomatic non-functioning G1-G2. For G3 metastatic non-functioning pNETs, temozolomide in combination with capecitabine can also be considered for upfront treatment[34].
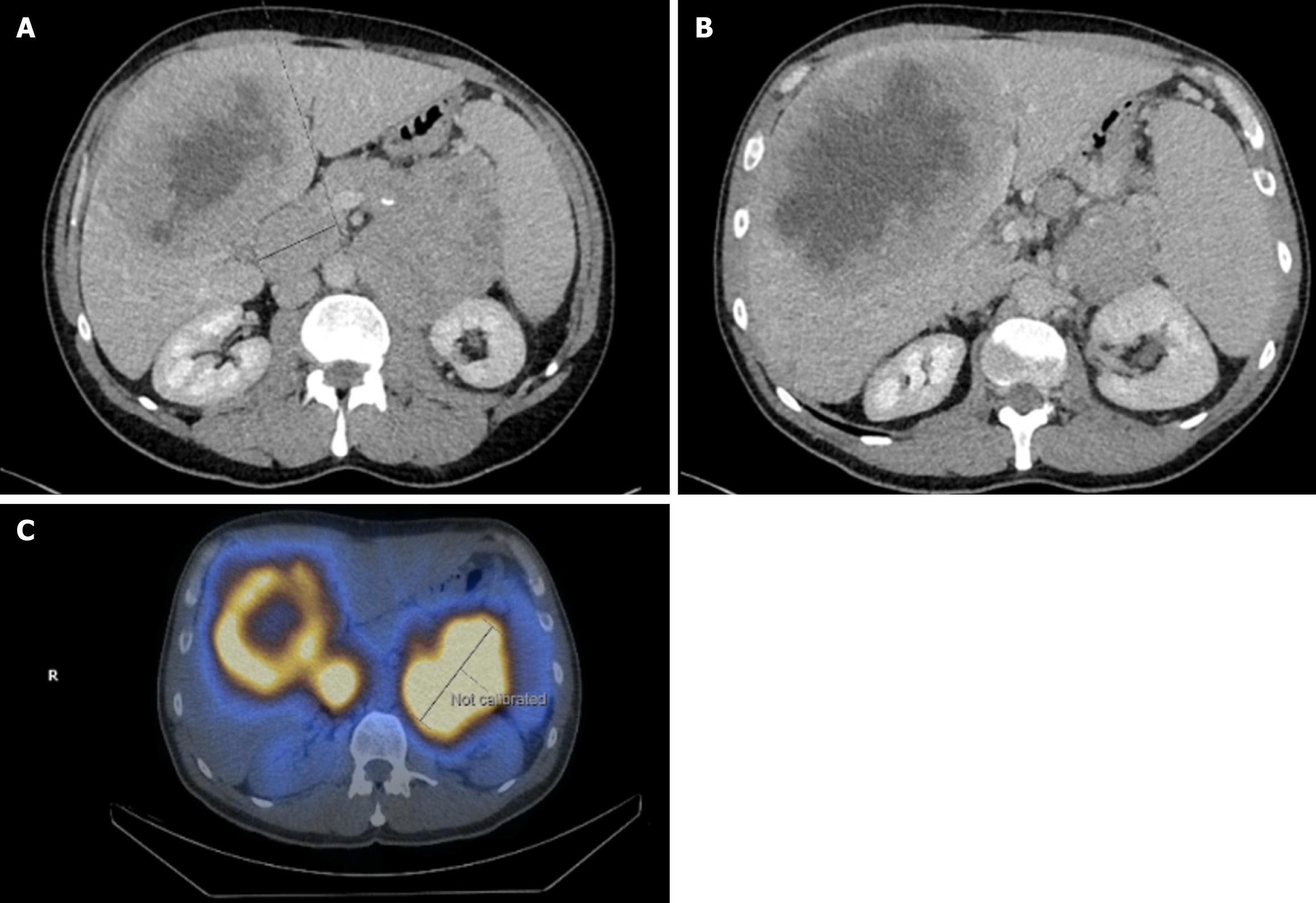
A 52-year-old male presented with new recurrent, severe hypoglycaemic episodes, including nocturnal symptoms and loss of consciousness. Relevant background is a 5-year history of weight gain (body mass index 33 kg/m2), fatigue, general malaise, loss of appetite and intermittent undiagnosed abdominal pain. Investigations revealed new-onset diabetes and a suspicious lesion on the liver and pancreas on ultrasound. The patient was prescribed insulin (Abasaglar and Humalog Kwikpen) and referred for a CT scan of the thorax, abdomen, and pelvis (TAP), which identified a large (10 cm × 9.5 cm) primary mass replacing the pancreatic tail (Figure 2A), extending diffusely into the pancreatic body. A fasting gut hormone profile suggested that the tumor was nonfunctional at the initial diagnosis. Bulky pathological lymph nodal disease was noted in the periportal territory (4.8 cm max diameter) around the superior mesenteric artery and coeliac axis. A large central necrotic mass was noted in segment 8 of the liver, measuring 15 cm (Figure 2B), with small benign cysts seen in the left hepatic lobe.
An ultrasound-guided liver biopsy of the right lobe of the liver, with histological analysis, confirmed Grade 2 NET, Ki67, 5%-10%. Planar imaging at 0004h, 0024h, and 0040h with SPECT CT Abdomen and Pelvis at 24 hours confirmed the pancreatic tail mass, periphery of large hepatic metastasis in segment 8, large portocaval node and additional 2 areas of nodal disease in the upper abdominal mesentery, with increased Octreotide uptake confirming SSTR positivity (Figure 2C). As per the guidelines, no detectable peptides on biopsy specimens and clinical picture at the time of diagnosis did not exhibit features of hormone hypersecretion syndrome, the diagnosis was non-functioning NET with metastasis[20].
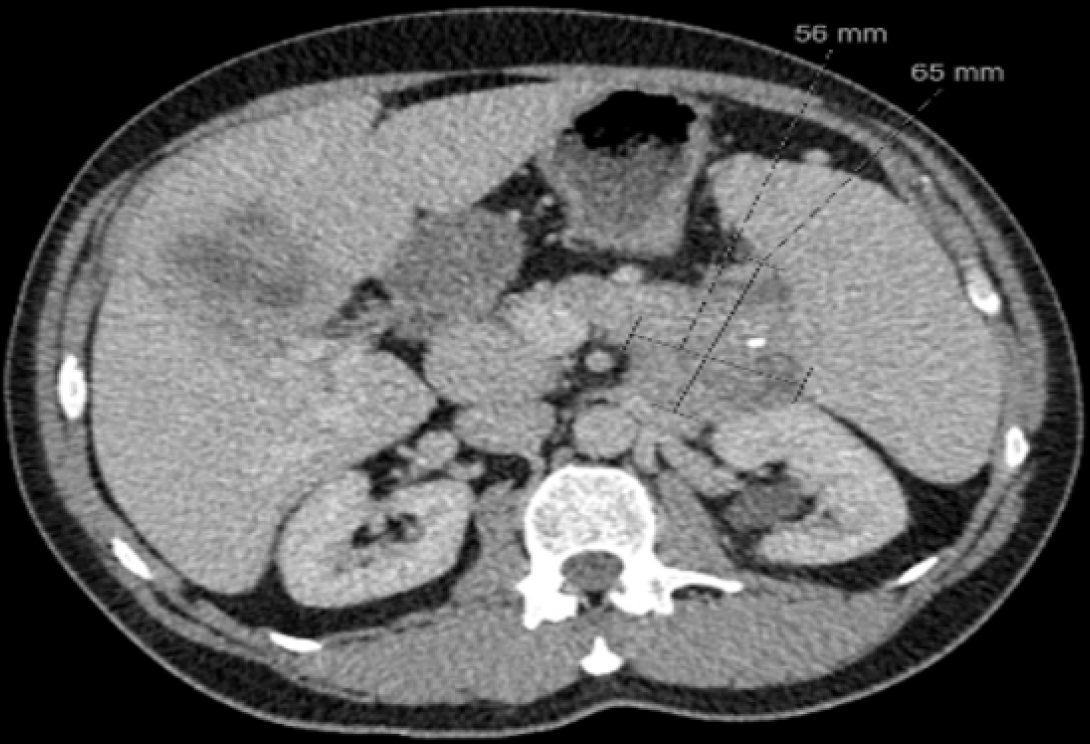
In line with literature, surgical resection was not performed due to widespread liver metastasis and the advanced disease stage[35,36]. Systemic therapy was initiated to prolong the survival and for symptomatic control. As per RADIANT-2 trial[37], SSA therapy (Octreotide LAR 30 mg, subcutaneously monthly) and targeted therapy (Everolimus 10 mg, oral, for 28-day cycles). The patient developed thrombocytopenia and a generalized itchy rash with maculopapular lesions, so Everolimus was subsequently changed to Sunitinib. 47 cycles of SSA and Everolimus were completed, and he was monitored bimonthly via CT-TAP (Figure 3, showing the smallest size of the primary tumor at 20 months post-treatment).
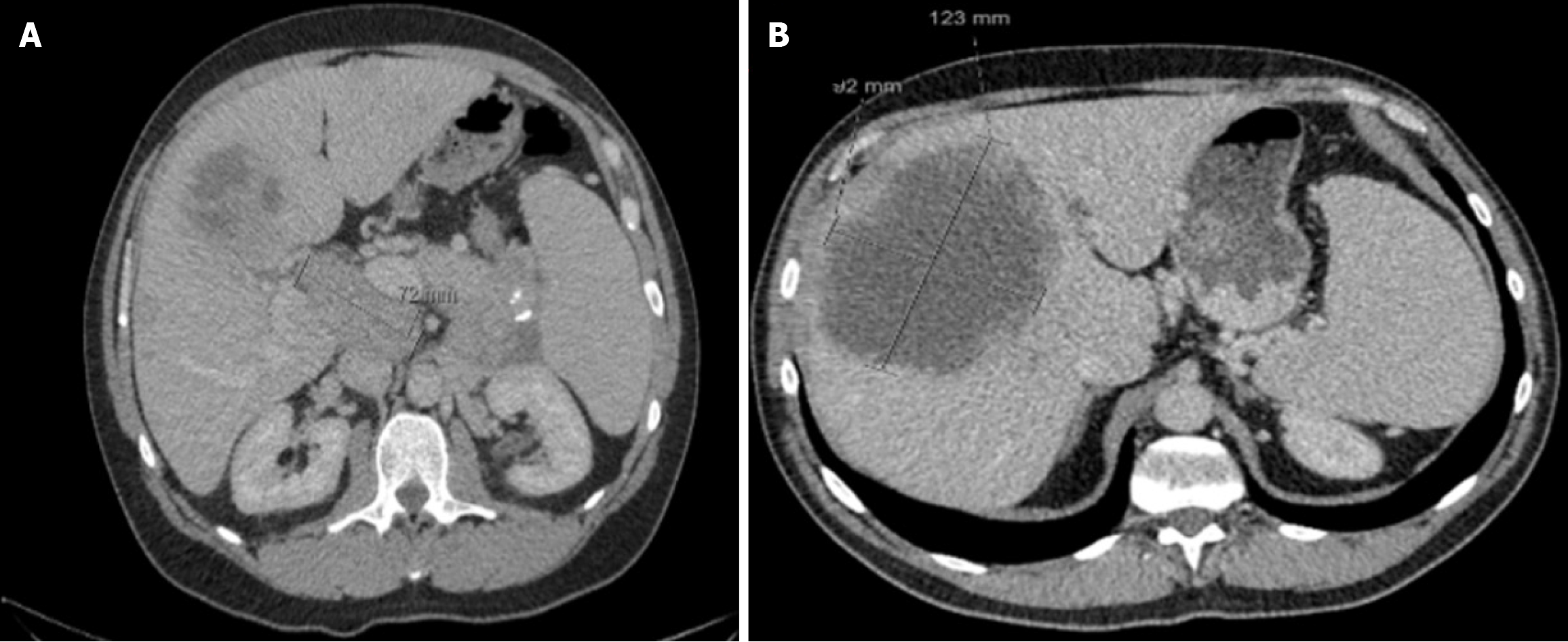
Disease progression was observed at 48 and 49 months (Figure 4), in line with a worsening clinical picture of new hypoglycaemic episodes (> 4 times weekly) and the development of biliary sepsis requiring hospitalization. Insulin was stopped due to frequent hypoglycaemic attacks during the most recent admission, but the hypoglycaemic episodes continued. He was treated with diazoxide up to 200 mg daily. Even with this and continuous intravenous glucose infusions, he developed recurrent hypoglycaemic episodes while in the hospital.
Further investigations were performed due to the evolving clinical picture from the initial presentation. A plasma C-peptide level of 6975 pmol/L with an insulin level 147 mU/L when plasma glucose was 2.4 mmol/L with a negative urine sulphonylurea screening test confirmed endogenous hyperinsulinism from a functional insulinoma. The patient was referred for peptide receptor radionuclide therapy (PRRT), in conjunction with SSA, due to disease progression despite systemic therapy, and died due to intractable hypoglycaemia and cardiac arrest.
The reported case highlights the initial presentation of non-functioning pNET subsequently turning out to become an insulinoma. The rate of transformation in case series is reported between 3.34% to 6.8%[38-40]. A review of the literature only highlights recent case reports with similar clinical pictures for comparison, highlighting the rarity and dilemma in clear management.
This case can be compared to that of a G3 Ki-67 index of 40% pNET with liver, lung and spinal metastasis, which was seen to turn into functional insulinoma 3 months post-diagnosis, post ineffective treatment with SSA (octreotide) to manage recurrent hypoglycaemia[40]. Glycaemic control was achieved to somewhat stable limits by Day 10 on diazoxide, octreotide and prednisolone. The case reflected reluctance from patients to try molecular-targeted therapies (everolimus/sunitinib) as a second line but also highlighted aggressive disease course in comparison to a median of 15 months in other reports[38]. The patient was given a one-off course of FOLFOX chemotherapy to manage the systemic oncological burden but had denied palliative chemotherapy and progressively declined.
A more recent case report documents a patient diagnosed with well-differentiated non-functional NET who underwent total pancreatectomy and started on an insulin regimen following surgery[41]. Two years post-surgery, the patient was identified to have recurrent disease in the mesentery and liver and received sunitinib along with PRRT. Symptoms of intermittent hypoglycaemia began a year post-treatment from recurrent disease, resulting in stopping the insulin regimen. Severe neuroglycopenic episodes and acute refractory hypoglycaemia in months following confirmed an insulinoma. Octreotide was trialled but not tolerated due to GI upset, thus, the patient’s hypoglycaemia was managed with diazoxide, dexamethasone and capecitabine (for palliation). No further chemotherapy, hormonal or immunologic therapy, was suitable and refractory hypoglycaemia was managed symptomatically as and when.
Overall, these cases, including our own, report the importance of recognizing new hypoglycaemia as a red-flag symptom for an investigation into malignant insulinoma developing from a previous nonfunctional pNET. Biochemical analysis is necessary and prompts a multidisciplinary care approach to tailor individualized treatment plans as the transformation from non-functioning to functioning pNET confers a poor prognosis and high symptomatic burden for patients. Ultimately, the prognosis is multifactorial and dependent on grade, stage and prognostic factors, but a coherent investigation can afford the most targeted treatment for tumor characteristics.
In conclusion, functioning neuroendocrine tumors are rare, and prompt management is difficult due to presenting signs and symptoms. Although extremely rare, a transformation of a nonfunctioning pNET to a functioning pNET (insulinoma) should be considered when unexplainable hypoglycaemia ensues in such a patient. Thus, this review and novel case highlight that further studies are warranted to understand the frequency of functional transformation of non-functioning pNETs. Moreover, managing refractory hypoglycaemia from malignant insulinomas is challenging, and there is an avenue for constant evidence-based recommendations of therapeutic efficacy.
| 1. | Alwan H, La Rosa S, Andreas Kopp P, Germann S, Maspoli-Conconi M, Sempoux C, Bulliard JL. Incidence trends of lung and gastroenteropancreatic neuroendocrine neoplasms in Switzerland. Cancer Med. 2020;9:9454-9461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Tan B, Zhang B, Chen H. Gastroenteropancreatic neuroendocrine neoplasms: epidemiology, genetics, and treatment. Front Endocrinol (Lausanne). 2024;15:1424839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 3. | Danek E, Kavnoudias H, McLean C, Gerstenmaier JF, Di Muzio B. Radiological Variability in Pancreatic Neuroendocrine Neoplasms: A 10-Year Single-Center Study on Atypical Presentations and Diagnostic Challenges. Biomedicines. 2025;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Magi L, Marasco M, Rinzivillo M, Faggiano A, Panzuto F. Management of Functional Pancreatic Neuroendocrine Neoplasms. Curr Treat Options Oncol. 2023;24:725-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Yan J, Yu S, Jia C, Li M, Chen J. Molecular subtyping in pancreatic neuroendocrine neoplasms: New insights into clinical, pathological unmet needs and challenges. Biochim Biophys Acta Rev Cancer. 2020;1874:188367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Sonbol MB, Mazza GL, Mi L, Oliver T, Starr J, Gudmundsdottir H, Cleary SP, Hobday T, Halfdanarson TR. Survival and Incidence Patterns of Pancreatic Neuroendocrine Tumors Over the Last 2 Decades: A SEER Database Analysis. Oncologist. 2022;27:573-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 7. | Das S, Dasari A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr Oncol Rep. 2021;23:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 256] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 8. | Uhlig J, Nie J, Gibson J, Cecchini M, Stein S, Lacy J, Kunz P, Kim HS. Epidemiology, treatment and outcomes of gastroenteropancreatic neuroendocrine neoplasms. Sci Rep. 2024;14:30536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 9. | Dasari A, Mehta K, Byers LA, Sorbye H, Yao JC. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162,983 cases. Cancer. 2018;124:807-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 10. | Lee CS, Perreault N, Brestelli JE, Kaestner KH. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 2002;16:1488-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 240] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 11. | Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA, Velculescu VE, Diaz LA Jr, Vogelstein B, Kinzler KW, Hruban RH, Papadopoulos N. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199-1203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1432] [Cited by in RCA: 1377] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 12. | Uccella S, La Rosa S, Metovic J, Marchiori D, Scoazec JY, Volante M, Mete O, Papotti M. Genomics of High-Grade Neuroendocrine Neoplasms: Well-Differentiated Neuroendocrine Tumor with High-Grade Features (G3 NET) and Neuroendocrine Carcinomas (NEC) of Various Anatomic Sites. Endocr Pathol. 2021;32:192-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 13. | Fernandez CJ, Agarwal M, Pottakkat B, Haroon NN, George AS, Pappachan JM. Gastroenteropancreatic neuroendocrine neoplasms: A clinical snapshot. World J Gastrointest Surg. 2021;13:231-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (3)] |
| 14. | Dromain C, Déandréis D, Scoazec JY, Goere D, Ducreux M, Baudin E, Tselikas L. Imaging of neuroendocrine tumors of the pancreas. Diagn Interv Imaging. 2016;97:1241-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103:153-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 1021] [Article Influence: 102.1] [Reference Citation Analysis (1)] |
| 16. | Granata V, Fusco R, Setola SV, Castelguidone ELD, Camera L, Tafuto S, Avallone A, Belli A, Incollingo P, Palaia R, Izzo F, Petrillo A. The multidisciplinary team for gastroenteropancreatic neuroendocrine tumours: the radiologist's challenge. Radiol Oncol. 2019;53:373-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Jensen RT, Berna MJ, Bingham DB, Norton JA. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer. 2008;113:1807-1843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 311] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 18. | Park MJ, Lee YH, Cho JH, Choi JH. Limited Palatal Muscle Resection for the Treatment of Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Ma ZY, Gong YF, Zhuang HK, Zhou ZX, Huang SZ, Zou YP, Huang BW, Sun ZH, Zhang CZ, Tang YQ, Hou BH. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J Gastroenterol. 2020;26:2305-2322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (5)] |
| 20. | Yang Z, Shi G. Comparative outcomes of pancreatic neuroendocrine neoplasms: A population-based analysis of the SEER database. Eur J Surg Oncol. 2022;48:2181-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 21. | Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M, Asa SL. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol. 2022;33:115-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 617] [Article Influence: 154.3] [Reference Citation Analysis (2)] |
| 22. | Yang M, Zeng L, Ke NW, Tan CL, Tian BL, Liu XB, Xiang B, Zhang Y. World Health Organization grading classification for pancreatic neuroendocrine neoplasms: a comprehensive analysis from a large Chinese institution. BMC Cancer. 2020;20:906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Bocchini M, Nicolini F, Severi S, Bongiovanni A, Ibrahim T, Simonetti G, Grassi I, Mazza M. Biomarkers for Pancreatic Neuroendocrine Neoplasms (PanNENs) Management-An Updated Review. Front Oncol. 2020;10:831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Moser E, Ura A, Vogel L, Steiger K, Mogler C, Evert M, Märkl B, Scheidhauer K, Martignoni M, Friess H, von Werder A, Marinoni I, Perren A, Klöppel G, Kasajima A. ARX, PDX1, ISL1, and CDX2 Expression Distinguishes 5 Subgroups of Pancreatic Neuroendocrine Tumors With Correlations to Histology, Hormone Expression, and Outcome. Mod Pathol. 2024;37:100595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 25. | Hackeng WM, Schelhaas W, Morsink FHM, Heidsma CM, van Eeden S, Valk GD, Vriens MR, Heaphy CM, Nieveen van Dijkum EJM, Offerhaus GJA, Dreijerink KMA, Brosens LAA. Alternative Lengthening of Telomeres and Differential Expression of Endocrine Transcription Factors Distinguish Metastatic and Non-metastatic Insulinomas. Endocr Pathol. 2020;31:108-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Sundin A, Arnold R, Baudin E, Cwikla JB, Eriksson B, Fanti S, Fazio N, Giammarile F, Hicks RJ, Kjaer A, Krenning E, Kwekkeboom D, Lombard-Bohas C, O'Connor JM, O'Toole D, Rockall A, Wiedenmann B, Valle JW, Vullierme MP; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine & Hybrid Imaging. Neuroendocrinology. 2017;105:212-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 324] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 27. | Pancreatic Neuroendocrine Tumor Stages [Internet]. ACS; 2018 [cited 2025 Feb 12]. Available from: https://www.cancer.org/cancer/types/pancreatic-neuroendocrine-tumor/detection-diagnosis-staging/net-staging.html#:~:text=Well%2Ddifferentiated%20tumors%20(which%20include,index%20of%20more%20than%2020%25. |
| 28. | Survival rates for pancreatic neuroendocrine tumor [Internet]. ACS; 2023 [cited 2025 Feb 12]. Available from: https://www.cancer.org/cancer/types/pancreatic-neuroendocrine-tumor/detection-diagnosis-staging/survival-rates.html. |
| 29. | Friebe L, Freitag MT, Braun M, Nicolas G, Bauman A, Bushnell D, Christ E, Wild D. Peptide Receptor Radionuclide Therapy Is Effective for Clinical Control of Symptomatic Metastatic Insulinoma: A Long-Term Retrospective Analysis. J Nucl Med. 2024;65:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1348] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 31. | Baum RP, Kluge AW, Kulkarni H, Schorr-Neufing U, Niepsch K, Bitterlich N, van Echteld CJ. [(177)Lu-DOTA](0)-D-Phe(1)-Tyr(3)-Octreotide ((177)Lu-DOTATOC) For Peptide Receptor Radiotherapy in Patients with Advanced Neuroendocrine Tumours: A Phase-II Study. Theranostics. 2016;6:501-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 32. | Antonella M, Laura O, Stefania D, Joniada D, Matteo S. Should peptide receptors radionuclide therapy (PRRT) be considered as a treatment of choice in functioning metastatic insulinomas? A review of literature and our center experience. Clin Transl Imaging. 2022;10:425-433. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Hofland J, Falconi M, Christ E, Castaño JP, Faggiano A, Lamarca A, Perren A, Petrucci S, Prasad V, Ruszniewski P, Thirlwell C, Vullierme MP, Welin S, Bartsch DK. European Neuroendocrine Tumor Society 2023 guidance paper for functioning pancreatic neuroendocrine tumour syndromes. J Neuroendocrinol. 2023;35:e13318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 142] [Reference Citation Analysis (0)] |
| 34. | Kos-Kudła B, Castaño JP, Denecke T, Grande E, Kjaer A, Koumarianou A, de Mestier L, Partelli S, Perren A, Stättner S, Valle JW, Fazio N. European Neuroendocrine Tumour Society (ENETS) 2023 guidance paper for nonfunctioning pancreatic neuroendocrine tumours. J Neuroendocrinol. 2023;35:e13343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 156] [Reference Citation Analysis (0)] |
| 35. | Gregoire E, Le Treut YP. Liver transplantation for primary or secondary endocrine tumors. Transpl Int. 2010;23:704-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Cloyd JM, Poultsides GA. Non-functional neuroendocrine tumors of the pancreas: Advances in diagnosis and management. World J Gastroenterol. 2015;21:9512-9525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Pavel ME, Hainsworth JD, Baudin E, Peeters M, Hörsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM, Öberg K, Van Cutsem E, Yao JC; RADIANT-2 Study Group. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 767] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 38. | Wynick D, Williams SJ, Bloom SR. Symptomatic secondary hormone syndromes in patients with established malignant pancreatic endocrine tumors. N Engl J Med. 1988;319:605-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 52] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | de Mestier L, Hentic O, Cros J, Walter T, Roquin G, Brixi H, Lombard-Bohas C, Hammel P, Diebold MD, Couvelard A, Ruszniewski P, Cadiot G. Metachronous hormonal syndromes in patients with pancreatic neuroendocrine tumors: a case-series study. Ann Intern Med. 2015;162:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Buddhavarapu VS, Dhillon G, Grewal HS, Soles B, Halbur L, Surani S, Kashyap R. Transformation of pancreatic nonfunctioning neuroendocrine tumor into metastatic insulinoma: A rare case report. Clin Case Rep. 2023;11:e8152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 41. | Barai R, Barai R, Kanin M, Grock S. #1697411 A New Diagnosis of Hypoglycemia: The Progression of a Non-functional Pancreatic Neuroendocrine Tumor to a Metastatic Insulinoma. Endocr Pract. 2024;30:S74-S75. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/