Published online May 22, 2021. doi: 10.4291/wjgp.v12.i3.51
Peer-review started: September 23, 2020
First decision: November 16, 2020
Revised: November 30, 2020
Accepted: February 25, 2021
Article in press: February 25, 2021
Published online: May 22, 2021
Processing time: 232 Days and 12 Hours
Cytomegalovirus (CMV) is the most common viral pathogen after liver trans
To determine the incidence of reactivated CMV prior to LT.
This was a prospective cohort study evaluating adult patients who underwent LT between 2014 and 2016. A plasma sample was obtained from all patients for CMV quantitative real-time PCR testing right before transplantation. Patients were followed for at least 1 year to assess the following outcomes: Incidence of CMV infection, organ rejection and overall mortality.
A total of 72 patients were enrolled. Four patients died before transplantation, thus 68 patients were followed up for a median of 44 mo (20-50 mo). In 23/72 patients (31.9%) CMV was reactivated before transplantation. Post-transplan
This study shows that CMV infection is common in patients with chronic liver disease just before LT, but the clinical impact of this infection seems to be negligible.
Core Tip: Cytomegalovirus (CMV) commonly reactivates before liver transplantation in patients with chronic liver conditions. This prospective cohort study demonstrates for the first time that although frequent, CMV reactivation has limited clinical impact when occurring just before liver transplantation.
- Citation: Stadnik CMB, Caurio CFB, Rodrigues-Filho EM, Nedel WL, Cantisani GP, Zanotelli ML, Pasqualotto AC. Impact of cytomegalovirus reactivation just before liver transplantation: A prospective cohort study. World J Gastrointest Pathophysiol 2021; 12(3): 51-58
- URL: https://www.wjgnet.com/2150-5330/full/v12/i3/51.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v12.i3.51
Cytomegalovirus (CMV) is the most common viral pathogen after liver transplantation (LT). Most infections occur between the 3rd and the 12th postoperative week, reaching the highest incidence around the 5th post-transplant week. The overall incidence of CMV infection is between 50%-60% in liver transplant recipients, with 20%-30% of patients demonstrating symptomatic infection[1]. The incidence of post-transplant CMV infection depends mainly on the recipient and donor serological profile. Accordingly, it is more frequent in the context of positive immunoglobulin G (IgG) CMV serology in donors, and negative recipients (i.e., D+/R- status), with more than half of these patients developing visceral disease, in the absence of antiviral prophylaxis[2]. The lowest-risk groups include positive serology for both donors and recipients (D+/R+ status) and a negative status for both donors and recipients (D-/R-). The incidence of CMV infection in such low-risk groups ranges between 5%-40%[3]. Intense immunosuppression and fulminant hepatitis transplantation are also important risk factors for infection.
Although reactivation of CMV infection is mostly described in the context of overt immunosuppression, reactivation may also occur in critically ill immunocompetent patients[4-7] associated with increased mortality[8,9]. A subgroup of particular interest is patients with chronic liver diseases[10,11]. Whether CMV reactivation in these individuals that are listed for LT has any impact on post-transplant outcomes has not been determined[12]. Therefore, here we investigate the frequency and impact of CMV reactivation in patients with chronic liver disease on the waiting list for LT. In particular, we were interested to study the impact of plasma circulating CMV DNA in terms of organ rejection, reactivation of CMV post-transplantation and overall mortality.
This was a prospective cohort study that evaluated adult (≥ 18 years of age) patients with chronic liver disease listed to undergo LT at Santa Casa de Misericórdia de Porto Alegre. Santa Casa is a referral hospital for organ transplantation in Latin America, and performs approximately 60 liver transplant procedures every year. Patients were non-consecutively enrolled between the years 2014 and 2016.
Clinical and demographic data obtained in this study included age, gender, presence of comorbidities, Model for End-Stage Liver Disease (MELD) score, donor and recipient IgG serostatus for CMV infection, presence of hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, renal insufficiency, hepatocarcinoma, fulminant hepatitis and re-transplantation.
Patients were followed for a minimum of 1 year after LT. During this period, all episodes of CMV reactivation [detected by either quantitative real-time PCR (qRT-PCR) and/or pp65 antigenemia] were documented, as well as events of CMV disease, organ rejection and overall mortality. Screening for CMV reactivation was performed monthly for the first three months after transplantation or whenever the patient presented with clinical symptoms such as fever, fatigue, organ rejection or in the case of diagnostic uncertainty (according to the institutional protocol of low resource countries). Antiviral prophylaxis was not used, instead preemptive treatment against CMV was applied to all patients, including sero-discordant patients.
At the time the enrolled participants were called in for LT, 4 mL of plasma was collected in an ethylene diamine tetraacetic acid tube centrifuged at 1300 g for 15 min and frozen at -80ºC until nucleic acid extraction for analysis of CMV qRT-PCR.
DNA was extracted using the Qiagen DNA Mini Kit (Qiagen Inc., Valencia, United States) following the manufacturer’s instructions. qRT-PCR reactions were performed using an in-house assay calibrated with the 1st WHO International Standard for Human Cytomegalovirus for Nucleic Acid Amplification Techniques NIBSC code: 09/162 that targets the genes UL 34 and UL 80.5. Primers and probes used in this study were described by Ho and Barry and the sequences are shown in the supplementary material with some modifications in the probe design[13]. The reagents and concentration of the qRT-PCR reaction are shown in the supplementary material. Amplification was performed in an 7500 real-time PCR system (Thermo Scientific, United States), the thermocycling conditions for the qRT-PCR reaction were: 1 cycle of 2 min at 50°C; 2 min at 95°C; followed by 40 cycles of 15 s at 95°C, and 1 min at 60°C. The results are reported in International Units (IU/mL) according to CMV World Health Organization standards[14]. The limit of detection and quantification of the test was 60.26 IU/mL, and the results were considered positive only above this cut-off value.
Statistical calculations were performed using SPSS 20.0 software. The Chi-square test or Fisher’s exact test compared categorical variables, as appropriate. For continuous variables, we used the Student t-test or Mann-Whitney test, as appropriate. Multi
Considering the primary endpoint of the study and based on studies showing that approximately 50% of cirrhotic patients have detectable plasma CMV DNA[15], 64 patients would need to be studied, considering an alpha error of 0.05 and 80% of power. Thus, respecting a confidence interval of 95%, and to account for possible losses (10%), we estimated to include 70 patients.
This study was approved by the Research Ethics Committee at Santa Casa de Misericordia of Porto Alegre, No. 294/2010. All patients signed an informed consent form and agreed to participate in the study.
A total of 72 patients were enrolled in the study. Four patients died before transplantation; thus, 68 patients were followed up for a median of 44 mo (25%-75% percentile: 20-50 mo). Clinical and demographic characteristics of the patients are shown in Table 1. The majority of patients were female (70.8%) had active chronic hepatitis C infection (63.9%) and hepatocellular carcinoma (58.3%). Only 5 patients (6.9%) were CMV sero-discordant (D+/R-).
| Total (%) | Reactivation (%) | RR (95%CI) | P value | |
| Number of patients (%) | 72 (100) | 23 (32) | ||
| Gender (male) | 21 (29.2) | 7 (33.3) | 1.09 (0.37-3.23) | 0.871 |
| Mean age, years (SD) | 56.3 (9.6) | 57.3 (9.2) | NA | 0.900 |
| MELD, median (IqR) | 12 (14) | 12 (12) | NA | 0.712 |
| Lymphocyte count, median (IqR) | 929 (808) | 929 (770) | NA | 0.471 |
| CMV receptor IgG-negative | 5 (8.7) | 2 (40) | 0.68 (0.11-4.40) | 0.652 |
| HCV | 46 (63.9) | 15 (32.6) | 1.09 (0.39-3.1) | 0.872 |
| HBV | 5 (6.9) | 1 (20) | 0.51 (0.05-4.9) | 1.000 |
| Hepatocarcinoma | 42 (58.3) | 14 (33.3) | 1.17 (0.42-3.2) | 0.765 |
| Fulminant hepatitis | 2 (3) | 0 | NA | NA |
| Diabetes mellitus | 24 (33.3) | 8 (33.3) | 1.1 (0.38-3.13) | 0.858 |
| Renal failure | 8 (11.1) | 5 (62.5) | 4.26 (0.92-19.7) | 0.100 |
| Re-transplant | 2 (3) | 0 | NA | NA |
CMV reactivation was demonstrated in 31.9% (23/72) of patients before transplantation. Median plasma CMV DNA concentration in these patients was 1.212 IU/mL (25%-75% percentile: 560-4.197 IU/mL). In addition, two IgG negative patients had CMV reactivation but none received treatment at that time (7.486 and 7.917 UI/mL). Following LT, CMV infection occurred in 16/67 patients (23.8%) including two patients with IgG negative/PCR positive. At univariate analysis, the only statistically significant factor associated with post-transplant CMV infection was a CMV negative recipient with a positive CMV donor (Table 2). Multivariate analysis confirmed this as the only statistically significant factor for the prediction of post-transplant CMV infection [Odds ratio (OR): 11.5; 95% confidence interval (CI): 1.1-120; P = 0.04].
| CMV (%) | No CMV (%) | RR (95%CI) | P value | |
| Number of patients (%) | 16/68 (23.5) | 52/68 (76.5%) | ||
| CMV reactivation before transplantation | 7/16 (43.8) | 15/52 (28.8) | 1.91 (0.6-6.1) | 0.265 |
| Quantitative PCR pre-transplant (IU/mL), mean (SD) | 2862 (5696) | 868 (2756) | NA | 0.154 |
| Gender (male) | 4/16 (25) | 16/52 (30.8) | 0.75 (0.2-2.7) | 0.762 |
| Mean age, years (SD) | 55 (10.3) | 57.3 (8) | NA | 0.373 |
| MELD score, median (IqR) | 11 (4) | 12 (11) | NA | 0.254 |
| Lymphocyte count, median (IqR) | 1101 (1109) | 918 (754) | NA | 0.580 |
| Organ rejection | 3/16 (18.7) | 8/52 (15.3) | 1.27 (0.3-5.5) | 0.716 |
| CMV-negative receptor | 3/16 (18.7) | 1/52 (1.9) | 11.7 (1.1-122.6) | 0.038 |
| Hepatitis C infection | 9/16 (56.2) | 34/52 (65.4) | 0.7 (0.2-2.1) | 0.508 |
| Hepatitis B infection | 1/16 (6.2) | 4/52 (7.7) | 0.8 (0.1-7.1) | 0.100 |
| Hepatocarcinoma | 9/16 (56.2) | 30/52 (57.7) | 0.9 (0.3-2.9) | 0.919 |
| Fulminant hepatitis | 0 | 1/52 (1.9) | NA | NA |
| Diabetes mellitus | 6/16 (37.5) | 16/52 (30.8) | 1.3 (0.4-4.3) | 0.615 |
| Renal failure | 2/7 (12.5) | 5/52 (9.6) | 1.3 (0.2-7.7) | 0.664 |
| Re-transplantation | 1/16 (6.2) | 1/52 (1.9) | 3.4 (0.2-57.7) | 0.418 |
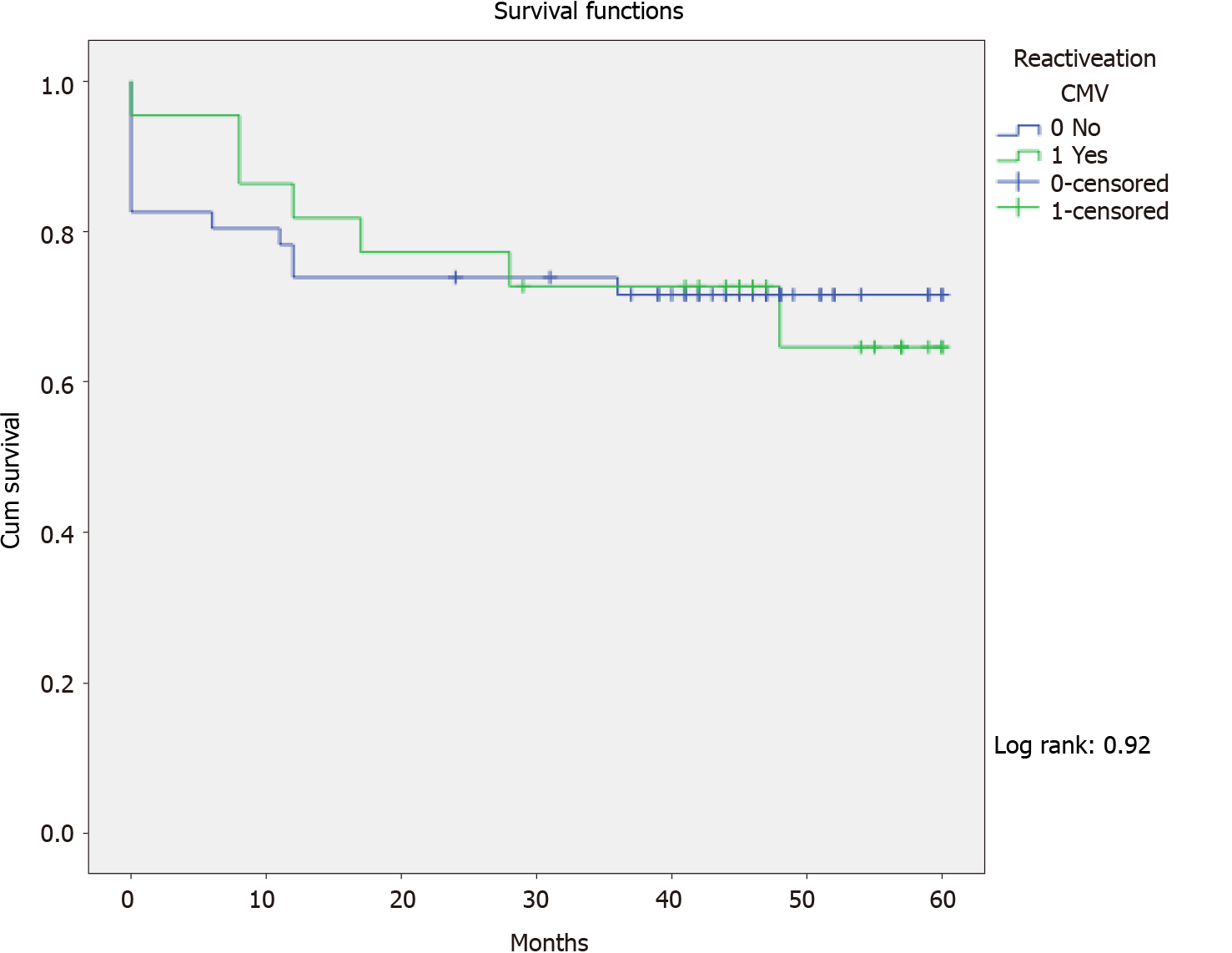
The crude mortality rate was 20/68 (29.4%), median 7.7 mo (perc 25-75: 1-12), and 7/22 (31.8%) in patients with pre-transplant CMV reactivation (P = 0.763). In Kaplan-Meier analyses, pre-transplant CMV reactivation had no impact on mortality following LT (log rank: 0.92) (Figure 1). Cox regression analysis also identified no statistically significant factor for mortality in this cohort.
This is the first study to document the frequency of CMV infection just before LT in patients with chronic liver disease, using a very sensitive diagnostic tool (qPCR). We observed a high frequency of CMV infection in these patients (31.9%), even though it had no impact on clinically significant variables in the post-transplant period, including CMV infection/disease, organ rejection and mortality. CMV viremic patients usually had a low CMV viral load (median: 1212 IU/mL).
Our results were probably influenced by the profile of patients being transplanted in our institution, which follows the modified Milan criteria[16], together with the proportion of patients with hepatocellular carcinoma (58.3%), as these patients usually have better performance with a lower MELD, which could induce a lower CMV reactivation rate. Nevertheless, in a similar study, a pre-LT reactivation incidence of 0.7% was found, much lower than that in our study[12]. Our findings were similar to the incidence of reactivation in intensive care patients (31%; 95%CI: 24%-39%) as shown in a recent meta-analysis[9].
When comparing with the findings in the literature, Lapiński et al[17] evaluated 123 patients with chronic HCV hepatitis for the presence of CMV infection, also determined by qPCR. CMV DNAemia, predominantly at low levels, was detected in 18 (14.6%) patients. Similar to our study, there was no correlation with HCV viral load, and detection of CMV DNA did not result in clinical and laboratory changes[17]. Bayram et al[15] quantitatively evaluated the presence of CMV infection in liver biopsy samples from 44 patients with chronic HBV and 25 patients with chronic HCV infection. CMV infection was demonstrated by qPCR in 52.3% of patients with HBV and in 36% of patients with HCV. Histological activity scores (necroinflammation and fibrosis) were worse in patients who were infected with CMV[15].
We observed that CMV was reactivated in 23% of patients in the post-transplant period, which is comparable to other studies[1-3] as most of them were low or moderate risk for infection (CMV receptor positive in 93%). Moreover, we did not find any association between reactivation before transplantation and reactivation after transplantation in both univariate and multivariate analyses. According to the literature, only a high risk for CMV infection (D+/R-) was statistically associated with CMV reactivation following LT (OR:11.5, 95%CI: 1.1-120, P = 0.04). We also did not identify pre-transplant CMV reactivation as a risk factor for organ rejection or overall mortality when both 30 d and 1-year mortality were considered.
This investigation has several limitations, including being a single-center study. In addition, patient selection occurred by convenience (sampling was not consecutive), which may have added some selection bias. Given that the reactivation rate was lower than initially expected (32% vs 50%), despite the sample calculation, we had small numbers of some of the events, which may have mainly affected the multivariate analysis.
The findings of this study suggest that pre-transplant CMV reactivation has no influence on LT results, and has no impact on post-transplant CMV reactivation or overall mortality. Based on this study, screening for CMV DNAemia before LT does not seem justified. A larger sample size, better quality and multicenter studies are required to fully elucidate this issue.
The overall incidence of cytomegalovirus (CMV) infection is between 50%-60% in liver transplant recipients, with 20%-30% of patients demonstrating a symptomatic infection[1]. The incidence of post-transplant CMV infection depends mainly on the recipient and donor serological profile. The lowest-risk groups include positive serology for both donors and recipients (D+/R+ status) and a negative status for both donors and recipients (D-/R-). Although reactivation of CMV infection is mostly described in the context of overt immunosuppression, reactivation may also occur in critically ill immunocompetent patients[4-7] associated with increased mortality[8,9].
A subgroup of particular interest is patients with chronic liver diseases[10,11]. Whether CMV reactivation in these individuals that are listed for liver transplantation has any impact on post-transplant outcomes has not been determined[12].
To determine the incidence of reactivated CMV prior to liver transplantation.
This was a prospective cohort study that evaluated adult (≥ 18 years of age) patients with chronic liver disease listed to undergo liver transplantation at a referral hospital for organ transplantation in Latin America. Patients were followed for a minimum of 1 year after liver transplantation. During this period, all episodes of CMV reactivation [detected by either quantitative real-time PCR (qRT-PCR) and/or pp65 antigenemia] were documented, as well as events of CMV disease, organ rejection and overall mortality. Screening for CMV reactivation was performed monthly for the first three months after transplantation or whenever the patient presented with clinical symptoms. At the time the enrolled participants were called in for liver transplan
A total of 72 patients were enrolled in the study. Four patients died before transplantation, thus 68 patients were followed up for a median of 44 mo (25%-75% percentile: 20-50 mo). CMV reactivation was demonstrated in 31.9% (23/72) of patients before transplantation. Median plasma CMV DNA concentration in these patients was 1.212 IU/mL (25%-75% percentile: 560-4.197 IU/mL). Following liver transplantation, CMV infection occurred in 16/67 patients (23.8%).
The crude mortality rate was 20/68 (29.4%), median 7.7 mo (perc 25-75: 1-12), and 7/22 (31.8%) in patients with pre-transplant CMV reactivation (P = 0.763). In Kaplan-Meier analyses, pre-transplant CMV reactivation had no impact on mortality following liver transplantation (log rank: 0.92) (Figure 1). Cox regression analysis also identified no statistically significant factor for mortality in this cohort.
The findings of this study suggest that pre-transplant CMV reactivation has no influence on liver transplantation results, and has no impact on post-transplant CMV reactivation or overall mortality.
Based on this study, screening for CMV DNAemia before liver transplantation does not seem justified. A larger sample size, better quality and multicenter studies are required to fully elucidate this issue.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Brazilian Society of Infectious Diseases, No. 24134.
Specialty type: Transplantation
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Akbulut S, Chiu KW S-Editor: Fan JR L-Editor: Webster JR P-Editor: Yuan YY
| 1. | Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, Humar A; The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102:900-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 870] [Article Influence: 124.3] [Reference Citation Analysis (1)] |
| 2. | Brasil IRC, Custodio-Lima J, Sampaio RL, Pierre AMM, Esmeraldo TM, Lima RVC, Lima LFA, Esmeraldo RM. Pre-emptive Therapy for Cytomegalovirus in Post-transplantation Liver Patients With Donor-Positive/Recipient-Negative Serostatus. Transplant Proc. 2017;49:871-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Mengelle C, Rostaing L, Weclawiak H, Rossignol C, Kamar N, Izopet J. Prophylaxis versus pre-emptive treatment for prevention of cytomegalovirus infection in CMV-seropositive orthotopic liver-transplant recipients. J Med Virol. 2015;87:836-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Jain M, Duggal S, Chugh TD. Cytomegalovirus infection in non-immunosuppressed critically ill patients. J Infect Dev Ctries. 2011;5:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | von Müller L, Klemm A, Weiss M, Schneider M, Suger-Wiedeck H, Durmus N, Hampl W, Mertens T. Active cytomegalovirus infection in patients with septic shock. Emerg Infect Dis. 2006;12:1517-1522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Kutza AS, Muhl E, Hackstein H, Kirchner H, Bein G. High incidence of active cytomegalovirus infection among septic patients. Clin Infect Dis. 1998;26:1076-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 142] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Jaber S, Chanques G, Borry J, Souche B, Verdier R, Perrigault PF, Eledjam JJ. Cytomegalovirus infection in critically ill patients: associated factors and consequences. Chest. 2005;127:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Ziemann M, Sedemund-Adib B, Reiland P, Schmucker P, Hennig H. Increased mortality in long-term intensive care patients with active cytomegalovirus infection. Crit Care Med. 2008;36:3145-3150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Li X, Huang Y, Xu Z, Zhang R, Liu X, Li Y, Mao P. Cytomegalovirus infection and outcome in immunocompetent patients in the intensive care unit: a systematic review and meta-analysis. BMC Infect Dis. 2018;18:289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Varani S, Lazzarotto T, Margotti M, Masi L, Gramantieri L, Bolondi L, Landini MP. Laboratory signs of acute or recent cytomegalovirus infection are common in cirrhosis of the liver. J Med Virol. 2000;62:25-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Brindley SM, Lanham AM, Karrer FM, Tucker RM, Fontenot AP, Mack CL. Cytomegalovirus-specific T-cell reactivity in biliary atresia at the time of diagnosis is associated with deficits in regulatory T cells. Hepatology. 2012;55:1130-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Bruminhent J, Razonable RR. Outcomes of patients with cytomegalovirus viremia at the time of liver transplantation. Liver Transpl. 2014;20:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Jones S, Webb EM, Barry CP, Choi WS, Abravaya KB, Schneider GJ, Ho SY. Commutability of Cytomegalovirus WHO International Standard in Different Matrices. J Clin Microbiol. 2016;54:1512-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Fryer JF, Heath AB, Minor PD; Collaborative Study Group. A collaborative study to establish the 1st WHO International Standard for human cytomegalovirus for nucleic acid amplification technology. Biologicals. 2016;44:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Bayram A, Ozkur A, Erkilic S. Prevalence of human cytomegalovirus co-infection in patients with chronic viral hepatitis B and C: a comparison of clinical and histological aspects. J Clin Virol. 2009;45:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Firl DJ, Sasaki K, Agopian VG, Gorgen A, Kimura S, Dumronggittigule W, McVey JC, Iesari S, Mennini G, Vitale A, Finkenstedt A, Onali S, Hoppe-Lotichius M, Vennarecci G, Manzia TM, Nicolini D, Avolio AW, Agnes S, Vivarelli M, Tisone G, Ettorre GM, Otto G, Tsochatzis E, Rossi M, Viveiros A, Cillo U, Markmann JF, Ikegami T, Kaido T, Lai Q, Sapisochin G, Lerut J; European Hepatocellular Cancer Liver Transplant Study Group; Aucejo FN. Charting the Path Forward for Risk Prediction in Liver Transplant for Hepatocellular Carcinoma: International Validation of HALTHCC Among 4,089 Patients. Hepatology. 2020;71:569-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Lapiński TW, Kovalchuk O, Parfieniuk A, Flisiak R. [Prevalence, clinical and therapeutical implications of active CMV infection in patients with chronic hepatitis C]. Przegl Epidemiol. 2009;63:305-309. [PubMed] |