Published online Jun 20, 2020. doi: 10.4291/wjgp.v11.i4.84
Peer-review started: January 7, 2020
First decision: February 19, 2020
Revised: April 4, 2020
Accepted: April 18, 2020
Article in press: April 18, 2020
Published online: June 20, 2020
Processing time: 160 Days and 17.5 Hours
The P2X7 receptor is expressed by enteric neurons and enteric glial cells. Studies have demonstrated that administration of a P2X7 receptor antagonist, brilliant blue G (BBG), prevents neuronal loss.
To report the effects of BBG in ileum enteric neurons immunoreactive (ir) following experimental ulcerative colitis in Rattus norvegicus albinus.
2,4,6-trinitrobenzene sulfonic acid (TNBS group, n = 5) was injected into the distal colon. BBG (50 mg/kg, BBG group, n = 5) or vehicle (sham group, n = 5) was given subcutaneously 1 h after TNBS. The animals were euthanized after 24 h, and the ileum was removed. Immunohistochemistry was performed on the myenteric plexus to evaluate immunoreactivity for P2X7 receptor, neuronal nitric oxide synthase (nNOS), choline acetyltransferase (ChAT), HuC/D and glial fibrillary acidic protein.
The numbers of nNOS-, ChAT-, HuC/D-ir neurons and glial fibrillary acidic protein-ir glial cells were decreased in the TNBS group and recovered in the BBG group. The neuronal profile area (μm2) demonstrated that nNOS-ir neurons decreased in the TNBS group and recovered in the BBG group. There were no differences in the profile areas of ChAT- and HuC/D-ir neurons.
Our data conclude that ileum myenteric neurons and glial cells were affected by ulcerative colitis and that treatment with BBG had a neuroprotective effect. Thus, these results demonstrate that the P2X7 receptor may be an important target in therapeutic strategies.
Core tip: This work aims to analyze the effects of experimental ulcerative colitis (EUC) in ileum myenteric neurons immunoreactive (ir) for P2X7 receptor, neuronal nitric oxide synthase, choline acetyltransferase, HuC/D and enteric glial cells immunoreactive for glial fibrillary acidic protein. The animals were treated with P2X7 receptor antagonist, brilliant blue G (BBG). The results showed that the numbers of neuronal nitric oxide synthase-, choline acetyltransferase-, HuC/D-ir neurons and glial fibrillary acidic protein-ir glial cells were decreased in the EUC group and recovered in the animals treated with BBG. BBG treatment demonstrated that the P2X7 receptor may be a possible therapeutic target in the treatment of the EUC.
- Citation: Souza RF, Evangelinellis MM, Mendes CE, Righetti M, Lourenço MCS, Castelucci P. P2X7 receptor antagonist recovers ileum myenteric neurons after experimental ulcerative colitis. World J Gastrointest Pathophysiol 2020; 11(4): 84-103
- URL: https://www.wjgnet.com/2150-5330/full/v11/i4/84.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v11.i4.84
The enteric nervous system (SNE) performs functions in gastrointestinal tract motility, control of gastric acid secretion, regulation of fluid movement through the epithelium, changes in local blood flow, and interactions with the endocrine and intestinal immune systems[1,2]. This system has two ganglionic plexuses, the myenteric plexus and the submucosal plexus. The myenteric plexus is located between the outer longitudinal muscular layer and the circular muscle layer, extending throughout the digestive tract from the esophagus to the rectum. The submucosal plexus is found predominantly in the small and large intestines and has a smaller ganglion, and its interconnected fibers are thinner compared to those of the myenteric plexus[1,2]. Enteric glial cells have functions to support neurons, regulate synaptic transmission, and release cytokines[3,4].
Inflammatory bowel diseases (IBDs) are disorders that affect the digestive tract. These problems include ulcerative colitis and Crohn's disease[5]. There are changes in SNE populations in ulcerative colitis of animals and humans[6-10]. Additionally, McCready et al[11] described the expansion of the inflammatory process from the distal ileum.
Studies have shown that the release of ATP by enteric neurons as noncholinergic and nonadrenergic neurotransmitters may be related to intestinal motility[12-15]. Purinergic receptors are classified into P1 and P2, where P1 receptors are activated by the adenine nucleoside and P2 receptors are activated by the nucleotide ADP (adenosine diphosphate) or ATP[16]. P2X receptors are ion channels with selective permeability to Ca²+, K+ and Na+ cations, and they can be found in the central nervous system, ENS and enteric glial cells[15,17,18]. Seven types of P2X receptors have been described: P2X1, P2X2, P2X3, P2X4, P2X5, P2X6 and P2X7[18-21].
The P2X7 receptor has been described in the ENS[22,23]. Studies show that brilliant blue G (BBG) is a P2X7 antagonist, and its low toxicity[24,25] and high selectivity make this compound an ideal candidate to block the adverse effects of P2X7 receptor activation[26]. P2X7 receptor-deficient animals have been shown to exhibit improvements in their overall condition when subjected to experimental ulcerative colitis[27]. Peng et al[28] demonstrated recovery of the rat spinal cord after mechanical injury following BBG administration. Additionally, Palombit et al[29] observed recovery of BBG-treated enteric neurons following an ischemia and reperfusion protocol.
This work aims to analyze the effects of experimental ulcerative colitis in neurons immunoreactive (ir) for neuronal nitric oxide synthase (nNOS), choline acetyltransferase (ChAT) which is marker for intrinsic primary afferent neurons (IPANs) and excitatory motor neuron, and HuC/D (a pan-neuronal marker) and enteric glial cells immunoreactive for glial fibrillary acidic protein (GFAP) in the ileum in animals treated with BBG.
The animal experiments in this study were conducted according to the current regulations of the Ethics Committee on Animal Use of the Biomedical Science Institute of the University of São Paulo. Furthermore, all protocols were approved by the Ethics Committee on Animal Use of the Biomedical Science Institute of the University of São Paulo (Protocol 68/2016). Young male Wistar rats (200–300 g body weight) were maintained under standard conditions at 21 °C with a 12-h light-dark cycle. All groups were supplied with water ad libitum.
The rats were anesthetized with a mixture of xylazine (20 mg/kg) and ketamine (100 mg/kg) administered subcutaneously. Inflammation was induced through the intrarectal insertion of a polypropylene 8 cm cannula. 2,4,6-trinitrobenzene sulfonic acid (TNBS, Sigma, Saint Louis, United States) was injected at a dose of 30 mg/kg in 600 μL of 30% ethanol in the colon lumen (n = 5). Sham animals (n = 5) were injected with vehicle. BBG (50 mg/kg, Sigma Aldrich, United Kingdom, n = 5) or saline was injected 1 h following TNBS injection (n = 5)[28,29]. The survival time after colitis induction was 24 h.
For macroscopic and microscopic analyses, colitis was assessed according to macroscopic colonic injury[30]. The scores were stratified as follows: 0 = normal, 1 = presence of hyperemia without ulcers, 2 = ulcerations without hyperemia, 3 = ulcerations at one site, 4 = two or more sites of ulcerations, 5 = sites of damage extending > 1 cm, and 6 to 10 = sites of damage extending > 2 cm, with the score increasing by 1 for each additional cm[30]. The microscopic colitis scores were assessed using a scoring system adapted from Erdogan et al[31] and Fabia et al[32]. The scores were categorized as follows according to the corresponding parameters. Ulcerations: 0 = no ulcer, 1 = single ulceration not exceeding the lamina muscularis mucosa, 2 = ulcerations not exceeding the mucosa, and 3 = ulcerations exceeding the submucosa. Edema (submucosa): 0 = no edema, 1 = mild edema, 2 = moderate edema, and 3 = severe edema. Inflammatory cell infiltration: 0 = no infiltration, 1 = mild infiltration, 2 = moderate infiltration, and 3 = dense infiltration.
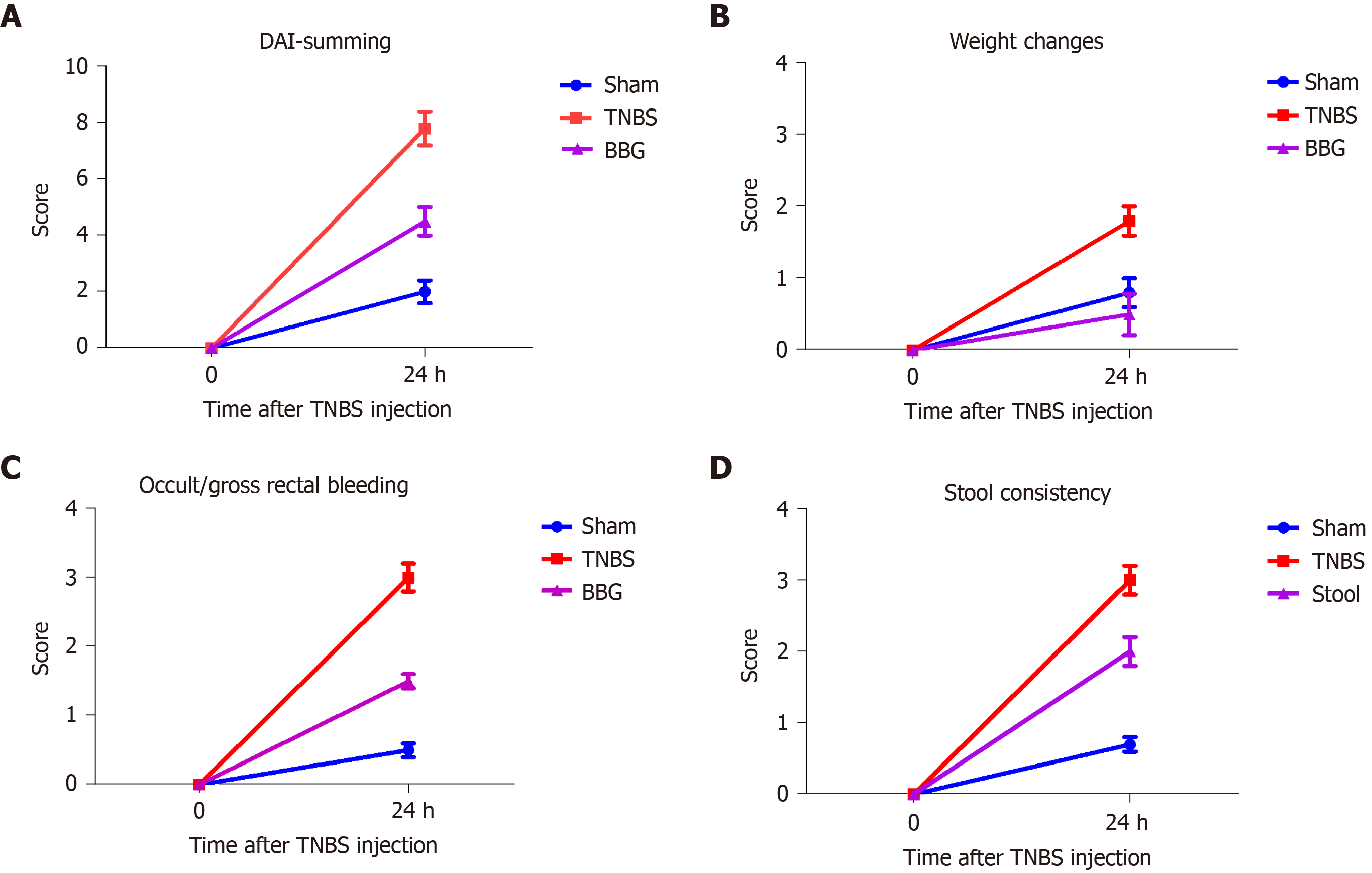
The scoring of the disease activity index (DAI) was analyzed in the control, sham and colitis rats. Percentage weight change, stool consistency and/or presence of occult bleeding were examined. The scores were categorized as follows according to the corresponding parameters. Weight change (%) score: 0 = 1%, 1 = 1%-5%, 2 = 5%-10%, 3 = 10%-15%, and 4 = > 15%. Stool consistency (%) score: 0 = normala (well-formed pellets), 1 = normal, 2 = loose stoolb (pasty and semiformed stools that do not stick to the anus), 3 = loose stool, and 4 = diarrheac (liquid stools that stick to the anus). Occult/gross rectal bleeding: 0 = normal, 1 = occult blood +, 2 = occult blood ++, 3 = occult blood +++, and 4 = gross bleeding. The disease activity index was calculated by summing the score parameters[7,33,34].
For immunohistochemistry, fresh segments of the ileum were dissected and placed in PBS containing nicardipine (10-6 mol/L, Sigma, United States) to inhibit tissue contraction. The segments were opened along the mesenteric border and cleaned with PBS. The tissues were then placed mucosal side down onto a sheet of balsa wood and fixed overnight at 4 °C with 4% paraformaldehyde in sodium phosphate buffer 0.2 mol/L (pH 7.3). The next day, the tissue was cleared of fixative with three 10-min washes in 100% dimethyl sulfoxide (DMSO), followed by three 10-min washes in PBS. All tissue was stored at 4 °C in PBS containing sodium azide (0.1%). The tissue collection was performed by the same researcher who placed the tissue onto the balsa board to be fixed (see material and methods). The processing maintained the same stretch between preparations.
The fixed tissue was subdissected to remove the mucosal and circular layers, producing only the longitudinal muscle layer with the myenteric plexus. For immunohistochemistry, the myenteric plexus of the ileum was preincubated with 10% normal horse serum in PBS containing 1.5% Triton X-100 for 45 min at room temperature. The antibodies used in this study are listed in Table 1. Double labeling was achieved using combinations of the antisera indicated in Table 1. After incubation with primary antisera, tissues were washed three times for 10 min each time in PBS and incubated with various secondary antibodies (Table 1). The PBS washes were repeated, and the tissue was mounted in buffered glycerol with 0.5 mol/L sodium carbonate (pH 8.6).
| Antigen | Host | Dilution | Source |
| P2X7 receptor | Rabbit | 1:200 | Millipore (AB5246) |
| nNOS | Sheep | 1:2000 | Millipore (AB1529) |
| ChAT | Goat | 1:50 | Chemicon (AB144P) |
| Anti-HuC/D | Mouse | 1:100 | Molecular probes (A-21271) |
| GFAP | Rabbit | 1:400 | DAKO (Z0334) |
| GFAP | Mouse | 1:200 | Sigma (G3893) |
| Secondary antibodies | |||
| Donkey anti-rabbit IgG 488 | 1:500 | Molecular probes (A21206) | |
| Donkey anti-sheep IgG 594 | 1:100 | Molecular probes (A11016) | |
| Donkey anti-mouse IgG 594 | 1:200 | Molecular probes (A21203) | |
The stained tissue specimens were examined using a Nikon 80i fluorescent microscope. The images were captured using a digital camera and the NIS Nikon software package. Additionally, the tissue specimens were analyzed using confocal microscopy on a Zeiss confocal scanning laser system installed on a Zeiss Axioplan 2 microscope. Images were taken at 512 × 512 pixels, and the thickness of each optical section was 0.5 µm. Z-stacks of ir cells were captured as a series of optical sections with a center spacing of 0.2 µm. The confocal images were collected using LSM 5 Image Zeiss processing software and were further processed using Corel Photo Paint and Corel Draw software.
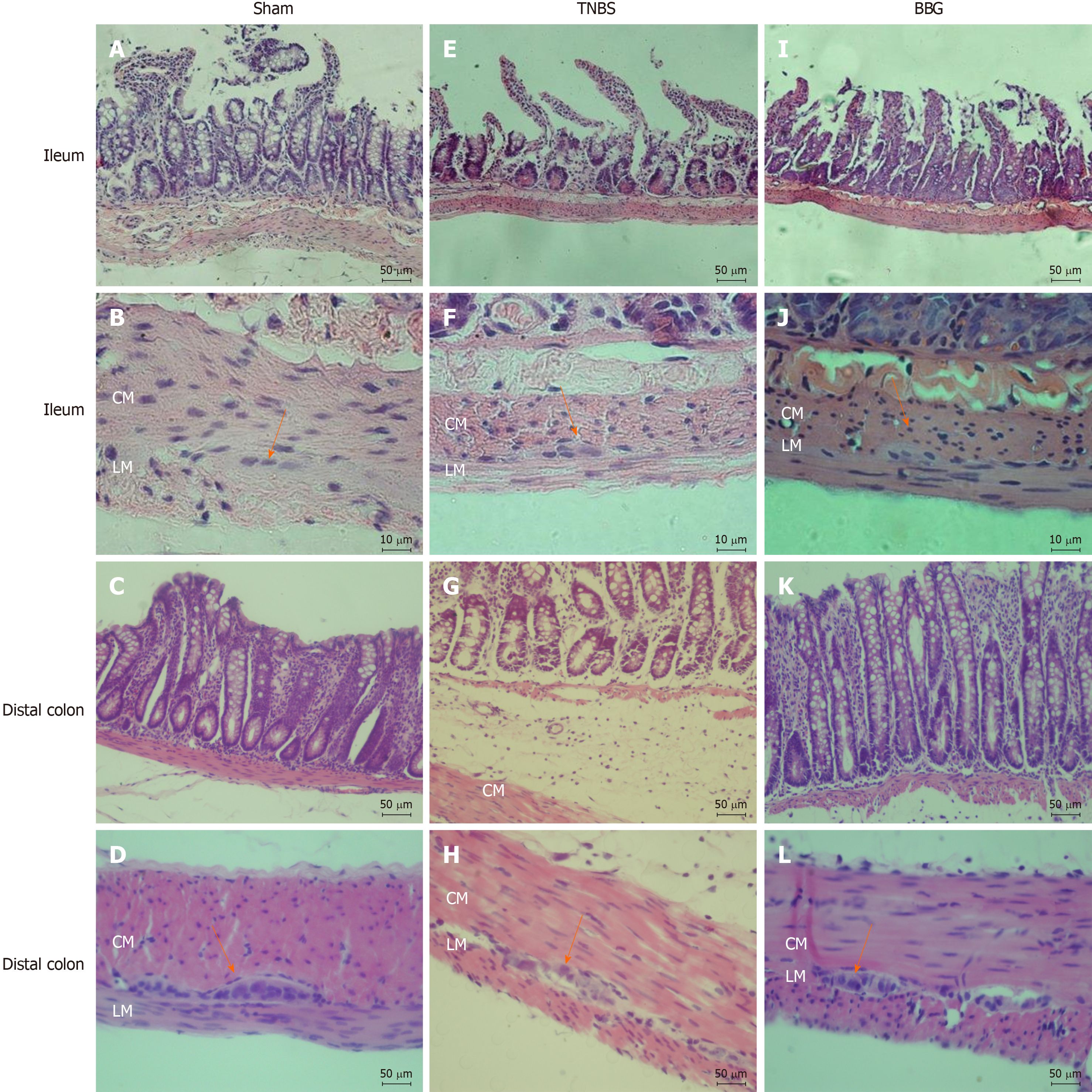
Samples of ileum and distal colon from the sham (n = 3), TNBS (n = 3) and BBG (n = 3) groups were washed in PBS, opened at the mesenteric border, placed on balsa wood and fixed in 4% paraformaldehyde for 48 h. The tissues were treated in increasing concentrations of alcohol, cleared in xylene and embedded in Paraplast Plus® (Sigma). The tissues were cut (5 mm) and stained with hematoxylin-eosin (HE). Qualitative analysis was performed to observe changes caused by experimental ulcerative colitis. For analyzes it was used a Nikon 80i microscope coupled to a camera with NIS-Elements AR 3.1 (Nikon) software.
The analyzes were also done by double marking the membrane preparations on the Nikon 80i fluorescence microscope. First, the neurons were located by the presence of the fluorophore that marks an antigen and then the filter was changed to determine whether or not the neuron was marked by a second antigen, located by a second fluorophore of a different color. The cohort size was 100 neurons, and the data were collected from preparations obtained from five animals. The percentages of double-ir neurons were calculated and expressed as the mean ± SE (n = number of preparations). In total, 100 neurons and 100 enteric glial cells from each membrane preparation were analyzed from each of the sham (n = 5), TNBS (n = 5) and BBG (n = 5) groups[7,29]. The density of neurons (neurons/cm2) ir for P2X7, nNOS, ChAT, anti-HuC/D (pan-neuronal marker) and GFAP (pan-glial cells) as well as the neuronal morphological profiles was measured by analyzing all of the samples at 100 × magnification. Counts were made in 40 microscopic fields (0.000379 cm2) for each antigen in a zig-zag pattern to avoid counting the same area more than once for each antigen in each animal, and a total of 200 microscopic fields were analyzed per immunoreactivity. Cell profile areas (µm2) were obtained for 100 randomly selected neurons in two whole-mount preparations per animal per nNOS, ChAT, anti-HuC/D immunoreactivity assay from 5 rats for each group. A total of 500 neurons per group were analyzed using a Nikon 80i microscope coupled to a camera with NIS-Elements AR 3.1 (Nikon) software and were measured using Image-Pro Plus software version 4.1.0.0. Data were compared by analysis of variance (ANOVA) and Tukey’s test for multiple comparisons, as appropriate. P < 0.05 was considered statistically significant.
On histological analysis, the ileum showed no lesions and had a normal appearance in the sham, TNBS and BBG groups (Table 2). However, the histological observations showed that in distal colon the edema and inflammatory cell infiltration in the TNBS group. The mucosa, the circular and longitudinal muscles and the distal colon enteric neurons in the Sham and BBG groups were preserved. Additionally, the microscopic scores did not indicate ulcerations, edema or inflammatory cell infiltration in the ileums of all groups (Table 2).
| Variables | Sham | TNBS | BBG |
| Macroscopic score | 0 | 0 | 0 |
| Microscopic score | |||
| Ulcerations | 0 | 0 | 0 |
| Edema (submucosa) | 0 | 0 | 0 |
| Inflammatory cell infiltration | 0 | 0 | 0 |
The DAI showed changes in weight (%), stool consistency (%) and occult/gross rectal bleeding (%) in the sham, TNBS and BBG groups (Figure 1). The results show that there was an increase in DAI scores, stool consistency and occult bleeding in the TNBS group and a recovery in the BBG group. Histological studies revealed that the mucosa, lamina propria and submucosal ganglia in all groups were not affected (Figure 2).
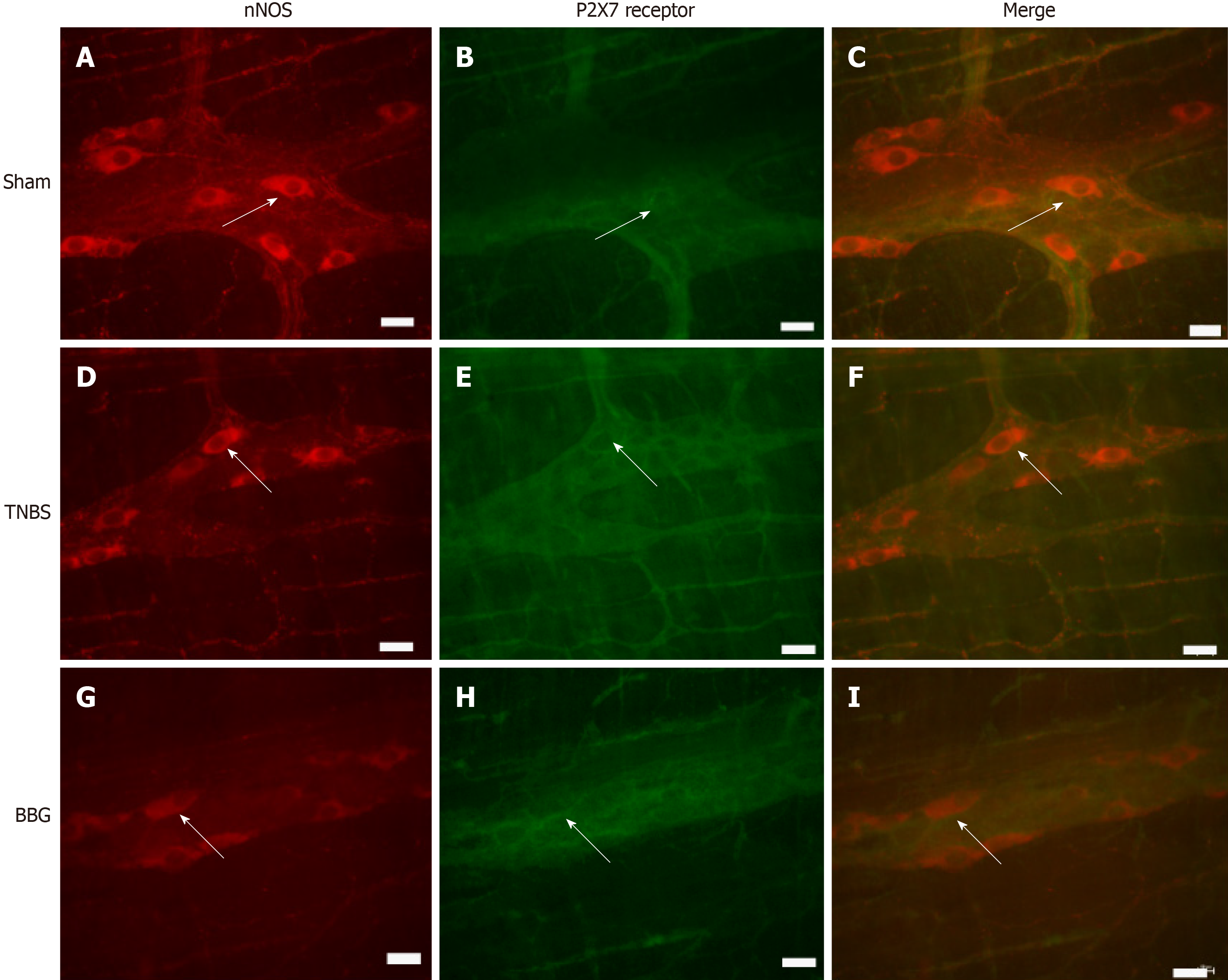
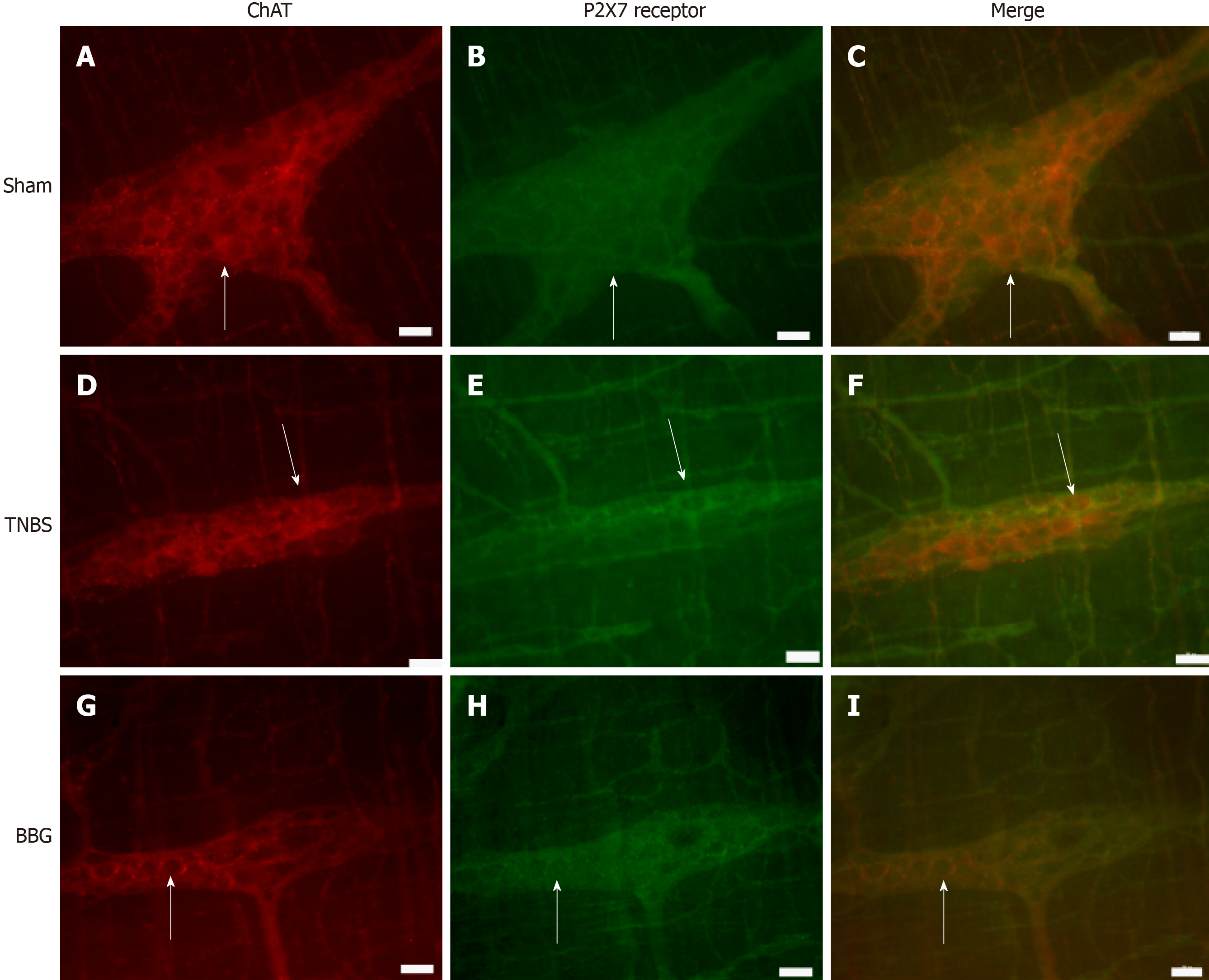
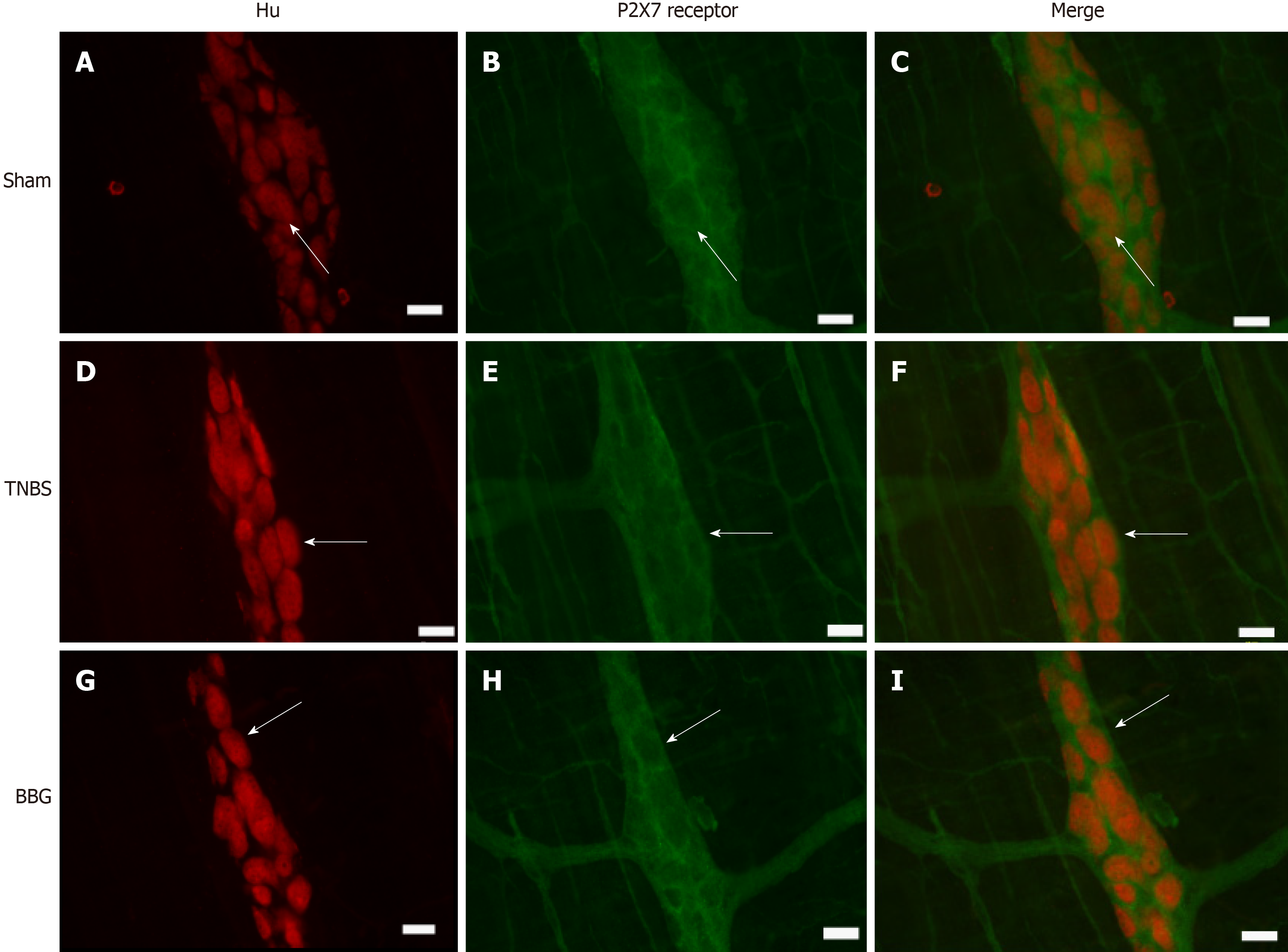
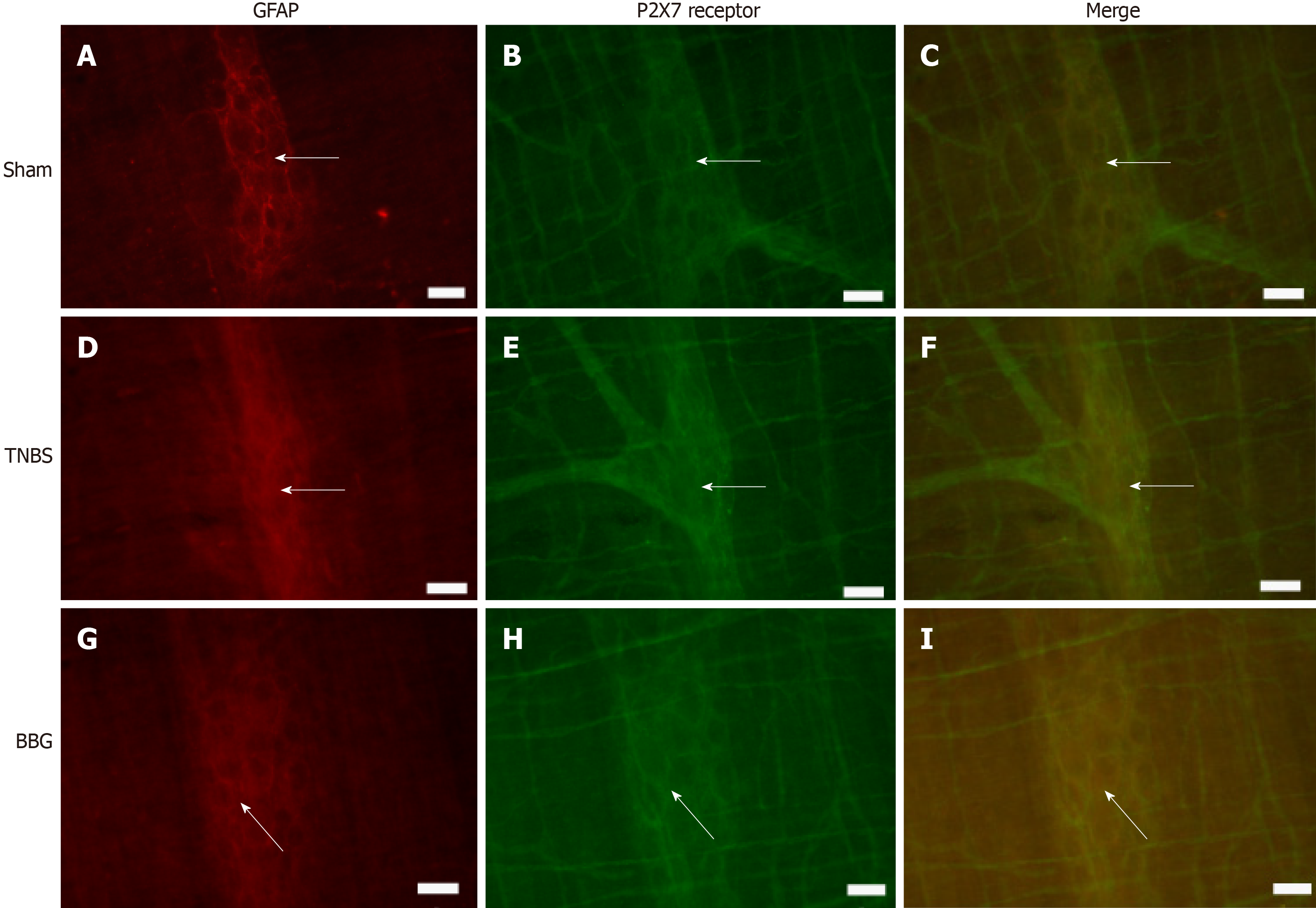
Immunohistochemical analysis showed that the P2X7 receptor was present in the myenteric neurons in the sham, TNBS and BBG groups. P2X7 receptor-ir neurons were labeled for HuC/D, nNOS, ChAT and GFAP in all groups studied (Figures 3, 4, 5 and 6). The P2X7 receptor immunoreactivity colocalized 100% with neurons positive for HuC/D, nNOS, ChAT and GFAP in all groups.
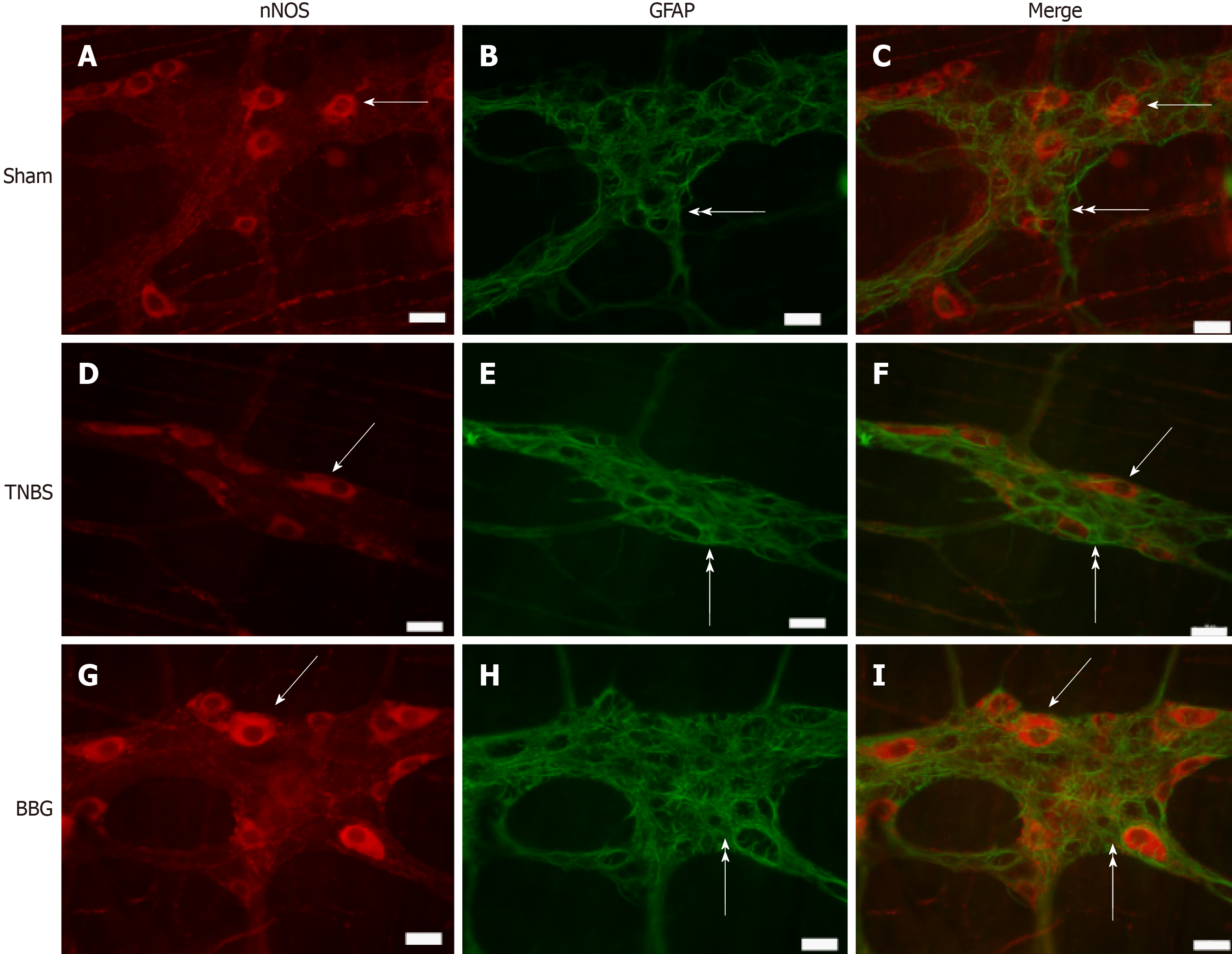
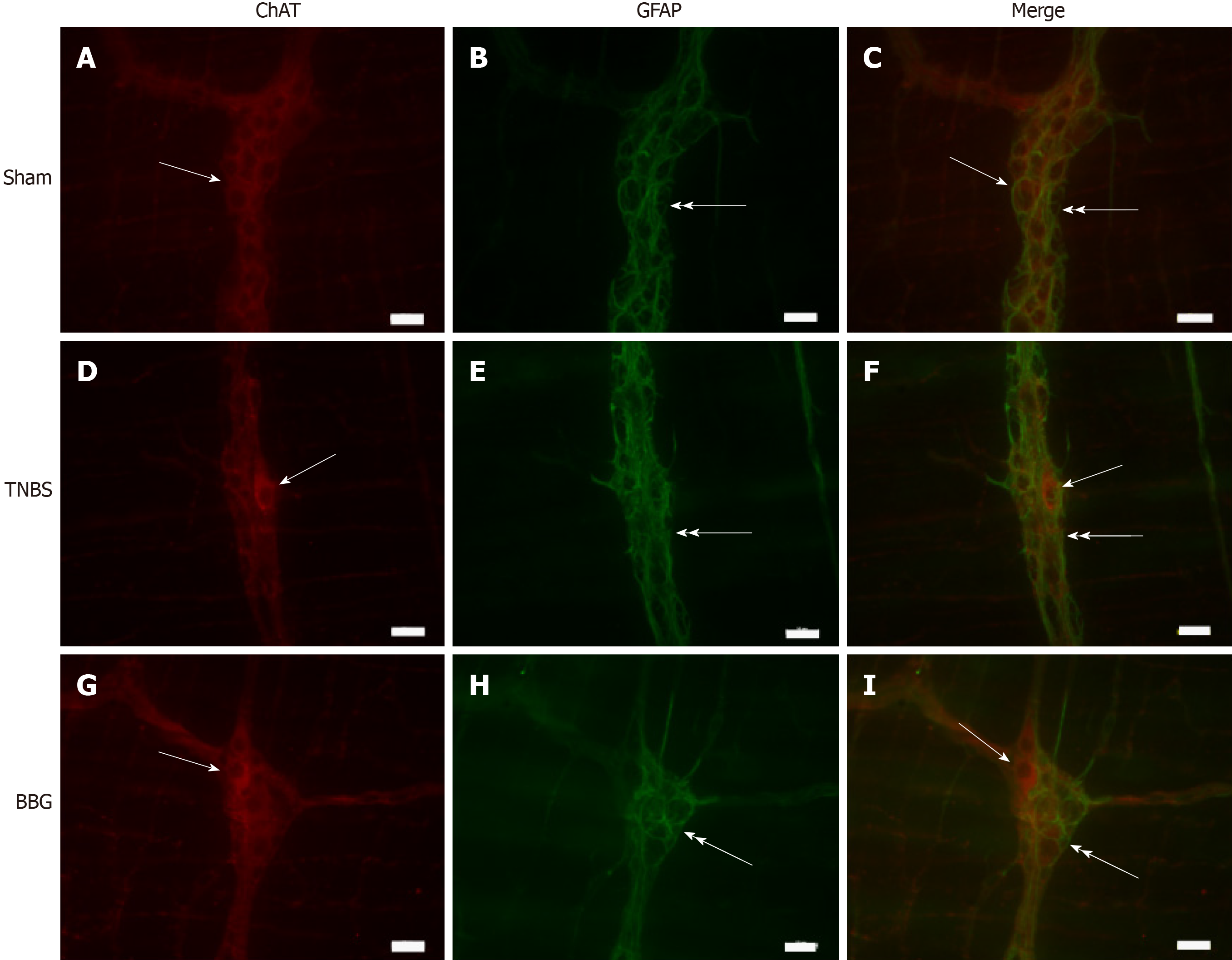
GFAP-positive glial cells were observed close to ChAT- and nNOS-immunoreactive neurons (Figures 7 and 8).
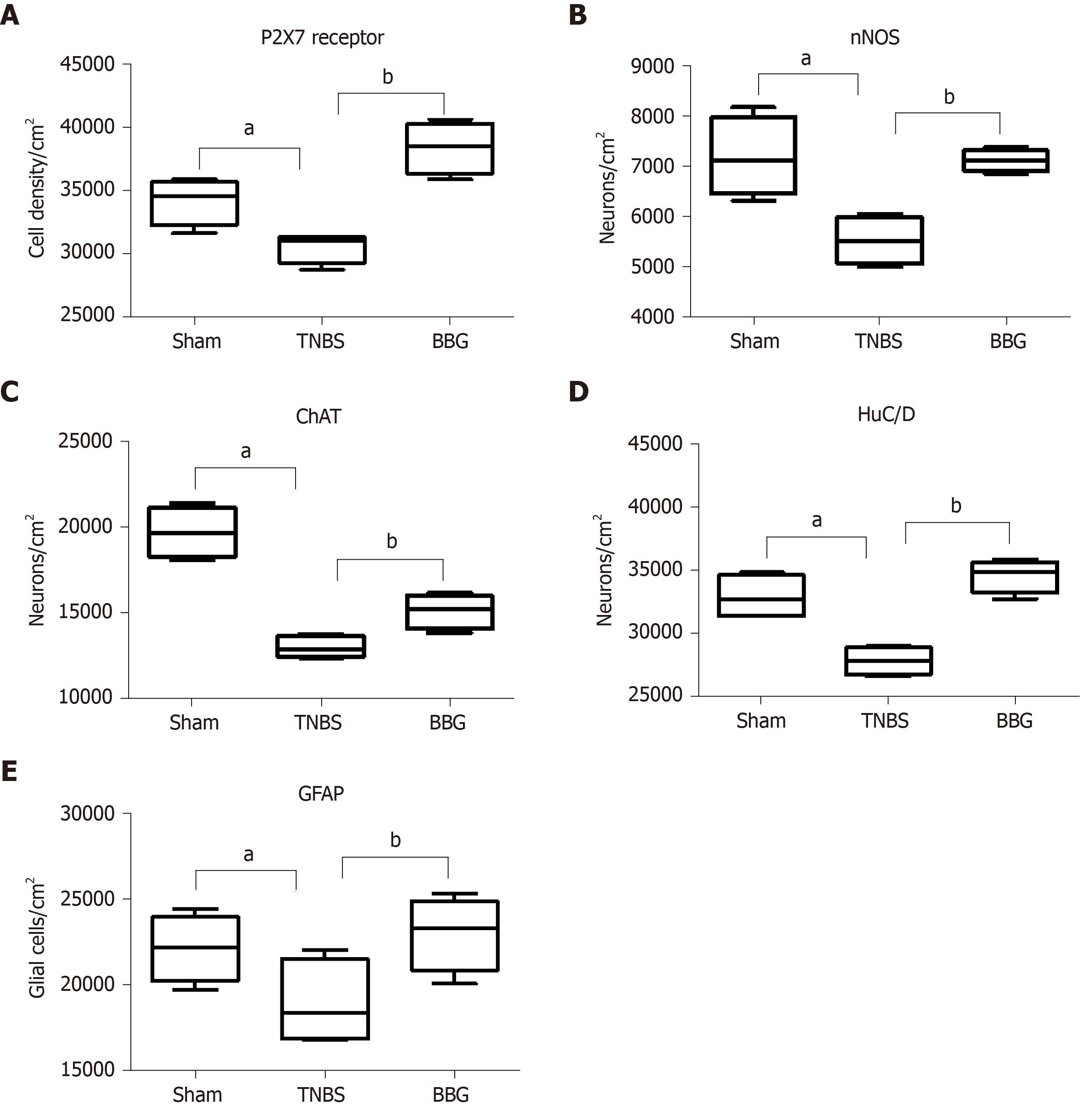
P2X7 receptor immunoreactivity per area of neurons decreased in the TNBS group by 10.6% compared to that in the sham group (P < 0.05). There was an increase of 20.4% in the BBG group compared to the TNBS group (P < 0.01) (Figure 9A).
nNOS-positive neurons per area decreased by 22.9% in the TNBS group compared to the sham group (P < 0.05). An increase of 22.2% was observed in the BBG group compared to the TNBS group (P < 0.01) (Figure 9B).
The ChAT-immunoreactive neurons per cm2 were reduced by 34.0% in the TNBS group compared to the sham group (P < 0.05), and they were increased by 13.9% in the BBG group compared to the TNBS group (P < 0.01) (Figure 9C).
HuC/D-immunoreactive neurons per area reduced by 15.4% in the TNBS group compared to the sham group (P < 0.05). Furthermore, there was an increase of 19.5% in the BBG group compared to the TNBS group (P < 0.01) (Figure 9D).
The GFAP-positive enteric glial cells per cm2 reduced by 14.4% in the TNBS group compared to the sham group (P < 0.05), and there was an increase of 17.7% in enteric glia in the BBG group compared to the TNBS group (P < 0.01) (Figure 9E).
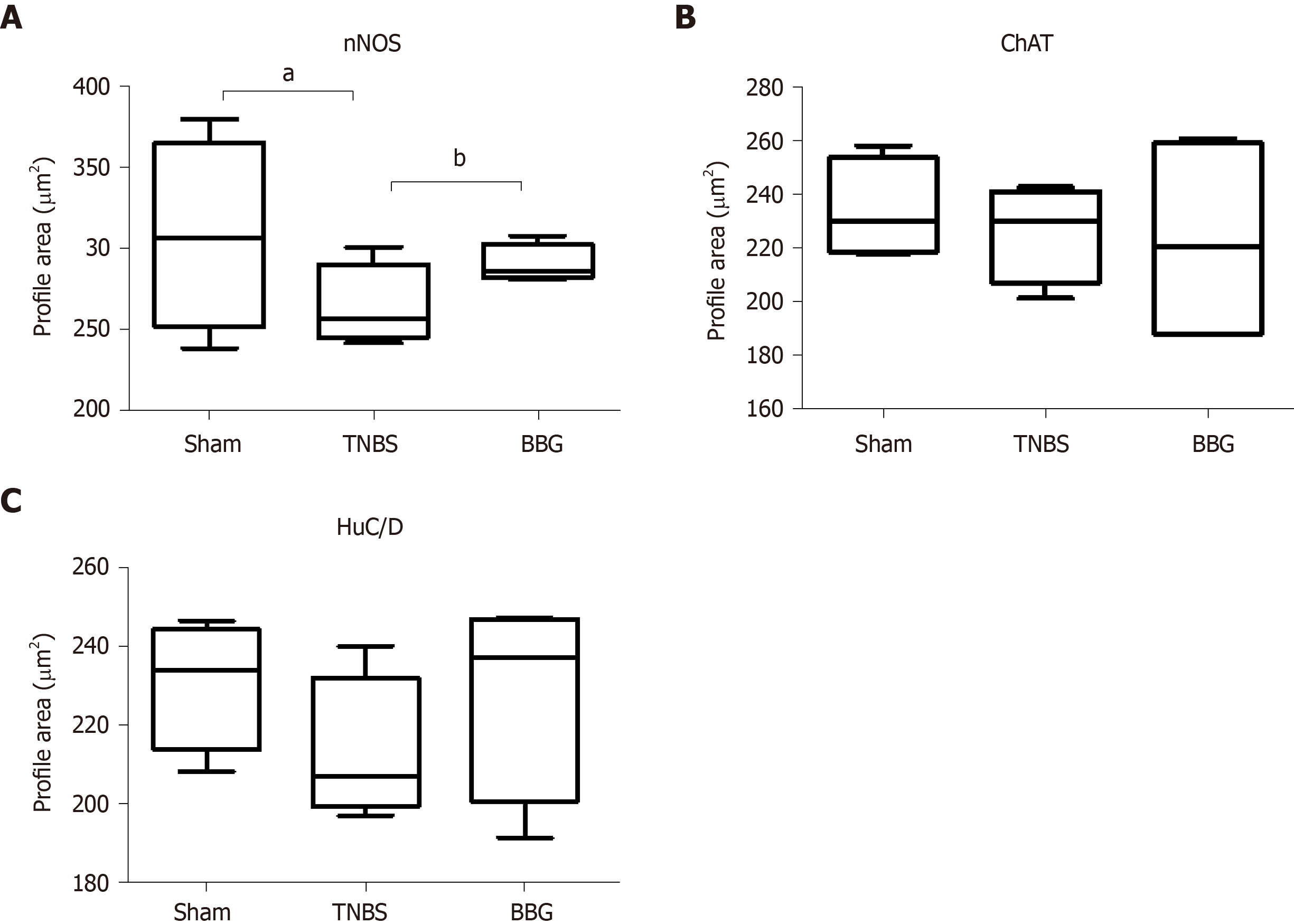
Regarding neuronal profile area, the nNOS profile area decreased by 12% in the TNBS group compared to the sham group (P < 0.05), and an increase of 8% was observed in the BBG group compared to the TNBS group (P < 0.05) (Figure 10A). No differences were observed between the ChAT- and HuC/D neuronal profile areas of the studied groups. Due to colocalization, the profile areas of P2X7-positive nerve cells in the myenteric plexus were not quantified (Figure 10).
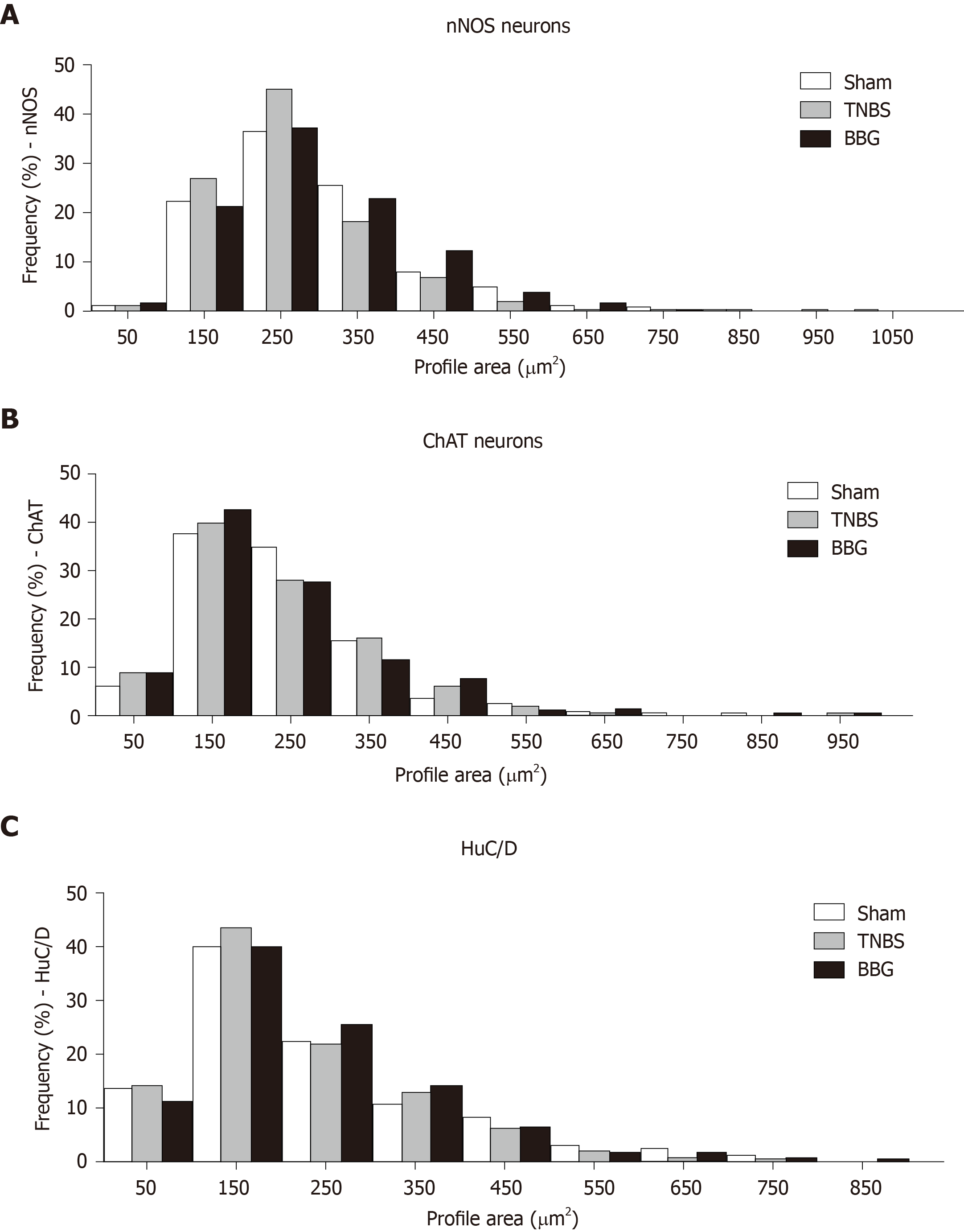
The distribution of nNOS-positive neurons showed that the size ranged from 50 μm2 to 1050 μm2 and that 22% to 25% of neurons were between 150 μm2 and 350 μm2 in the sham, TNBS and TNBS groups (Figure 11A).
The immunoreactive ChAT neurons demonstrated that the range size ranged from 50 μm2 to 950 μm2, with 27% to 37% being between 150 μm2 and 250 μm2 in all groups (Figure 11B).
The distribution of HuC/D-positive neurons showed that the size ranged from 50 μm2 to 850 μm2, with 21% to 43% between 150 μm2 and 250 μm2 in all groups (Figure 11C).
The experimental ulcerative colitis model affected ileum myenteric plexus neurons, and these neurons recovered with the use of BBG. The colitis model established by injecting TNBS in ethanol solution is considered effective and is widely used in the literature to produce experimental ulcerative colitis[10,35]. From the DAI, it was possible to observe that the experimental ulcerative colitis affected the weight, change in stool consistency and occult/gross rectal bleeding, and the reduction in these parameters showed an improvement in the condition of the group treated with BBG.
In our work, macroscopic and microscopic analysis of the ileum did not show that the mucosa or submucosa were affected. However, the literature has shown that experimental ulcerative colitis presents superficial inflammation, limited to mucosal and submucosal regions in the distal colon[6,7,36].
Enteric neurons of the distal colon are affected by experimental ulcerative colitis and Crohn’s disease[37-39]. In our study, we observed a decrease in nNOS, ChAT and HuC/D-positive neurons in the ileum, thus demonstrating that experimental colitis in the distal colon may affect neurons in locations distant from the origin of the lesion.
Immunohistochemical studies have demonstrated the expression of P2X7 receptors in the SNE[6,7,22]. Gulbransen et al[40] observed activation of P2X7 receptors during colitis. In our study, we observed P2X7 receptor immunoreactivity in ileum myenteric plexus cells in all groups. We also observed a reduction in the number of P2X7 receptor immunoreactive cells in the TNBS group compared to that in the sham group and recovery of the neurons in the BBG group. da Silva et al[6,7] observed a decrease in P2X7 receptor-positive cells in the distal colon following experimental ulcerative colitis.
Purinergic mechanisms may be involved in the etiology of many conditions that affect the nervous system, due the large extracellular release of ATP[41]. Changes in purinergic receptor expression in neurons are observed in neuronal maturation, differentiation, acute CNS injuries, such as hypoxia ischemia, mechanical stress and inflammation.
It was observed that experimental ulcerative colitis affected neuronal classes in the ileum. In this study, a decrease in nNOS-, ChAT- and HuC/D-immunoreactive neurons per µm2 was observed, and the recovery of these neurons per µm2 was observed with BBG treatment. It has been observed that several classes of enteric neurons are affected in Crohn's disease and experimental ulcerative colitis[8,38,39,42]. Studies have demonstrated that ischemia and reperfusion decrease the number of enteric neurons[23] and treatment with BBG, a P2X7 antagonist, recovers rat enteric neurons[29]. Additionally, transient receptor potential channel vanilloid 2 (TRPV2) and the release of nitric oxide (NO) are related to intestinal motility[43].
Enteric glial cells have different functions in the face of gastrointestinal disorders[44,45]. In our study, we observed a decrease in GFAP immunoreactive glia in the ileum of the TNBS group compared to that in the sham group and recovery in the BBG group.
The evaluation of morphometric changes (measurement of cell profile area) has been widely studied in several experimental protocols. In the present work, the analysis of the profile of the nNOS-, ChAT- and HuC/D-ir neurons was performed to determine changes in the profile areas in these neuronal classes. However, only nitrergic neurons of the TNBS group showed a decrease, and those treated with BBG demonstrated recovery. The increased neuronal profile area could be explained as a compensatory mechanism due to neuronal death in the TNBS group[6,29].
The literature elucidates the neuroprotective role of P2X7 receptor blockade, as well as a possible increase in the expression of anti-inflammatory factors IL-10 and TGF-β1 by KO P2X7 mice, expressly helping to control inflammation[27,29,46].
Studies have described that gastrointestinal epithelia release ATP and Transient Receptor Potential Vanilloid 4 (TRPV4) is expressed throughout the gastrointestinal epithelia[47].
The importance of this work is to demonstrate that experimental ulcerative colitis affects distant organs such as ileum myenteric neurons that express the P2X7 receptor. In addition, BBG treatment was shown to be effective in the recovery of ileum myenteric neurons, thus demonstrating that the P2X7 receptor may be a possible therapeutic target in the treatment of the effects of experimental ulcerative colitis. Also, has been described that the expansion of the inflammatory process from the distal neck to the distal ileum.
The enteric nervous system performs functions in gastrointestinal tract such as motility, control of gastric acid secretion, regulation of fluid movement through the epithelium. This system has two ganglionic plexuses, the myenteric plexus and the submucosal plexus. Inflammatory bowel diseases (IBDs) are disorders that include ulcerative colitis and Crohn's disease. In experimental ulcerative colitis, there are changes in enteric neurons. The P2X7 receptor has been described in the ENS.
Studies have demonstrated that P2X7 antagonist, brilliant blue G (BBG) recovers neurons following injuries.
The topics of this work were to analyze the effects of experimental ulcerative colitis in enteric neurons and enteric glial cells in the ileum in animals treated with P2X7 antagonist (BBG).
The rats were anesthetized with a mixture of xylazine (20 mg/kg) and ketamine (100 mg/kg) administered subcutaneously. Inflammation was induced through the intrarectal insertion of a polypropylene 8 cm cannula. 2,4,6-trinitrobenzene sulfonic acid (TNBS, Sigma, Saint Louis, United States) was injected at a dose of 30 mg/kg in 600 μL of 30% ethanol in the colon lumen (n = 5). Sham animals (n = 5) were injected with vehicle. BBG (50 mg/kg, Sigma Aldrich, United Kingdom, n = 5) or saline was injected 1 h following TNBS injection (n = 5). The survival time after colitis induction was 24 h. For immunohistochemistry, fresh segments of the ileum were dissected after fixed. Double labeling has been done of P2X7 receptor with neuronal nitric oxide synthase (nNOS), choline acetyltransferase (ChAT), and HuC/D (a pan-neuronal marker) and enteric glial cells immunoreactive for glial fibrillary acidic protein (GFAP). The stained tissue specimens were examined using a Nikon 80i fluorescent and Confocal microscope. The counting of the neurons per area and glial cell were done in fluorescent microscope.
The numbers of nNOS-, ChAT-, HuC/D- immunoreactive (ir) neurons and GFAP-ir glial cells were decreased in the TNBS group and recovered in the BBG group. The neuronal profile area (μm2) demonstrated that nNOS-ir neurons decreased in the TNBS group and recovered in the BBG group. There were no differences in the profile areas of ChAT- and HuC/D-ir neurons. Our data conclude that ileum myenteric neurons and glial cells were affected by ulcerative colitis and that treatment with BBG had a neuroprotective effect. Thus, these results demonstrate that the P2X7 receptor may be an important target in therapeutic strategies.
Ileum myenteric neurons and glial cells were affected by experimental ulcerative colitis and that treatment with P2X7 receptor antagonist, BBG had a neuroprotective effect. The results demonstrate that the P2X7 receptor may be an important target in therapeutic strategies. P2X7 receptor may be a possible therapeutic target in the treatment of the effects of experimental ulcerative colitis Ileum myenteric neurons and glial cells were affected by experimental ulcerative colitis and treatment with BBG may recover enteric neurons. P2X7 receptor may be a possible therapeutic target in the treatment of the experimental ulcerative colitis. Injection of BBG (50 mg/kg, Sigma Aldrich, United Kingdom) for experimental ulcerative colitis and effects in the distal ileum. Inflammation was induced through the intrarectal insertion of a polypropylene 8 cm cannula. 2,4,6-trinitrobenzene sulfonic acid (TNBS, Sigma, Saint Louis, United States) was injected at a dose of 30 mg/kg in 600 μL of 30% ethanol in the colon lumen. There was affected the distal ileum. Additionally, injection of BBG recover enteric neurons distal ileum. Studies show that BBG is a P2X7 antagonist, and its low toxicity and high selectivity make this compound an ideal candidate to block the adverse effects of P2X7 receptor activation. BBG treatment was shown to be effective in the recovery of ileum myenteric neurons, thus demonstrating that the P2X7 receptor may be a possible therapeutic target in the treatment of the effects of experimental ulcerative colitis.
Study of effects of the experimental ulcerative colitis in the ileum and may use of the P2X7 receptor for therapeutic target. Additionally, study effects of BBG in the distal colon following experimental ulcerative colitis. The direction of the future research will be study effects of the experimental ulcerative colitis of myenteric neurons in the P2X7 receptor-deficient animals. The best method will be use P2X7 receptor-deficient animals.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Mihara H, Yamamoto S S-Editor: Wang YQ L-Editor: A E-Editor: Qi LL
| 1. | Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 1134] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 2. | Furness JB. The Enteric Nervous System. MA: Blackwell Publishing, 2006. |
| 3. | Gabella G. Neuron size and number in the myenteric plexus of the newborn and adult rat. J Anat. 1971;109:81-95. [PubMed] |
| 4. | Bassotti G, Villanacci V, Fisogni S, Rossi E, Baronio P, Clerici C, Maurer CA, Cathomas G, Antonelli E. Enteric glial cells and their role in gastrointestinal motor abnormalities: introducing the neuro-gliopathies. World J Gastroenterol. 2007;13:4035-4041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Kawada M, Arihiro A, Mizoguchi E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J Gastroenterol. 2007;13:5581-5593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 128] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | da Silva MV, Marosti AR, Mendes CE, Palombit K, Castelucci P. Submucosal neurons and enteric glial cells expressing the P2X7 receptor in rat experimental colitis. Acta Histochem. 2017;119:481-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | da Silva MV, Marosti AR, Mendes CE, Palombit K, Castelucci P. Differential effects of experimental ulcerative colitis on P2X7 receptor expression in enteric neurons. Histochem Cell Biol. 2015;143:171-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Linden DR, Couvrette JM, Ciolino A, McQuoid C, Blaszyk H, Sharkey KA, Mawe GM. Indiscriminate loss of myenteric neurones in the TNBS-inflamed guinea-pig distal colon. Neurogastroenterol Motil. 2005;17:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Neunlist M, Aubert P, Toquet C, Oreshkova T, Barouk J, Lehur PA, Schemann M, Galmiche JP. Changes in chemical coding of myenteric neurones in ulcerative colitis. Gut. 2003;52:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Poli E, Lazzaretti M, Grandi D, Pozzoli C, Coruzzi G. Morphological and functional alterations of the myenteric plexus in rats with TNBS-induced colitis. Neurochem Res. 2001;26:1085-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | McCready FJ, Bargen JA. Involvement of the ileum in chronic ulcerative colitis. N Engl J Med. 1949;240:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 44] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Antonioli L, Blandizzi C, Giron MC. Enteric purinergic signaling: Shaping the "brain in the gut". Neuropharmacology. 2015;95:477-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Antonioli L, Colucci R, Pellegrini C, Giustarini G, Tuccori M, Blandizzi C, Fornai M. The role of purinergic pathways in the pathophysiology of gut diseases: pharmacological modulation and potential therapeutic applications. Pharmacol Ther. 2013;139:157-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Antonioli L, Giron MC, Colucci R, Pellegrini C, Sacco D, Caputi V, Orso G, Tuccori M, Scarpignato C, Blandizzi C, Fornai M. Involvement of the P2X7 purinergic receptor in colonic motor dysfunction associated with bowel inflammation in rats. PLoS One. 2014;9:e116253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 483] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 16. | Burnstock G, Straub RW, Bolis L. A basis for distinguishing two types of purinergic receptor. In: Straub RW, Bolis L. Cell membrane receptors for drugs and hormones: a multidiciplinary aproach. Straub RW, Bolis L. New York: Raven Press, 1978: 107-118. |
| 17. | Burnstock G. The journey to establish purinergic signalling in the gut. Neurogastroenterol Motil. 2008;20 Suppl 1:8-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 569] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 19. | Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 639] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 20. | Di Virgilio F, Chiozzi P, Falzoni S, Ferrari D, Sanz JM, Venketaraman V, Baricordi OR. Cytolytic P2X purinoceptors. Cell Death Differ. 1998;5:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 215] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Volonté C, D'Ambrosi N. Membrane compartments and purinergic signalling: the purinome, a complex interplay among ligands, degrading enzymes, receptors and transporters. FEBS J. 2009;276:318-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Hu HZ, Gao N, Lin Z, Gao C, Liu S, Ren J, Xia Y, Wood JD. P2X(7) receptors in the enteric nervous system of guinea-pig small intestine. J Comp Neurol. 2001;440:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Palombit K, Mendes CE, Tavares-de-Lima W, Silveira MP, Castelucci P. Effects of ischemia and reperfusion on subpopulations of rat enteric neurons expressing the P2X7 receptor. Dig Dis Sci. 2013;58:3429-3439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Remy M, Thaler S, Schumann RG, May CA, Fiedorowicz M, Schuettauf F, Grüterich M, Priglinger SG, Nentwich MM, Kampik A, Haritoglou C. An in vivo evaluation of Brilliant Blue G in animals and humans. Br J Ophthalmol. 2008;92:1142-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Burnstock G, Volonté C. Pharmacology and therapeutic activity of purinergic drugs for disorders of the nervous system. CNS Neurol Disord Drug Targets. 2012;11:649-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol Pharmacol. 2000;58:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 326] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 27. | Figliuolo VR, Savio LEB, Safya H, Nanini H, Bernardazzi C, Abalo A, de Souza HSP, Kanellopoulos J, Bobé P, Coutinho CMLM, Coutinho-Silva R. P2X7 receptor promotes intestinal inflammation in chemically induced colitis and triggers death of mucosal regulatory T cells. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1183-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci USA. 2009;106:12489-12493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 346] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 29. | Palombit K, Mendes CE, Tavares-de-Lima W, Barreto-Chaves ML, Castelucci P. Blockage of the P2X7 Receptor Attenuates Harmful Changes Produced by Ischemia and Reperfusion in the Myenteric Plexus. Dig Dis Sci. 2019;64:1815-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Bell CJ, Gall DG, Wallace JL. Disruption of colonic electrolyte transport in experimental colitis. Am J Physiol. 1995;268:G622-G630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Erdogan B, Isiksoy S, Dundar E, Pasaoglu O, Bal C. The effects of sodium phosphate and polyethylene glycol-electrolyte bowel preparation solutions on 2,4,6-trinitrobenzenesulfonic acid-induced colitis in the rat. Exp Toxicol Pathol. 2003;55:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Fabia R, Ar'Rajab A, Johansson ML, Willén R, Andersson R, Molin G, Bengmark S. The effect of exogenous administration of Lactobacillus reuteri R2LC and oat fiber on acetic acid-induced colitis in the rat. Scand J Gastroenterol. 1993;28:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 119] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-249. [PubMed] |
| 34. | Nooh HZ, Nour-Eldien NM. The dual anti-inflammatory and antioxidant activities of natural honey promote cell proliferation and neural regeneration in a rat model of colitis. Acta Histochem. 2016;118:588-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Jacobson K, McHugh K, Collins SM. The mechanism of altered neural function in a rat model of acute colitis. Gastroenterology. 1997;112:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Elson CO, Sartor RB, Targan SR, Sandborn WJ. Challenges in IBD Research: updating the scientific agendas. Inflamm Bowel Dis. 2003;9:137-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Sanovic S, Lamb DP, Blennerhassett MG. Damage to the enteric nervous system in experimental colitis. Am J Pathol. 1999;155:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 148] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Boyer L, Ghoreishi M, Templeman V, Vallance BA, Buchan AM, Jevon G, Jacobson K. Myenteric plexus injury and apoptosis in experimental colitis. Auton Neurosci. 2005;117:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Winston JH, Li Q, Sarna SK. Paradoxical regulation of ChAT and nNOS expression in animal models of Crohn's colitis and ulcerative colitis. Am J Physiol Gastrointest Liver Physiol. 2013;305:G295-G302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med. 2012;18:600-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 358] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 41. | Franke H, Krügel U, Illes P. P2 receptors and neuronal injury. Pflugers Arch. 2006;452:622-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Geboes K, Collins S. Structural abnormalities of the nervous system in Crohn's disease and ulcerative colitis. Neurogastroenterol Motil. 1998;10:189-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 171] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 43. | Mihara H, Boudaka A, Shibasaki K, Yamanaka A, Sugiyama T, Tominaga M. Involvement of TRPV2 activation in intestinal movement through nitric oxide production in mice. J Neurosci. 2010;30:16536-16544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 44. | Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2012;9:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 293] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 45. | Gulbransen BD, Christofi FL. Are We Close to Targeting Enteric Glia in Gastrointestinal Diseases and Motility Disorders? Gastroenterology. 2018;155:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Hofman P, Cherfils-Vicini J, Bazin M, Ilie M, Juhel T, Hébuterne X, Gilson E, Schmid-Alliana A, Boyer O, Adriouch S, Vouret-Craviari V. Genetic and pharmacological inactivation of the purinergic P2RX7 receptor dampens inflammation but increases tumor incidence in a mouse model of colitis-associated cancer. Cancer Res. 2015;75:835-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 47. | Mihara H, Boudaka A, Tominaga M, Sugiyama T. Transient Receptor Potential Vanilloid 4 Regulation of Adenosine Triphosphate Release by the Adenosine Triphosphate Transporter Vesicular Nucleotide Transporter, a Novel Therapeutic Target for Gastrointestinal Baroreception and Chronic Inflammation. Digestion. 2020;101:6-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |